Abstract
Our recent studies have revealed that among the 10 different commonly used AAV serotypes, AAV3 vectors transduce human liver cancer cells extremely efficiently because these cells express high levels of human hepatocyte growth factor receptor (hHGFR), and AAV3 utilizes hHGFR as a cellular co-receptor for viral entry. In this report, we provide further evidence that both extracellular as well as intracellular kinase domains of hHGFR are involved in AAV3 vector entry and AAV3-mediated transgene expression. We also document that AAV3 vectors are targeted for degradation by the host cell proteasome machinery, and that site-directed mutagenesis of surface exposed tyrosine (Y) to phenylalanine (F) residues on AAV3 capsids significantly improves the transduction efficiency of Y701F, Y705F and Y731F mutant AAV3 vectors. The transduction efficiency of the Y705+731F double-mutant vector is significantly higher than each of the single-mutants in liver cancer cells in vitro. In immuno-deficient mouse xenograft models, direct intra-tumor injection of AAV3 vectors also led to high-efficiency transduction of human liver tumor cells in vivo. We also document here that the optimized tyrosine-mutant AAV3 vectors lead to increased transduction efficiency following both intra-tumor and tail-vein injections in vivo. The optimized tyrosine-mutant AAV3 serotype vectors containing pro-apoptotic genes should prove useful for the potential gene therapy of human liver cancers.
Keywords: AAV vectors, tyrosine mutants, human hepatocyte growth factor receptor, human liver cancer, gene therapy
Introduction
Adeno-associated virus 2 (AAV2), a non-pathogenic human parvovirus, contains a single-stranded DNA genome, and possesses a wide tissue-tropism that transcends the species barrier1. Recombinant AAV2 vectors have gained attention as a promising vector system for the potential gene therapy of a variety of human diseases, and are currently in use in a number of gene therapy clinical trials2. More recently, several additional AAV serotypes have been isolated, and have been shown to transduce specific cell types efficiently3-8. Whereas various steps in the life cycle of AAV2 are reasonably well understood9-26, less is known about the other serotypes.
Of the 10 commonly used AAV serotypes, AAV3 has been reported to transduce cells and tissues poorly27,28. However, recent studies from our laboratory revealed that AAV3 vectors transduce established human hepatoblastoma (HB) and human hepatocellular carcinoma (HCC) cell lines as well as primary human hepatocytes extremely efficiently29. Subsequently, we documented that AAV3 infection was strongly inhibited by hepatocyte growth factor (HGF), HGF receptor (HGFR) specific siRNA, and anti-HGFR antibody, which suggested that AAV3 utilizes HGFR as a cellular receptor/co-receptor for viral entry30. The precise underlying molecular mechanisms of HGFR-mediated viral entry as well as additional steps in the life cycle of AAV3 remain unexplored.
Others and we have previously reported that the ubiquitin-proteasome pathway plays a crucial role in intracellular trafficking of AAV vectors15,31,32. We have also reported that intact AAV2 capsids can be phosphorylated at tyrosine residues by epidermal growth factor receptor protein tyrosine kinase (EGFR-PTK), and that tyrosine-phosphorylation of AAV capsids negatively affects viral intracellular trafficking and transgene expression. These observations led to the suggestion that tyrosine-phosphorylation is a signal for ubiquitination of AAV capsids followed by proteasome-mediated degradation32,33. This led to the hypothesis that mutations of the surface-exposed tyrosine residues (Y) to phenylalanine (F) might allow the vectors to evade phosphorylation, ubiquitination and proteasome-mediated degradation. Indeed, mutations of the surface-exposed tyrosine residues in AAV2 vectors led to high-efficiency transduction at lower doses both in HeLa cells in vitro and murine hepatocytes in vivo34. Therapeutic levels of expression of human factor IX have been obtained in several different strains of mice using the single and multiple tyrosine-mutant AAV2 vectors34,35. Additional studies have corroborated that similar Y-F mutations in AAV serotypes 6, 8 and 9 also lead to augmented transgene expression36-38. Six of 7 surface-exposed tyrosine residues in AAV2 are also conserved in AAV3, but their involvement in AAV3-mediated transduction has not been evaluated.
In this study, we report that: (i) AAV3 vector-mediated transduction is dramatically increased in T47D cells, a human breast cancer cell line that expresses undetectable levels of the endogenous hHGFR39, following stable transfection and over-expression of hHGFR; (ii) the tyrosine kinase activity associated with hHGFR negatively affects the transduction efficiency of AAV3 vectors; (iii) the use of proteasome inhibitors significantly improves AAV3 vector-mediated transduction; (iv) site-directed mutagenesis of three surface-exposed tyrosine residues on the AAV3 capsid leads to improved transduction efficiency; (v) a specific combination of two tyrosine-mutations further improves the extent of transgene expression; and (vi) AAV3 vectors efficiently transduce human HB and HCC tumors in a murine xenograft model in vivo, following both intratumoral or systemic administration. These optimized AAV3 vectors may be useful for the potential gene therapy of liver cancer in humans.
Results
Human HGFR is required for AAV3 infectivity
We recently provided preliminary evidence that AAV3 utilizes human hepatocyte growth factor receptor (HGFR) as a cellular co-receptor30. To unequivocally corroborate this contention, we used a human breast cancer cell line, T47D, which expresses undetectable levels of hHGFR39, as well as T47D cells stably transfected with a hHGFR expression plasmids (T47D+hHGFR)39. The expression of hHGFR protein in the established cell line, T47D+hHGFR, was confirmed by Western blot analysis (data shown below in Fig 2C). Equivalent numbers of T47D and T47D+hHGFR cells were transduced with various multiplicities-of-infection (MOI) of self-complementary (sc) AAV3-CBAp-EGFP vectors under identical conditions and transgene expression was determined 72 hrs post-transduction. These results, shown in Fig. 1A, document that the transduction efficiency of AAV3 vectors is ~8-13-fold higher in cells that express hHGFR than those that do not. AAV3 vector-mediated transduction of T47D+hHGFR cells could be completely blocked in the presence of 5 μg/ml of hHGF (Fig. 1B). Taken together, these data provide conclusive evidence that cell surface expression of hHGFR is required for successful transduction by AAV3 vectors.
Figure 2. The effect of BMS-777607 on AAV3-mediated transgene expression.
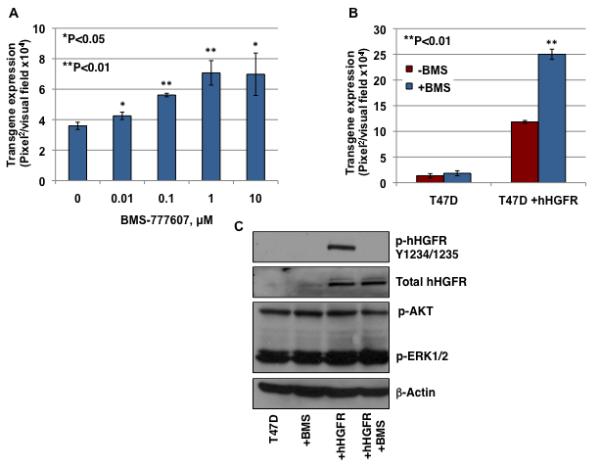
(A) T47D+ hHGFR cells, either mock-treated or treated with various concentration of BMS-777607, were infected with 2,000 vgs/cell of scAAV3-CBAp-EGFP vectors. Transgene expression was determined by fluorescence microscopy 72 hrs post-infection. (B) T47D and T47D+hHGFR cells were infected with 10,000 vgs/cell of scAAV3-CBAp-EGFP vectors in the absence or the presence of 1 μM of BMS-777607. (C) T47D and T47D+hHGFR cells were mock-treated or pretreated with BMS-777607 for 2 hrs. Whole-cell lysates were prepared and analyzed on Western blots using various indicated primary antibodies. β-actin was used as a loading control.
