Abstract
Aim:
Sister chromatid exchange (SCE) test is a sensitive, biomarker of genotoxic substances. The present study was conducted to observe the frequency of SCEs in peripheral blood lymphocytes of 30 males with and without the habit of cigarette smoking and alcohol consumption.
Materials and Methods:
Subjects for this study were males aged between 25-50 years and were selected from the students, employees and the patients attending the outpatient department of Ragas dental college and Hospital, Chennai.
Results:
Controls, smokers, and smokers with alcohol habit were divided into two age groups as ≤30 years and ≥30 years. In controls the mean frequency of SCEs/cell in ≤30 years and ≥30 year's age group was 5.80 and 6.05, respectively. In Smokers SCEs/cell in ≤30 years and ≥30 year's age group was 7.7 and 8.8, respectively. In Smokers with alcohol habit SCEs/cell in ≤30 years and ≥30 years age group was 10.1 and 12.8, respectively.
Conclusions:
In this study, the duration of the smoking habit has shown a positive correlation with the mean SCE frequency. Whereas, frequency of the habit did not show any influence on the SCE levels. In smokers with alcohol habit, both the duration and frequency of their smoking habit has shown a significant effect on the SCE levels suggesting a synergistic effect of alcohol and smoking leading to excessive DNA damage.
Keywords: Biomarker, genotoxic, sister chromatid exchange
INTRODUCTION
Majority of human cancers may be due to exposure to mutagens like tobacco, alcohol, diet and occupational hazards. Sister chromatid exchange (SCE) is a popular method in genetic toxicology and human population cytogenetic monitoring.[1] It is considered to be a sensitive measure of chromosomal (DNA) damage and possible repair, which offers a means of ascertaining the possible susceptibility to the effects of mutagens and carcinogens and of detecting the possible high tendency to malignancy in a few instances, as in Bloom's syndrome. It has been suggested that SCE may serve as a preclinical marker for early detection of cancer.[2] An abnormal SCE may imply a defect in the DNA repair mechanism, a predisposing factor to neoplasia.[3–5] SCE was first demonstrated in 1957 by Taylor et al. SCE is the reciprocal interchange of DNA between two chromatids of a single chromosome that can be visualized in metaphase chromosomes during the S phase of the cell cycle.[6,7] SCEs are commonly scored in lymphocytes, which replicate their DNA twice in the presence of the thymidine analogue 5-bromodeoxyuridine (BrdUrd). Normally, 5-8 SCEs per cell are present in a healthy individual who is not exposed to carcinogens and mutagens.[8] Tobacco smoke is considered to be mutagenic to humans and there are several determinants of tobacco carcinogen exposure and cancer risk. And alcoholic beverages are known to be human carcinogens (International Agency for research on Cancer 1ARC 1988, Longnecker and Eenger 1996) and are related to cancers of the mouth, pharynx, larynx, and esophagus. As SCE reflects the mutational events compatible with cell survival, we choose to conduct this study on SCE analysis to know whether this test is potent enough to assess the risk and possibly detect early and helps in prevention.[3,9]
MATERIALS AND METHODS
Peripheral blood samples were obtained with informed consent from 10 male subjects who had smoking habit, 10 male subjects who had smoking and drinking habits, 10 male subjects without smoking and drinking habits attending the outpatient department of Ragas dental college and Hospital, Chennai.
Peripheral blood samples were collected in heparinized syringe under aseptic conditions. Whole blood lymphocytes from individuals were cultured in RPMI- 1640 (rosewell park memorial institute), supplemented with fetal bovine serum and phytohemagglutinin. After 24 h BrdU (5-bromo-2–deoxyuridine) was added at a concentration of 5 μg/ml and cells were grown in dark at 37°C at 69th h colchicines was added after hypotonic treatment by potassium chloride and fixation done in 3:1 methanol-acetic acid and slides were prepared by air drying. Later, these slides were stained with Hoechst 33258 (Bisbenzimide) at a concentration of 5 μg/ml in a dark for 30 min. Now the slides were mounted by 2 × sodium saline citrate buffer (SSC) solution and placed in bright sunlight for 1 h, then washed in distilled water, the slides are aged by keeping them for one day at room temperature. Next day, slides were washed with distilled water stained with Giemsa (2 ml 4% Giemsa+2 ml buffer+46 ml distilled water) for 5 min washed with water and air dried. The slides were studied systematically at magnification 40 × objective and cells judged suitable for analysis were scored at high magnification (100 × objective). Chromosomes having unilateral homogenous linear bands of alternative dark and light stain were considered to have taken up the Hoechst and Giemsa stain. These alternative dark and light bands represents sister chromatid exchanges. Only spreads with 46 chromosomes were included in the analysis. Cells with shattered or pulverized chromosomes were excluded from the study. The frequencies of SCEs were analyzed in 25 metaphase plates per sample/subject, each plate containing 46 chromosomes i.e., a total of 750 metaphase plates were scored in 30 individuals for this study.
