Abstract
The objective of this investigation was to examine the pharmacokinetics of isoflavone concentrations over a 24-hour period among healthy adults consuming either soy foods or soy isoflavone tablets at different doses. This randomized, cross-over trial was conducted with twelve generally healthy adults. The three phases of the intervention included: isoflavone tablets at 1) 144 mg/day or 2) 288 mg/day, and 3) soy foods designed to provide a calculated 96 mg isoflavones/day (doses in aglycone equivalents). Doses were spread out over three meals/day. After 6 days on each study phase, plasma isoflavone concentrations were determined on the seventh day at 0, 4, 8, 10, 12 and 24 hours. Average levels of total isoflavone concentrations at 8, 10 and 12 hours were >4 μmol/L for the soy food phase and for the higher dose tablet phase. Genistein concentrations were higher overall in the soy food vs. both the lower and the higher dose supplement phases of the study (p≤0.05). When comparing plasma concentrations for the two doses of tablets, saturation appeared more evident for genistein than daidzein at the higher dose level. In conclusion, we observed important differences in the pharmacokinetics of genistein and daidzein contrasting the sources and doses of isoflavones when administered three times daily, including a possible advantage for increasing serum concentrations of isoflavones from consuming soy foods relative to isoflavone supplements.
Keywords: Isoflavones, soy foods, supplements, pharmacokinetics, humans
INTRODUCTION
Epidemiological and mechanistic evidence suggest that consumption of either soy foods or specific components of soy foods may result in a lower risk of cardiovascular disease, various cancers, osteoporosis, and menopausal symptoms.1–6 However, there has been significant heterogeneity in the reports of presence vs. absence of health benefits.7–10 A particularly interesting group of molecules found almost uniquely in soy beans are the isoflavones, whose structural similarity to estrogen allows them to bind to the estrogen receptors of various types of cells.11–14 The two predominant isoflavones are genistein and daidzein. These isoflavones are thought to be at least partially responsible for the potential health benefits of soy consumption.
In the last decade many important factors related to isoflavone pharmacokinetics have been elucidated. One consistent finding has been that peak blood concentrations of genistein and daidzein occur approximately 4–8 hours after ingestion of a single dose.15–19 It has also been consistently reported that genistein appears in higher concentrations than daidzein in serum or plasma, but urinary recovery of daidzein is consistently higher than genistein.16–18, 20, 21 Other areas are more controversial. Evidence for a saturation level of isoflavone absorption at upper intake levels is inconsistent.17, 22–24 Another area of inconsistency involves differences, or lack of, in metabolism of aglycone vs. glycoside forms of the isoflavones. This is relevant because many soy isoflavone supplements and food products contain primarily the glycoside forms vs. pure isoflavones in their aglycone forms.15, 19, 25–31 Therefore, questions regarding isoflavone pharmacokinetics such as saturability and differential effects between different sources remain unresolved. In addition, virtually all of the pharmacokinetic work to date has been performed by exposure to a single bolus, while multiple doses spread out over the day have not been adequately studied.11–13, 15, 17–19, 22–28 In the current investigation we have addressed some of these gaps.
As part of a larger project to examine the effects of soy isoflavones on breast cancer, prostate cancer and bone health, our research group did preliminary developmental work to identify the timing of the peak in plasma isoflavone concentrations in response to isoflavone intake under conditions relevant to the design of that protocol. Study participants in the larger, follow-up project would be taking isoflavone supplements with their meals, three times a day. The purpose of the current experiment was to examine plasma isoflavone concentrations over the course of 24 hours in response to different sources and doses of soy isoflavones, when taken three times a day with meals.
SUBJECTS AND METHODS
Subjects
Participants were recruited from the local community and University employees primarily through flyers, letters to previous study participants, and e-mail lists. Adults 18 years and older were invited to enroll if they were generally healthy, were not and had not been taking antibiotics within the past three months, and were willing to avoid consuming soy products other than those provided for the 42-day duration of the protocol. Exclusion criteria, determined by self-administered questionnaire, included hypertension (except for those stable on anti-hypertension medications), type I or II diabetes mellitus, heart, renal or liver disease, cancer or active neoplasms, hyperthyroidism unless treated and under control, alcohol intake ≥3 drinks/day, and pregnancy/lactation. All study participants provided written informed consent and the study was approved annually by the Stanford University Human Subjects Committee.
Design
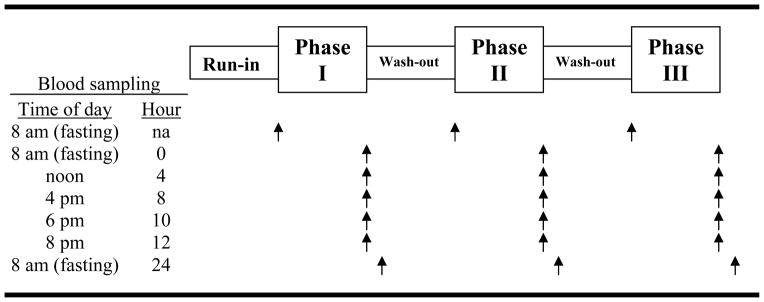
The trial design included three 7-day treatment phases: 1) a single Novasoy® tablet, three times/day (3 tablets total), 2) two Novasoy tablets, three times/day (6 tablets total), or 3) soy foods taken three times a day (Figure 1). Tablets and soy foods were taken with meals at breakfast, lunch and dinner. The protocol included a 7-day run-in, and wash-outs of ≥7 days between phases.
Figure 1.