Figure 1. Analysis of AAV3-mediated transgene expression in T47D and T47D+hHGFR cells.
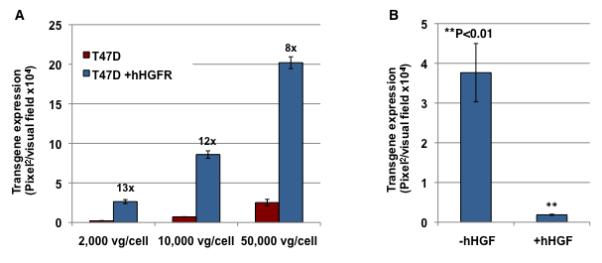
(A) Equivalent numbers of T47D and T47D+hHGFR cells were infected with various indicated multiplicity-of-infection (MOI) of scAAV3-CBAp-EGFP vectors under identical conditions. Transgene expression was determined by fluorescence microscopy 72 hrs post-infection. (B) T47D+hHGFR cells were transduced with 2,000 vgs/cell of scAAV3 vectors in the absence or the presence of 5 μg/ml of hHGF. Transgene expression was determined by fluorescence microscopy 72 hrs post-infection.
Inhibition of HGFR protein tyrosine kinase activity enhances the transduction efficiency of AAV3 vectors
We next wished to examine whether in addition to the extracellular domain, the intracellular domain of HGFR, which contains protein tyrosine kinase activity, is also involved in AAV3 infection. Binding of its ligand, HGF, results in dimerization of the receptor and intermolecular trans-phosphorylation of multiple tyrosine residues in the intracellular domain40. T47D+hHGFR cells were treated for 2 hrs with increasing concentrations of a specific HGFR kinase inhibitor, BMS-77760707 (BMS)41,42. Cells were subsequently infected with scAAV3 vectors at 2,000 vgs/cell. These results are shown in Fig. 2A. It is evident that BMS-777607-treatment led to ~2-fold increase in AAV3 transduction efficiency. Although the p-value is higher when BMS-777607 was used at the highest concentration of 10 μM, compared with the lower concentration of 1 μM, this change is most likely due to drug toxicity. In previous studies, it has been reported that BMS-777607 treatment had no significant effect on cell growth at doses ≤1 μM. However, doses of 10 μM did result in significant reduction in cell proliferation, which suggests that this concentration is toxic to cells43. In the next experiment, to rule out any possible non-specific nature of this drug, the parental T47D cells were included as a control. Both cell types were treated with 1 μM BMS-777607 for 2 hrs and then infected with scAAV3 vectors at 10,000 vg/cell. The results, shown in Fig. 2B, indicated that whereas BMS-777607-treatment significantly enhances AAV3 infectivity in T47D+hHGFR cells, it has no effect in T47D cells that lack expression of hHGFR.
We also wished to examine whether inhibition of the HGFR kinase led to alterations in the phosphorylation status of specific cellular proteins involved in the downstream signaling pathway. Total and phosphorylation levels of the HGFR protein in both T47D and T47D+hHGFR lysates were determined following a 2-hr drug-incubation period. Activation of signaling pathways downstream from HGFR kinase, ERK1/2 and Akt, were analyzed using phosphorylation-specific antibodies. These results, shown in Fig. 2C, confirmed that whereas little expression of hHGFR occurs in T47D cells, the level of expression is significantly higher in T47D+hHGFR cells for both total HGFR and phosphorylated HGFR, which is consistent with previously published reports39. Treatment of T47D+hHGFR cells with BMS-777607 completely blocked the phosphorylation of HGFR but not total HGFR. In addition, BMS-777607-treatment had no effect on the expression of phosphorylated AKT and ERK1/2. These results suggest that the enhancement of AAV3 vector infectivity by the BMS-777607-treatment is due to inhibition of HGFR kinase.
To date, only AAV2 has been reported to use hHGFR as a co-receptor44. The roles of hHGFR and hHGFR kinase inhibitor on other AAV serotypes are not known. To rule out any non-specific enhancement of transduction by BMS-777607, other serotypes of AAV, which are not dependent on HGFR, as well as AAV2 vectors were compared for transduction efficiency following treatment of cells with BMS-777607. These results, shown in Fig. 3, indicate that whereas AAV2 and AAV3 vectors can efficiently transduce T47D+hHGFR cells, other serotypes (AAV4-AAV9) can only transduce these cells at a very low efficiency. This result suggests that hHGFR is not involved in the life cycle of these AAV serotypes. Treatment of cells with BMS-777607 significantly increased the transduction efficiency of both AAV2 and AAV3 vectors, but not the other AAV serotypes, which suggests that the effect of the BMS-777607-treatment is AAV serotype-specific.
Figure 3. The effect of BMS-777607 on various AAV serotype-mediated transgene expression.
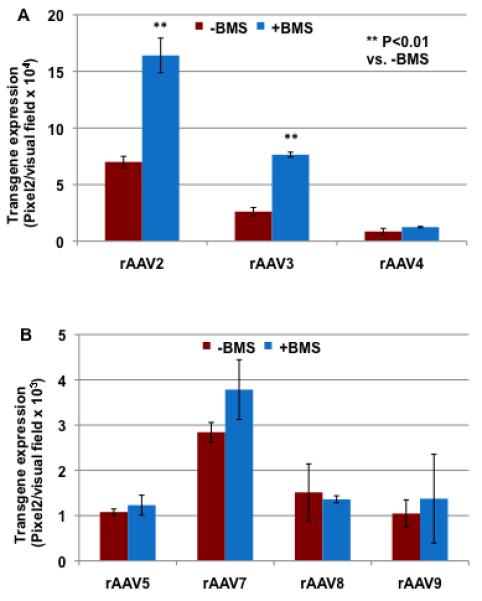
(A) T47D+ hHGFR cells, either mock-treated or treated with 1 μM of BMS-777607, were infected with 2,000 vgs/cell of either scAAV2-, scAAV3- or scAAV4-CBAp-EGFP vectors. (B) T47D+ hHGFR cells, either mock-treated or treated with 1 μM of BMS-777607, were infected with 2,000 vgs/cell of either scAAV5-, scAAV7-, scAAV8- or scAAV9-CBAp-EGFP vectors. Transgene expression was determined by fluorescence microscopy 72 hrs post-infection.
Proteasome inhibitors increase the transduction efficiency of AAV3 vectors
Previous studies by others and us have shown that proteasome inhibitors, such as MG132, can significantly enhance the transduction efficiency of AAV2 vectors by facilitating intracellular trafficking31,45. To evaluate whether MG132 can also improve AAV3 trafficking in target cells, Huh7, a well-established human hepatocellular carcinoma cell line46, and Hep293TT, a recently established human hepatoblastoma cell line47, were either mock-treated or treated with increasing concentrations of MG132. Following a two-hour treatment, cells were infected with scAAV3-EGFP vectors. HeLa cells, treated with 5 μM MG132 and transduced with scAAV2 vectors, were included as a positive control. Transgene expression was determined by fluorescence microscopy 72 hrs post-transduction. These data are shown in Fig. 4A and 4B. As can be seen, pretreatment with MG132 significantly increased the transduction efficiency of scAAV2 vectors in HeLa cells, which is consistent with our previously published studies31. Interestingly, a dose-dependent increase in the transduction efficiency of scAAV3 vectors in both Huh7 and Hep293TT cells occurred following MG132-treatment, suggesting that AAV3 vectors also undergo ubiquitination followed by proteasome-mediated degradation.
Figure 4. Comparative analyses of AAV3-mediated transduction efficiency in Huh7 and Hep293TT cells with or without treatment with MG132.
