Abstract
Introduction:
In India, it is estimated that 2.5 million people are currently living with Human Immunodeficiency virus infection (HIV) of which one million are women. Given the occurrence of oral lesions in our population, we studied the patter of these lesions with respect to the role played by gender.
Materials and Methods:
3729 consecutive patients seen over a period of 10 years (from 1998 to 2008) attending the YRG CARE (Center for AIDS Research and Education), at Chennai, India constituted the study group. The oral lesions were diagnosed and the findings were entered into a database and analysed using the SPSS package SPSS11.
Results:
3724 adult patients (71% males 29% females) were recruited in this study. 95% and 92% of males and females respectively acquired the infection through the heterosexual route. 69% of them presented with at least one oral lesion. There was a significant difference in the occurrence of oral candidiasis (OC) (18.8% males 10.3% females, P = 0.00) and oral hairy leukoplakia (OHL) (1.2% males 0.4% females, P = 0.023) between gender. The mean CD4 counts in males (n = 1908) was 284.48 ± 222.45 and in females (n = 1087) it was 394.51 ± 274.56. Males had 2.2 times higher risk of getting OC, 3.1 times higher risk of OHL and over all males had 1.58 times of having any oral lesion compared to females. Multivariate logistic regression that the odds of having OC (OR = 1.7, 95%CI 1.2-2.2, P = 0.001) and OHL (OR = 3.1, 95%CI 1.1-8.9; P = 0.03) were significantly higher for males than for females after controlling for duration of being HIV positive, CD4 count and HAART. 1412 patients had their spouses HIV status also as HIV positive and 769 patients had their spouse HIV status as negative. 858 patients were on HARRT (627 males and 231 females) The partial correlation analysis, done between gender and CD4 counts, when controlling for HAART was r = 0.2028 (P = 0.00).
Conclusion:
Our study confirms that males had a higher risk of oral lesions, especially OC and OHL, than females. The females in this study had a significantly higher mean CD4 counts than males. This different immunological status of the females compared to males should be taken in to consideration in the evaluation and management of HIV positive patients in our country.
Keywords: CD4 counts, gender, HIV, India, oral
INTRODUCTION
In India, it is estimated that 2.5 million people are currently living with human Immunodeficiency virus infection (HIV) of which one million are women. Women in the age group of 15-24 years are more vulnerable than men and men in the age group of 30 years and above are more vulnerable than others.[1] Gender differences in the pattern of opportunistic infections and the impact of antiretroviral therapy (ART) were described from our center earlier.[2] Manifestations of viral infections have been reported to be different between women and men and significant gender differences have been reported in HIV patients.[3] It has also been documented that women infected with HIV have lower HIV RNA levels than comparable men[4] and progress at a faster rate to acquired immunodeficiency syndrome (AIDS) for a particular given viral load than men.[5,6]
Oral manifestations are diagnostic and prognostic indicators of HIV infection.[7–10] The role played by gender in the occurrence of oral lesions have been explored in the western population and notably significant differences have been found in some studies and some did not report any gender differences. In our cohort of HIV infected patients, the number of males were more than females. The purpose of this article is to study the oral lesions in our cohort of HIV infected patients and to analyze their prevalence with respect to gender. Since oral lesions are an important constellation of HIV infection and given the paucity of data with respect to gender and oral lesions from our country, information ascertained from this study would help us to understand the progression of the disease and their management.
MATERIALS AND METHODS
Three thousand, seven hundred and twenty-nine consecutive patients seen over a period of 10 years (from 1998 to 2008) attending the YRG CARE (Center for AIDS Research and Education), at Chennai, India constituted the study group. History and details with respect to the source of HIV infection and ART was recorded by trained counselors. Confirmation of all HIV sero- status for all patients was by ELISA and Western blot. Trained physicians and dental surgeons performed systemic examination and clinical oral examination, respectively, and the findings were recorded in the same format. The format included description of each lesion with respect to colour, character, location and number. The definitions and criteria used for diagnosis of the oral lesions were done based on work of USA, Oral AIDS Collaborative group[11] and EC- clearing house on oral problems related to HIV infection.[12]
Statistical analysis
Data processing and analysis were carried out using the statistical package SPSS 11. Chi-squared test was used for comparisons of the prevalence of oral lesions between genders. Student's t-test was used to analyze the differences between the means and standard deviation (shown as mean ± SD). In determining the association between oral lesions and gender a direction was established in which females were considered as the reference group and males were considered as the test group. Odds ratio and 95% confidence interval are presented. Correlation coefficient was calculated with the aim of studying the strength of the association between the variables. Multiple logistic regression was performed to assess the risk factors for oral candidiasis (OC and OHL). Results were considered statistically significant when the P value was ≤0.05.