Study Design
For each 7-day phase, there were 7 blood draws over three separate days: a fasting sample on the morning of the first day of the 7-day phase (after having had no soy foods or tablets for at least the 7 preceding days), five samples on day #7 (fasting, then 4, 8, 10 and 12 hours later), and a final sample the next day after an overnight fast (24 hours after the fasting day #7 sample).
Participants were randomized to one of the six possible sequences of the three phases (i.e., ABC, ACB, BAC, BCA, CAB, CBA). Two participants were randomly assigned to each of these 6 sequences by drawing from a hat with 12 slips of paper, two with each sequence written on them.
Isoflavone tablets
A single lot of soy isoflavone tablets (Novasoy) were generously provided by the Archer Daniel Midlands Corporation. Notably, at the onset of the study, an analysis of study tablets provided by the supplier indicated an isoflavone content (aglycone equivalents) of 32 mg/tablet. At the end of the study, the tablets were re-analyzed by co-investigator AF for stability and/or possible degradation. During this analysis, the isoflavone content of the tablets was determined to be 50% higher than originally represented by the supplier. Re-analysis by co-investigator AF and by a separate and independent laboratory (Plant Bioactives Research Institute, Orem, Utah) confirmed the higher value to be correct. Isoflavone composition of the tablets as determined at the conclusion of the trial is presented in Table 1. The isoflavone content of the tablets was 98.1% glycoside, with just 1.9% aglycone. Genistein and daidzein were the major components, with a 1.0:1.2 genistein:daidzein ratio by weight.
Table 1.
Isoflavone content (mg) per tablet of Novasoy
| Aglycone equivalents† | Glycosides + Aglycones | |
|---|---|---|
| Genistein | 19.9 +/− 2.4 | 31.7 +/− 3.9 |
| Daidzein | 24.9 +/− 1.0 | 40.6 +/− 1.6 |
| Glycitein | 3.4 +/− 1.1 | 5.3 +/− 1.8 |
|
| ||
| Total | 48.1 +/− 4.5 | 77.6 +/− 7.2 |
conversion of each isoflavone conjugate into its respective aglycone weight
Results were determined from separate analyses, at two different time points, of 10 tablets chosen at random from the single lot used during the study
Isoflavone containing soy foods
Isoflavone content of the foods used in these menu items were obtained from Reinli and Block 32 and/or from the USDA soy isoflavone database accessed at http://www.nal.usda.gov/fnic/foodcomp/Data/isoflav/isoflav.html. The average total aglycone isoflavone content of the soy food menu items was estimated to be 32 +/− 0.4 mg/meal. The average estimated aglycone content of genistein and daidzein in the meals was 17 +/− 2 and 13 +/− 2 mg/meal, respectively, a 1.0:0.8 genistein:daidzein ratio by weight. The relatively small contribution of isoflavones other than genistein and daidzein to the total isoflavone level was not available in the databases used to design the menus. Approximately 95% of the isoflavone in non-fermented foods are known to occur as glycosides.33
The soy foods included a variety of single soy items and several recipes. A three-day cycle of menus was prepared and provided by the research kitchen of the General Clinical Research Center (GCRC) at Stanford University Hospital. Soy items were purchased locally over the duration of the study in amounts as needed; in other words, they were not purchased as single large batches at the onset of the study. Each single item soy product, combination of items, or soy food recipe was designed to yield ~32 mg soy isoflavones per meal, such that when consumed 3 times/day, at breakfast lunch and dinner, the total isoflavone intake for the day would be ~96 mg/day of aglycone equivalents. This amount was intended, by design, to match the lower level of tablets (1 tablet/meal, 3 tablets/day). However, as noted above, the tablets were determined by analysis at the close of the study to contain 48 rather than 32 mg isoflavone/tablet. Therefore, the calculated level of intake from foods was substantially lower than that provided by either the low or the high dose tablets.
On average, the combined energy contribution of the soy-containing breakfast, lunch and dinner items was calculated to provide ~1,200 Kcal/day. Standardized and identical amounts were provided to all participants, regardless of body size or different levels of habitual energy expenditure. During the soy food phase, participants were responsible for providing and consuming the non-soy containing portion of their daily intake and for these choices they were instructed to select typical food and beverage items from their habitual diet. They were also instructed to maintain their habitual diets as closely as possible during the two soy tablet phases of the study.
The three-day cycle of menus is presented in Table 2. Notably, tempeh was the only fermented soy food provided (other than a small amount of miso included in Day #1), and was included in only one of the 9 meals from the three-day cycle. The menu for Day #1 was provided to all participants on the last of the 7 days on soy foods, the day of the serial blood draws, so as to minimize the inter-individual variability on the day of pharmacokinetic assessment. Tempeh was not included in the Day #1 menu.
Table 2.
Three-day menu cycle of soy foods provided to participants
| Day 1 | Day 2 | Day 3 | |
|---|---|---|---|
| Breakfast | Cereal with soynuts & raisins | Muesli with soy nuts & dried fruit | Blueberry muffins with soynuts raisins & soy milk |
| Lunch | Soy falafels pita bread tomato & lettuce ranch dressing Trail mix w/soy nuts |
Tempeh with pasta | Confetti salad with canned soy beans Trail mix w/soy nuts |
| Dinner | Tofu cutlets and rice w/miso | Soy burger with tomato & lettuce and condiments Trail mix w/soy nuts |
Three-bean chili (including soybean) with crackers |
Menus were designed to provide 32 mg isoflavones per meal, and 96 mg isoflavones per day calculated as aglycone equivalents as estimated from Reinli and Block (27) and/or from the USDA soy isoflavone database http://www.nal.usda.gov/fnic/foodcomp/Data/isoflav/isoflav.html. All foods and menus items listed were provided to participants for the 7 days they were on the “soyfood” phase of the study. All foods not listed above that were consumed by participants were selected, purchased, prepared, and consumed on their own.