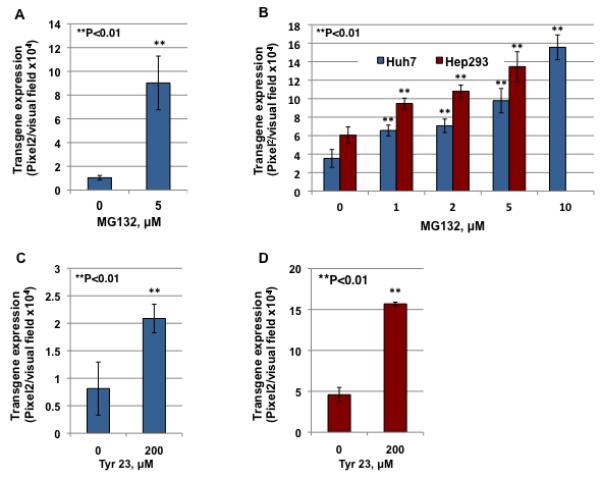
(A) HeLa cells, either mock-treated or treated with 5 μM of MG132, were infected with scAAV2-CBAp-EGFP vectors. (B) Huh7 and Hep293TT cells, either mock-treated or treated with various concentration of MG132, were infected with scAAV3-WT-CBAp-EGFP vectors. (C) HeLa cells, either mock-treated or treated with 200 μM of Tyr23, were infected by scAAV2-CBAp-EGFP vectors. (D) Hep293TT cells, either mock-treated or treated with Tyr23, were infected by scAAV3-CBAp-EGFP vectors. Transgene expression was determined 72 hrs post-transduction.
Previous studies from our laboratory have also shown that inhibition of EGFR-PTK signaling by Tyrphostin 23 (Tyr23), a specific inhibitor of EGFR-PTK48, modulates the Ub/proteasome pathway, which in turn, facilitates intracellular trafficking and transgene expression mediated by AAV2 vectors31. Hep293TT cells were mock-treated or treated with Tyr23 for 2 hrs and transduced with scAAV3 vectors. HeLa cells, pretreated with Tyr23 and transduced with scAAV2 vectors, were included as appropriate controls. Transgene expression was determined 72 hrs post-transduction. These results, shown in Fig. 4C and 4D, indicate that Tyr23-treatment led to a significant increase in the transduction efficiency of both scAAV2 and scAAV3 vectors. The increased transgene expression was independent of vector entry, since there was no significant difference in the amounts of internalized viral DNA in the presence or absence of either MG132 or Tyr23 (data not shown). These results further corroborate the involvement of the host cell Ub/proteasome machinery in the life cycle of AAV3 vectors as well.
Site-directed mutagenesis of surface-exposed tyrosine residues significantly improves the transduction efficiency of scAAV3 vectors
Recent studies from our laboratory have shown that there are 7 surface-exposed tyrosine residues (Y252, Y272, Y444, Y500, Y700, Y704 and Y730) on AAV2 capsids that are phosphorylated by EGFR-PTK and negatively affect the transduction efficiency of AAV2 vectors34. Alignment of amino acid sequences from AAV2 and AAV3 capsids indicated that six of seven tyrosine residues (Y252, Y272, Y444, Y701, Y705 and Y731) are conserved in AAV3 capsid (Table 1). One tyrosine residue, Y500 in AAV2, is present as F501 in AAV3. Since we and others have shown that Y-F mutations in several AAV serotypes enhance transgene expression by circumventing ubiquitination and proteasome-mediated degradation34,36-38, we reasoned that mutation of F501 back to a tyrosine residue would reduce the transduction efficiency of AAV3 vectors. This hypothesis was tested by generating a mutant AAV3 vector in which the phenylalanine residue was substituted with a tyrosine residue (F501Y). The transduction efficiency of the mutant vector was compared with its wild-type (WT) AAV3 counterpart using Huh7 cells under identical conditions. As can be seen in Fig. 5A, the extent of the transgene expression mediated by the F501Y mutant vector was reduced by ~50% compared with the WT AAV3 vector.
Table 1.
Surface-exposed tyrosine residues on AAV capsids, and site-directed mutagenesis to phenylalanine residues.
| AAV2 | AAV3 |
|---|---|
| Y252 | Y252→F |
| Y272 | Y272→F |
| Y444 | Y444→F |
| Y500 | F501 |
| Y700 | Y701→F |
| Y704 | Y705→F |
| Y730 | Y731→F |
The surface-exposed tyrosine (Y) residues on AAV2 and AAV3 capsids are shown; red arrows denote the site-directed mutations from Y to phenylalanine (F) residues on AAV3 capsids.
Figure 5. Site-directed mutational analyses of surface-exposed tyrosine residues on AAV3 capsids.
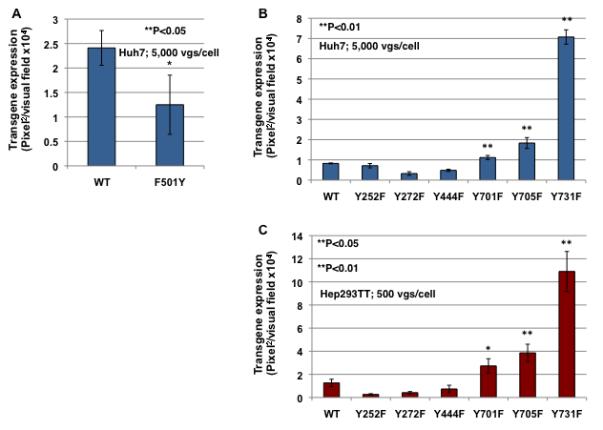
Huh7 cells were transduced with WT or F501Y scAAV3-CBAp-EGFP vectors under identical conditions, and transgene expression was determined 72 hrs post-transduction (A). Transduction efficiency of WT and various Y-F scAAV3-mediated transgene expression in Huh7 (B) and Hep293TT (C) cells. Transgene expression was determined 72 hrs post-transduction.
To further test our hypothesis that tyrosine-mutations on AAV3 capsids would lead to decreased EGFR-PTK-mediated phosphorylation followed by reduced ubiquitination and impaired proteasome-mediated degradation resulting in increased transgene expression, we modified all six surface-exposed tyrosine residues on AAV3 capsids and substituted with phenylalanine residues (tyrosine-phenylalanine, Y-F). Each of the single tyrosine-mutant vectors encapsidating scAAV2-CBAp-EGFP genomes could be successfully packaged. Vector titers for each of the mutants were determined by both quantitative DNA slot blots and qPCR, and no significant differences in the packaging efficiency were observed (data not shown). The transduction efficiency of each of the tyrosine-mutant vectors was analyzed and compared with the WT scAAV3-CBAp-EGFP vector in both Huh7 (Fig. 5B) and Hep293TT (Fig. 5C) cells under identical conditions. From these results, it is evident that, the transduction efficiency of three of the tyrosine-mutant vectors (Y701F, Y705F and Y731F) is significantly higher compared with the WT scAAV3 vector. Specifically, the transduction efficiency of Y731F vector was ~8-fold higher than the WT vector, followed by Y705F (~3-fold) and Y701F (~2-fold) vectors.
