Abstract
Aspergillosis of the nasal and paranasal sinuses is recognized as being second to candidiasis, among opportunistic fungal infections in immunocompromised patients. However, invasive variant in normal and mildly immunocompromised hosts is a very rare occurrence. We report one such case of aspergillosis involving paranasal sinuses in mildly immunocompromised patient.
Keywords: Aspergillosis, fruiting body, hyphae, methanamine silver stain
INTRODUCTION
Aspergillus is a ubiquitous fungus and belongs to the ascomycete molds, which together with penicillium form the family of Aspergillaceae. They are commonly found in humid areas, damp soil or agricultural environment, on grain, cereal, moldy flour, and organic decaying or decomposing matter. Aspergillus grows by budding or branching. The former or conidium, 1–3 μm in diameter, is carried by air and easily inhaled in the lungs. The branching hyphae are 2–5 μm in diameter, split dichotomously at 45° angle, and are best recognized by methanamine silver stain.[1] Aspergillus is most common fungal pathogen in sinus disease. Spores are ubiquitous, usually introduced by inhalation, and are frequent inhabitants of the human upper respiratory tract.[2]
An infection commonly isolated in the lungs of immunocompromised patients and occasionally as a destructive lesion of the maxillary sinuses, anterior palate, and nasal passages is the result of the inhalation of spores of Aspergillosis fumigates and Aspergillosis flavus.[3]
A number of studies and experiences emphasize that aspergillosis of the paranasal sinuses also develops in people who are otherwise healthy.[4,5]
Aspergillus sinusitis occurs in normal hosts but invasive and fulminant types are common in immunocompromised patients. However, an invasive form in normal host is very rare.[6]
The purpose of this paper is to draw attention to the clinical presentation, radiographic investigations, review of literature, and to emphasize the need for high index of suspicion in its histopathological diagnosis of this condition in maxillofacial region.
CASE REPORT
A 70-year-old male patient presented with a chief complaint of slow growing swelling on left side of the face since 6 months.Patient used to consume alcohol occasionally and was also hypertensive, for which he was receiving medication.
Dental history revealed extraction of left maxillary cuspid area 6 months back and then he noticed a nonhealing lesion in the same area.
On inspection, extra-oral examination revealed a diffuse swelling that was present on the left side of the face involving the periorbital region and left maxillary region with slight edema of upper lip, with a deviation of face to the right side, from ala of the nose to the tragus anteroposteriorly, measuring around 3×4 cm. The skin on the center of the swelling was erythematous with no pus discharge. No scar or redness, but mild hyper pigmentation of left maxillary region was seen. Decreased vision on left side with diplopia was observed in the patient [Figure 1].
Figure 1.
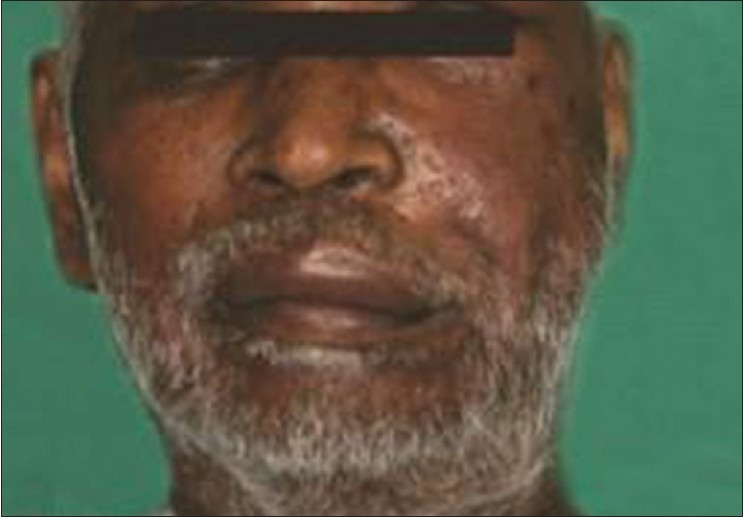
Extra-oral photograph showing swelling on left side of face
Intraorally, small papillomatous mass was found. On palpation, apart from confirming the above findings, firm and nontender swelling was observed on left maxillary region. Temperature was not raised. “Step deformity” with firm zygomatic bone over the infra orbital region was noted.