RESULTS
The present study was conducted to observe the frequency of SCEs in peripheral blood lymphocytes of 30 males with and without the habit of cigarette smoking and alcohol consumption. Subjects were divided into three groups. Controls (10), Smokers (10), and Smokers with alcohol habit (10) and all the subjects of this study were within the age group of 25-55 years [Table 1]. They were divided into two age groups as ≤30 years and ≥30 years. In controls, the mean frequency of SCEs/cell in ≤30 years and ≥30 years age group was 5.80 and 6.05, respectively. In smokers SCEs/cell in ≤30 years and ≥30 year's age group was 7.7 and 8.8, respectively. In smokers with alcohol habit SCEs/cell in ≤30 years and ≥30 year's age group was 10.1 and 12.8, respectively. The mean SCE frequency was statistically significant between ≤30 and ≥30 years age groups . Data on the mean frequency of SCEs of controls, smokers and smokers with alcohol habit are presented in Table 2 and Figure 1. Subjects having the combination of cigarette smoking and alcohol consumption had a mean SCE frequency of 11.48 ± 1.85 SD, whereas, smokers without drinking habit had a mean SCE frequency 8.30 ± 0.77 SD. These values are significantly higher than the frequency of value of 5.91 ± 0.31 SD found in controls. The mean SCE/cell difference between controls and smokers (2.392), controls smokers with alcohol habit (5.576*) and smokers and smokers with alcohol habit was statistically significant [Table 3] When the duration of the smoking habit in smokers was compared to smokers cigarettes habit, the mean SCEs/cell were found to be more in the latter group [Table 4 and Figure 2]. When the frequency of smoking habit in smokers was compared to smokers with alcohol habit, the mean SCE/cell was found to be more in the latter group [Table 5 and Figure 3]. In this study, age of the subjects in controls (r = 0.632*, P = <0.05), smokers (r = 0.879**, P = 0.001) and smokers with alcohol habit (r = 0.770**, P = 0.009) had a positive correlation and was directly proportional to the mean SCE frequency. The duration of the smoking habit in smokers (r = 0.985**, P = 0.000) and smokers with alcohol habit (r = 0.905**, P = 0.000) has shown a positive correlation and was directly proportional to the mean SCE frequency. The frequency of smoking habit in smokers with alcohol habit (r = 0.867**, P = 0.001) has shown a positive correlation with the mean SCE frequency, whereas, in smokers (r = 0.607, P = 0.063) it has not shown any correlation [Table 6].
Table 1.
Age distribution among controls, smokers and smokers using alcohol
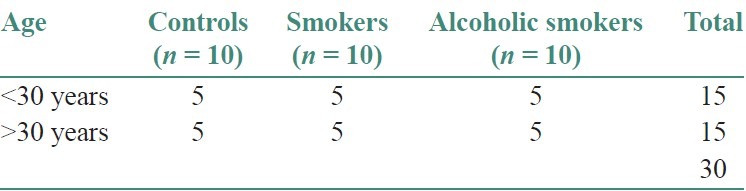
Table 2.
Mean sce/cell in controls, smokers and smokers using alcohol
Figure 1.
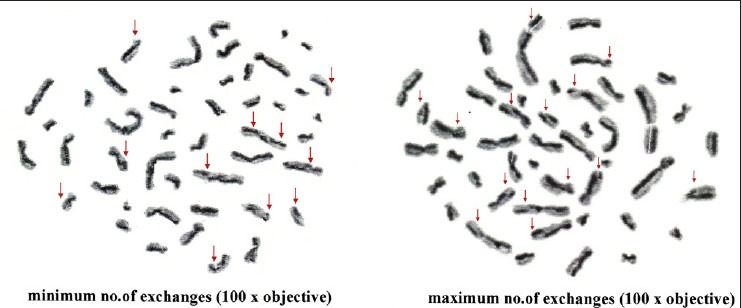
Metaphase plate showing sister chromatid exchanges in smokers with alcohol habit
Table 3.
Mean difference of sce/cell between the groups
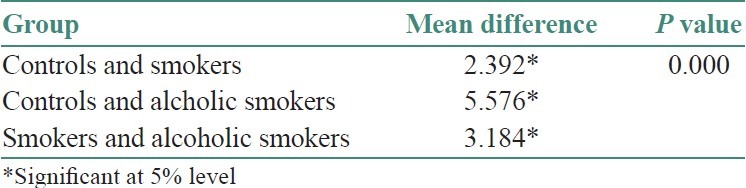
Table 4.
