Abstract
Parry Romberg syndrome is a rare disorder of unknown etiology, seen most commonly as an asymmetry of the face, rarely affecting the limbs. Trophic malfunction of sympathetic system has been proposed as a cause. The syndrome presents with characteristic skeletal, dental, and soft tissue changes in the affected half of the face, with or without neurological signs and symptoms. Imaging studies sometimes reveal lesions in the brain corresponding with the neurological defects. The disfiguring nature of the disease Results in psychological disturbance and communication disorders like speech defects, as also dental anomalies. The present article reports such a case of an 8-year-old girl who presented with mainly hard tissue changes, without neurological or intraoral soft tissue changes. There has to be prompt multi-disciplinary management of such cases keeping in mind development, aesthetics, speech, and masticatory function, along with symptomatic treatment of neurological deficits.
Keywords: Parry Romberg syndrome, hemifacial atrophy, progressive facial hemiatrophy
INTRODUCTION
Parry Romberg syndrome is a rare disorder of unknown etiology. First described by Parry in 1825 and Romberg in 1846, the term progressive facial hemiatrophy (PFH) was coined by Eulenberg in 1871.[1] The syndrome is characterized by slowly progressive atrophy involving one side of the face. The affected individuals are morphologically normal at birth, the atrophy being insidious, occurring within the first two decades of life. This is usually limited to the face, but rarely involves unilateral limbs. It exhibits similarity to localized scleroderma, with atrophy of skin and subcutaneous fat, and rarely muscles and bone. After a progressive phase which may span up to 20 years, the process stabilizes. The deformities suffered are usually permanent. Seen more often in females, it commonly affects the eyes and is sometimes (15%) associated with neurological disorders like trigeminal neuralgia, facial paresthesia, headache, and focal epilepsy.[2]
The disease is thought to be a unilateral inflammatory process associated with chronic vascular disturbance or neurogenic cause. A few cases have been reported with a genetic or hereditary character. Other postulated causes include autoimmunity, trauma, and endocrine and metabolic disorders. Sporadic cases have been observed with Lyme disease and positive serology for Borrelia burgdorferi.[3]
Diagnosis is based on history and clinical features. Histopathological evidence of epidermal atrophy and dermal fibrosis is akin to scleroderma. Imaging studies like cranial computed tomography (CT) and magnetic resonance images (MRI) show numerous neurological and vascular lesions.
PFH is usually self-limiting. Immunosuppressants and corticosteroids are the treatment of choice in active disease state or if occurring concomitant with auto-immune disorders. The facial deformities may be corrected by reconstructive techniques involving grafting and other plastic surgical methods.
We report a unique case of Parry Romberg syndrome in an 8-year old girl, with no neurological lesions, presenting with delayed dental development and arrested root formation.
CASE REPORT
An 8-year-old girl presented with complaint of facial deformity and crooked teeth. History from her mother revealed that until the age of 5 years, the patient's face was quite normal, which was confirmed by photographs [Figure 1]. After the age of 5, a slow “shrinking” of the right side of the face was noted by her mother. She suffered no other systemic problems. There were no symptoms of neurological disturbances, and her vision was normal. There was no history of family members with similar complaints, and no incidences of trauma or infection were observed to coincide with or precede the onset of the deformity.
Figure 1.