Computed tomography (CT) scan shows abnormal hypodense soft tissue in the left buccal and premaxillary spaces extending into the left buccal pad. Abnormal soft tissue with air pockets that is seen in left maxillary antrum widening the left osteomeatal unit, extending into the left half of the nasal cavity, left frontal sinus through the frontoethmoid recess. Air locules were seen in the left buccal region within the soft tissue.
There was an erosion of the alveolar cortex of the maxilla in the region of the central incisors, posterolateral wall of the left maxillary sinus, and inferior orbital wall (orbital floor) with extension of the soft tissue within the maxillary sinus, contiguous with the left buccal space and mild extension into the left orbital cavity with associated retrobulbar fat stranding [Figure 2].
Figure 2.
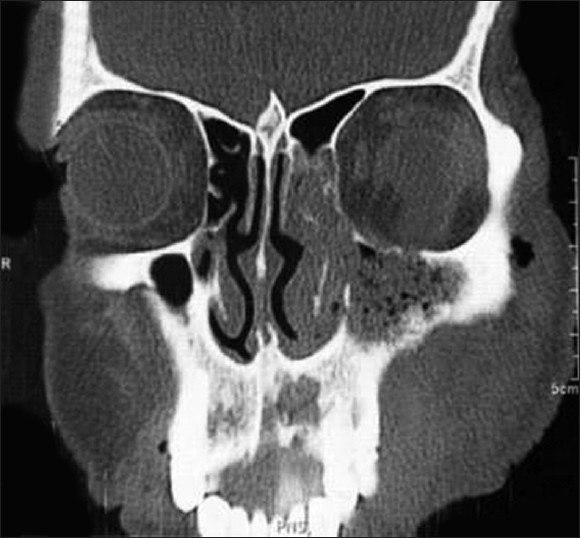
CT PNS showing there is erosion of alveolar cortex of the maxilla in the region of maxillary central incisors, posterolateral wall of the left maxillary sinus and inferior orbital wall (orbital floor) with extension of the soft tissue within the maxillary sinus, contiguous with buccal space and mild extension into orbital cavity
On performing the Caldwell-Luc surgery, the surgical exploration showed a considerable amount of greenish-black necrotic debris filling the sinus, which was cleared, and the specimen was sent for histopathological examination.
HISTOPATHOLOGY
Excisional biopsy was taken from left maxillary region. The specimen obtained for histopathological examination consisted of necrotic tissue and mucosa from margin of lesion. On gross anatomical finding, multiple soft tissues were received that were irregular in shape, cheesy in consistency, and brownish-black in color.
Examination of hematoxylin and eosin-stained sections revealed a pseudostratified ciliated columnar epithelium about 3–4 cell layer in thickness. Basement membrane was intact all over. Underlying connective tissue was fibrocellular with chronic inflammatory cell infiltrate. Another tissue section showed acute and chronic inflammatory infiltrate. Branching septate hyphae of aspergillosis were seen. Some of them were showing branching at acute angles [Figure 3]. These histopathological features were suggestive of fungal infection (aspergillosis) with tissue necrosis. Fruiting bodies and spores were also seen [Figures 4 and 5].
Figure 3.
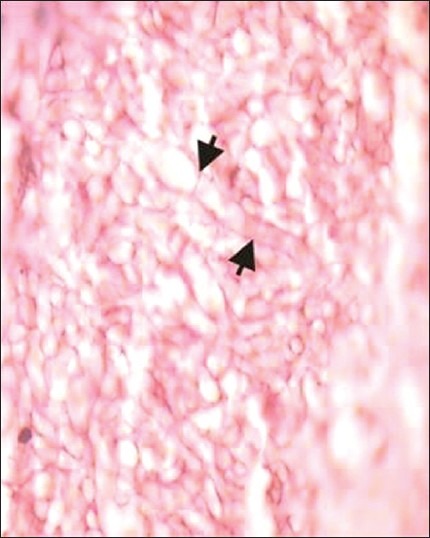
Photomicrograph reveals characteristic septate fungal hyphae of an Aspergillus species (H and E, ×100)
Figure 4.