Tablet and food adherence
Adherence to study tablets was determined by pill counting. Each participant received a single bottle of tablets for each of the two phases on soy isoflavone supplements; either 21 tablets (3 tablets/day × 7 days) or 42 tablets (6 tablets/day × 7 days).
Adherence to study foods was determined by a self-report questionnaire at the end of the 7-day food phase for each participant, asking them what meals or portions of meals they had missed during the week.
Participants were given instructions and lists of isoflavone-containing foods to avoid during all phases of the study. Adherence to non-study isoflavone intake was assessed by measuring levels of plasma isoflavones at the start of each 7-day soy food or soy tablet phase (i.e., the end of the run-in or each wash-out phase).
Plasma isoflavone analyses
Plasma samples were analyzed for genistein and daidzein by LC/MS34 as recently revised.35, 36 In brief, plasma was mixed with triethylamine acetate (pH7; 0.2M), beta-glucuronidase and arylsulfatase (both enzymes from Roche Applied Science, Indianapolis, IN) and incubated for 12–17 hours at 37°C. Triply 13C labeled isoflavonoids (obtained from the University of St. Andrews, Scotland) were added as internal standards followed by addition of acetonitrile for protein precipitation. This mixture was extracted three times with diethyl ethyl ether. The combined ether phases were dried under a stream of dry nitrogen, redissolved in 0.1 mL methanol followed by addition of 0.1 mL 0.2M acetate buffer (pH4) and analyzed immediately by injecting 20 μL into the LC system or stored at −20 °C until analyzed. LC/PDA/ESI-MS analyses were performed by injecting 10–20 μL of this solution onto a HydroBond PS 18 (100 × 3.0 mm; 5μm) column (MAC-MOD Analytical Inc., Chadds Ford, PA), followed by separation with a flow rate of 0.25 mL using a linear gradient of methanol/acetonitrile/water from 20/20/60 to 45/45/10. Mass spectrometric measurements were performed in the negative mode after electrospray ionization with mass screening covering the range 180–350 amu. Individual analytes were monitored by screening M-1 masses (mass of molecular ion minus one proton) of analytes alone or in combination with selected reaction monitoring of first generation product ions applying collision energies of 40%–42%. Lower limits of quantitation for analyses of genistein and daidzein in plasma samples were 20 and 3 nM, respectively. The validated range of assessment for all four analytes was from the lower limit of quantitation to 2000 nM. The CVs for interassay variation were 2.9 and 5.6%, respectively.34
Statistical analyses
Differences among treatments in the patterns of plasma genistein and daidzein concentrations when participants consumed soy foods and the two doses of soy isoflavone tablets were tested by ANOVA using SAS Proc Mixed specifying an autoregressive correlation structure between repeated measures on the same participant. When the ANOVA findings were statistically significant, pair-wise testing was conducted to determine the source of the differences with Bonferonni adjustment for multiple testing. Separate analyses were conducted to test for differences in total isoflavone concentrations at individual time points within each of the three treatment phases using a series of dependent t-tests. These latter, descriptive analyses were done without adjusting for multiple testing. All statistical tests were two-tailed using a significance level (alpha) of 0.05.
RESULTS
Thirteen participants, including five postmenopausal women, four premenopausal women, and four men, were randomly assigned to one of six sequences of the three soy products. One participant discontinued participation after the first week of the study due to unanticipated scheduling conflicts. Twelve participants completed the entire protocol. The average age of the participants was 42 +/− 12 years (range 28 to 74).
Adherence to study tablets and study foods was determined to be high; 10 of 12 participants consumed >90% of study tablets in both tablet phases and all 12 participants consumed >80% of study tablets in both phases. Adherence to study foods was 100% for 7 of the 12 participants, >90% for all but two participants and >80% for all but one participant. One participant consumed, on average, 65% of the study foods, and on several occasions substituted soy foods they obtained themselves for some of the provided soy food menu items, in violation of specific study instructions. All participant results were included on an intention-to-treat basis. On the final day of the 7-day soy foods phase, when serial blood samples were taken, all participants consumed all of their soy study foods. Similarly, on the final day of the 7-day tablet phases, all participants consumed all assigned tablets.
Adherence to the avoidance of non-study soy products was also high. Isoflavone levels were negligible for all participants at the end of the run-in and the end of the two wash-out phases (data not shown).
Plasma isoflavone concentrations
The total isoflavone levels after the 7 day washouts averaged 0.05 ± 0.08 μmol (mean ± sd) and were virtually identical for the three study phases, indicating that the 7-day wash out periods between treatments were sufficient.
Considerable individual variability was observed for total plasma concentrations of isoflavonoids. On average, the difference between the lowest and highest concentration within each phase and across all non-fasting time points, was approximately 3-fold for soy foods, 5-fold for low-dose tablets, and 9-fold for high-dose tablets (individual data not presented).
