Abstract
Purpose:
The purpose of this study was to estimate the prevalence of exfoliation syndrome (XFS) and its association with ocular disease in patients attending the eye clinic of the University College Hospital (UCH) in Ibadan, Nigeria.
Materials and Methods:
A total of 448 consecutive new patients, aged 30-90 years who presented to the eye clinic of UCH between December 2009 and November 2010 were evaluated. Each patient had a complete ophthalmic examination. Patients with exfoliative material on the anterior lens surface and/or pupillary margin in either or both eyes were considered to have XFS. Means, standard deviation, and 95% confidence intervals were calculated.
Results:
All the patients examined were from the southern part of Nigeria. Majority (94.2%) were of the Yoruba tribe from southwestern Nigeria, while 5.8% were from southeastern Nigeria. The mean age of the study cohort was 58.5 ± 13.8, 54.8% were males, 12 (2.7%) had XFS. All patients with XFS were of the Yoruba tribe, with a mean age 65.6 ± 5.6 years. There was a male predilection (66.7%). All eyes with XFS had lenticular opacities. XFS was bilateral in eight patients (66.7%) of whom seven patients (87.5%) had glaucoma and lenticular opacities bilaterally.
Conclusion:
This is the first report of the existence of XFS in Nigeria. Larger studies are necessary in this population to further investigate the disease.
Keywords: Exfoliation Syndrome, Glaucoma, Nigeria, Yoruba Tribe
INTRODUCTION
Exfoliation syndrome (XFS) is an age-related generalized disorder of the extracellular matrix characterized by the production and progressive accumulation of a fibrillar extracellular material in many ocular tissues.1
XFS is characterized clinically by the deposition of small, whitish exfoliative material (XFM) in the anterior segment, most commonly on the pupillary border and anterior lens capsule. XFS is the most common identifiable cause of glaucoma, accounting for the majority of cases in some countries.2 It has been estimated that between 60 and 70 million people are affected, making it a leading cause of blindness, and a disease of global importance.3
Glaucomatous damage in XFS progresses more rapidly, has a more serious clinical course, and worse prognosis than primary open-angle glaucoma.4 At the time of diagnosis, there is a significantly higher severity of optic nerve damage, worse visual field damage, poorer response to medications, and more frequent necessity for surgical intervention. The reported prevalence of XFS both with and without glaucoma has varied widely. Previously thought to be rare in Africa, recent reports suggest that it is common in Ethiopia,5 where 25% of open-angle glaucoma patients had XFS and in the Bantus of South Africa.6 It has also been reported in a retrospective clinic-based study in the Congo7 and population studies in Lesotho.8 In Zimbabwe,9 a prevalence of 7.4% has been reported among blind patients. However, in West Africa, it has been reported only in a few patients in the Gambia,10 and there have been no reports of XFS from other nations of West Africa. XFS was not found in a series of glaucoma patients in Ghana.11 XFS remains poorly understood among West Africans and there is lack of data from this subregion. The aim of this study was to estimate the prevalence of XFS and its association with ocular disease in patients attending the eye clinic of the University College Hospital in Ibadan, Nigeria.
MATERIALS AND METHODS
Consecutive new patients, aged 30-90 years, who attended the eye clinic of the University College Hospital between December 2009 and November 2010 were included. Each patient had a complete history and ophthalmic evaluation, consisting of uncorrected and best-corrected visual acuity, slit-lamp examination, intraocular pressure by Goldmann applanation tonometry, and gonioscopy using a 4-mirror Posner lens. Automated full-threshold visual fields for subjects with best-corrected visual acuity better than 6/60 using the 24-2 SITA standard program on the Humphrey 740 Visual Field Analyzer (Dublin, California, USA) was performed. All patients with open angles were dilated and stereoscopic examination of the vitreous, retina, and optic nerve head was done with the 78-diopter lens. Patients with narrow angles were only dilated after a peripheral laser iridotomy was performed. This study adhered to the tenents of the Declaration of Helsinki.
Assessment of exfoliation syndrome
Prior to dilation, the corneal endothelium, angle, iris, and pupillary margins were evaluated for deposits of exfoliation material (XFM) using a slit-lamp. After dilation, the anterior lens surface was examined using a narrow slit-lamp beam under full illumination and high magnification. The anterior lens capsule was scanned from left to right, focusing on the detection of early signs of XFS, including pre-granular radial lines and established granular deposits. Patients with XFM on the anterior lens surface and/or pupillary margin in either or both eyes were considered to have XFS.