Mean sce/cell and duration of smoking habits between smokers and smokers using alcohol
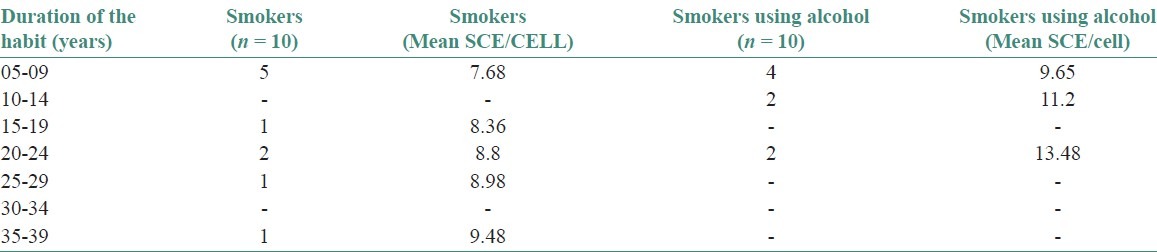
Figure 2.
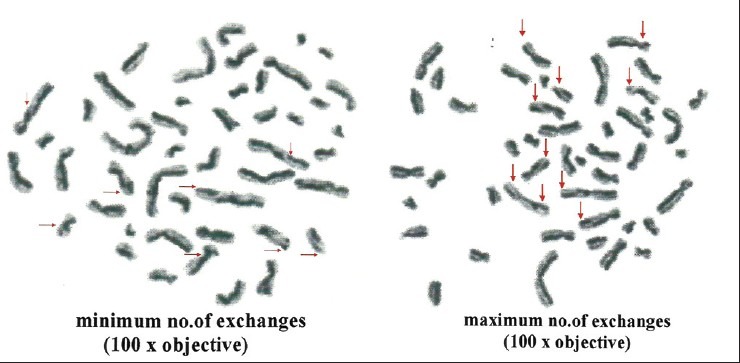
Metaphase plate showing sister chromatid exchanges in smokers
Table 5.
Frequency of smoking habit and mean sce/cell in smokers and using alcohol

Figure 3.
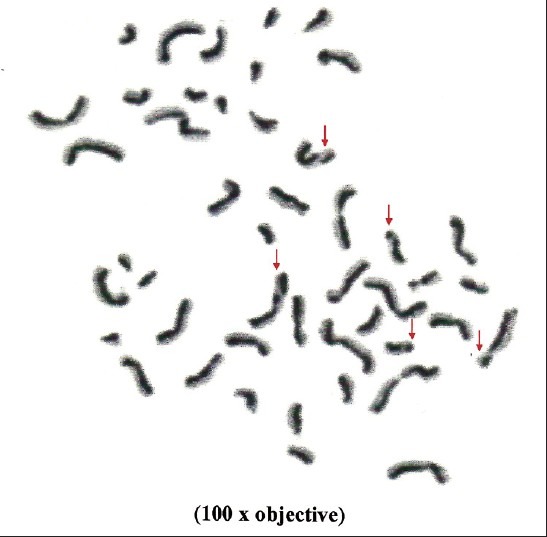
Metaphase plate showing sister chromatid exchanges in controls
Table 6.