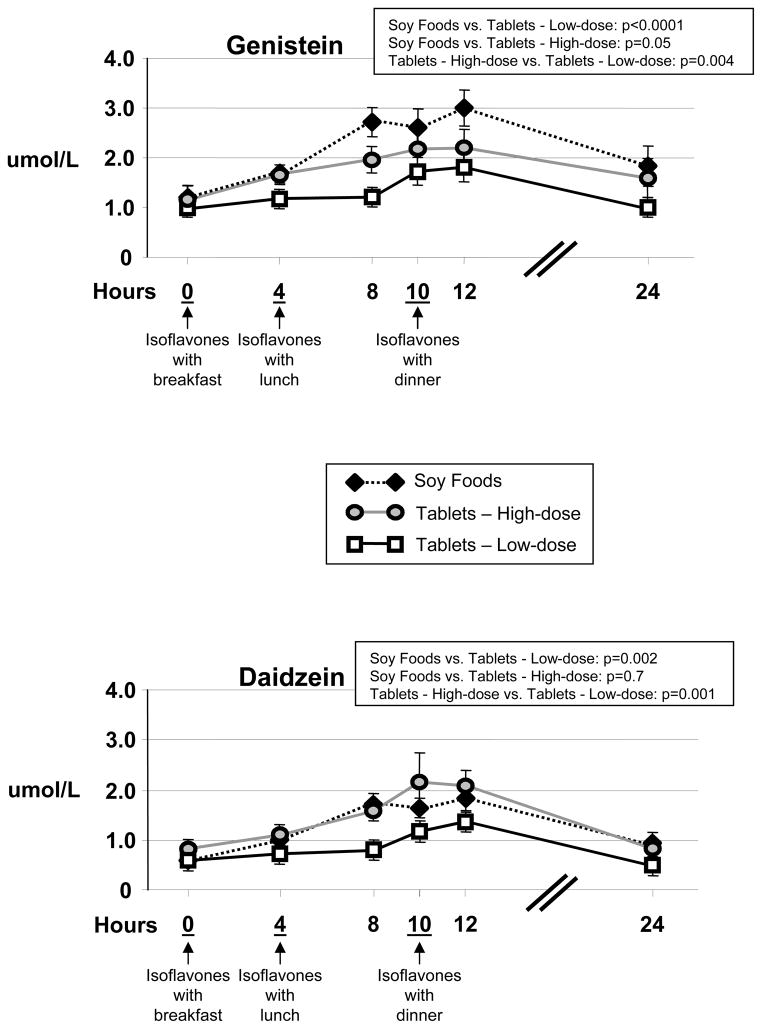
Average genistein concentrations over the 24-hour period of serial blood sampling were highest at the 12-hour time point for all three phases of the study vs. other time points (Figure 2). The repeated measures ANOVA testing for treatment differences was significant (F=4.3, p=0.02). Over all time points average genistein concentrations were highest for the soy foods phase of the study relative to both the lower-dose and higher-dose tablet phases, despite the higher-dose level of genistein intake being >2-fold higher than that of the calculated soy food intake level (50 mg/day for soy foods on the Day #1 menu vs. 119 mg/day for the 6 tablet/day phase of soy isoflavone tablets). Plasma concentrations of genistein, when averaged across the four time points of 4, 8, 10 and 12 hours, were only 39% higher for the high-dose than for the low-dose tablet, despite being twice the dose ingested.
Figure 2.
Average genistein and daidzein concentrations on the last day of 7 days of dosing for 12 adults who each followed three separate regimens in random order. The six time points were 0, 4, 8, 10, 12 and 24 hours after initiating isoflavone intake with breakfast. Isoflavone intake occurred at three times/day (with breakfast, lunch and dinner) and the three separate regimens of isoflavones were: Soy Foods (96 mg/day, 32 mg/dose – estimated from database isoflavone values), Tablets - Low dose (144 mg/day, 48 mg/tablet, 1 tablet/meal – determined by direct chemical analysis) or Tablets - High dose (288 mg/day, 48 mg/tablet, 2 tablets/meal – determined by direct chemical analysis). Differences between sources/doses for genistein and daidzein were determined by repeated measures ANOVA. Pair-wise comparisons are presented as Bonferroni adjusted p-values.
Average daidzein concentrations over the 24-hour serial blood sampling period were highest at the 10-hour and 12-hour sampling for the lower- and higher-dose tablet phases, and highest across the 8, 10, and 12-hour blood samplings for the soy foods phase (Figure 2). The repeated measures ANOVA testing for treatment differences was significant (F=5.8, p=0.007). Average daidzein concentrations were similar between the higher dose tablet phase and the soy foods phase at all time points except the 10-hour time point, when the average daidzein concentration was modestly higher for the higher dose tablet phase. Notably different than the observed results for genistein, for daidzein the plasma concentrations were substantially higher for the 6 tablet/day phase relative to the 3 tablet/day phase - 72% higher as averaged across the four time points of 4, 8, 10 and 12 hours.
Total isoflavonoid concentrations are presented in Table 3. One of the primary purposes of this developmental project was to confirm that total plasma isoflavonoid concentrations would be elevated and relatively similar during the 8, 10 and 12 hour time points among participants consuming isoflavones at breakfast, lunch and dinner. In order to examine this issue, statistical testing was done separately within each treatment and among the different time points in a series of dependent t-tests. Concentrations peaked, on average, at the 10-hour and 12-hour time points for the two tablet phases, and throughout the 8- through 12-hour time points for the soy foods phase. Average concentrations of total isoflavones reached a high of more than 5 μmol/L in both the higher dose tablet phase and the soy foods phase.
Table 3.
Total plasma isoflavonoid concentrations over 24 hours (mean +/− SD, μmol/L)
| Hours
|
||||||
|---|---|---|---|---|---|---|
| 0† | 4† | 8 | 10† | 12 | 24 | |
| Soy foods | 2.4a +/− 1.4 | 3.1b +/− 1.1 | 5.1c +/− 1.3 | 4.9c,d +/− 1.9 | 5.6c +/− 1.4 | 3.4a,b,d +/− 1.9 |
| 3 tablets/day (144 mg/day) | 2.1a,c +/− 1.2 | 2.2a,c +/− 1.1 | 2.5a +/− 0.9 | 3.3b +/− 1.5 | 3.7b +/− 1.5 | 2.0c +/− 0.6 |
| 6 tablets/day (288 mg/day) | 2.7a +/− 1.8 | 3.3a,c +/− 1.2 | 4.2b,d +/− 1.8 | 5.3b,c +/− 4.1 | 5.1b +/− 2.4 | 3.2a,d +/− 2.4 |
Isoflavones from tablets or foods were consumed at hours 0, 4 and 10.