Diagnosis of glaucoma
Diagnosis of glaucoma was based on the International Society of Geographic and Epidemiologic Ophthalmology (ISGEO) classification,12 which uses three levels of evidence.
The highest level of evidence is eyes with optic disc abnormalities {vertical cup to disc ratio (VCDR)} 97.5th percentile of the hyper-normal population) and visual field (VF) defects compatible with glaucoma, i.e., eyes with VCDR ≥0.7 and or VCDR asymmetry ≥0.2 or a neuro-retinal rim width reduced to ≤0.1 CDR (between 11:00 and 1:00 o’clock or 5:00 and 7:00 o’clock) that also shows definite visual field defect consistent with glaucoma.
A severely damage optic disc (VCDR 99.5th percentile of the hyper-normal population) if a VF test could not be performed satisfactorily. This means eyes with VCDR ≥0.9 in which a visual field could not be done satisfactorily.
Lastly, if the optic disc could not be examined because of media opacity (hence, no VF test was possible), then a visual acuity 20/400, IOP exceeding the 99.5th percentile of the hyper-normal population (26 mmHg), or evidence of previous glaucoma filtering surgery was taken as sufficient for a diagnosis of glaucoma.
Means, standard deviation and 95% confidence intervals (CI) were calculated for the variables under study.
RESULTS
A total of 448 consecutive new patients, aged 30-90 years, who attended the eye clinic of the University College Hospital between December 2009 and November 2010 were examined. All were from southern Nigeria. The majority (94.2%) were of the Yoruba tribe from southwestern Nigeria, while 5.8% were from southeastern Nigeria. Of the 448 patients (mean age 58.5 ± 13.8,54.8% males), 12 (2.7%) had XFS. All patients with XFS were Yoruba with mean age 65.6 ± 5.6 years. There was a male predilection (66.7%). Of the 448 patients, 225 (50.22%) had glaucoma. In patients with glaucoma, 29 had angle closure glaucoma (12.9%) and 196 patients (87.1%) had open angle glaucoma.
All eyes with XFS had lenticular opacities. XFS was bilateral in 8 patients (66.7%). Of the bilateral cases, 7 patients (87.5%) had glaucoma and lenticular opacities in both eyes. One patient had bilateral XFS with lenticular opacities but did not have glaucoma. One patient with unilateral XFS (25%) had bilateral glaucoma which was worse in the eye with the XFM, while the remaining 3 patients with unilateral XFS (75%) did not have glaucoma in both eyes. XFS with glaucoma was found in 3.5% of all the patients with glaucoma and 3.6% of patients with open angle glaucoma (OAG).
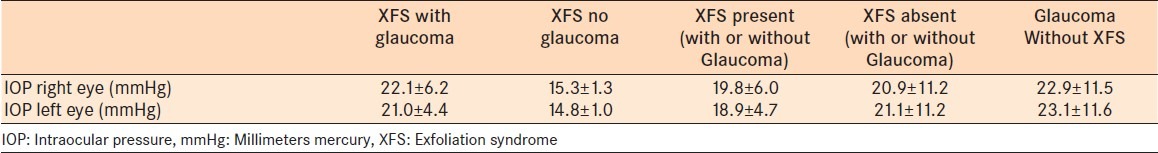
Glaucoma was present in 66.7% of all patients with XFS. Exfoliation material was found on the anterior lens capsule of 9 patients (75%) while 3 had XFM on both their anterior lens capsule [Figure 1] and pupillary margin [Figure 2]. None of the 12 patients with XFM were pseudophakic or aphakic. On gonioscopy, one patient (8.3%) had an anatomically narrow angle, while 91.7% had open angles. The mean IOP in patients with XFS was 19.8 ± 6.0 mmHg (95% CI, 16.0-23.6) for right eyes and 18.9±4.7 mmHg (95% CI, 15.9-21.9) for left eyes. In patients without XFS, however, the mean IOP was 20.9 ± 11.2 mm Hg (95% CI, 19.8–21.9) for right eyes and 21.1 ± 11.2 mmHg (95% CI,20.1–22.2) for left eyes [Table 1].
Figure 1.