RESULTS
Three thousand, seven hundred and twenty-nine adult patients were recruited in this study. Two thousand six hundred and thirty-seven (70.8%) were males and 1087 were females (29.2%) The maximum number of cases was in the 25-35 age group, (males: 46%, females: 50%). There were no significant differences in the economic status between males and females. Majority of the males (61%) and females (62%) were from middle class.
Of the 3729 HIV-positive patients, details of source of infection was available only for 3647 patients. Ninety-two percent of the study subjects acquired the infection through the heterosexual route. The main source of infection for males and females (95% and 92%) was through the heterosexual route. This was followed by blood transfusion (males 1.5% and females 5%).
Table 1 shows the prevalence of HIV related oral lesions by gender. Of the 3729 patients, 5 patients were not included for comparison of oral lesions, as their gender category was not specific. Of the 3724 patients included for gender analysis, 69% of them presented at least with one oral lesion and 15% of the males and 22% of the females presented without any lesions (P = 0.00). The oral lesions and conditions included OC, OHL, pigmentation, conventional periodontitis and gingivitis, oral submucous fibrosis (OSMF) and leukoplakia. There was a significant difference in the occurrence of oral lesions between gender except for hyperplastic candidiasis (HC) and ulcers.
Table 1.
Prevalence of Oral lesions between gender (n=3724)
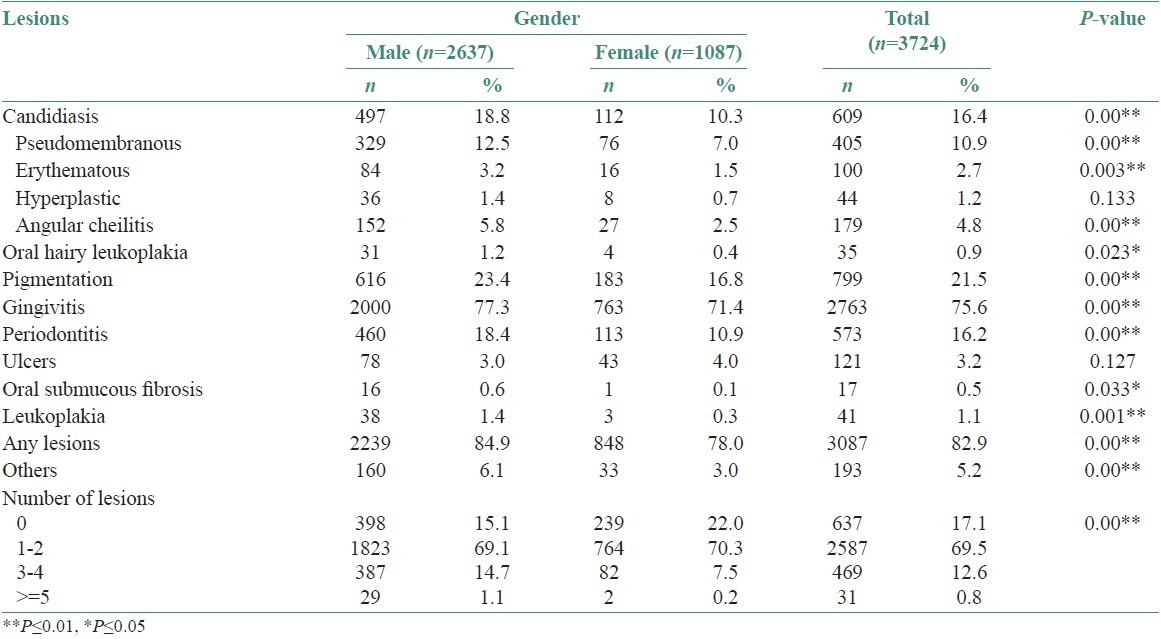
Seven hundred and ninety-nine patients (616 males, 23.4% and 183 females, 16.8%, P = 0.00) presented with pigmentation. We observed this feature in the buccal mucosa and in the palate. Unlike the racial pigmentation, the patients presented with pigmented areas which varied from dark brown to brownish-black in color and were diffuse or in irregular patches.The mean hemoglobin percentage (Hb%) in males with pigmentation was 11.8% ± 2.38 and in females it was 10.95% ± 2.05 (P = 0.00). The mean Hb% in males without pigmentation was 12.34% ± 2.50 and in females it was 11.33% ± 1.93 (P = 0.00). Over all of the 799 patients, who had pigmentation, 45.6% of them had the habit of smoking (58.8% males n = 362, 1.1% females, n = 2), OC was present in 16.4% patients (males: 18.8%, females:10.3% P = 0.00). 10.9% patients presented with pseudomembranous candidiasis (PC) (males 12.5%, females 7% P = 0.00), 2.7% (males 3.2%, females 1.5%, P = 0.003) had erythematous candidiasis (EC), 1.2% (males1.4%,1 females 0.7%, P = 0.113) had hyperplastic candidiasis (HC). OHL was seen in 0.9% of the patients (males1.2%; females 0.4%, p = 0.023). Ulcers were seen in 3.2% patients (males 3% females 4%, P = 0.127). Seventeen patients had (males 0.6%, females 0.1%) had OSMF, 41 (males1.4% and females 1.1%) had leukoplakia.