Within a row, time points that do not share a similar superscript differ significantly at p<0.05. The sampling hours of 0, 4, 8, 10, 12 and 24 hours corresponded roughly to 8 am, noon, 4, 6 and 8 pm, and 8 am the following morning, respectively.
The 8-, 10- and 12-hour are highlighted/set-apart above to reflect one of the primary purposes of the study – to confirm that plasma would be enriched with isoflavones and that the plasma concentrations would be relatively stable over this time span, corresponding to ~4–8 pm.
DISCUSSION
The primary objective of this study was to determine the optimal timing for blood sampling in order to obtain isoflavone-rich samples at an hour of the day that was practical for future repeated sampling for a separate, larger project. This aspect of the project was easily resolved; blood sampled between 4 and 8 pm when isoflavones are being consumed with breakfast, lunch and dinner yields high concentrations of plasma isoflavones that are relatively stable over that 4-hour time span. Secondary objectives included examining the extent to which different sources or doses had an impact on isoflavone concentrations. Isoflavones from soy foods appeared to be more bioavailable than those from supplements. We also observed possible saturation of bioavailability of genistein at doses of 288 vs. 144 mg total isoflavones/day; there was less evidence for saturation of daidzein bioavailability.
As expected, plasma concentrations over time in this study followed a very different pattern from the typical one-dose protocol16–18, 30, 31 due to cumulative effects caused by the multiple dosing protocol. The breakfast, lunch and dinner dosing caused uptake of isoflavones from the latter meals before the dose from the previous meal was eliminated. Assuming an average half-life of 6–8 hours, as has been reported by various investigators,37 there would be very little or even a negligible amount of the maximum isoflavone level left after 24 hours, as observed. These findings are consistent with previous suggestions that the optimum steady state serum isoflavone concentrations would be expected from consuming relatively small doses of soy regularly throughout the day, as opposed to a single dose at one time in the day.17, 38
Several studies have compared the pharmacokinetics of aglycone vs. glycoside forms of isoflavones. Faughnan et al. and Cassidy et al. have reported differences between soy milk, textured vegetable protein and tempeh, the tempeh being substantially higher in aglycone content than the soy milk and TVP.15, 30 Other investigators have contrasted supplements containing purified aglycone isoflavone forms vs. mixtures of glycoside and aglycone forms.19, 26–28 One study contrasted isoflavone kinetics when consumed as untreated soy milk vs. treated soy milk (i.e., aglycone vs. glycosides).31 In general the aglycone forms were found to be more readily and more quickly absorbed.15, 26, 28, 30, 31 However, at least two of the studies have reported only modest or negligible differences19, 27 Beyond the influence of the aglycone vs. glycoside form of the isoflavone, the matrix in which it is delivered is likely to have an effect on pharmacokinetics.39
Our data are among the first to directly contrast plasma concentrations of isoflavones from foods to those from supplements. We observed that the plasma concentrations resulting from food intake were high compared to those from tablet intake. This is in agreement with a recent finding that urinary isoflavone excretion rates were higher from soy isoflavones delivered in a food matrix (i.e., soy foods) than from a processed soy beverage when similar isoflavone doses were given.39
There were at least two important limitations to the comparison of isoflavone sources. First, the doses achieved from food intake and the lower-dose tablet intake were designed to be matched, but it was later determined that the isoflavone content of the tablets was substantially higher than originally believed. We anticipated this differential would have contributed to lower observed plasma concentrations of isoflavones from the food vs. supplement sources, when in fact we observed the opposite. Second, the isoflavone doses provided by study soy foods were not chemically analyzed, but rather were estimated from existing databases. Published databases on the isoflavone content of foods may or may not provide accurate data for the foods consumed in the current study; the isoflavone content of soy foods can vary substantially according to growing location and conditions, manufacturing processes, etc.40 However, given that several different food sources of isoflavones were used, and that these were purchased at several different times over the course of the study (i.e., different lots), it is likely that some soy items contained lower and some contained higher isoflavone levels than the published database values and that the overall calculated values of ~90 mg isoflavones/day from soy food sources were reasonably accurate. Even if the calculated values were somewhat in error, it is unlikely that the doses obtained from foods in this study were as high as even the 144 mg/day dose of isoflavone in the “low-dose” supplement phase. If the calculated values from food sources were actually overestimates, and the true isoflavone content from food sources was <90 mg/day, this would suggest that we have underestimated the advantage of food sources of isoflavones vs. supplement sources in raising plasma isoflavone concentrations.