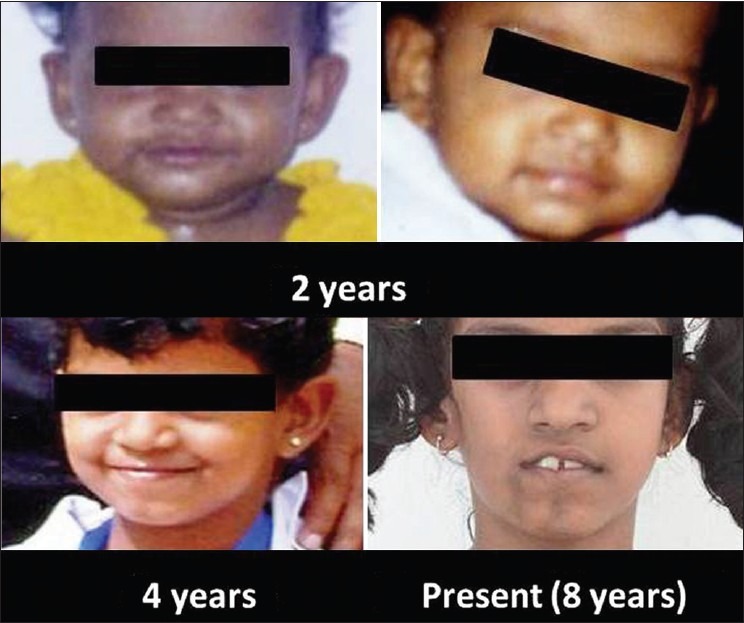
Photographs from ages 2 to 8 revealing the progressive nature of the defect
Clinical examination revealed a healthy girl with normal vital signs and systemic presentation. She was alert and cheerful, with no signs of mental or psychological instability. Speech and hearing were normal. The face was asymmetric due to a right-sided deformity. The right eyes were at a lower level compared to left resulting in an interpupillary cant. The right maxillary and zygomatic region appeared hypoplastic compared with the other side. The right half of upper and lower lip exhibited contracture with increased incisor show along with slight commissural lift. Nose and chin were deviated to the right side. There was a scar-like defect, particularly prominent in the symphyseal region (coup de sabre). Atrophy at all the facial levels (eyes, malar region, lips, and mandible) was observed. Ears were symmetric and normal. Skin and hair appeared normal except for the “scar” defect and a hyperpigmented patch just below the right commissure [Figure 2]. Intraoral examination revealed mixed dentition stage (dental age corresponded to chronological age) with class I molar relation, flared upper incisors (so-called ugly duckling stage), crowded lower anterior teeth, and unilateral cross bite in the right side [Figure 3]. The occlusal plane demonstrated canting down towards the left. Intraoral soft tissue examination, particularly of tongue, revealed no significant symmetry.
Figure 2.
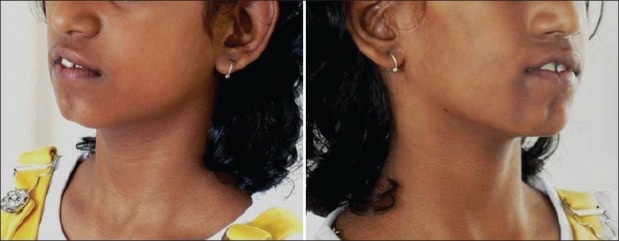
Clinical photograph of the patient showing facial defects
Figure 3.
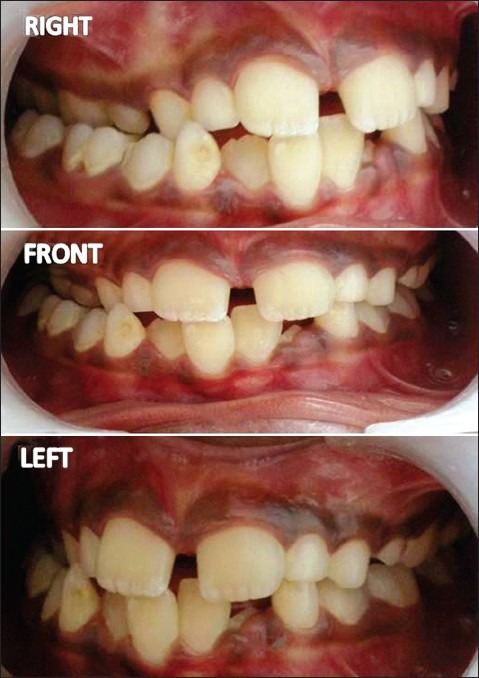
Intraoral photograph showing dental changes
A preliminary diagnosis of hemifacial atrophy was given prior to investigations. Routine blood tests, genetic testing, antibody testing, electromyography, radiographs and ultrasound/CT scans were performed. Genetic testing revealed no chromosomal abnormalities. Patient was negative for antinuclear antibodies. Ultrasound scan and CT of brain showed no significant abnormality. Ocular electromyography detected motor atrophy in right orbicularis oculi. CT scan of the face revealed atrophy of the zygomatic bone, maxillary bone, and its sinus on the right side. Right orbital floor was more inferior compared with the left. Mandible, however, revealed no gross asymmetry but for the smaller coronoid process on the right [Figures 4 and 5].