CD4 counts were available for 2651 patients. 1022 patients had C4 counts less than 200 and 1629 patients had CD4 counts greater than 200. There were significant differences between oral lesions between the groups. OC (19.8% in ≤ 200, 9% in > 200, P = 0.00), Gingivitis (81.6% in CD4 ≤ 200, 76.3% in > 200 P = 0.001), Pigmentation (26.4% in CD4 counts ≤ 200 16.5% in > 200, P = 0.00), ulcers (4.6% CD4 counts ≤ 200, 2.1% > 200, P = 0.00). When oral lesions were compared between gender, in those with CD4 counts ≤ 200, though there were no significant differences in oral lesions between gender females had lesser prevalence of oral lesions than males. But when the occurrence of oral lesions were compared between gender with CD4 counts > than 200 there were significant differences in the occurrence of OC (males: 10.8%, females 5.6%, P = 0.00), pigmentation (males: 18.9%, females 12.1%, P = 0.00), periodontitis (males: 14%, females: 7.9% P = 0.000).
Eight hundred and fifty-eight patients were on HAART (627 males, 231 females) and 2866 (2010 males, 656 females) were not on HAART. Between the patients on HAART and non-HAART, there significant differences in the occurrence of OC (non-HAART: 18.2%, HAART: 10.1%, P = 0.00), gingivitis (non-HAART: 81.1%, HAART 73.9%, P = 0.00), periodontitis (non-HAART: 11.9%, HAART: 10.7% P = 0.00) and OHL (non-HAART group: 1.2%, HAART:0.1%, P = 0.002). Even though female patients had lesser lesions than males, there were no significant differences between the occurrences of oral lesions between gender in HIV patients who were on HAART. In patients not on HAART, there were significant differences in the occurrence of OC (males: 21.2%, females: 11.1%, P = 0.00), gingivitis (males: 75.9%, females: 69.2%, P = 0.00), pigmentation (males: 23.3 %, females: 16.1%, P = 0.00), periodontitis (males: 20.3% females: 11.9%, P = 0.00) and OHL (males: 1.5%, females: 0.5%, P = 0.00).
The mean CD4 counts in males (n = 1908) was 284.48 ± 222.45 and in females (n = 1087) it was 394.51 ± 274.56.
We computed the odds ratio, with reference to the females, the risk of males acquiring each oral lesion [Figure 1]. Males had 2.2 times,1.5 times, 3.2 times higher risk of getting OC, pigmentation, OHL respectively and over all males had 1.58 times greater risk of having any oral lesion compared to females.
Figure 1.
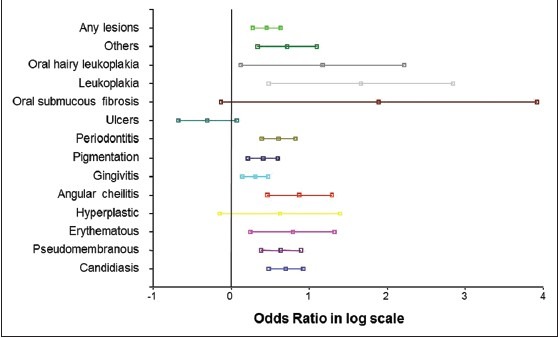
Odds Ratio for oral lesions in males with refence to females
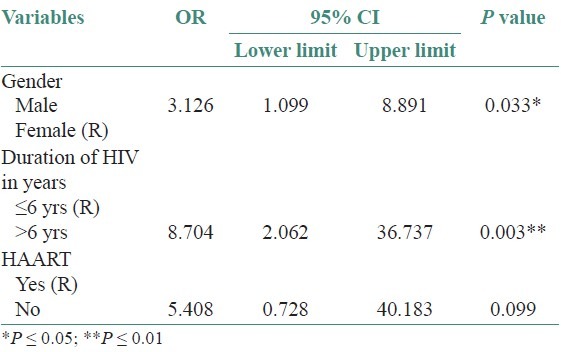
Table 2 shows multivariate logistic regression for OC. The odds of having OC was 1.7 times higher for males than for females after controlling for duration of being HIV positive, CD4 counts and HAART. Table 3 shows multivariate logistic regression for OHL. The odds of having OHL was 3.1 times higher for males than for females after controlling for duration of being HIV positive, and HAART. Since the difference in mean CD4 counts was not significant between genders for OHL, in the OHL model, we included only gender, duration of being HIV positive and HAART. This difference between gender was statistically significant in OC (OR = 1.7, 95%CI 1.2-2.2; P = 0.001) and OHL (OR =3.1, 95%CI 1.1- 8.89; P = 0.03) and showed a strong association between gender and occurrence of OC and OHL.