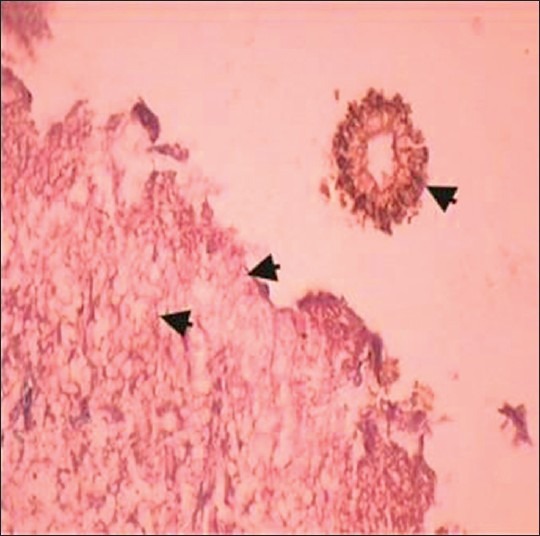
Photomicrograph reveals fungal hyphae and a fruiting body of an Aspergillus species (H and E, ×40)
Figure 5.
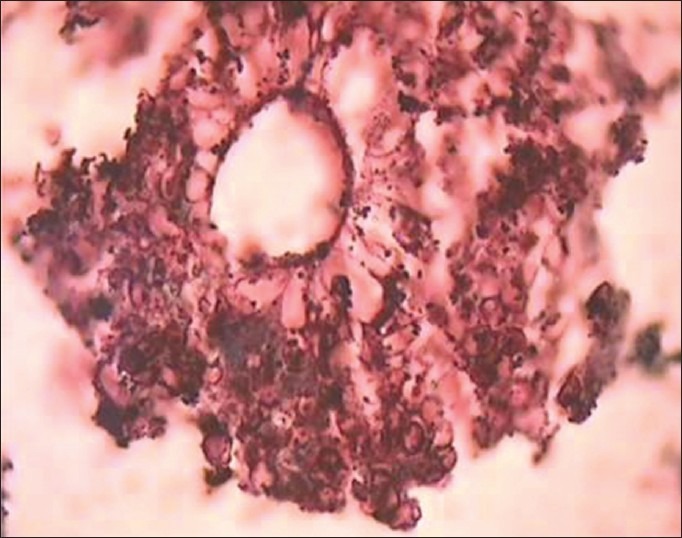
Photomicrograph reveals fruiting body of an Aspergillus species (H and E, ×100)
Further staining with methanamine silver stain showed septate dichomatous branching hyphae, and spores suggestive of Aspergillus fungus [Figures 6 and 7].
Figure 6.
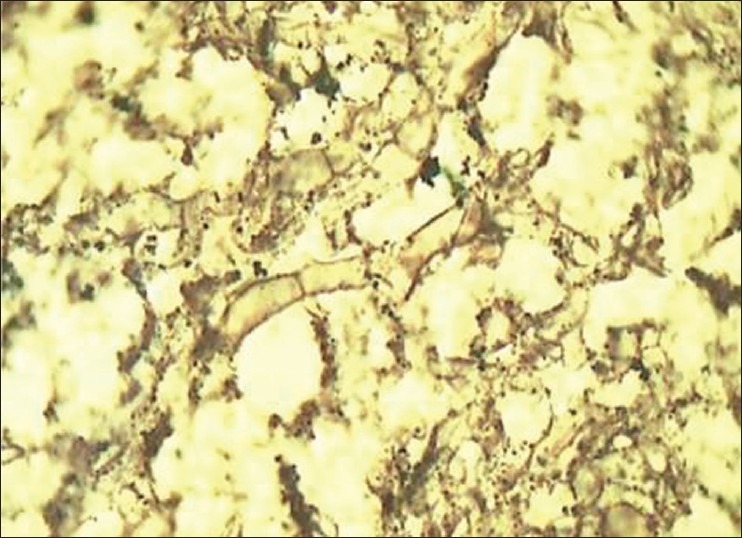
Photomicrograph reveals characteristic septate fungal hyphae of an Aspergillus species (Gnott-Gomori methanamine silver stain, ×100)
Figure 7.
