Abstract
Purpose:
To assess the regression rate of conductive keratoplasty (CK) in patients with or without previous laser-assisted in situ keratomileusis (LASIK) or photorefractive keratectomy (PRK).
Setting:
University of Utah, Medical School, John A. Moran Eye Center, Salt Lake City, Utah.
Materials and Methods:
A retrospective, age-matched chart review identified records of 6 patients who underwent CK after refractive surgery and 12 patients who underwent CK without prior refractive surgery. The main outcome measures were postoperative uncorrected and corrected visual acuities and refraction changes over time.
Results:
Preoperatively, the mean manifest refraction spherical equivalent (MRSE) of the 15 eyes (12 patients) that underwent CK without refractive surgery was 0.83 diopters (D) and the 7 eyes (6 patients) that underwent CK after refractive surgery had an average MRSE of 0.27 D. Postoperatively, the mean MRSE of the refractive surgery patients was -0.86 D at 6 months, regressing to -0.67 D at 12 months. The postoperative MRSE in the eyes without refractive surgery was -0.58 D. at 6 months, regressing to -0.38 D at 12 months. The rate of regression was linear in both groups, calculated at 0.033 D per month in all patients.
Conclusions:
Patients with previous LASIK or PRK showed a greater treatment response to CK but regressed at a similar rate as those eyes without prior LASIK or PRK. Overall CK is a safe procedure that inevitably regresses.
Keywords: Conductive Keratoplasty, Laser-assisted in situ Keratomileusis, Overcorrection, Photorefractive Keratectomy, Regression
INTRODUCTION
Conductive keratoplasty (CK) utilizes radiofrequency energy delivered at 8–24 locations based on a patient's refraction to cause localized collagen shrinkage. This steepens the central cornea to correct hyperopic refractive errors up to 3.25 D of hyperopia.1 CK is also a popular option to treat presbyopic patients by correcting the dominant eye for distance vision and the non-dominant eye for near or intermediate vision.2 After the US Food and Drug Administration approval for hyperopia in 2002, it was hoped that CK would become the standard treatment for both presbyopia and hyperopia. However, it became apparent that CK was similar to other methods of hyperopic correction in that regression to pretreatment manifest refraction was observed.1
CK is also effective in correcting refractive surgery-induced hyperopia, especially when a thin residual stromal bed precludes further corneal ablation treatment. Interestingly, CK after refractive surgery requires a reduced number of treatment spots compared to eyes without previous refractive surgery.3,4 In this study, we compare the refractive outcomes and rates of regression in patients that underwent CK after laser-assisted in situ keratomileusis (LASIK) or photorefractive keratectomy (PRK) and a control group of eyes that underwent CK without prior refractive surgery.
MATERIALS AND METHODS
Study design
In a retrospective, single-center study, a chart review was conducted of 52 patients who underwent CK at the John Moran Eye, Department of Ophthalmology and Visual Sciences, University of Utah, between 2004 and 2011. Eligibility requirements for CK to treat hyperopia were patients with +0.75 to +3.25 D of refractive error, with no more than 1.0 D of astigmatism. Patients were eligible for CK treatment of presbyopia if the desired correction was between 1 and 2.25 D to achieve a myopic endpoint of –1.25 to –1.75 D in their nondominant eye.
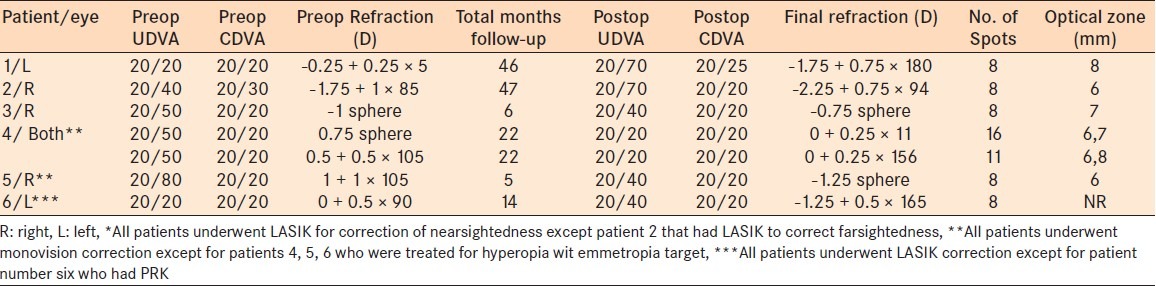
Of the 52 patients, 6 patients (7 eyes) had CK after LASIK or PRK. All patients underwent LASIK for myopic correction except one patient who had LASIK to correct hyperopia [Table 1]. The average time since LASIK surgery was 31 months with a range of 6–90 months. Of the 46 patients who underwent CK without prior refractive surgery, only 12 age-matched controls (15 eyes) had adequate clinic data for analysis [Tables 2 and 3]. Of the refractive surgery patients, four eyes underwent CK to treat hyperopic overcorrection with an emmetropic target and three eyes for monovision correction. Of the patients without refractive surgery, 13 eyes underwent monovision correction and 2 eyes for hyperopia correction with an emmetropia target.