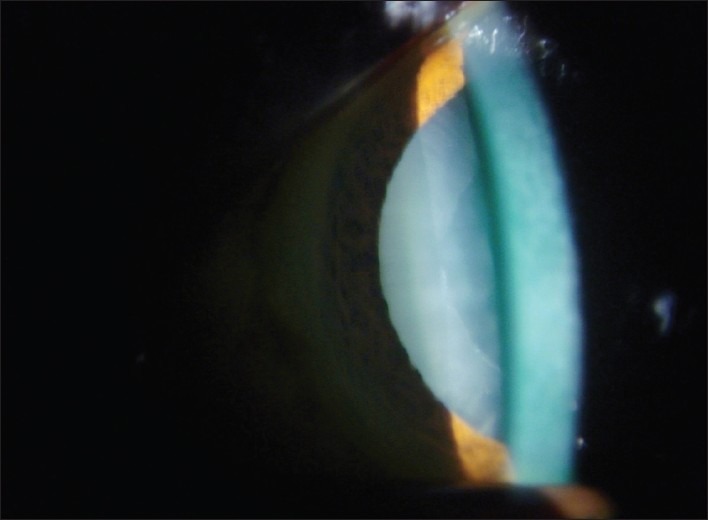
Exfoliation material on the anterior lens capsule
Figure 2.
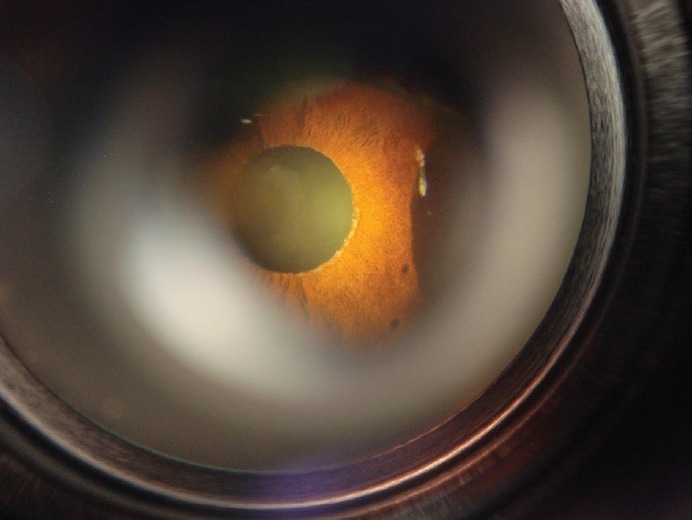
Exfoliatiosn material on the pupillary margin
Table 1.
Intraocular pressure distribution in eyes with or without exfoliation syndrome in a teaching hospital in Southern Nigeria
DISCUSSION
This study confirms that XFS exists in Nigeria and may not be as rare as previously documented.11 A recent study in Ghana11 did not find XFS in their cohort whereas a study in Gambia10 noted XFS in 5.5% of the cohort. In South Africa,6 a higher prevalence has been reported in population studies. Teshome et al.13 also reported a high prevalence of XFS among patients scheduled for cataract surgery in Ethiopians in North Africa. However, a population-based survey in rural Tanzania, East Africa yielded no cases of XFS among a sample of 3268 subjects older than 40 years.14 This is surprising because the Bantu populations of South Africa, who have a high prevalence of XFS, are thought to have been migrated from East Africa.6 The Barbados eye study15 also did not find XFS in their sample population. Prevalence of XFS has been reported to be low among African Americans; Gradle and Sugar16 reported only two cases among black Americans in their clinic in Chicago. In a series of 500 patients with open-angle glaucoma (OAG) in Southern Louisiana, XFS was present in only 0.4% of black patients compared with 2.7% among white patients.17
Previous reports of XFS showed an age-related increase, with XFS typically being less common below the age of 60 years and increasing thereafter.18,19 This is also suggested in our study, where the mean age of patients with XFS was higher than the mean age of those without exfoliation syndrome. XFS was more common among males in this case series; however there have been conficting results about gender predilection.1 Many studies have found no sex predilection,20,21 while some others have reported a female preponderance.22 In a study done among Ethiopian patients with XFS attending a glaucoma clinic,5 there was a marked male preponderance with a ratio of almost 3:1. A true sex difference however can only be assessed through population-based studies.