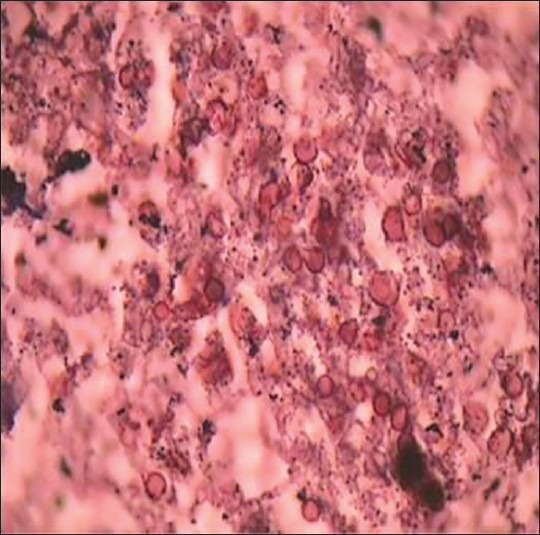
Photomicrograph reveals spores of fungal hyphae of an Aspergillus species (Gnott-Gomori methanamine silver stain, ×100)
After reviewing clinical, radiographic, and histopathological findings, diagnosis of chronic invasive aspergillosis of paranasal sinuses was made.
DISCUSSION
Since its first description as an opportunistic infection in 1953, there has been a substantial increase in the number of cases documented at autopsy in all developed nations. Furthermore, there has been a substantial increase in the number of patients at the risk of developing invasive aspergillosis.[7] Various researchers had reported about on aspergillosis [Table 1].
Table 1.
Milestone descriptions and reports of diseases caused by Aspergillus species

Aspergillus is pathogenic in birds, animals, and human beings. Eight of 350 species of Aspergillus have been associated with human disease.[4]
The route of infection is through either the gastrointestinal tract or the respiratory tract, and infections may occur anywhere along these routes.[8] Our case also showed respiratory tract involvement.
Invasive forms are the most severe and aggressive types, and cause gross morbid soft tissue and bone destruction and death.[9] Veress et al. reported that two factors may play an important role in paranasal sinus aspergillosis as follows:
Secretion of a toxic substance by fungi that penetrates into tissues under appropriate conditions and
Induction of tissue necrosis caused by an immune mechanism.[10]
Aspergillosis is usually seen in immunocompromised patients. However, invasive aspergillosis is usually seen in healthy or mildly immunocompromised host, as seen in our case who was an old patient with history of hypertension, which could be considered as mid immunocompromised state.[6,7] Stevens emphasized that functional obstruction of the sinus osteum may result in Aspergillus infection and that the fungus favors hypoxic or anaerobic conditions. Aspergillosis developing in tissues of resistance or in an antrum such as the nasal sinuses will ideally be ball-shaped (fungus ball) or necrotic material that is green-black and of a cheesy consistency or oral aspergillosis lesions, which are yellow or black in color.[4,11]
Most recent classification has been put forth by Rowe-Jones in 1994.[12]
Three main types
Noninvasive
Invasive
-
Destructive noninvasive types, which is further classified into:
- Aspergilloma
- Fungus ball
- Mycetoma (usually affecting one sinus) or Aspergillus sinusitis (involving more than one sinus).