Comparision of sce/cell with duration and frequency of smoking habit bewteen smokers and smokers using alcohol
DISCUSSION
Taylor et al. in 1957 were the first to show the Watson-Crick model of DNA - replication at the level of chromosomes and an unexpected exchange of labeled DNA between sister chromatids.[10] Since then studies on SCEs as an indicator for mutagenic or carcinogenic effects of environmental chemicals suggested a positive linear correlation exist between the SCE induction and transformation.[11] Few studies suggest that the SCE assays could be used to study DNA damage and repair.[10] Park et al. in 1992 and Lazutka in 1994 showed a linear increase in the mean SCE values in lymphocytes of 142 healthy Koreans, age ranging from newborn infants to 50 s. The values were newborn infant's 4.98/cell, small children 6.22/cell and older age group 8.56/cell. They also observed a 5% (O.42 SCE/cell) excess of SCE frequency in females over males.[12,5] Our study was done to determine the effects of tobacco smoking and alcohol consumption habits in the peripheral blood lymphocytes by assessing the frequencies of SCEs. We observed a linear increase in the mean SCE frequency with the age of the subjects in controls, smokers and smokers using alcohol. This increase may be associated within a sub population having a heavily damaged DNA, possibly accompanied by reduced efficiency in DNA repair. Such repair deficient cells may accumulate more DNA damage and eventually reflects as SCEs. These findings were consistent with that of Ganguly[13] and many others.[12,14,15] Lambert et al. (1978) were the first to report that the frequency of SCEs is increased in lymphocytes of smokers.[16] Livingston et al. (1983) were the pioneers to observe a significant correlation between cigarette smoking and SCE frequency.[17] The smokers group in our study has shown a duration dependent linear increase in the mean SCE frequency. Results of previous studies have suggested that this might be due to the polymorphisms in carcinogen metabolisms and DNA repair. These individuals with inherited variant metabolic enzyme activities and repair capacities might have altered the risks of cigarette smoke-induced DNA damage.[18] Similar findings were observed by Ghosh and Ghosh[19] Kirsti, Sorsa, Hilkka et al.[16] Lambert, Bredberg, Mckenzie et al.,[11] Livingston and Finernan[14], Sarto, MustariI et al. In the same group, we did not observe any correlation between the frequency of the habit (No. of cigarettes smoked/day) and the mean SCE frequency. In contrast to the result, Ghosh and Ghosh[19] (n = 36, No. of cigs/day = 20-40), Lambert, Bredberg, Mckenzie et al.,[11] (n = 41, No. of cigs/day = >20) Vijayalaxmi and Evans[20] (n = 55, No. of cigs/day = 20) have found a significant correlation between the frequency of the habit and mean SCE frequency. This difference in the result of our study could be due to the sample size (n = 10) or the number of cigarettes smoked per day (20) by the subjects in this group. Obe and Ristow (1977) found that acetaldehyde, a metabolic derivative of alcohol induces SCEs by itself irrespective of the subjects smoking habit. In our study, in smokers using alcohol, both the duration and frequency of their smoking habit showed a positive correlation with the mean SCE frequency. This may be due to the synergistic effect of alcohol. Vijayalaxmi and Evans[20] and Wong, Wang, Hsieh et al., have also supported that the synergistic effect of smoking and exposure to other environmental carcinogens like ethyl methane sulphonate or vinyl chloride monomer could also induce SCEs.
SUMMARY AND CONCLUSION
In the present study, the levels of SCEs were measured in peripheral blood lymphocytes in 30 subjects, to assess chromosomal damage due to smoking and drinking. The mean SCE frequency of cigarette smokers and smokers with alcohol habit was significantly increased when compared to the normal controls. In this study, in smokers, the duration of the smoking habit has shown a positive correlation with the mean SCE frequency, whereas, frequency of the habit did not show any influence on the SCE levels. In smokers with alcohol habit, both the duration and frequency of their smoking habit has shown a significant effect on the SCE levels suggesting a synergistic effect of alcohol and smoking leading to excessive DNA damage and finally reflecting as an increase in the SCE frequency. SCE assay is one of the most sensitive markers of DNA damage and can be used to investigate the genotoxicity of cigarette smoking and alcohol consumption. We consider SCE analysis is of a great value with respect to risk assessment early detection and possibly prevent oral cancer in individuals having the habit of smoking and alcohol consumption.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Lazutka JR. Sister chromatid exchanges (SCE'S) and high frequency cells (HFC'S) in human population studies: Principles of their analysis. Mutat Res. 1995;331:229–31. doi: 10.1016/0027-5107(95)00071-p. [DOI] [PubMed] [Google Scholar]