Figure 4.
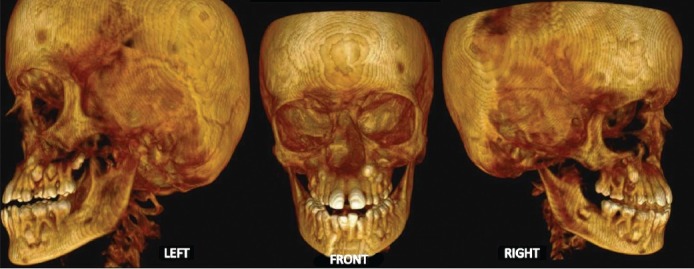
CT scan of the face showing skeletal defects
Figure 5.
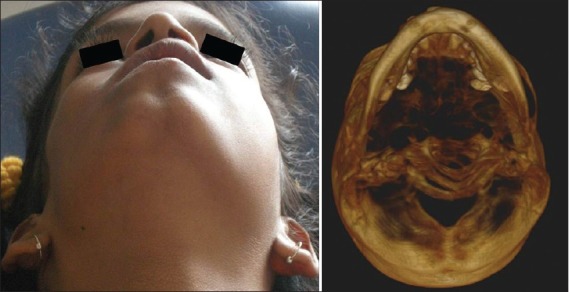
Photograph and CT scan showing skeletal defects
Radiographically, there was a clear discordance in development of the right and left maxillary teeth. Premolars and second molars were less developed in the upper right quadrant compared with their counterparts in the upper left quadrant. Mandibular teeth showed no such significant differences in development. Mandibular incisors on the right side, especially the central incisor, showed arrested root development [Figure 6].
Figure 6.
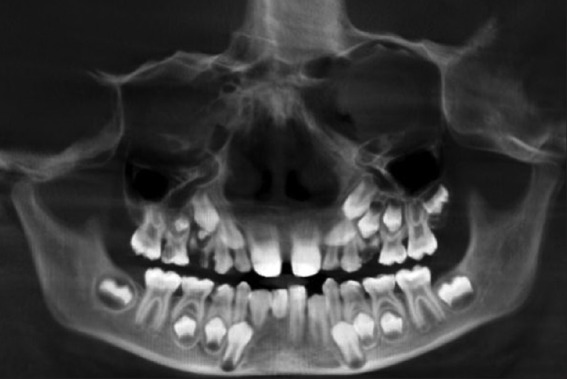
Orthopantomograph showing skeletal and dental findings
Masseter muscle biopsy on the right side revealed focal mononuclear infiltration in the dermis and surrounding hair follicles with mild vasculitis. Skin and muscle showed no significant abnormalities.
Based on the clinical and radiographic findings, the diagnosis of progressive hemifacial atrophy was made. Since the patient was in remarkable health, her dental and facial deformity became the major concern for treatment. It is currently planned to correct cross-bite with a slow palatal expansion appliance and then proceed to a hybrid appliance for correction of occlusal cant. Since the right premolars and molars showed severe retardation; eruption may be delayed or unlikely. Tooth extrusion or replacement options for premolars are to be considered at the next stage. Surgical procedures for facial deformity, once major growth completes, are being considered, keeping a close look on disease progression.