Invasive type represents true fungal tissue invasion that can be either slowly progressive or destructive (nonfulminant) or highly aggressive and lethal fulminant.[6] Invasive Aspergillus sinusitis is characterized by rapid spread of fungus from the sinus airspace into the adjacent structures.[13] Rarely, invasive aspergillosis has been reported to affect the paranasal sinuses of apparently normal immunocompetent individuals, which was seen in our case.[14]
Thus, most patients with chronic invasive aspergillosis have no discernible immunocompromising factors, although substantial majorities are diabetics, drink alcohol to excess, or have acquired immune deficiency syndrome (AIDS). As the disease progresses, usually over months, the clinical features of local involvement become apparent.
Visual symptoms are common and include diplopia, headaches, loss of impairment of smell, and features of chronic sinusitis (e.g. nasal stuffness). Fever is almost universally absent; all these hallmarks of chronic invasive aspergillosis were present in our case.
Radiological features of this disease are similar to those of acute disease. Bone destruction may occur without direct fungal invasion, apparently due either to a “pressure” effect or to a local toxin production (speculative).[7]
Histopathologically, invasive lesions comprise chronic granulomatous reactions and appear like any of the granulomas of sarcoidoses, midline lethal granuloma, or foreign body granuloma. Though hyphal forms can be seen, in the center of an area of necrosis, they are faintly seen in hematoxylin and eosin stained sections, they may go unnoticed unless selective special stains like methanamine silver are employed. They appear as septate hyphae, with branching at 45° angles and are about 2–4 mm in diameter.[6] Condiospores are also seen. This fungus differentiated from mucormycosis were broader non-septate hyphae with dichomatous brancing at 90° angles is observed.[3,8] Similar features were evident in our patient. However, bone invasion by hyphae may be under-recognized histopathologically because of the nature of the surgery.
Culture is difficult and is often negative. No skin sensitivity or serum reactivity tests are useful, because most of the affected patients are immunodeficient.[3] Case fatality rate of 66% was recorded for sinus aspergillosis studied in a systemic review by Lin et al.[15]
CONCLUSION
The diagnosis of aspergillosis in humans relies heavily on the clinical circumstances of the infection, the anatomic site, and the microscopic findings. Early recognition of these diseases and especially differentiating it from malignant lesions and other granulomas of the sinuses are very important as its presentation is very deceptive and misleading. However, from a practical standpoint treatment may need to be initiated immediately to prevent the patient demise.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Sharma OP, Chwogule R. Many faces of pulmonary aspergillosis. Eur Respir J. 1998;12:705–15. doi: 10.1183/09031936.98.12030705. [DOI] [PubMed] [Google Scholar]
- 2.Warder FR, Chikes PG, Hudson WR. Aspergillosis of the paranasalsinusis. Arch Otolaryngol. 1975;101:683–5. doi: 10.1001/archotol.1975.00780400041011. [DOI] [PubMed] [Google Scholar]
- 3.Sapp JP, Eversole LR, Wysocki GP. Contemporary Oral and Maxillofacial Pathology. 2nd ed. Missouri: Mosby - An Affiliate of Elsevier; 2004. Oral infections; pp. 207–51. [Google Scholar]
- 4.Kwon J, Park KH, Park SI, Jin SY. Aspergillosis of the paranasal sinuses–diagnostic significance of the computed tomography. Yonsei Med J. 1989;30:294–7. doi: 10.3349/ymj.1989.30.3.294. [DOI] [PubMed] [Google Scholar]