Table 1.
Patient demographics and preoperative refraction of both groups
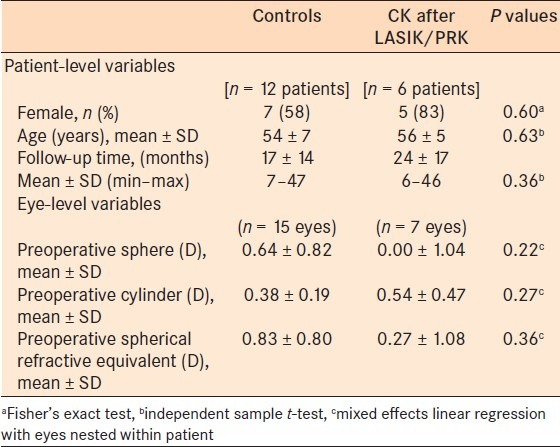
Table 2.
Preoperative and postoperative UDVA, CDVA, and manifest refraction of CK patients without prior refractive surgery
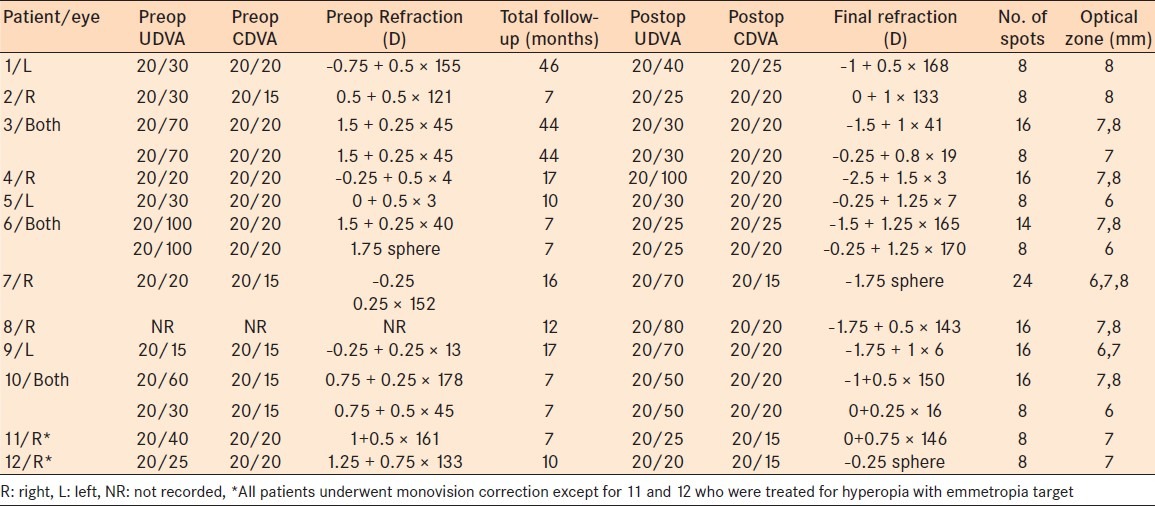
Table 3.
Preoperative and postoperative UDVA, CDVA, and manifest refraction of CK patients with prior refractive surgery
The main outcome measures were postoperative-uncorrected and corrected Snellen visual acuities and refraction changes over time. Complications were recorded from follow-up visit notes.
Surgical procedure
Two surgeons (MM and MDM) performed all CK procedures using a ViewPoint CK system (Refractec, Inc.) and standard parameters (0.6 W, 0.6-s exposure time per spot). The target refraction was myopia or emmetropia. A standardized nomogram was used to determine the number and placement of treatment spots on the cornea, using a light touch technique.2 The light touch technique is a modification of the number of spots, the diameter of the spot placement, and the amount of pressure used by the surgeon.