We also observed a potential minor differential in saturation of bioavailability for genistein and daidzein. Plasma concentrations of genistein were only ~39% higher for the high-dose tablets relative to the low-dose tablets despite the delivery of twice as much genistein. For daidzein the higher dose yielded plasma concentrations that were ~72% higher than that of the lower dose tablets and statistically significant. Neither of these indicated a linear increase in plasma concentrations after a doubling of the dose, but these results do suggest less of a saturation of daidzein bioavailability relative to genistein at the doses of tablets used. Notably this observation occurred under conditions of relatively high intake levels of isoflavones (144 vs. 288 mg/d), greater than would be realistic from typical intake of isoflavones from food sources.41 In studies of men22 and women23 where increasing doses of isoflavones were provided in a range from moderate to extremely high (1–16 mg/kg body weight), no apparent saturation of total isoflavone absorption was reported. However, a separate breakdown of specific isoflavones was not reported in these studies, and it may have been that genistein appearance in the blood did reach saturation levels at higher doses, while other isoflavones did not. Setchell et al. reported that increasing the daily dose of 13C genistein or 13C daidzein from ~25 to ~50 mg/day led to a non-linear increase in plasma concentrations, suggesting some saturation of bioavailability at the higher level of intake17 Not only did the doses used by Setchell differ from those used in the current study, but the source of isoflavones used in that study was a more purified source, which could partly explain the different reported outcomes in these studies.
Large individual variation in plasma concentrations of isoflavones has been reported.20 In the study by Wiseman et al., among 38 healthy adults receiving ~100 mg/day of total isoflavones, blood concentrations of specific isoflavones were reported to vary by over 1000-fold. The variation among the 12 participants in this study was high, but not nearly as wide as reported by Wiseman, and was in the range of 3- to 9-fold differences for non-fasting samples in the current study. There have been several investigations that have examined whether gender differences account for a portion of this inter-individual variation. To date most of these studies have indicated no gender effects on isoflavone metabolism and kinetics, while just a few have reported modest gender effects.15, 30, 42–45 In our trial the numbers of men and women, both pre-menopausal and post-menopausal, were too small to effectively assess this potential source of variability.
The majority of recent isoflavone trials and pharmacokinetic studies have used a single bolus of isoflavones, and have usually delivered these in tablet form, rather than foods.11–13, 15, 17–19, 22–25, 28 The findings from this investigation suggest that bioavailability may be higher from food sources of isoflavones than from tablets, and that spreading the intake out in multiple doses over the course of the day will lead to more constant steady-state plasma concentrations. Future studies are warranted where the doses between the foods and the tablets are matched, the isoflavone composition of both tablets and foods is directly determined through chemical analysis, and intake is compared for a single bolus vs. multiple intake time points over the course of the day. A greater understanding of isoflavone pharmacokinetics that may differ by source and dose could help to explain some of the heterogeneity of results among the large and growing number of trials testing the effects of soy isoflavones on a variety of potential health outcomes.
Acknowledgments
Sources of Financial Support:
This investigation was supported by NIH grants R01 000486 and P30 CA71789, and by Human Health Service grant M01-RR00070 from the National Center for Research Resources, National Institutes of Health.
Laurie Custer is acknowledged for her technical assistance in isoflavonoid analysis. This investigation was supported by NIH grants R01 000486 and P30 CA71789, and by Human Health Service grant M01-RR00070 from the National Center for Research Resources, National Institutes of Health.
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
LITERATURE CITED
- 1.Clarkson TB. Soy, soy phytoestrogens and cardiovascular disease. J Nutr. 2002 Mar;132(3):566S–569S. doi: 10.1093/jn/132.3.566S. [DOI] [PubMed] [Google Scholar]
- 2.Messina MJ, Persky V, Setchell KD, Barnes S. Soy intake and cancer risk: a review of the in vitro and in vivo data. Nutr Cancer. 1994;21(2):113–131. doi: 10.1080/01635589409514310. [DOI] [PubMed] [Google Scholar]
- 3.Messina M, Hughes C. Efficacy of soyfoods and soybean isoflavone supplements for alleviating menopausal symptoms is positively related to initial hot flush frequency. J Med Food. 2003 Spring;6(1):1–11. doi: 10.1089/109662003765184697. [DOI] [PubMed] [Google Scholar]
- 4.Messina MJ. Emerging evidence on the role of soy in reducing prostate cancer risk. Nutr Rev. 2003 Apr;61(4):117–131. doi: 10.1301/nr.2003.apr.117-131. [DOI] [PubMed] [Google Scholar]