Multiple-mutations in surface-exposed tyrosine residues further improve the transduction efficiency of AAV3 vectors
In our recently published studies with Y-F mutant AAV2 vectors, we observed that specific combinations of the most efficient single-mutations of surface-exposed tyrosine residues further augmented the transduction efficiency of AAV2 vectors35. To examine whether a similar enhancement could be achieved with AAV3 vectors, we generated the following double- and triple-mutant AAV3 vectors: Y701+731F, Y705+731F, and Y701+705+731F. Each of these mutant vectors was packaged to similar titers, as determined by both quantitative DNA slot blots and qPCR (data not shown). The transduction efficiency of these multiple-mutants was compared with the WT and the Y731F single-mutant AAV3 vectors in Huh7 cells under identical conditions. These results are shown in Fig. 6A. As can be seen, whereas the Y731F mutation significantly increased the transduction efficiency of AAV3 vectors, as observed before, only one of the double-mutations (Y705+731F) led to an additional significant increase in transgene expression. Interestingly, the transduction efficiency of both the double-mutant (Y701+731F) and the triple-mutant (Y701+705+731F) vectors was reduced to levels similar to the WT AAV3 vector. The best performing single and multiple tyrosine-mutants on human liver cancer cells were then evaluated for transduction of T47D and T74D+hHGFR cells (Fig. 6B). Similar to human liver cancer cells, the tyrosine-mutant rAAV3 vectors led to high-efficiency transduction of both cell types, with or without hHGFR expression.
Figure 6. Transduction efficiency of WT and single, double, and triple tyrosine-mutant AAV3 vectors.
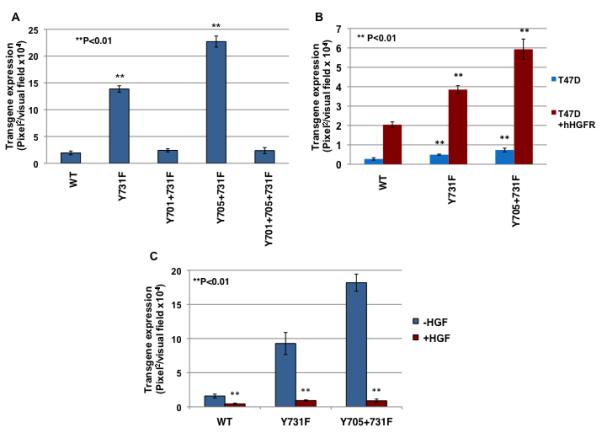
(A) Huh7 cells were transduced with WT or various indicated Y-F mutant scAAV3-CBAp-EGFP vectors under identical conditions. Transgene expression was determined 72 hrs post-transduction. (B) Huh7 cells were transduced with 5,000 vgs/cell of WT or Y-F mutant scAAV3 vectors in the absence or the presence of 5 μg/ml of hHGF. Transgene expression was determined by fluorescence microscopy 72 hrs post-infection.
To examine the possibility whether the observed enhanced transduction efficiency of the Y-F mutant vectors was due to the involvement of one or more additional putative cellular receptor/co-receptor functions, the WT, Y731F, and Y705+731F mutant scAAV3-CBAp-EGFP vectors were used to transduce Huh7 cells in the absence or the presence of 5 μg/ml hHGF under identical conditions. These results are shown in Fig. 6C. As is evident, the presence of hHGF dramatically inhibited the transduction efficiency and transgene expression of all three AAV3 vectors, which is consistent with the interpretation that the tyrosine-mutant vectors also utilize hHGFR as a cellular receptor/co-receptor for viral entry.
AAV3 vectors transduce human liver tumors in murine xenograft models in vivo
It was important to obtain proof-of-principle whether AAV3 vectors could also transduce human HB and HCC tumors in a xenograft mouse model in vivo. To this end, ~5×106 HCC (Huh7) or HB (Hep293TT) cells were injected sub-cutaneously in NOD/Scid gamma (NSG) mice. Four-weeks later, when tumors were clearly visible and palpable in both groups of animals, ~2×1010 vgs of scAAV3-CBAp-EGFP vectors were injected directly into tumors. Four-days post-vector injections, tumors were excised, and thin sections were examined under a fluorescence microscope. These results, shown in Fig. 7, indicate that AAV3 provides an effective means to transduce both human HCC (Fig. 7A) and HB (Fig. 7B) tumors in vivo. Consistent with the in vitro data, the transduction efficiency of AAV3 vectors was higher in Hep293TT cell-derived tumors than that in Huh7 cell-derived tumors.
Figure 7. Transduction efficiency of AAV3 vectors in vivo following direct intra-tumor injections.
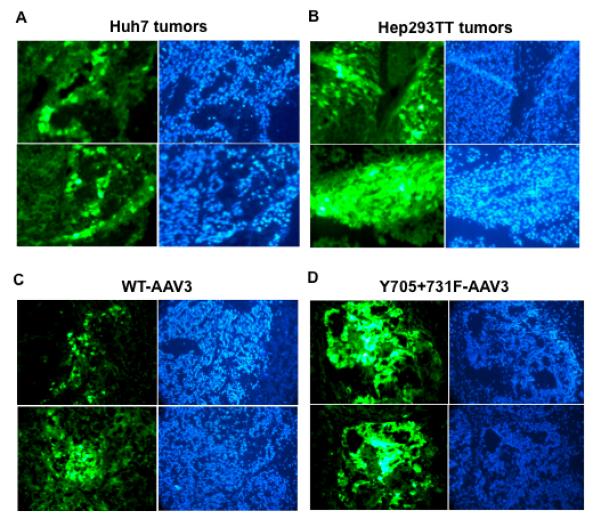
Transduction efficiency of WT-AAV3 vectors in (A) Huh7- and (B) Hep293TT-derived tumors in NSG mice. Transduction efficiency of (C) WT- and – (D) Y705+731F-AAV3 vectors in Hep293TT-derived tumors in NSG mice. EGFP fluorescence (green) and DAPI staining (blue) of two representative tumor sections from each set of mice is shown.
Optimized tyrosine-mutant AAV3 vectors are highly efficient in transducing human liver tumors in a murine xenograft model in vivo
Next, the best performing double tyrosine-mutant AAV3 vectors were further evaluated in vivo for xenograft human liver tumors gene transfer. In the first set of experiments, ~5×1010 vgs of either the wild-type (WT) scAAV3- or Y705+731F-AAV3-CBAp-EGFP vectors were intratumorally injected in NSG mice bearing human HB (Hep293TT) tumors. Four-days post-vector injections, tumors were excised, and thin sections were examined under a fluorescence microscope (Fig. 7C). As can be seen, tumors injected with the WT-AAV3 vectors exhibited detectable levels expression of EGFP. The transduction efficiency of the double tyrosine-mutant AAV3 vectors was significantly higher compared with the WT AAV3 vectors, which is consistent with our in vitro data.
In the second set of experiments, ~5×1011 vgs of either the WT-scAAV3- or the Y705+731F-scAAV3-CBAp-EGFP vectors were injected via the tail-vein in NSG mice bearing human HB (Hep293TT) tumors. Phosphate-buffered saline (PBS) injections were used as an appropriate control. As can be seen in Fig. 8, whereas little trangene expression occurred in tumors from mice injected with pBS (Fig. 8A), direct tumor-targeting could be achieved following systemic administration of AAV3 vectors. The transduction efficiency of the optimized tyrosine-mutant AAV3 vectors (Fig. 8C), once again, was significantly higher than that of the WT AAV3 vectors (Fig. 8B). These data suggest that the observed increased transduction efficiency of tyrosine-mutant AAV3 vectors is independent of viral administration route. Additional studies are warranted with the Y705+731F mutant scAAV3 vectors containing a therapeutic gene to further evaluate their safety and efficacy these vectors for the potential gene therapy of HB and HCC.
Figure 8. Transduction efficiency of WT- and Y705+731F-AAV3 vectors in Hep293TT-derived tumors in NSG mice following tail-vein injections.
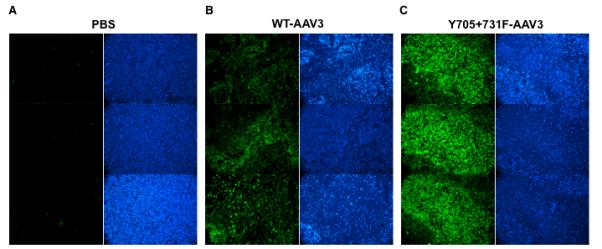
EGFP fluorescence (green) and DAPI staining (blue) of tumor in three representative tumor sections from each set of mice injected with (A) PBS, or (B) WT-AAV3, or (C) Y705+731F-AAV3 vectors is shown.