Exfoliation syndrome has been reported as the most common identifiable cause for open-angle glaucoma.23 This study confirms that the presence of XFS increases the risk of having OAG in our population. About two-thirds of the patients with XFS in our study had glaucoma. However, XFS was present in 3.6% of the open-angle glaucoma cases in our study. This is lower than the 13.4% reported from the Blue Mountains eye study24 and approximately 50% reported in Turkey.25 In a study of South African blacks,6 XFS was the most frequently associated secondary cause of glaucoma, accounting for 24.3% (9/37) and 23.1% (9/39) of cases of OAG. All our patients had XFM on their anterior lens capsule. Therefore the possibility of XFM being clinically visible only on the lens emphasizes the need for dilated lens examinations. Although IOP measurements were not reflective of either peak IOP or duration of IOP elevation, because this was a cross-sectional study, there was no significant difference in the mean IOP of eyes with XFS and those without XFS. This is contrary to some published reports where the IOP of patients with XFS was significantly higher than in patients without XFS.24,26 However our finding is similar to a study done in India.27
This study was undertaken to report the presence of XFS in Nigeria. Observations among ancestrally related populations in the Caribbean14 and United States15,16 show that it is a very rare form of secondary open angle glaucoma among blacks. However, XFS may be more common among certain ethnic groups in the West African region than has been reported. This may be due to genetic or environmental influences. The need for routine dilated lens examination however cannot be overemphasized especially in patients with cataracts and glaucoma above the age of 40 years. A program to instruct eye care workers in the West African region to recognize the salient and overt features of the disease is valuable.
This was not a population-based study; therefore, we are unable to determine the prevalence or the relative proportions of XFS in this population. A population-based study would be of great value to further define the prevalence of XFS in this region and to compare its characteristics with XFS in other parts of the world.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Ritch R, Schlötzer-Schrehardt U. Exfoliation syndrome. Surv Ophthalmol. 2001;45:265–315. doi: 10.1016/s0039-6257(00)00196-x. [DOI] [PubMed] [Google Scholar]
- 2.Ritch R. Exfoliation syndrome - The most common identifiable cause of open-angle glaucoma. J Glaucoma. 1994;3:176–7. [PubMed] [Google Scholar]
- 3.Ritch R, Schlötzer-Schrehardt U, Konstas AG. Why is glaucoma associated with exfoliation syndrome? Prog Retinal Eye Res. 2003;22:253–75. doi: 10.1016/s1350-9462(02)00014-9. [DOI] [PubMed] [Google Scholar]
- 4.Leske MC, Heijl A, Hussein M, Bengtsson B, Hyman L, Komaroff E, et al. Factors for glaucoma progression and the effect of treatment: The early manifest glaucoma trial. Arch Ophthalmol. 2003;121:48–56. doi: 10.1001/archopht.121.1.48. [DOI] [PubMed] [Google Scholar]
- 5.Bedri A, Alemu B. Pseudoexfoliation syndrome in Ethiopian glaucoma patients. East Afr Med J. 1999;76:278–80. [PubMed] [Google Scholar]
- 6.Rotchford AP, Kirwan JF, Johnson GJ, Roux P. Exfoliation syndrome in black South Africans. Arch Ophthalmol. 2003;121:863–70. doi: 10.1001/archopht.121.6.863. [DOI] [PubMed] [Google Scholar]
- 7.Kaimbo Wa, Kaimbo D, Missotten L. Glaucoma in Congo. Bull Soc Belge Ophtalmol. 1997;267:21–6. [PubMed] [Google Scholar]
- 8.Gordon YJ, Mokete M. Survey of ophthalmic conditions in rural Lesotho. Doc Ophthalmol. 1980;49:285–91. doi: 10.1007/BF01886621. [DOI] [PubMed] [Google Scholar]