Table 2.
Multivariate logistic regression for OC
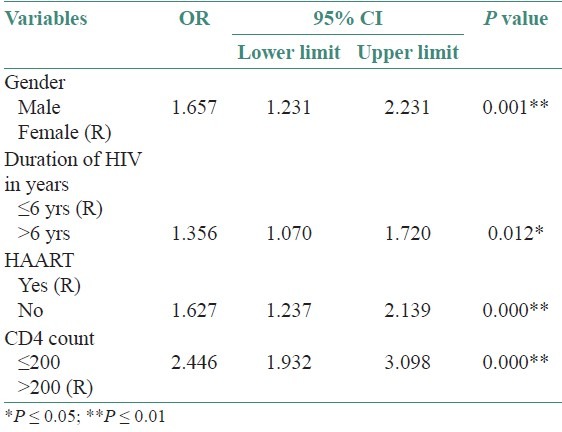
Table 3.
Multivariate logistic regression for OHL
Given the occurrence of oral lesions significantly greater in males than in the females and higher mean CD4 counts in females, we further analyzed the following to understand the gender difference with reference to the spouse HIV status, duration of HIV infection and HAART.
Of the 3724 HIV-positive patients, HIV status of the spouses was available for 2886 patients. 1412 patients had their spouses HIV status also as HIV positive and 769 patients had their spouse HIV status as negative. Six hundred and eighty-four (35%) spouses of males and 728 (78.7%) spouses of females were tested HIV-positive, 689 (35%) spouses of males and 80 (9%) spouses of females tested HIV negative, 588 spouses of males (30%) and 117 (12.6%) spouses of females were not tested.
Of the 1412 HIV-positive patients, whose spouse were also HIV positive, source of infection was available for 1393 patients (672 males and 721 females). Ninety-seven percent and 96.9% of males spouse and females spouse respectively acquired HIV infection through the heterosexual route. Of the 769 HIV-positive patients whose spouses were HIV negative, information about source of HIV infection was available only for 754 patients (675 males and 79 females). Ninety-four percent of males and 62% of females got the infection through heterosexual route and 34% of the females got the infection through blood transfusion.
The mean duration of HIV infection in males since detected (n = 2636) was 6.56± 2.75 years and in females (n = 1087), it was 6.36± 2.89 years. Clinically significant difference was not present with respect to duration of HIV infection between gender and their spouse HIV status.
For male HIV-positive patients, when their spouse is HIV positive the mean CD4 count was 261 ± 201 and when the spouse is HIV negative it was 305 ± 231 (P = 0.002). For female HIV-positive patients, when their spouse is HIV positive the mean CD4 count was 407 ± 276 and when the spouse is HIV negative the mean CD4 count was 409 ± 251, (P = 0.152). Given this finding, we further analyzed if this difference with respect to mean CD4 counts and spouse HIV status was influenced by the duration of being HIV positive. Correlation between the duration of being HIV positive and CD4 counts was done to study if there was any association. The correlation was r = 0.005; (P = 0.787) and it shows that there was no significant difference between duration of HIV infection and CD4 counts.
The mean CD4 counts of the group on HAART (n = 841) and not on HAART (n = 1810) was 267 ± 213 and 338 ± 253 (P = 0.00), respectively. We stratified the same for gender.CD4 counts for males and females who were on HAART was 243 ± 195 and 332 ± 243 (P = 0.00) and not on HAART it was 304 ±232 and 422 ± 283 (P = 0.00), respectively. This denotes that, the female patients in our study group were having significantly higher CD4 counts than males even when stratified for HAART and this finding helps to understand that, HAART does not explain the association between gender and CD4 counts.
When the corelation was done between males and females with respect to CD4 counts, the r-value was 0.203 (P = 0.00). The partial correlation analysis done between gender and CD4 counts, when controlling for HAART was r = 0.2028 (P = 0.00).