Discussion
Recombinant vectors based on AAV serotype 2 are currently in use in a number of gene therapy clinical trials2, and have recently shown remarkable efficacy in the treatment of Leber’s congenital amaurosis26,49,50. However, concerns have been raised with reference to the humoral response to AAV2 vectors based on the high prevalence of sero-positivity in the general population (~80 to 90%)51-53. The discovery of many novel AAV serotypes has prompted the development of AAV vectors to circumvent this potential problem3-8. For example, recombinant AAV8 vectors were recently reported to be therapeutic in a mouse model of liver cancer54. However, several groups have described various strategies to target human liver cancer cells in murine models using AAV2 vectors55-63. In our pursuit to identify the most efficient AAV serotype to target human liver cancer cells, we observed that three different human liver cancer cell lines could be transduced extremely efficiently by AAV3 vectors29. We subsequently identified human hepatocyte growth factor receptor (hHGFR) as a cellular co-receptor for AAV3 infection30. However, the precise role of hHGFR, especially the role of tyrosine kinase activity associated with the intracellular domain of hHGFR, in AAV3-mediated transduction remained unclear. Here, we present a more detailed account of the AAV3-hHGFR interactions, and describe the development of an optimal AAV3 vector for its potential use in targeting human liver cancer cells.
HGFR is a trans-membrane receptor tyrosine kinase, and binding of its ligand, HGF, results in dimerization of the receptor and intermolecular trans-phosphorylation of multiple tyrosine residues in the intracellular domain64. Whereas it is clear that AAV3 capsid interacts with the extracellular domain of hHGFR, it is less clear whether AAV3-binding to hHGFR also triggers its activation and phosphorylation of the downstream target proteins. Our data do indeed demonstrate that suppression of the hHGFR-PTK activity leads to a modest increase in AAV3 vector-mediated transgene expression. In this context, it is of interest to note that the transduction efficiency of AAV3 vectors is significantly higher in a more recently established human hepatoblastoma (HB) cell line, Hep293TT, compared with that in a HB cell line, Huh6, which was established nearly three decades ago. Although subtle differences might exist between the two cell lines, we have identified specific mutations in the tyrosine kinase domain of hHGFR in Hep293TT cells, which render it inactive, and that the hHGFR-specific kinase inhibitor, BMS-777607, which augments the transduction efficiency in Huh6 cells, has little effect on AAV3 transduction efficiency in Hep293TT cells (data not shown).
Despite the utilization of two distinct cellular growth factor receptors as co-receptors by AAV2 (hFGFR1) and AAV3 (hHGFR), the two serotypes appear to share certain post-receptor entry and intracellular trafficking pathways. For example, both capsids become phosphorylated at tyrosine residues by EGFR-PTK, presumably in the late endosomes, followed by ubiquitination, which leads to proteasome-mediated degradation33. However, although 6 of 7 surface-exposed tyrosines in AAV2 are conserved in AAV3, the patterns of behavior of the corresponding Y-F mutants are somewhat divergent. For example, Y730F (for AAV2) and Y731F (for AAV3) are the most efficient single-mutants, followed by Y444F (for AAV2), and Y705F (for AAV3), the transduction efficiency of Y444F (for AAV3) remains unaltered. Similarly, whereas the transduction efficiency of the Y730+444F double-mutant (for AAV2) is not significantly different from that of Y730F, the transduction efficiency of the Y705+731F double-mutant (for AAV3) is significantly higher than Y731F. Furthermore, the Y730+500+444F triple-mutant (for AAV2) is the most efficient, the Y731+501+705F triple-mutant (for AAV3) is the most efficient, the Y501 residue having already been mutated in the WT AAV3 capsid. The molecular basis for these observed differences are not readily apparent, and further studies are warranted to gain a better understanding of the underlying molecular mechanisms. Interestingly, even the WT AAV3 vectors were able to transduce human liver tumors reasonably well in a mouse xenograft model in vivo following intratumor injection. However, we showed evidence that the tyrosine-mutant vector resulted in higher gene transfer efficiency in vivo. It is tempting to speculate that the use of the optimized tyrosine-mutant AAV3 vectors containing a pro-apoptotic gene would lead to a therapeutic effect in human liver tumors in vivo.
Human liver cancer, especially hepatocellular carcinoma (HCC), is one of the most aggressive malignant tumors. The major obstacle to survival with HCC is recurrence after HCC resection65. Thus, transduction of 100% of target cells is desirable in order to totally eliminate the tumor. In our previous studies, we have observed that melittin, a toxic peptide derived from bee venom, inhibits the viability and motility of HCC cells both in vitro and in vivo via the suppression of Rac1-dependent pathway66 and up-regulation of mitochondria membrane protein 7A667. We have also demonstrated that melittin can induce apoptosis of HCC cells potentially by activating CaMKII/TAK1/JNK/p38 signaling pathway68. Based on our previous studies with recombinant adenovirus vectors containing the melittin gene driven by a liver cancer cell-specific promoter to achieve specific killing of liver cancer cells both in vitro and in vivo69, we now wish to develop optimized tyrosine-mutant AAV3-melittin vectors under the control of a liver cancer cell-specific promoter to selectively target both primary and metastatic liver cancer.
Material and Methods
Cell lines and cultures
Human cervical cancer (HeLa) and hepatocellular carcinoma (Huh7) cell lines were purchased from American Type Culture Collection (Manassas, VA, USA), and maintained in complete DMEM medium (Mediatech Inc., Manassas, VA, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Sigma-Aldrich, St. Louis, MO, USA), 1% penicillin and streptomycin (P/S, Lonza, Walkersville, MD). A newly established human hepatoblastoma (Hep293TT) cell line47 was generously provided by Dr. Gail E. Tomlinson, University of Texas Health Science Center at San Antonio, San Antonio, TX, USA), and was maintained in complete RPMI medium 1640 (Invitrogen, Camarillo, CA) supplemented with 15% heat-inactivated FBS (Sigma-Aldrich, St. Louis, MO, USA), 1% penicillin and streptomycin (P/S, Lonza, Walkersville, MD). Cells were grown as adherent cultures in a humidified atmosphere at 37°C in 5% CO2 and were sub-cultured after treatment with trypsin-versene mixture (Lonza, Walkersville, MD) for 2-5 min at room temperature, washed and re-suspended in complete medium. A human breast cancer cell line, T47D, and T47D cells stably transfected with a hHGFR expression plasmid (T47D+hHGFR), were maintained in complete DMEM medium (Mediatech Inc., Manassas, VA, USA) with or without 600 μg/ml of G418, supplemented with 10% heat-inactivated fetal bovine serum (FBS, Sigma-Aldrich, St. Louis, MO, USA), 1% penicillin and streptomycin (P/S, Lonza, Walkersville, MD).
Recombinant AAV plasmids and vectors
Recombinant AAV3 packaging plasmid and recombinant AAV2-CBAp-EGFP vector plasmid were generously provided respectively by Drs. R. Jude Samulski and Xiao Xiao, University of North Carolina at Chapel Hill, Chapel Hill, NC. Highly purified stocks of scAAV2 and scAAV3 vectors containing the enhanced green fluorescence protein (EGFP) gene driven by the chicken β-actin promoter (CBAp) were packaged by the calcium phosphate triple-plasmid transfection protocol described previously70,71. The physical particle titers of recombinant vector stocks were determined by quantitative DNA slot-blot analyses71.