- 5.Nestel P. Isoflavones: their effects on cardiovascular risk and functions. Curr Opin Lipidol. 2003 Feb;14(1):3–8. doi: 10.1097/00041433-200302000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Setchell KD, Lydeking-Olsen E. Dietary phytoestrogens and their effect on bone: evidence from in vitro and in vivo, human observational, and dietary intervention studies. Am J Clin Nutr. 2003 Sep;78(3 Suppl):593S–609S. doi: 10.1093/ajcn/78.3.593S. [DOI] [PubMed] [Google Scholar]
- 7.Cheong JM, Martin BR, Jackson GS, et al. Soy isoflavones do not affect bone resorption in postmenopausal women: a dose-response study using a novel approach with 41Ca. J Clin Endocrinol Metab. 2007 Feb;92(2):577–582. doi: 10.1210/jc.2006-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kreijkamp-Kaspers S, Kok L, Grobbee DE, et al. Effect of soy protein containing isoflavones on cognitive function, bone mineral density, and plasma lipids in postmenopausal women: a randomized controlled trial. Jama. 2004 Jul 7;292(1):65–74. doi: 10.1001/jama.292.1.65. [DOI] [PubMed] [Google Scholar]
- 9.Sacks FM, Lichtenstein A, Van Horn L, Harris W, Kris-Etherton P, Winston M. Soy protein, isoflavones, and cardiovascular health: an American Heart Association Science Advisory for professionals from the Nutrition Committee. Circulation. 2006 Feb 21;113(7):1034–1044. doi: 10.1161/CIRCULATIONAHA.106.171052. [DOI] [PubMed] [Google Scholar]
- 10.St Germain A, Peterson CT, Robinson JG, Alekel DL. Isoflavone-rich or isoflavone-poor soy protein does not reduce menopausal symptoms during 24 weeks of treatment. Menopause. 2001 Jan-Feb;8(1):17–26. doi: 10.1097/00042192-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Chen XW, Garner SC, Anderson JJ. Isoflavones regulate interleukin-6 and osteoprotegerin synthesis during osteoblast cell differentiation via an estrogen-receptor-dependent pathway. Biochem Biophys Res Commun. 2002 Jul 12;295(2):417–422. doi: 10.1016/s0006-291x(02)00667-8. [DOI] [PubMed] [Google Scholar]
- 12.Kostelac D, Rechkemmer G, Briviba K. Phytoestrogens modulate binding response of estrogen receptors alpha and beta to the estrogen response element. J Agric Food Chem. 2003 Dec 17;51(26):7632–7635. doi: 10.1021/jf034427b. [DOI] [PubMed] [Google Scholar]
- 13.Mitropoulou TN, Tzanakakis GN, Nikitovic D, Tsatsakis A, Karamanos NK. In vitro effects of genistein on the synthesis and distribution of glycosaminoglycans/proteoglycans by estrogen receptor-positive and -negative human breast cancer epithelial cells. Anticancer Res. 2002 Sep-Oct;22(5):2841–2846. [PubMed] [Google Scholar]
- 14.Morito K, Hirose T, Kinjo J, et al. Interaction of phytoestrogens with estrogen receptors alpha and beta. Biol Pharm Bull. 2001 Apr;24(4):351–356. doi: 10.1248/bpb.24.351. [DOI] [PubMed] [Google Scholar]
- 15.Faughnan MS, Hawdon A, Ah-Singh E, Brown J, Millward DJ, Cassidy A. Urinary isoflavone kinetics: the effect of age, gender, food matrix and chemical composition. Br J Nutr. 2004 Apr;91(4):567–574. doi: 10.1079/BJN20041087. [DOI] [PubMed] [Google Scholar]
- 16.King RA, Bursill DB. Plasma and urinary kinetics of the isoflavones daidzein and genistein after a single soy meal in humans. Am J Clin Nutr. 1998;67(5):867–872. doi: 10.1093/ajcn/67.5.867. [DOI] [PubMed] [Google Scholar]
- 17.Setchell KD, Faughnan MS, Avades T, et al. Comparing the pharmacokinetics of daidzein and genistein with the use of 13C-labeled tracers in premenopausal women. Am J Clin Nutr. 2003 Feb;77(2):411–419. doi: 10.1093/ajcn/77.2.411. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe S, Yamaguchi M, Sobue T, et al. Pharmacokinetics of soybean isoflavones in plasma, urine and feces of men after ingestion of 60 g baked soybean powder (kinako) J Nutr. 1998;128(10):1710–1715. doi: 10.1093/jn/128.10.1710. [DOI] [PubMed] [Google Scholar]
- 19.Zubik L, Meydani M. Bioavailability of soybean isoflavones from aglycone and glucoside forms in American women. Am J Clin Nutr. 2003 Jun;77(6):1459–1465. doi: 10.1093/ajcn/77.6.1459. [DOI] [PubMed] [Google Scholar]
- 20.Wiseman H, Casey K, Bowey EA, et al. Influence of 10 wk of soy consumption on plasma concentrations and excretion of isoflavonoids and on gut microflora metabolism in healthy adults. Am J Clin Nutr. 2004 Sep;80(3):692–699. doi: 10.1093/ajcn/80.3.692. [DOI] [PubMed] [Google Scholar]
- 21.Xu X, Wang HJ, Murphy PA, Cook L, Hendrich S. Daidzein is a more bioavailable soymilk isoflavone than is genistein in adult women. J Nutr. 1994 Jul;124(6):825–832. doi: 10.1093/jn/124.6.825. [DOI] [PubMed] [Google Scholar]
- 22.Bloedon LT, Jeffcoat AR, Lopaczynski W, et al. Safety and pharmacokinetics of purified soy isoflavones: single-dose administration to postmenopausal women. Am J Clin Nutr. 2002 Nov;76(5):1126–1137. doi: 10.1093/ajcn/76.5.1126. [DOI] [PubMed] [Google Scholar]
- 23.Busby MG, Jeffcoat AR, Bloedon LT, et al. Clinical characteristics and pharmacokinetics of purified soy isoflavones: single-dose administration to healthy men. Am J Clin Nutr. 2002 Jan;75(1):126–136. doi: 10.1093/ajcn/75.1.126. [DOI] [PubMed] [Google Scholar]