DISCUSSION
Parry Romberg syndrome/PFH is an uncommon condition comprising unilateral atrophy of the face, which rarely even affects the trunk and limbs. More strict definitions of the syndrome include contralateral jacksonian epilepsy, trigeminal neuralgia, and changes in the eyes and hair.[1] The onset of the disease is in the first two decades, with a progressive phase lasting 2–20 years, and then a stable phase sets in, usually culminating in resolution. The facial features may be similar to limited scleroderma (coup de sabre lesion), and many workers suggest that the two disorders are part of the same entity. However, there is no consensus on this classification.[2–5]
Etiology is not clear, although the primary cause appears to be a cerebral lesion. Viral infections, endocrine disturbances, trauma, auto-immunity, and hereditary causes have also been suggested. Isolated cases have been observed with positive serology for Lyme disease (Borrelia burgdorferi).[6,7]
Many authors propose trophic malfunction of sympathetic nervous system as a cause, since normal development of skin, muscle, and bone require trophic stimulation. Experimental studies, which performed unilateral sympathectomy or ganglion ablation, have produced clinical features of the syndrome in lower animals but not in humans. Similar symptoms have been attributed to lesions of the mesencephalotruncal and superior cervical sympathetic ganglia. Cory et al. postulated that there is a diffuse self-limiting inflammatory process associated with the cervical sympathetic ganglia and carotid plexus, resulting in hyperactivity of the sympathetic system. They described a case of a 5 year old with initial migraine-type headaches accompanied by sensorimotor symptoms, followed by development of typical facial changes.[8] On the other hand, Blitstein and Vecchione suggested that the primary event is a neurovasculopathy and associated it with the systemic vasculitis of scleroderma. Their finding of cerebral micro-hemorrhages has been supported by others.[7,9]
The syndrome presents with a unilateral atrophy of the skin, subcutaneous fat, and rarely muscle and bone, resulting in facial asymmetry. Earliest change is observed as a cleft near the facial midline. Skin is tensed or fibrosed with loss or gain of pigmentation. Hair defects when present, manifests as focal baldness and facial hair loss. The changes involve the dermatome at one or more branches of the trigeminal nerve. Ocular changes include enophthalmos due to loss of periorbital fat, and rarely uveitis and retinal vasculitis.[10] Ear on the affected side may be smaller, although no functional defects in the eye and ear are observed.[11]
The oral mucosa and tongue can be affected, as also are jaws, salivary glands, and teeth. There is deviation of the mouth and nose toward the affected side. There may be a unilateral reduction in tongue size (not observed in the present case) along with jaw hypoplasia.[6] Roots of teeth are often poorly developed or resorbed, resulting in delayed eruption of teeth on the affected side. This may cause a unilateral posterior open bite. Because in the present case predominant atrophy was in the right maxilla, cross-bite was the result.
Lip atrophy and unilateral exposure of teeth are common. Tooth size, vitality, and facial muscular functions are usually normal, although muscle wasting has been described in certain cases.[11]
Osseous defects are usually seen when the atrophy manifests before 15 years of age. Fronto-maxillary defects are seen in before-5-year onsets; mandibular defects in 5–15 year onsets, and later onsets (>15 years) have almost exclusively soft tissue changes. Jaws are smaller unilaterally in all dimensions, resulting in a midline shift towards the affected side, along with delay in mandibular angle development. Bony healing is affected after exodontia in the affected side.[12]
Neurological symptoms include trigeminal neuralgia, migraine-type headache, facial paresthesia, and focal epilepsy/neurological deficits. Imaging studies reveal ipsilateral cerebral changes. CT of the brain reveals focal cerebral hypodensities and intracranial calcifications; and MRI show focal white matter enhancement, cortical thickening and meningeal enhancement. This has been described as meningocortical dysmorphism. A few workers have found ipsilateral cerebral atrophy but that has not been supported by other studies. Vascular anomalies like intracranial aneurysms, reversible vasoconstriction, and vascular malformations have also been reported. Electroencephalographs (EEG) do not reveal obvious epileptiform changes.[8,13–15] It is to be noted in the present case there are no neurological signs or symptoms, and no lesions in the brain.