Construction of surface-exposed tyrosine residue mutant AAV3 capsid plasmids
A two-stage procedure, based on QuikChange II site-directed mutagenesis (Stratagene) was performed by using plasmid pAAV3 as described previously29,30. Briefly, in stage one, two PCR extension reactions were performed in separate tubes for each mutant. One tube contained the forward PCR primer and the other contained the reverse primer (Table 2). In stage two, the two reactions were mixed and a standard PCR mutagenesis assay was carried out as the manufacturer’s instructions. PCR primers were designed to introduce changes from tyrosine to phenylalanine residues and a silent change to create a new restriction endonuclease site for screening purposes (Table 2). All mutants were screened with the appropriate restriction enzyme and were sequenced before use.
Table 2.
Nucleotide sequences of primers used for site-directed mutagenesis.
| Mutants | Primer sequences (5′-3′) |
|---|---|
| Y252F | ACCAGAACCTGGGCTCTGCCCACTTTCAACAACCATCTCTACAAG -ApaI Tyr→Phe |
| Y272F | CAATCAGGAGCTTCGAACGACAACCACTTCTTTGGCTACAGCACC +BstBI Tyr→Phe |
| Y444F | CTTATCGATCAGTATCTGTACTTCCTGAACAGAACGCAAGGAACA +ClaI Tyr→Phe |
| F501Y | GCTAACGACAACAACAACAGTAACTATCCATGGACAGCGGCCAGCAAA Phe→Tyr +NcoI |
| Y701F | TGGAATCCAGAGATTCAGTTCACGTCCAACTACAACAAGTCTGTT Tyr→Phe +BmgBI |
| Y705F | GAGATTCAGTACACGTCCAACTTCAACAAGTCTGTTAATGTGGAC +AflIII Tyr→Phe |
| Y731F | GTGAACCTCGCCCTATTGGAACCCGGTTTCTCACACGAAACTTG Tyr→Phe |
The codon triplets are shown in bold; red fonts denote the mutations from tyrosine to phenylalanine residues, and green fonts indicate the silent mutations to eliminate/create the restriction enzyme sites (underlined), which were used to identify the desired clones.
AAV vector transduction assays
Huh7 or HeLa cells were seeded in 96-well plates at a concentration of 5,000 cells per well in complete DMEM medium. AAV infections were performed in serum- and antibiotic-free DMEM medium. Hep293TT cells were seeded in 96-well plates at a concentration of 10,000 cells per well in complete RPMI medium. The infections were performed in serum- and antibiotic-free RPMI medium. The expression of EGFP was analyzed by direct fluorescence imaging 72 hrs post-transduction.
Western blot analyses
Cells were harvested and disrupted in a radio-immunoprecipitation assay (RIPA) lysis buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.1% SDS, 1% NP-40, 0.25% sodium deoxycholate and 1 mM EDTA with protease inhibitor cocktail, 1 mM NaF and 1 mM Na3VO4). Total protein concentration was measured using a Bradford reagent (Bio-Rad, Hercules, CA) and equal amounts (50 μg) of whole cell lysates were resolved by SDS-PAGE. After electrophoresis, samples were electro-transferred to a nitrocellulose membrane (Bio-Rad), probed with relevant primary antibodies at 4°C overnight, incubated with horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch, West Grove, PA), and detected with an enhanced chemi-luminescence substrate (Amersham, Piscataway, NJ). Antibodies against phospho-c-Met (Y1234/1235), total c-Met, phospho-Akt (S473) and phospho-ERK (T202/Y204) were purchased from Cell Signaling (Danvers, MA), and anti-β-actin (AC-74) antibody was obtained from Sigma (St. Louis, MO).
Recombinant AAV3 vector transduction studies in mouse xenograft models in vivo
Groups of 6-weeks old NSG mice (Jackson Laboratories, Bar Harbor, ME) were injected subcutaneously with 5×106 Hep293TT or Huh7 cells. Four-week post-injection, indicated numbers of AAV3 vector genomes (vgs) were administered either intratumorally or through tail-vein. Four days post-vector administration, tumors were resected, cross-sectioned and evaluated for EGFP expression using a fluorescent microscope. Sections were also stained with DAPI to visualize the cell nucleus. All animal experiments were conducted in accordance with the University of Florida Institutional Animal Care and Use Committee guidelines.
Statistical analysis
Results are presented as mean ± standard deviation (SD). Differences between groups were identified using a grouped-unpaired two-tailed distribution of Student’s T test. P values <0.05 were considered statistically significant.
Acknowledgments
We thank Drs. R. Jude Samulski and Xiao Xiao for their kind gifts of recombinant AAV3 and AAV2-CBAp-EGFP plasmids, respectively, and Dr. Gail E. Tomlinson for generously providing the Hep293TT cell line. We also thank Drs. Regino Gonzalez-Peralta and Satish K. Walia for a critical review of the manuscript as well as for their general counsel. The expert technical assistance of Mr. Baozheng Li and Ms. Wenqin Ma is gratefully acknowledged. This research was supported in part by a Junior Investigator Award from the University of Florida Shands Cancer Center, American Cancer Society Chris DiMarco Institutional Research Grant, (to GVA),Institutional American Cancer Society grant # (to GVA), and Public Health Service grants R01 HL-076901, R01 HL-097088, and P01 DK-058327 (Project 1) from the National Institutes of Health (to AS). BC was supported by a State-Sponsored Program for Graduate Students from China Scholarship Council, Govt. of P.R. China.
Footnotes
Conflict of interest None of the authors declares any conflict of interest.
References
- 1.Muzyczka N. Use of adeno-associated virus as a general transduction vector for mammalian cells. Current topics in microbiology and immunology. 1992;158:97–129. doi: 10.1007/978-3-642-75608-5_5. [DOI] [PubMed] [Google Scholar]
- 2.Daya S, Berns KI. Gene therapy using adeno-associated virus vectors. Clinical microbiology reviews. 2008;21:583–593. doi: 10.1128/CMR.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muramatsu S, Mizukami H, Young NS, Brown KE. Nucleotide sequencing and generation of an infectious clone of adeno-associated virus 3. Virology. 1996;221:208–217. doi: 10.1006/viro.1996.0367. [DOI] [PubMed] [Google Scholar]
- 4.Chiorini JA, Yang L, Liu Y, Safer B, Kotin RM. Cloning of adeno-associated virus type 4 (AAV4) and generation of recombinant AAV4 particles. Journal of virology. 1997;71:6823–6833. doi: 10.1128/jvi.71.9.6823-6833.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiorini JA, Kim F, Yang L, Kotin RM. Cloning and characterization of adeno-associated virus type 5. Journal of virology. 1999;73:1309–1319. doi: 10.1128/jvi.73.2.1309-1319.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rutledge EA, Halbert CL, Russell DW. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. Journal of virology. 1998;72:309–319. doi: 10.1128/jvi.72.1.309-319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X, et al. Clades of Adeno-associated viruses are widely disseminated in human tissues. Journal of virology. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Summerford C, Samulski RJ. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. Journal of virology. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qing K, Mah C, Hansen J, Zhou S, Dwarki V, Srivastava A. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nature medicine. 1999;5:71–77. doi: 10.1038/4758. [DOI] [PubMed] [Google Scholar]
- 11.Summerford C, Bartlett JS, Samulski RJ. AlphaVbeta5 integrin: a co-receptor for adeno-associated virus type 2 infection. Nature medicine. 1999;5:78–82. doi: 10.1038/4768. [DOI] [PubMed] [Google Scholar]