- 24.Setchell KD, Brown NM, Desai PB, et al. Bioavailability, disposition, and dose-response effects of soy isoflavones when consumed by healthy women at physiologically typical dietary intakes. J Nutr. 2003 Apr;133(4):1027–1035. doi: 10.1093/jn/133.4.1027. [DOI] [PubMed] [Google Scholar]
- 25.Hutchins AM, Slavin JL, Lampe JW. Urinary isoflavonoid phytoestrogen and lignan excretion after consumption of fermented and unfermented soy products. J Am Diet Assoc. 1995 May;95(5):545–551. doi: 10.1016/S0002-8223(95)00149-2. [DOI] [PubMed] [Google Scholar]
- 26.Izumi T, Piskula MK, Osawa S, et al. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J Nutr. 2000 Jul;130(7):1695–1699. doi: 10.1093/jn/130.7.1695. [DOI] [PubMed] [Google Scholar]
- 27.Richelle M, Pridmore-Merten S, Bodenstab S, Enslen M, Offord EA. Hydrolysis of isoflavone glycosides to aglycones by beta-glycosidase does not alter plasma and urine isoflavone pharmacokinetics in postmenopausal women. J Nutr. 2002 Sep;132(9):2587–2592. doi: 10.1093/jn/132.9.2587. [DOI] [PubMed] [Google Scholar]
- 28.Setchell KD, Brown NM, Desai P, et al. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J Nutr. 2001 May;131(4 Suppl):1362S–1375S. doi: 10.1093/jn/131.4.1362S. [DOI] [PubMed] [Google Scholar]
- 29.Zheng Y, Sun-Ok L, Verbruggen MA, Murphy PA, Hendrich S. The apparent absorptions of isoflavone glucosides and aglucons are similar in women and are increased by rapid gut transit time and low fecal isoflavone degradation. Journal of Nutrition. 2004;134:2534–2539. doi: 10.1093/jn/134.10.2534. [DOI] [PubMed] [Google Scholar]
- 30.Cassidy A, Brown JE, Hawdon A, et al. Factors affecting the bioavailability of soy isoflavones in humans after ingestion of physiologically relevant levels from different soy foods. J Nutr. 2006 Jan;136(1):45–51. doi: 10.1093/jn/136.1.45. [DOI] [PubMed] [Google Scholar]
- 31.Kano M, Takayanagi T, Harada K, Sawada S, Ishikawa F. Bioavailability of isofavones after ingestion of soy beverages in healthy adults. J Nutr. 2006 Sep;136(9):2291–2296. doi: 10.1093/jn/136.9.2291. [DOI] [PubMed] [Google Scholar]
- 32.Reinli K, Block G. Phytoestrogen content of foods--a compendium of literature values. Nutr Cancer. 1996;26(2):123–148. doi: 10.1080/01635589609514470. [DOI] [PubMed] [Google Scholar]
- 33.Franke AA, Hankin JH, Yu MC, Maskarinec G, Low SH, Custer LJ. Isoflavone levels in soy foods consumed by multiethnic populations in Singapore and Hawaii. J Agric Food Chem. 1999 Apr;47(3):977–986. doi: 10.1021/jf9808832. [DOI] [PubMed] [Google Scholar]
- 34.Franke AA, Custer LJ, Wilkens LR, et al. Liquid chromatographic-photodiode array mass spectrometric analysis of dietary phytoestrogens from human urine and blood. J Chromatogr B Analyt Technol Biomed Life Sci. 2002 Sep 25;777(1–2):45–59. doi: 10.1016/s1570-0232(02)00216-7. [DOI] [PubMed] [Google Scholar]
- 35.Cline JM, Franke AA, Register TC, Golden DL, Adams MR. Effects of dietary isoflavone aglycones on the reproductive tract of male and female mice. Toxicol Pathol. 2004 Jan-Feb;32(1):91–99. doi: 10.1080/01926230490265902. [DOI] [PubMed] [Google Scholar]
- 36.Blair RM, Appt SE, Franke AA, Clarkson TB. Treatment with antibiotics reduces plasma equol concentration in cynomolgus monkeys (Macaca fascicularis) J Nutr. 2003 Aug;133(7):2262–2267. doi: 10.1093/jn/133.7.2262. [DOI] [PubMed] [Google Scholar]
- 37.Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005 Jan;81(1 Suppl):230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 38.Frank RA, van der Klaauw NJ. The contribution of chemosensory factors to individual differences in reported food preferences. Appetite. 1994;22(2):101–123. doi: 10.1006/appe.1994.1011. [DOI] [PubMed] [Google Scholar]
- 39.Franke AA, Custer LJ, Hundahl SA. Determinants for urinary and plasma isoflavones in humans after soy intake. Nutr Cancer. 2004;50(2):141–154. doi: 10.1207/s15327914nc5002_3. [DOI] [PubMed] [Google Scholar]
- 40.Lee SJ, Ahn JK, Kim SH, et al. Variation in isoflavone of soybean cultivars with location and storage duration. J Agric Food Chem. 2003 May 21;51(11):3382–3389. doi: 10.1021/jf0261405. [DOI] [PubMed] [Google Scholar]
- 41.Arai Y, Watanabe S, Kimira M, Shimoi K, Mochizuki R, Kinae N. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J Nutr. 2000 Sep;130(9):2243–2250. doi: 10.1093/jn/130.9.2243. [DOI] [PubMed] [Google Scholar]
- 42.Kelly GE, Nelson C, Waring MA, Joannou GE, Reeder AY. Metabolites of dietary (soya) isoflavones in human urine. Clin Chim Acta. 1993 Dec 31;223(1–2):9–22. doi: 10.1016/0009-8981(93)90058-c. [DOI] [PubMed] [Google Scholar]
- 43.Kirkman LM, Lampe JW, Campbell DR, Martini MC, Slavin JL. Urinary lignan and isoflavonoid excretion in men and women consuming vegetable and soy diets. Nutr Cancer. 1995;24(1):1–12. doi: 10.1080/01635589509514388. [DOI] [PubMed] [Google Scholar]
- 44.Lu LJ, Anderson KE. Sex and long-term soy diets affect the metabolism and excretion of soy isoflavones in humans. Am J Clin Nutr. 1998;68(6 Suppl):1500S–1504S. doi: 10.1093/ajcn/68.6.1500S. [DOI] [PubMed] [Google Scholar]
- 45.Setchell KD, Borriello SP, Hulme P, Kirk DN, Axelson M. Nonsteroidal estrogens of dietary origin: possible roles in hormone-dependent disease. Am J Clin Nutr. 1984 Sep;40(3):569–578. doi: 10.1093/ajcn/40.3.569. [DOI] [PubMed] [Google Scholar]