The postoperative target refraction determined the number of CK treatment spots (8 or 16) and optical zone used (6, 7, or 8 mm). Because the effect of CK is exaggerated in patients who have had previous PRK or LASIK, the intended CK correction was reduced by 30–50% in the post-LASIK/PRK eyes.3,5
Statistical analysis
All statistical comparisons were performed using Stata version 12 statistical software (College Station, TX; StateCorp LP). All reported P values are from a two-sided comparison.
RESULTS
The average age of the patients with LASIK/PRK induced hyperopia was 56 years and the average age of control patients was 54 years. The gender distribution was 5 males and 7 females in the controls and 1 male and 5 females in the refractive surgery group [Table 1]. Prior to refractive surgery, the mean manifest refraction spherical equivalent (MRSE) in the seven eyes with refractive surgery was –3.73 D (range –7.5 to 0.625 D, SD 2.7 D). Prior to CK, the average MRSE of these eyes (post-LASIK or PRK/pre-ck) was 0.27 D (range –1 to 2 D, SD 1.08 D). The 15 control eyes had a preoperative MRSE of 0.83D (range –2.625 to 1 D, SD 0.80D) [Tables 1–3].
Safety was measured by a loss of more than two lines of best spectacle-corrected visual acuity (BSCVA) in treated eyes. We found that no eye had lost two or more Snellen lines at 6 months follow-up and all eyes had a BSCVA of 20/25 or better [Figure 1]. Complaints after CK surgery included minor pain (three patients), foreign body sensation (one patient), tearing (one patient), and burning sensation (two patients). All complaints resolved by 4 weeks postoperatively.
Figure 1.
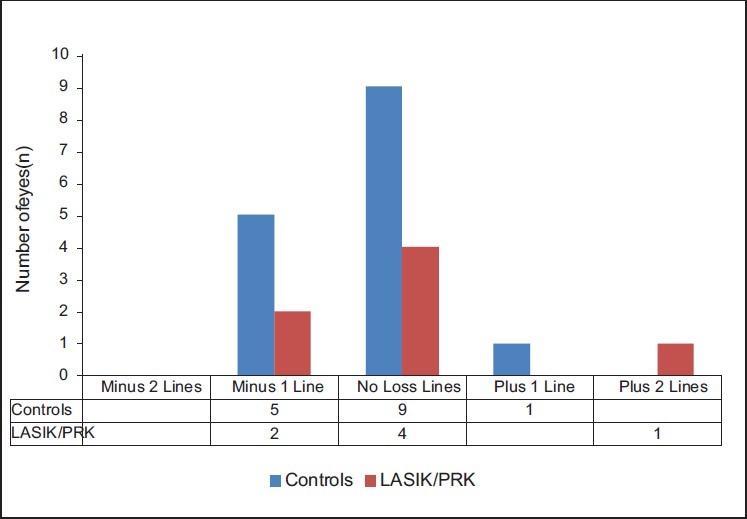
Safety: change in BSCVA compared with preoperative BSCVA (n= 22 eyes)
Regression of conductive keratoplasty
The mean follow-up was 18.6 months (range 5–47 months) in patients with prior refractive surgery and 16.7 months (range 7–46 months) in the control eyes.
Postoperatively, the mean MRSE of patients with previous LASIK/PRK surgery was –0.86 D at 6 months, regressing to –0.66 D at 12 months [Figure 2]. The postoperative MRSE in the control patients was –0.57D at 6 months regressing to -0.38 D at 12 months [Figure 2].
Figure 2.
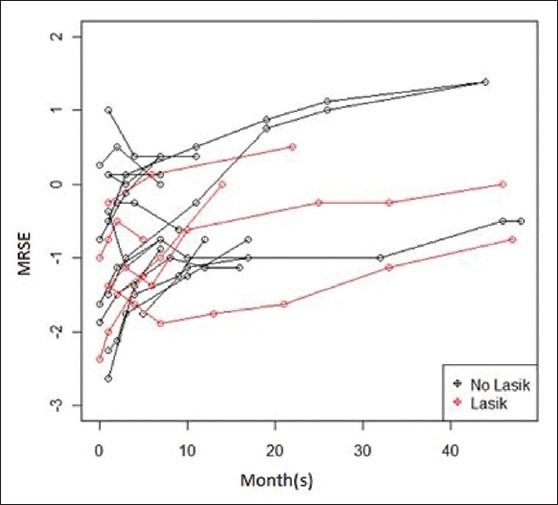
MRSE regression in CK patients with and without prior LASIK/PRK at 50 months (n= 22 eyes)
Conductive keratoplasty treatment effect
The mean number of CK treatment spots used for eyes with refractive surgery was 9.6 ± 3.0 with a pre- to postoperative MRSE change of 1.43 D at 1 month [Table 4]. The average number of CK treatment spots used for the controls was 12.1 ± 5.0 with a pre- to postoperative MRSE change of 1.73 D [Table 4]. Using mixed effects linear regression and controlling for the difference in number of treatment spots and preoperative refractive error, the prior refractive surgery patients showed a greater treatment effect of 0.42 D of MRSE at 1 month [Table 5].