- 2.Frentz G, Wulf HC, Munch-Petersen B, Niebuhr E. Normal sister chromatid exchange in lymphocytes from patients with multiple epidermal cancer. Arch Dermatol Res. 1987;279:180–3. doi: 10.1007/BF00413254. [DOI] [PubMed] [Google Scholar]
- 3.Nohutcu RM, Emre S, Sakizli M, Gürsel B, Eratalay YK, Hosal N. Sister chromatids exchange in lymphocytes of patientswith cancer of the larynx. Am J Otolaryngol. 1991:101–3. doi: 10.1016/0196-0709(91)90044-g. [DOI] [PubMed] [Google Scholar]
- 4.Murty VV, Mitra AB, Luthra UK, Singh IP. Sister chromatid exchanges in patients with precancerous and cancerous lesions of cervix uteri. Hum Genet. 1986;72:37–42. doi: 10.1007/BF00278815. [DOI] [PubMed] [Google Scholar]
- 5.Park EH, Kim YJ, Byun DH, Lee JY, Lee JS. Baseline frequency of sister chromatid exchanges in 142 persons of general Korean population. Mutat Res. 1992;268:239–46. doi: 10.1016/0027-5107(92)90230-y. [DOI] [PubMed] [Google Scholar]
- 6.Murthy MK, Bhargava MK, Augustus M. Sister chromatid exchange studies in oral patients. Indian J Cancer. 1997;34:49–58. [PubMed] [Google Scholar]
- 7.Nagaya T, Toriumi H. Spontaneous and induced sister chromatid exchanges in lymphocytes of healthy persons. Environ Res. 1986;40:181–7. doi: 10.1016/s0013-9351(86)80094-9. [DOI] [PubMed] [Google Scholar]
- 8.Bender MA, Preston RJ, Leonard RC, Pyatt BE, Gooch PC, Shelby MD. Chromosomal aberration and sister chromatid exchange frequencies in peripheral blood lymphocytes of a human population sample. Mutat Res. 1988;204:421–33. doi: 10.1016/0165-1218(88)90038-9. [DOI] [PubMed] [Google Scholar]
- 9.Obe G, Göbel D, Engeln H, Herha J, Natarajan AT. Chromosomal aberration in peripheral lymphocytes of alcoholics. Mutat Res. 1980;73:377–86. doi: 10.1016/0027-5107(80)90202-x. [DOI] [PubMed] [Google Scholar]
- 10.Deen DF, Morgan WF, Tofilon PJ, Barcellos-Hoff MH. Measurement of sister chromatid exchanges and their relationship to DNA damage, repair and cell killing. Pharmacol Ther. 1998;42:349–60. doi: 10.1016/0163-7258(89)90030-2. [DOI] [PubMed] [Google Scholar]
- 11.Lambert B, Bredberg A, Mckenzie W, Sten M. Sister chromatids exchanges in human population: The effect of smoking, drug treatment and occupational exposure. Cytogenet Cell Genet. 1982;33:62–7. doi: 10.1159/000131727. [DOI] [PubMed] [Google Scholar]
- 12.Lazutka JR, Dedonyte V, Krapavickaite D. Sister chromatid exchanges and their distribution in human lymphocytes in relation to age, sex and smoking. Mutat Res. 1995;306:173–80. doi: 10.1016/0027-5107(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh R, Ghosh PK. Sister chromatid exchanges in betel and tobacco chewers. Mutat Res. 1984;139:79–81. doi: 10.1016/0165-7992(84)90107-6. [DOI] [PubMed] [Google Scholar]
- 14.Livingston GK, Fineman RM. Correlation of human lymphocytes SCE frequency with smoking history. Mutat Res. 1983;119:59–64. doi: 10.1016/0165-7992(83)90038-6. [DOI] [PubMed] [Google Scholar]
- 15.Sarto F, Faccioli MC, Cominato I, Levis AG. Ageing and smoking increase the frequency of sister chromatid exchanges (SCE) in man. Mutat Res. 1985;144:183–7. doi: 10.1016/0165-7992(85)90137-x. [DOI] [PubMed] [Google Scholar]
- 16.Husgafvel-Pursiainen K, Sorsa M, Järventaus H, Norppa H. Sister chromatid exchanges in lymphocytes of smokers in experimental study. Mutat Res. 1984;138:197–203. doi: 10.1016/0165-1218(84)90044-2. [DOI] [PubMed] [Google Scholar]
- 17.Livingston GK, Cannon LA, Bishop DT, Johnson P, Fineman RM. Sister chromatid exchange: Variation by age, sex, smoking and breast cancer status. Cancer Genet Cytogenet. 1982;9:289–99. doi: 10.1016/0165-4608(83)90013-4. [DOI] [PubMed] [Google Scholar]
- 18.Lei YC, Hwang SJ, Chang CC, Kuo HW, Luo JC, Chang MJ, et al. Effects of sister chromatid exchange frequency of polymorphisms in DNA repair gene XRCC1 in smokers. Mutat Res. 2002;519:93–101. doi: 10.1016/s1383-5718(02)00127-4. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh R, Ghosh PK. The effect of tobacco smoking on the frequency of sister chromatid exchanges in human lymphocyte chromosome. Cancer Genet cytogenet. 1987;27:15–9. doi: 10.1016/0165-4608(87)90254-8. [DOI] [PubMed] [Google Scholar]
- 20.Vijaylakshmi, Evans HJ. In vivo and in vitro effects of cigarette smoke on chromosomal damage and sister chromatid exchange in human peripheral blood lymphocytes. Mutat Res. 1996;351:187–92. doi: 10.1016/0027-5107(82)90234-2. [DOI] [PubMed] [Google Scholar]