- 5.Stammberger H, Jakse R, Beaufort F. Aspergillosis of the paranasal sinuses; X-ray diagnosis, histopathology, and clinical aspects. Ann Otol Rhinol Laryngol. 1984;93:251–6. doi: 10.1177/000348948409300313. [DOI] [PubMed] [Google Scholar]
- 6.Dayananda BC, Vandana R, Rekha K, Kumar GS. Aspergillosis of the maxillary antrum: A case report. J Oral Maxillofac Pathol. 2002;1:26–9. [Google Scholar]
- 7.Denning DW. Invasive aspergillosis. Clin Infect Dis. 1998;26:781–803. doi: 10.1086/513943. [DOI] [PubMed] [Google Scholar]
- 8.Regezi JA, Sciubba JJ, Jordan RC, editors. Oral pathology-Clinical Pathologic Correlations. 4th ed. Missouri: Saunders - An Imprint of Elsevier Science; 2003. Ulcerative conditions; pp. 23–74. [Google Scholar]
- 9.Chambers MS, Lyzak WA, Martin JW, Lyzak JS, Toth BB. Oral complications associated with aspergillosis in patients with a hematologic malignancy. Presentation and treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79:559–63. doi: 10.1016/s1079-2104(05)80095-2. [DOI] [PubMed] [Google Scholar]
- 10.Veress B, Malik OA, el-Tayeb AA, el-Daoud S, Mahgoub ES, el-Hassan AM. Further observations on the primary paranasal aspergillus granuloma in the Sudan: A morphological study of 46 cases. Am J Trop Med Hyg. 1973;22:765–72. doi: 10.4269/ajtmh.1973.22.765. [DOI] [PubMed] [Google Scholar]
- 11.Samaranayake LP, Keung Leung W, Jin L. Oral mucosal fungal infections. Periodontol 2000. 2009;49:39–59. doi: 10.1111/j.1600-0757.2008.00291.x. [DOI] [PubMed] [Google Scholar]
- 12.Rowe-Jones JM, Moore-Gillon V. Destructive noninvasive paranasal sinus aspergillosis: Component of a spectrum of disease. J Otolaryngol. 1994;23:92–6. [PubMed] [Google Scholar]
- 13.Myoken Y, Sugata T, Fujita Y, Fujihara M, Iwato K, Murayama SY, et al. Early diagnosis and successful management of atypical invasive aspergillus sinusitis in a hematopoietic cell transplant patient: a case report. J Oral Maxillofac Surg. 2006;64:860–3. doi: 10.1016/j.joms.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 14.Neville BW, Damm DD, Allen CM, Bouquot JE. Oral and Maxillofacial Pathology. 2nd ed. Pennsylvania: Saunders - An Imprint of Elsevier; 2005. Fungal and protozoal diseases; pp. 189–211. [Google Scholar]
- 15.Lin SJ, Schranz J, Teutsch SM. Aspergillosis case-fatality rate: Systematic review of the literature. Clin Infect Dis. 2001;32:358–66. doi: 10.1086/318483. [DOI] [PubMed] [Google Scholar]
- 16.Nikolaizik WH, Weichel M, Blaser K, Crameri R. Intracutaneous tests with recombinant allergens in cystic fibrosis patients with allergic bronchopulmonary aspergillosis and aspergillus allergy. Am J Respir Crit Care Med. 2002;165:916–21. doi: 10.1164/ajrccm.165.7.2109008. [DOI] [PubMed] [Google Scholar]
- 17.Hartl D, Latzin P, Zissel G, Krane M, Krauss-Etschmann S, Griese M. Chemokines indicate allergic bronchopulmonary aspergillosis in patients with cystic fibrosis. Am J Respir Crit Care Med. 2006;173:1370–6. doi: 10.1164/rccm.200508-1271OC. [DOI] [PubMed] [Google Scholar]
- 18.Kraemer R, Deloséa N, Ballinari P, Gallati S, Crameri R. Effect of allergic bronchopulmonary aspergillosis on lung function in children with cystic fibrosis. Am J Respir Crit Care Med. 2006;174:1211–20. doi: 10.1164/rccm.200603-423OC. [DOI] [PubMed] [Google Scholar]
- 19.Frisvad JC, Rank C, Nielsen KF, Larsen TO. Metabolomics of Aspergillus fumigatus. Med Mycol. 2009;47(Suppl 1):S53–71. doi: 10.1080/13693780802307720. [DOI] [PubMed] [Google Scholar]