Table 4.
Postoperative refraction changes at 1 month postoperatively of both groups
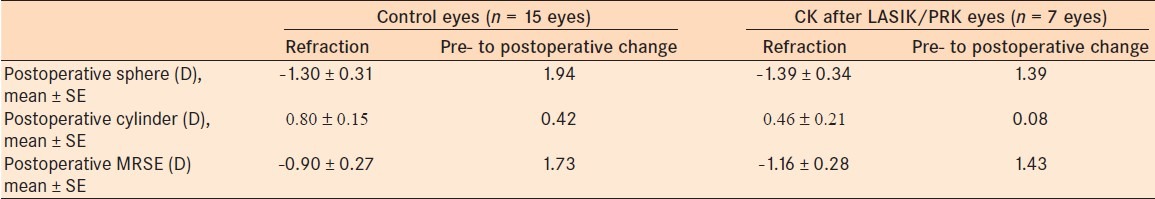
Table 5.
Difference of CK treatment effect among the controls and refractive surgery patients
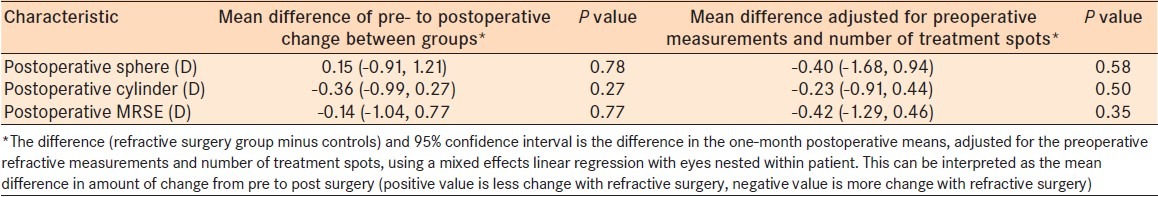
Regression analysis
Considering the follow-up visits could be nested within eyes, repeated measure analysis using a hierarchical model was performed. All preoperative and postoperative variables including demographics, pre- and postoperative refraction, the length of time from refractive surgery to CK, the number of CK treatment spots, and the optical zone(s) used for the CK treatment were analyzed. This regression indicates that time since surgery was the only variable found to be significantly related to MSRE when all data points were used up to 50 months (P = 0.000). When controlling for whether or not an individual had refractive surgery, every 1 month increase in time since CK surgery led to an increase in MSRE of 0.033 D. These results suggest that both groups had equal, linear rates of regression at each month. In addition, on average at any given month the MRSE for eyes that have had LASIK/PRK is 0.38 D lower than non-LASIK/PRK eyes. This 0.38 D of difference is explained in part by a lower mean preoperative MRSE of 0.56 D in the LASIK/PRK group compared to the controls. Indeed, with a P-value of 0.274, there is insufficient evidence to conclude the difference is statistically significant. Analysis of data collected up to 6, 12, and 30 months also found no significance between refractive surgery and control patients.
DISCUSSION
The purpose of this study was to examine the regression rate after CK in patients with or without prior refractive surgery. Regression rates after CK have a reported range of 0.02 D (1) to 0.04D (2) of MRSE per month. Our patients demonstrated a similar rate of regression at 0.03 D per month (P = 0.00).