DISCUSSION
Gender differences have been demonstrated in HIV positive patients presenting for the initiation of ART in diverse parts of the world with respect to clinical, immunological and viral characteristics.[2,13] It has also been documented that in females with HIV infection, significantly low viral load than males is seen and they have 1.6 times higher risk of developing AIDS as compared to the males with a similar viral load.[5,6,14–17]
As nearly half of the people living with HIV globally are females,[18] there is a need for studies focusing on gender based differences, especially in the era of ART.[19] Our center, in an earlier study has shown that the female patients had higher median CD4 lymphocyte counts and greater survival rates than the male patients.[20] We have also established that there are significant physiological, immunological, and clinical differences between males and females initiated on HAART.[2,21]
Oral lesions have established, diagnostic and prognostic value in HIV infection.[7,22] Though many epidemiological studies of HIV-related oral lesions have been done in different centers and clinical settings globally,[23–26] only a few have focused on the role played by gender in the occurrence of such diseases.[27–30]
In this study, females constituted 29% compared with 26% in our earlier study.[9] Studies from various parts of the world have reported diverse gender distribution. In a gender based study from Mexico,[31] Italy[32] and America[30] females constituted 13%, 33% and 51%, respectively. This difference could be attributed to the differences in source of acquiring the infection. It was predominantly heterosexual route among males (95%) and females (92%) in our study, whereas in the Italian study it was intravenous drug usage (41% in females, 61% in males) followed by heterosexual and homosexual route. In the Mexican study it was predominantly by blood transfusion in females (63%) whereas in the males it was the heterosexual route (90%).
In the present cohort the percentage of HIV-positive status of the spouses was more among the females than the males and only 9% of the spouses of the females were HIV negative. When we looked into the route of transmission for those who had their spouse status as HIV positive, 97% of males and females acquired it through heterosexual route. Supporting this, our earlier report states that, for the Indian females, being married and monogamous were the major risk factors for acquiring HIV infection, implying the high-risk activity among their spouses.[33]
The occurrence of all the oral lesions (except ulcers) were significantly higher in males compared to females. OC has been associated with HIV infection and is one of the earliest clinical manifestation of immunosuppression and indicator of HIV disease.[34] The prevalence of OC in both the genders in this study were reduced compared to our previous study due to early antifungal therapy and OC was also significantly associated with CD4 counts lower than 200 cells/μl.
In three epidemiological cohorts studied by Shiboski et al.[30] to investigate the influence of gender in the occurrence of oral lesions in HIV infected patients, OC was higher in males (24%) than in females (13%). Arendorf et al.[35] in a South African study of 600 HIV positive patients reported a higher prevalence (46%) in females and a lower prevalence (36%) in males. Similar findings of significantly higher prevalence in females (35%) than males (19%) was also seen in the Italian study by Campisi et al.[32] At the other end of the spectrum Ramirez-Amador et al.[31] did not observe any difference in the prevalence of OC between males and females in a cohort of 436 HIV infected patients in Mexico. In this study, the prevalence of PC was higher than EC overall and PC was significantly higher in males than in females consistent with our previous studies.[9,10] In the Mexican study,[31] EC was significantly higher than PC and was also associated with blood transfusion. OC was also significantly associated with CD4 counts lower than 200 cells/μl in our study.
Males in our cohort had 1.7 times higher risk of getting OC than females after controlling for duration of being HIV positive, CD4 counts and HAART. Similar trend in gender differences was found in the study by Shiboski et al.[30] where the adjusted odds ratio for males was 1.8 times higher than the females irrespective of the length of follow-up.
OHL was seen in less than 1% of our cohort and its occurrence was significantly higher in males than in females. Shiboski et al. reported that the odds of presenting with OHL was 2.5 times higher for males than females and accounted this finding to the difference in the mode of expression of Epstein-Barr virus. In our study, males had 3.1 times higher odds for getting OHL compared to females after controlling for duration of being HIV-positive and HAART.
Pigmentation (other than racial) also exhibited gender difference in our study with a higher prevalence in males than in females. However, smoking habit, anemia, antiretrovirals, were confounding factors that need further longitudinal studies.
Significant differences with respect to conventional periodontitis and gingivitis were seen between genders. However, the high prevalence of these lesions in our normal population, limits the significance of occurrence of this lesion, in the context of immunosuppression.
Seventeen patients (16 males and 1 female) had oral submucous fibrosis and 41 patients had (38 males and 3 males) had leukoplakia, due to the habit of chewing arecanut, tobacco and smoking tobacco.
There were significant differences in the occurrence of OC and OHL between those on and not on HAART. This finding was consistent with our earlier study.[36] In patients on HAART we did not observe any significant difference between genders, but in patients not on HAART there was significant increased occurrence of OC and OHL in males compared to females.
Our study confirms that males had a higher risk of oral lesions, especially OC and OHL, than females. Irrespective of the duration of being HIV positive, on HAART, and the HIV status of the spouse, the females in this study had a significantly higher mean CD4 counts than males.