- 9.Masanganise R. Pseudo-exfoliation syndrome in Chivi District: A disease with no geographical or racial boundaries. Cent Afr J Med. 1997;43:229–31. [PubMed] [Google Scholar]
- 10.Chuka-Okosa CM, Faal HB, Ogunro A, Duke R. Types of Glaucoma and recent trends applied in treatment: Observations from a Glaucoma Training Workshop in the Gambia. Niger Postgrad Med J. 2005;12:203–9. [PubMed] [Google Scholar]
- 11.Herndon LW, Challa P, Ababio-Danso B, Boateng JO, Broomer B, Ridenhour P, et al. Survey of glaucoma in an eye clinic in Ghana, West Africa. J Glaucoma. 2002;11:421–5. doi: 10.1097/00061198-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86:238–42. doi: 10.1136/bjo.86.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teshome T, Regassa K. Prevalence of Pseudoexfoliation syndrome in Ethiopian patients scheduled for cataract surgery. Acta Ophthalmol Scand. 2004;82:254–8. doi: 10.1111/j.1395-3907.2004.00263.x. [DOI] [PubMed] [Google Scholar]
- 14.Buhrmann RR, Quigley HA, Barron Y, West SK, Oliva MS, Mmbaga BB. Prevalence of glaucoma in a rural East African population. Invest Ophthalmol Vis Sci. 2000;41:40–8. [PubMed] [Google Scholar]
- 15.Leske MC, Connell AM, Schachat AP, Hyman L The Barbados Eye Study. Prevalence of open angle glaucoma. Arch Ophthalmol. 1994;112:821–9. doi: 10.1001/archopht.1994.01090180121046. [DOI] [PubMed] [Google Scholar]
- 16.Gradle HS, Sugar HS. Glaucoma capsulare. Am J Ophthalmol. 1947;30:12–9. doi: 10.1016/0002-9394(47)91331-7. [DOI] [PubMed] [Google Scholar]
- 17.Ball SF. Exfoliation syndrome prevalence in the glaucoma population of South Louisiana. Acta Ophthalmol Suppl. 1988;184:93–8. doi: 10.1111/j.1755-3768.1988.tb02636.x. [DOI] [PubMed] [Google Scholar]
- 18.Lumme P, Laatikainen L. Exfoliation syndrome and cataract extraction. Am J Ophthalmol. 1993;116:51–5. doi: 10.1016/s0002-9394(14)71743-x. [DOI] [PubMed] [Google Scholar]
- 19.Drolsum L, Haaskjold E, Davanger M. Pseudoexfoliation syndrome and extracapsular cataract extraction. Acta Ophthalmol (Copenh) 1993;71:765–70. doi: 10.1111/j.1755-3768.1993.tb08597.x. [DOI] [PubMed] [Google Scholar]
- 20.Moreno Montañeś J, Alcolea Paredes A, Campos García S. Prevalence of pseudoexfoliation syndrome in the northwest of Spain. Acta Ophthalmol (Copenh) 1989;67:383–5. [PubMed] [Google Scholar]
- 21.Summanen P, Tönjum AM. Exfoliation syndrome among Saudis. Acta Ophthalmol Suppl. 1988;184:107–11. doi: 10.1111/j.1755-3768.1988.tb02639.x. [DOI] [PubMed] [Google Scholar]
- 22.Ekstrom C. Prevalence of pseudoexfoliation in a population of 65-74 years of age. Acta Ophthalmol Scand. 1987;182:9–10. [Google Scholar]
- 23.Prince AM, Ritch R. Clinical signs of the pseudoexfoliation syndrome. Ophthalmology. 1986;93:803–7. doi: 10.1016/s0161-6420(86)33664-9. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell P, Wang JJ, Hourihan F. The relationship between glaucoma and pseudoexfoliation: The Blue Mountains Eye Study. Arch Ophthalmol. 1999;117:1319–24. doi: 10.1001/archopht.117.10.1319. [DOI] [PubMed] [Google Scholar]
- 25.Yalaz M, Othman I, Nas K, Eroğlu A, Homurlu D, Cikintas Z, et al. The frequency of pseudoexfoliation syndrome in the eastern Mediterranean area of Turkey. Acta Ophthalmol (Copenh) 1992;70:209–13. doi: 10.1111/j.1755-3768.1992.tb04125.x. [DOI] [PubMed] [Google Scholar]
- 26.Davanger M, Ringvold A, Blika S. Pseudo-exfoliation, IOP and glaucoma. Acta Ophthalmol (Copenh) 1991;69:569–73. doi: 10.1111/j.1755-3768.1991.tb04841.x. [DOI] [PubMed] [Google Scholar]
- 27.Krishnadas R, Nirmalan PK, Ramakrishnan R, Thulasiraj RD, Katz J, Tielsch JM, et al. Pseudoexfoliation in a rural population of southern India: The Aravind Comprehensive Eye Survey. Am J Ophthalmol. 2003;135:830–7. doi: 10.1016/s0002-9394(02)02271-7. [DOI] [PubMed] [Google Scholar]