Histopathological examination of skin reveals atrophy of the epithelium and dermal tissue with fibrosis (if clinically resembling linear scleroderma) and loss of subcutaneous fat, along with atrophy of hair follicles. Perivascular chronic inflammation is variable, and distinguishes from localized scleroderma where the perivascular inflammation is massive, especially in the early stages. Dermal elastic tissue is preserved in PFH but lost in scleroderma. Silver stain may be performed to rule out Borrelia burgdorferi infection.[7,8,13]
Diagnosis of Parry Romberg syndrome is based on patient history and clinical examination and is supported by imaging and histopathological studies. The severity of the condition depends on the age of onset and may or may not correlate with the extent of cerebral pathology.[9,16,17]
The disease is self-limiting, and has no definite cure. The active stage of the disease is usually treated with corticosteroids and immunosuppressant therapy. Cases associated with Lyme disease have been treated with antibiotics like parenteral penicillin and ceftriaxone. Phototherapy with UV-A radiation (340–400 nm) has been tried with success as it has been known to induce matrix metalloproteinase 1 (MMP-1) to reverse the fibrosis, although use is limited due to unpleasant adverse effects. Once the deformities have set in, plastic and reconstructive surgeries are recommended. Orthodontic treatment with hybrid appliances may be designed to manage the dental malocclusion.[7,18]
Jun et al. described two cases of the syndrome in the third and fourth decades of life where grafting, flap surgery, bone distraction, and steroid injections were attempted for the aesthetic management of the patients. They reported lack of success in surgical correction at such relatively advanced ages. Lazaridou et al. reported a case of a 14 year old, treated with topical steroids, and discussed the treatment protocols in young age. They pointed out the lack of sufficient literature regarding efficacy and safety of steroids and other drugs used to treat the syndrome.[19,20]
CONCLUSION
Parry Romberg syndrome is a disfiguring disease of uncertain origin where early diagnosis and prompt management is essential for optimal quality of life. The present case was diagnosed at 8 years of age and is undergoing treatment. If diagnosed much earlier in life, the developing facial deformity could have been prevented. More research is necessary to assess safety and efficacy of management of this incurable disease.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Regezi JA, Scuibba JJ, Jordan RC. Clinical pathological correlations. 4th ed. USA: Saunders: Elsevier Science; 2003. Oral pathology. [Google Scholar]
- 2.Buonaccorsi S, Leonardi A, Covelli E, Indrizzi E, Perdicchi A, Fini G. Parry-Romberg syndrome. J Craniofac Surg. 2005;16:1132–5. doi: 10.1097/01.scs.0000183466.08332.be. [DOI] [PubMed] [Google Scholar]
- 3.Kumar AA, Kumar RA, Shantha GP, Aloogopinathan G. Progressive hemi facial atrophy - Parry Romberg syndrome presenting as severe facial pain in a young man: A case report. Cases J. 2009;2:6776. doi: 10.4076/1757-1626-2-6776. (online) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stone J. Parry-Romberg syndrome: A global survey of 205 patients using the Internet. Neurology. 2003;61:674–6. doi: 10.1212/wnl.61.5.674. [DOI] [PubMed] [Google Scholar]