Napravnik et al.,[4] in their meta-analysis of gender differences in HIV RNA levels stated that females tend to have a lower plasma HIV RNA levels than males, but were unable to explain a biological mechanism for this gender difference. Hormonal differences could contribute to sex-related differences in viral load.[5,36,37] It has also been stated that females generally experience more effective cell-mediated immunity than males, which could probably explain the greater control of virus production in the early stages of HIV disease and the difference in the immunity pattern between gender.[13] Earlier reports[20] from our center have shown that female patients presented with a greater median CD4 counts and survival rates than male patients and it was also confirmed in our later study.[2] A similar trend was seen in those females for up to one year after initiation of HAART. The probable explanation given for this finding was that asymptomatic Indian women always almost accompanied their spouses for medical care. Thus they had the opportunity to know their HIV status at an earlier stage, unlike their spouses who were diagnosed in the context of medical care for a persistent opportunistic infection at a more advanced stage of the disease.
It has also been postulated that,[38] Toll-like receptors which could modify innate and adaptive immunity to viruses, could be involved in the sex-related variability in response to viral infections and it has been established that sex differences in TLR-mediated activation of plasmacytoid dendritic cells (pDC’ cells)2 could account for higher immune activation in females compared to males at a given HIV–viral load. This may facilitate a mechanism by which HIV disease progresses faster in females compared to males.
The findings of the present study must be interpreted in the context that this is a cross-sectional study and we were unable to perform CD4 counts for all the patients, due to resource constraints. The different immunological status of the females compared to males should be taken in to consideration in the evaluation and management of these patients. Treatment protocols need to factor in these differences to enable optimum management of these HIV positive patients.
ACKNOWLEDGMENTS
We thank the management and Principal, Ragas Dental College and Hospital for their support. R. Hemalatha, Bio-statistician for her inputs in this manuscript. Support of Dr. S. Balasundaram is gratefully acknowledged by authors.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.HIV Sentinel Surveillance and HIV Estimation in India 2007. A Technical Brief published by National AIDS Control Organization, Ministry of Health and Family Welfare, Government of India. [Last accessed on 2012 Jan]. Available from: https://www.nacoonline.org .
- 2.Kumarasamy N, Venkatesh KK, Cecelia AJ, Devaleenol B, Saghayam S, Yepthomi T, et al. Gender-based differences in treatment and outcome among HIV patients in South India. J Women's Health. 2008;17:1–5. doi: 10.1089/jwh.2007.0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meier A, Chang J, Chan ES, Pollard RB, Sidhu HK, Kulkarni S, et al. Sex differences in the TLR-mediated response of pDCs to HIV-1 are associated with higher immune activation in infected women. Nat Med. 2009;15:955–9. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naravink S, Poole C, Thomas JC, Eron JJ., Jr Gender differences in HIV RNA levels. J Acquir Immune Defic Syndr. 2002;31:11–9. doi: 10.1097/00126334-200209010-00002. [DOI] [PubMed] [Google Scholar]
- 5.Farzadegan H, Hoover DR, Astemborski J, Lyles CM, Margolick JB, Markham RB, et al. Sex differences in HIV-1 viral load and progression to AIDS. Lancet. 1998;352:1510–4. doi: 10.1016/S0140-6736(98)02372-1. [DOI] [PubMed] [Google Scholar]
- 6.Lyles CM, Dorrucci M, Vlahov D, Pezzotti P, Angarano G, Sinicco A, et al. Longitudinal human immunodeficiency virus type 1 load in the Italian seroconversion study: Correlates and temporal trends of virus load. J Infect Dis. 1999;180:1018–24. doi: 10.1086/314980. [DOI] [PubMed] [Google Scholar]