- 12.Hansen J, Qing K, Kwon HJ, Mah C, Srivastava A. Impaired intracellular trafficking of adeno-associated virus type 2 vectors limits efficient transduction of murine fibroblasts. Journal of virology. 2000;74:992–996. doi: 10.1128/jvi.74.2.992-996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen J, Qing K, Srivastava A. Adeno-associated virus type 2-mediated gene transfer: altered endocytic processing enhances transduction efficiency in murine fibroblasts. Journal of virology. 2001;75:4080–4090. doi: 10.1128/JVI.75.9.4080-4090.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanlioglu S, Benson PK, Yang J, Atkinson EM, Reynolds T, Engelhardt JF. Endocytosis and nuclear trafficking of adeno-associated virus type 2 are controlled by rac1 and phosphatidylinositol-3 kinase activation. Journal of virology. 2000;74:9184–9196. doi: 10.1128/jvi.74.19.9184-9196.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Douar AM, Poulard K, Stockholm D, Danos O. Intracellular trafficking of adeno-associated virus vectors: routing to the late endosomal compartment and proteasome degradation. Journal of virology. 2001;75:1824–1833. doi: 10.1128/JVI.75.4.1824-1833.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao W, Zhong L, Wu J, Chen L, Qing K, Weigel-Kelley KA, et al. Role of cellular FKBP52 protein in intracellular trafficking of recombinant adeno-associated virus 2 vectors. Virology. 2006;353:283–293. doi: 10.1016/j.virol.2006.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas CE, Storm TA, Huang Z, Kay MA. Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. Journal of virology. 2004;78:3110–3122. doi: 10.1128/JVI.78.6.3110-3122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong L, Li W, Yang Z, Qing K, Tan M, Hansen J, et al. Impaired nuclear transport and uncoating limit recombinant adeno-associated virus 2 vector-mediated transduction of primary murine hematopoietic cells. Human gene therapy. 2004;15:1207–1218. doi: 10.1089/hum.2004.15.1207. [DOI] [PubMed] [Google Scholar]
- 19.Ferrari FK, Samulski T, Shenk T, Samulski RJ. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. Journal of virology. 1996;70:3227–3234. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher KJ, Gao GP, Weitzman MD, DeMatteo R, Burda JF, Wilson JM. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. Journal of virology. 1996;70:520–532. doi: 10.1128/jvi.70.1.520-532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qing K, Hansen J, Weigel-Kelley KA, Tan M, Zhou S, Srivastava A. Adeno-associated virus type 2-mediated gene transfer: role of cellular FKBP52 protein in transgene expression. Journal of virology. 2001;75:8968–8976. doi: 10.1128/JVI.75.19.8968-8976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong L, Qing K, Si Y, Chen L, Tan M, Srivastava A. Heat-shock treatment-mediated increase in transduction by recombinant adeno-associated virus 2 vectors is independent of the cellular heat-shock protein 90. The Journal of biological chemistry. 2004;279:12714–12723. doi: 10.1074/jbc.M310548200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong L, Li W, Yang Z, Chen L, Li Y, Qing K, et al. Improved transduction of primary murine hepatocytes by recombinant adeno-associated virus 2 vectors in vivo. Gene therapy. 2004;11:1165–1169. doi: 10.1038/sj.gt.3302283. [DOI] [PubMed] [Google Scholar]
- 24.Zhong L, Zhou X, Li Y, Qing K, Xiao X, Samulski RJ, et al. Single-polarity recombinant adeno-associated virus 2 vector-mediated transgene expression in vitro and in vivo: mechanism of transduction. Mol Ther. 2008;16:290–295. doi: 10.1038/sj.mt.6300376. [DOI] [PubMed] [Google Scholar]
- 25.McCarty DM, Young SM, Jr., Samulski RJ. Integration of adeno-associated virus (AAV) and recombinant AAV vectors. Annual review of genetics. 2004;38:819–845. doi: 10.1146/annurev.genet.37.110801.143717. [DOI] [PubMed] [Google Scholar]
- 26.Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. The New England journal of medicine. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 27.Zincarelli C, Soltys S, Rengo G, Koch WJ, Rabinowitz JE. Comparative cardiac gene delivery of adeno-associated virus serotypes 1-9 reveals that AAV6 mediates the most efficient transduction in mouse heart. Clinical and translational science. 3:81–89. doi: 10.1111/j.1752-8062.2010.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008;16:1073–1080. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
- 29.Glushakova LG, Lisankie MJ, Eruslanov EB, Ojano-Dirain C, Zolotukhin I, Liu C, et al. AAV3-mediated transfer and expression of the pyruvate dehydrogenase E1 alpha subunit gene causes metabolic remodeling and apoptosis of human liver cancer cells. Molecular genetics and metabolism. 2009;98:289–299. doi: 10.1016/j.ymgme.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ling C, Lu Y, Kelsi JK, Jayandharan GR, Li B, Ma W, et al. Human Hepatocyte Growth Factor Receptor is a Cellular Co-Receptor for AAV3. Human gene therapy. doi: 10.1089/hum.2010.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhong L, Zhao W, Wu J, Li B, Zolotukhin S, Govindasamy L, et al. A dual role of EGFR protein tyrosine kinase signaling in ubiquitination of AAV2 capsids and viral second-strand DNA synthesis. Mol Ther. 2007;15:1323–1330. doi: 10.1038/sj.mt.6300170. [DOI] [PubMed] [Google Scholar]
- 32.Duan D, Yue Y, Yan Z, Yang J, Engelhardt JF. Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. The Journal of clinical investigation. 2000;105:1573–1587. doi: 10.1172/JCI8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhong L, Li B, Jayandharan G, Mah CS, Govindasamy L, Agbandje-McKenna M, et al. Tyrosine-phosphorylation of AAV2 vectors and its consequences on viral intracellular trafficking and transgene expression. Virology. 2008;381:194–202. doi: 10.1016/j.virol.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhong L, Li B, Mah CS, Govindasamy L, Agbandje-McKenna M, Cooper M, et al. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7827–7832. doi: 10.1073/pnas.0802866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Markusic DM, Herzog RW, Aslanidi GV, Hoffman BE, Li B, Li M, et al. High-efficiency Transduction and Correction of Murine Hemophilia B Using AAV2 Vectors Devoid of Multiple Surface-exposed Tyrosines. Mol Ther. doi: 10.1038/mt.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrs-Silva H, Dinculescu A, Li Q, Min SH, Chiodo V, Pang JJ, et al. High-efficiency transduction of the mouse retina by tyrosine-mutant AAV serotype vectors. Mol Ther. 2009;17:463–471. doi: 10.1038/mt.2008.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qiao C, Zhang W, Yuan Z, Shin JH, Li J, Jayandharan GR, et al. AAV6 capsid tyrosine to phenylalanine mutations improve gene transfer to skeletal muscle. Human gene therapy. doi: 10.1089/hum.2010.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor J, Ussher JE. Optimized Transduction of Human Monocyte-Derived Dendritic Cells by Recombinant Adeno-Associated Virus Serotype 6 (rAAV6) Human gene therapy. doi: 10.1089/hum.2010.087. [DOI] [PubMed] [Google Scholar]
- 39.Abella JV, Peschard P, Naujokas MA, Lin T, Saucier C, Urbe S, et al. Met/Hepatocyte growth factor receptor ubiquitination suppresses transformation and is required for Hrs phosphorylation. Molecular and cellular biology. 2005;25:9632–9645. doi: 10.1128/MCB.25.21.9632-9645.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen L, Holgado-Madruga M, Maroun C, Fixman ED, Kamikura D, Fournier T, et al. Association of the multisubstrate docking protein Gab1 with the hepatocyte growth factor receptor requires a functional Grb2 binding site involving tyrosine 1356. The Journal of biological chemistry. 1997;272:20811–20819. doi: 10.1074/jbc.272.33.20811. [DOI] [PubMed] [Google Scholar]