- 5.Tollefson MM, Witman PM. En coup de sabre morphea and Parry-Romberg syndrome: A retrospective review of 54 patients. J Am Acad Dermatol. 2007;56:257–63. doi: 10.1016/j.jaad.2006.10.959. [DOI] [PubMed] [Google Scholar]
- 6.Pinheiro TP, da Silva CC, de Silveira CS, Botelho PC, Pinheiro MG, Pinheiro Jde J. Progessive hemifacial atrophy – a case report. Med Oral Patol Oral Cir Bucal. 2006;11:E112–4. [PubMed] [Google Scholar]
- 7.Padmavathy J, Rao LL. Unilateral linear pansclerotic morphea affecting face and limbs. Indian J Dermatol Venereol Leprol. 2005;71:192–4. doi: 10.4103/0378-6323.16237. [DOI] [PubMed] [Google Scholar]
- 8.Cory RC, Clayman DA, Faillace WJ, McKee SW, Gama CH. Clinical and radiologic findings in progressive facial hemiatrophy (Parry-Romberg syndrome) AJNR Am J Neuroradiol. 1997;18:751–7. [PMC free article] [PubMed] [Google Scholar]
- 9.Blitstein MK, Vecchione MJ. Parry Romberg syndrome. [Last accessed on 2011 Sept 28];Appl Radiol. 2011 40:34–6. Available from: http://www.appliedradiology.com/Issues/2011/01/Cases/Parry-Rombergsyndrome.aspx . [Google Scholar]
- 10.Ong K, Billson FA, Pathirana DS, Clifton Bligh P. A case of progressive hemifacial atrophy with uveitis and retinal vasculitis. Aust N Z J Ophthalmol. 1991;19:295–8. doi: 10.1111/j.1442-9071.1991.tb00675.x. [DOI] [PubMed] [Google Scholar]
- 11.Mazzeo N, Fisher JG, Mayer MH, Mathieu GP. Progressive hemifacial atrophy (Parry-Romberg syndrome) Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:30–5. doi: 10.1016/s1079-2104(05)80069-1. [DOI] [PubMed] [Google Scholar]
- 12.Gómez-Diez S, Gallego-López L, López-Escobar M, Junquera- Gutiérrez L, Pérez-Oliva N. Progressive facial hemiatrophy with associated osseous lesions. Med Oral Patol Oral Cir Bucal. 2007;12:E602–4. [PubMed] [Google Scholar]
- 13.Bergler-Czop B, Święty AL, Brzezińska-Wcisło L. Scleroderma linearis: Hemiatrophia faciei progressiva (Parry-Romberg syndrome) without any changes in CNS and linear scleroderma “en coup de sabre” with CNS tumor. BMC Neurol. 2009;9:39. doi: 10.1186/1471-2377-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maletic J, Panos T, Dimitrios I, Taskos KN. Parry-Romberg syndrome associated with localized scleroderma. Case Rep Neurol. 2010;2:57–62. doi: 10.1159/000314927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pichiecchio A, Uggetti C, Eggito MG, Zappoli F. Parry Romberg syndrome with migraine and intracranial aneurysm. Neurology. 2002;59:606–8. doi: 10.1212/wnl.59.4.606. [DOI] [PubMed] [Google Scholar]
- 16.Okumura A, Ikuta T, Tsuji T, Kato T. Parry-Romberg syndrome with a clinically silent white matter lesion. AJNR Am J Neuroradiol. 2006;27:1729–31. [PMC free article] [PubMed] [Google Scholar]
- 17.Matute RG, Alonso RE. Parry Romberg syndrome. Med Clin (Barc) 2012;139:323. doi: 10.1016/j.medcli.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 18.Sudarshan R, Annigeri RG. Parry Romberg syndrome. Wien Klin Wochensch. 2010;122:606. doi: 10.1007/s00508-010-1482-2. [DOI] [PubMed] [Google Scholar]
- 19.Jun JH, Kim HY, Jung HJ, Lee WJ, Lee SJ, Kim do W, et al. Parry-Romberg syndrome with en coup de sabre. Ann Dermatol. 2011;23:342–7. doi: 10.5021/ad.2011.23.3.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lazaridou E, Giannopoulou C, Apalla Z, Fotiadou C, Trigoni A, Ioannides D, et al. Parry-Romberg syndrome. J Dermatol Case Rep. 2010;4:30–2. doi: 10.3315/jdcr.2010.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]