- 7.Greenspan JS. Sentinels and signposts: The epidemiology significance of oral manifestations of HIV disease. Oral Dis. 1997;3(Suppl 1):S13–7. doi: 10.1111/j.1601-0825.1997.tb00344.x. [DOI] [PubMed] [Google Scholar]
- 8.Shiboski CH, Hilton JF, Greenspan D, Westenhouse JL, Derish P, Vranizan K, et al. HIV-related oral manifestations in two cohorts of women in San Francisco. J Acquir Immune Defic Syndr Hum Retrovirol. 1994;7:964–71. [PubMed] [Google Scholar]
- 9.Ranganathan K, Reddy BV, Kumarasamy N, Solomon S, Vishwanathan R, Johnson NW. Oral lesions and conditions associated with human immunodeficiency virus infection in 300 South Indian patients. Oral Dis. 2000;6:152–7. doi: 10.1111/j.1601-0825.2000.tb00326.x. [DOI] [PubMed] [Google Scholar]
- 10.Ranganathan K, Umadevi M, Saraswathi TR, Kumarasamy N, Solomon S, Johnson N. Oral lesions and conditions associated with human immunodeficiency virus infection in 1000 South Indian patients. Ann Acad Med Singapore. 2004;33(Suppl):37S–42S. [PubMed] [Google Scholar]
- 11.Greenspan JS, Barr CE, Sciubba JJ, Winkler JR. Oral manifestations of HIV infection: Definitions, diagnostic criteria and principles of therapy. Oral Surg Oral Med Oral Pathol. 1992;73:142–4. doi: 10.1016/0030-4220(92)90185-s. [DOI] [PubMed] [Google Scholar]
- 12.Classification and diagnostic criteria for oral lesions in HIV infection and WHO collaborating center on oral manifestations of the immunodeficiency virus. J Oral Pathol Med. 1993;22:289–91. [PubMed] [Google Scholar]
- 13.Grinsztejn B, Smeaton L, Barnett R, Klingman K, Hakim J, Flanigan T, et al. Sex-associated differences in pre-antiretroviral therapy plasma HIV-1 RNA in diverse areas of the world vary by CD4 cell count. Antivir Ther. 2011;16:1057–62. doi: 10.3851/IMP1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans JS, Nims T, Cooley J, Bradley W, Jagodzinski L, Zhou S, et al. Serum levels of virus burden in early-stage human immunodeficiency virus type 1 disease in women. J Infect Dis. 1997;175:795–800. doi: 10.1086/513973. [DOI] [PubMed] [Google Scholar]
- 15.Gandhi M, Bacchetti P, Miotti P, Quinn TC, Veronese F, Greenblatt RM. Does patient sex affect human immunodeficiency virus levels? Clin Infect Dis. 2002;35:313–22. doi: 10.1086/341249. [DOI] [PubMed] [Google Scholar]
- 16.Katzenstein DA, Hammer SM, Hughes MD, Gundacker H, Jackson JB, Fiscus S, et al. The relation of virologic and immunologic markers to clinical outcomes after nucleoside therapy in HIV-infected adults with 200 to 500 CD4 cells per cubic millimetre. AIDS Clinical Trials Group Study 175 Virology Study Team. N Engl J Med. 1996;335:1091–8. doi: 10.1056/NEJM199610103351502. [DOI] [PubMed] [Google Scholar]
- 17.Sterling TR, Lyles CM, Vlahov D, Astemborski J, Margolick JB, Quinn TC. Sex differences in longitudinal human immunodeficiency virus type 1 RNA levels among seroconverters. J Infect Dis. 1999;180:666–72. doi: 10.1086/314967. [DOI] [PubMed] [Google Scholar]
- 18.UNAIDS. UNAIDS/ WHO AIDS epidemic update. [Last accessed on 2012 Jan]. Available from: http://www.unaids.org/epidemic.update/
- 19.Gandhi M, Aweeka F, Greenblatt RM, Blasche TF. Sex differences in pharmacokinetics and pharmacodynamics. Ann Rev Pharmacol Toxicol. 2004;44:499–523. doi: 10.1146/annurev.pharmtox.44.101802.121453. [DOI] [PubMed] [Google Scholar]
- 20.Kumarasamy N, Solomon S, Flanigan TP, Hemalatha R, Thyagarajan SP, Mayer KH. Natural history of human immunodeficiency virus disease in Southern India. Clin Infect Dis. 2003;36:79–85. doi: 10.1086/344756. [DOI] [PubMed] [Google Scholar]
- 21.Patton LL, Mckaig RG, Eron JJ, Lawrence HP, Strauss RP. Oral hairy leukoplakia and oral candidiasis as predictors of HIV viral load. J Oral Pathol Med. 1999;28:173–7. doi: 10.1097/00002030-199910220-00026. [DOI] [PubMed] [Google Scholar]
- 22.Hodgson TA, Greenspan D, Greenspan JS. Oral lesions of HIV disease and HAART in industrialized countries. Adv Dent Res. 2006;19:57–62. doi: 10.1177/154407370601900112. [DOI] [PubMed] [Google Scholar]
- 23.Ranganathan K, Hemalatha R. Oral lesions in HIV infection in developing countries: An overview. Adv Dent Res. 2006;19:63–8. doi: 10.1177/154407370601900113. [DOI] [PubMed] [Google Scholar]