By studying corneal histological changes after CK, Esquenazi et al. have demostrated the regression of CK is due to a wound healing response.6 Initially there is a rapid activation of myofibroblasts in the CK treatment areas. It is likely that these contractile cells contribute to the purse-string tightening and steepening of the cornea. However, over time as the numbers of myofibroblasts in the wound site diminish, so does the refractive correction of the procedure.6
Although the LASIK/PRK eyes regressed at a similar rate as the controls, there was a greater treatment effect in the LASIK/PRK eyes. After controlling for the number of spots and preoperative measurements, the LASIK/PRK eyes demonstrated a greater myopic shift of 0.42 D after 1 month. Tomita et al. reported similar findings despite setting the refractive target to 20–30% less than the standard nomogram.5 This greater refractive outcome may be attributed to structural changes caused by flap creation or excimer ablation. As the central corneal thickness is more reduced than the peripheral regions, the corneal elasticity is decreased.4,6 This induces a strong steepening effect, which results in an approximate doubling effect of CK when compared with eyes without a refractive surgery history.5
We found CK to be a safe procedure with no eye losing two or more Snellen lines at 6 months of follow-up and all eyes had a BSCVA of 20/25 or better. The most common complaints were blurry vision and minor pain which resolved by 4 weeks postoperatively without sequela.
Due to CK's safety profile, CK remains an appealing option for several patient populations. As LASIK has become the most popular choices for the correction of refractive error, we expect that the number of patients seeking treatment after LASIK will increase. In patients with refractive surgery, CK avoids flap manipulation and further laser ablation. CK is especially useful in patients with thin corneas, thin flaps, multiple previous eye surgeries, or epithelial basement membrane dystrophy where further LASIK or PRK may expose the patient to unnecessary risk.7
CK may also be preferable to LASIK and PRK for low hyperopes who have very flat corneas or dry eyes. In addition, CK may be a reasonable bridge for low hyperopes with early cataracts with a refractive error too small to consider clear lens extraction. Finally, CK remains a viable option for presbyopic near-vision correction.
A major weakness of this study is the small sample size, which lacks the power required to demonstrate significant differences between the control and refractive surgery patients. Another weakness is the inclusion of both right and left eyes in three patients, which are not independent variables. The limited number of patients with different targets of emmetropia versus presybiopic targets made it difficult to match these characteristics between the two groups.
Despite these limitations, patients with previous LASIK or PRK showed a better response with similar regression after CK compared to those without refractive surgery. Overall, we found CK to be a safe procedure that inevitably regresses.
Footnotes
Source of Support: This investigation was supported by the University of Utah Study Design and Biostatistics Center, with funding in part from the Public Health Services research grant numbers UL1-RR025764 and C06-RR11234 from the National Center for Research Resources
Conflict of Interest: None declared
REFERENCES
- 1.Ehrlich JS, Manche EE. Regression of effect over long-term follow-up of conductive keratoplasty to correct mild to moderate hyperopia. J Cataract Refract Surg. 2009;35:1591–6. doi: 10.1016/j.jcrs.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 2.McDonald MB, Davidorf J, Maloney RK, Manche EE, Hersh P. Conductive keratoplasty for the correction of low to moderate hyperopia: 1-year results on the first 54 eyes. Ophthalmology. 2002;109:637–49. doi: 10.1016/s0161-6420(01)01022-3. [DOI] [PubMed] [Google Scholar]
- 3.Alio JL, Ramzy MI, Galal A, Claramonte PJ. Conductive keratoplasty for the correction of residual hyperopia after LASIK. J Refract Surg. 2005;21:698–704. doi: 10.3928/1081-597X-20051101-07. [DOI] [PubMed] [Google Scholar]
- 4.Du TT, Fan VC, Asbell PA. Conductive keratoplasty. Curr Opin Ophthalmol. 2007;18:334–7. doi: 10.1097/ICU.0b013e3281df2cf0. [DOI] [PubMed] [Google Scholar]
- 5.Tomita M, Watabe M, Ito M, Tsuru T. Conductive keratoplasty for the treatment of presbyopia: Comparative study between post- and non-LASIK eyes. Clin Ophthalmol. 2011;5:231–7. doi: 10.2147/OPTH.S16791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esquenazi S, He J, Kim DB, Bazan NG, Bui V, Bazan HE. Wound-healing response and refractive regression after conductive keratoplasty. J Cataract Refract Surg. 2006;32:480–6. doi: 10.1016/j.jcrs.2005.12.077. [DOI] [PubMed] [Google Scholar]
- 7.Hersh PS, Fry KL, Chandrashekhar R, Fikaris DS. Conductive keratoplasty to treat complications of LASIK and photorefractive keratectomy. Ophthalmology. 2005;112:1941–7. doi: 10.1016/j.ophtha.2005.05.017. [DOI] [PubMed] [Google Scholar]