- 24.Phelan JA, Saltzman BR, Friedland GH, Klein RS. Oral findings in patients with acquired immunodeficiency syndrome. Oral Surg Oral Med Oral Pathol. 1987;64:50–6. doi: 10.1016/0030-4220(87)90116-2. [DOI] [PubMed] [Google Scholar]
- 25.Feigal DW, Katz MH, Greenspan D, Westenhouse J, Winkelstein W, Jr, Lang W, et al. The prevalence of oral lesions in HIV-infected homosexual and bisexual men: Three San Francisco epidemiologic cohorts. AIDS. 1991;5:519–25. doi: 10.1097/00002030-199105000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Barone R, Ficarra G, Gaglioti D, Orsi A, Mazzotta F. Prevalence of oral lesions among HIV-infected intravenous drug abusers and other risk groups. Oral Surg Oral Med Oral Pathol. 1990;69:169–73. doi: 10.1016/0030-4220(90)90322-j. [DOI] [PubMed] [Google Scholar]
- 27.Imam N, Carpenter CC, Meyer KH, Fisher A, Stein M, Danforth SB. Hierarchical pattern of mucosal candida infections in HIV-seropositive women. Am J Med. 1990;89:142–6. doi: 10.1016/0002-9343(90)90291-k. [DOI] [PubMed] [Google Scholar]
- 28.Shiboski CH, Hilton JF, Greenspan D, Westenhouse JL, Derish P, Vranizan K, et al. HIV related oral manifestations in two cohorts of women in San Francisco. J Acquir Immune Defic Syndr. 1994;7:964–71. [PubMed] [Google Scholar]
- 29.Wanzala P, Manji F, Pindborg JJ, Plummer F. Low prevalence of oral mucosal lesions in HIV-1 seropositive African women. J Oral Pathol Med. 1989;18:416–8. doi: 10.1111/j.1600-0714.1989.tb01574.x. [DOI] [PubMed] [Google Scholar]
- 30.Shiboski CH, Hilton JF, Neuhaus JM, Canchola A, Greenspan D. Human immunodeficiency virus-related oral manifestations and gender. The University of California, San Fransisco Oral AIDS Centre Epidemiology Collaborative Group. Arch Intern Med. 1996;156:2249–54. doi: 10.1001/archinte.156.19.2249. [DOI] [PubMed] [Google Scholar]
- 31.Ramirez-Amador V, Esquivel-Pedraza L, Sierra-Madero J, Ponce-de-Leon S, Ponce-de-Leon S. Oral manifestations of HIV infection by gender and transmission category in Mexico City. J Oral Pathol Med. 1998;27:135–40. doi: 10.1111/j.1600-0714.1998.tb01929.x. [DOI] [PubMed] [Google Scholar]
- 32.Campisi G, Pizzo G, Mancuso S, Margiotta V. Gender differences in human immunodeficiency virus-related oral lesions: An Italian study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:546–51. doi: 10.1067/moe.2001.113548. [DOI] [PubMed] [Google Scholar]
- 33.Newmann S, Sarin P, Kumarasamy N, Amalraj E, Rogers M, Madhivanan P, et al. Marriage, monogamy and HIV: A profile of HIV-infected women in south India. Int J STD AIDS. 2000;11:250–3. doi: 10.1258/0956462001915796. [DOI] [PubMed] [Google Scholar]
- 34.Egusa H, Soysa NS, Ellepola AN, Yatani H, Samaranayake LP. Oral Candidosis in HIV-Infected Patients. Curr HIV Res. 2008;6:485–99. doi: 10.2174/157016208786501445. [DOI] [PubMed] [Google Scholar]
- 35.Arendorf TM, Bredekamp B, Cloete CA, Sauer G. Oral manifestations of HIV infection in 600 South African patients. J Oral Pathol Med. 1998;27:176–9. doi: 10.1111/j.1600-0714.1998.tb01936.x. [DOI] [PubMed] [Google Scholar]
- 36.Umadevi KM, Ranganathan K, Pavithra S, Hemalatha R, Saraswathi TR, Kumarasamy N, et al. Oral lesions among persons with HIV disease with and without highly active antiretroviral therapy in Southern India. J Oral Pathol Med. 2007;36:136–41. doi: 10.1111/j.1600-0714.2006.00505.x. [DOI] [PubMed] [Google Scholar]
- 37.Touloumi G, Pantazis N, Babiker AG, Walker SA, Katsarou O, Karafoulidou A, et al. CASCADE collaboration differences in HIV RNA levels before the initiation of antiretroviral therapy among 1864 individuals with known HIV-1 seroconversion dates. AIDS. 2004;18:1697–705. doi: 10.1097/01.aids.0000131395.14339.f5. [DOI] [PubMed] [Google Scholar]
- 38.Torcia MG, Nencioni L, Clemente AM, Civitelli L, Celestino I, Limongi D, et al. Sex differences in the response to viral infections: TLR8 and TLR9 ligand stimulation induce higher IL10 production in males. PLoS ONE. 2012;7:e39853. doi: 10.1371/journal.pone.0039853. [DOI] [PMC free article] [PubMed] [Google Scholar]