- 41.Schroeder GM, An Y, Cai ZW, Chen XT, Clark C, Cornelius LA, et al. Discovery of N-(4-(2-amino-3-chloropyridin-4-yloxy)-3-fluorophenyl)-4-ethoxy-1-(4-fluor ophenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide (BMS-777607), a selective and orally efficacious inhibitor of the Met kinase superfamily. Journal of medicinal chemistry. 2009;52:1251–1254. doi: 10.1021/jm801586s. [DOI] [PubMed] [Google Scholar]
- 42.Dai Y, Siemann DW. BMS-777607, a small-molecule met kinase inhibitor, suppresses hepatocyte growth factor-stimulated prostate cancer metastatic phenotype in vitro. Molecular cancer therapeutics. 9:1554–1561. doi: 10.1158/1535-7163.MCT-10-0359. [DOI] [PubMed] [Google Scholar]
- 43.Dai Y, Siemann DW. BMS-777607, a small-molecule met kinase inhibitor, suppresses hepatocyte growth factor-stimulated prostate cancer metastatic phenotype in vitro. Molecular cancer therapeutics. 2010;9:1554–1561. doi: 10.1158/1535-7163.MCT-10-0359. [DOI] [PubMed] [Google Scholar]
- 44.Kashiwakura Y, Tamayose K, Iwabuchi K, Hirai Y, Shimada T, Matsumoto K, et al. Hepatocyte growth factor receptor is a coreceptor for adeno-associated virus type 2 infection. Journal of virology. 2005;79:609–614. doi: 10.1128/JVI.79.1.609-614.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan Z, Zak R, Luxton GW, Ritchie TC, Bantel-Schaal U, Engelhardt JF. Ubiquitination of both adeno-associated virus type 2 and 5 capsid proteins affects the transduction efficiency of recombinant vectors. Journal of virology. 2002;76:2043–2053. doi: 10.1128/jvi.76.5.2043-2053.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakabayashi H, Taketa K, Miyano K, Yamane T, Sato J. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer research. 1982;42:3858–3863. [PubMed] [Google Scholar]
- 47.Chen TT, Rakheja D, Hung JY, Hornsby PJ, Tabaczewski P, Malogolowkin M, et al. Establishment and characterization of a cancer cell line derived from an aggressive childhood liver tumor. Pediatric blood & cancer. 2009;53:1040–1047. doi: 10.1002/pbc.22187. [DOI] [PubMed] [Google Scholar]
- 48.Mah C, Qing K, Khuntirat B, Ponnazhagan S, Wang XS, Kube DM, et al. Adeno-associated virus type 2-mediated gene transfer: role of epidermal growth factor receptor protein tyrosine kinase in transgene expression. Journal of virology. 1998;72:9835–9843. doi: 10.1128/jvi.72.12.9835-9843.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cideciyan AV, Aleman TS, Boye SL, Schwartz SB, Kaushal S, Roman AJ, et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:15112–15117. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr., Mingozzi F, Bennicelli J, et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. The New England journal of medicine. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boutin S, Monteilhet V, Veron P, Leborgne C, Benveniste O, Montus MF, et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Human gene therapy. 21:704–712. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- 52.Mendell JR, Campbell K, Rodino-Klapac L, Sahenk Z, Shilling C, Lewis S, et al. Dystrophin immunity in Duchenne’s muscular dystrophy. The New England journal of medicine. 363:1429–1437. doi: 10.1056/NEJMoa1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nature medicine. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 54.Kota J, Chivukula RR, O’Donnell KA, Wentzel EA, Montgomery CL, Hwang HW, et al. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell. 2009;137:1005–1017. doi: 10.1016/j.cell.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Su H, Chang JC, Xu SM, Kan YW. Selective killing of AFP-positive hepatocellular carcinoma cells by adeno-associated virus transfer of the herpes simplex virus thymidine kinase gene. Human gene therapy. 1996;7:463–470. doi: 10.1089/hum.1996.7.4-463. [DOI] [PubMed] [Google Scholar]
- 56.Peng D, Qian C, Sun Y, Barajas MA, Prieto J. Transduction of hepatocellular carcinoma (HCC) using recombinant adeno-associated virus (rAAV): in vitro and in vivo effects of genotoxic agents. Journal of hepatology. 2000;32:975–985. doi: 10.1016/s0168-8278(00)80102-6. [DOI] [PubMed] [Google Scholar]
- 57.Su H, Lu R, Ding R, Kan YW. Adeno-associated viral-mediated gene transfer to hepatoma: thymidine kinase/interleukin 2 is more effective in tumor killing in non-ganciclovir (GCV)-treated than in GCV-treated animals. Mol Ther. 2000;1:509–515. doi: 10.1006/mthe.2000.0073. [DOI] [PubMed] [Google Scholar]
- 58.Ma H, Liu Y, Liu S, Xu R, Zheng D. Oral adeno-associated virus-sTRAIL gene therapy suppresses human hepatocellular carcinoma growth in mice. Hepatology (Baltimore, Md. 2005;42:1355–1363. doi: 10.1002/hep.20918. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y, Huang F, Cai H, Zhong S, Liu X, Tan WS. Potent antitumor effect of TRAIL mediated by a novel adeno-associated viral vector targeting to telomerase activity for human hepatocellular carcinoma. The journal of gene medicine. 2008;10:518–526. doi: 10.1002/jgm.1177. [DOI] [PubMed] [Google Scholar]
- 60.Tse LY, Sun X, Jiang H, Dong X, Fung PW, Farzaneh F, et al. Adeno-associated virus-mediated expression of kallistatin suppresses local and remote hepatocellular carcinomas. The journal of gene medicine. 2008;10:508–517. doi: 10.1002/jgm.1180. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Y, Ma H, Zhang J, Liu S, Liu Y, Zheng D. AAV-mediated TRAIL gene expression driven by hTERT promoter suppressed human hepatocellular carcinoma growth in mice. Life sciences. 2008;82:1154–1161. doi: 10.1016/j.lfs.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 62.Malecki M, Swoboda P, Pachecka J. Recombinant adeno-associated virus derived vectors (rAAV2) efficiently transduce ovarian and hepatocellular carcinoma cells--implications for cancer gene therapy. Acta poloniae pharmaceutica. 2009;66:93–99. [PubMed] [Google Scholar]
- 63.Wang Y, Huang F, Cai H, Wu Y, He G, Tan WS. The efficacy of combination therapy using adeno-associated virus-TRAIL targeting to telomerase activity and cisplatin in a mice model of hepatocellular carcinoma. Journal of cancer research and clinical oncology. 136:1827–1837. doi: 10.1007/s00432-010-0841-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu X, Yao W, Newton RC, Scherle PA. Targeting the c-MET signaling pathway for cancer therapy. Expert opinion on investigational drugs. 2008;17:997–1011. doi: 10.1517/13543784.17.7.997. [DOI] [PubMed] [Google Scholar]
- 65.Tang ZY. Hepatocellular carcinoma surgery--review of the past and prospects for the 21st century. Journal of surgical oncology. 2005;91:95–96. doi: 10.1002/jso.20291. [DOI] [PubMed] [Google Scholar]
- 66.Liu S, Yu M, He Y, Xiao L, Wang F, Song C, et al. Melittin prevents liver cancer cell metastasis through inhibition of the Rac1-dependent pathway. Hepatology (Baltimore, Md. 2008;47:1964–1973. doi: 10.1002/hep.22240. [DOI] [PubMed] [Google Scholar]
- 67.Zhang C, Li B, Lu SQ, Li Y, Su YH, Ling CQ. [Effects of melittin on expressions of mitochondria membrane protein 7A6, cell apoptosis-related gene products Fas and Fas ligand in hepatocarcinoma cells] Zhong xi yi jie he xue bao = Journal of Chinese integrative medicine. 2007;5:559–563. doi: 10.3736/jcim20070517. [DOI] [PubMed] [Google Scholar]
- 68.Wang C, Chen T, Zhang N, Yang M, Li B, Lu X, et al. Melittin, a major component of bee venom, sensitizes human hepatocellular carcinoma cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis by activating CaMKII-TAK1-JNK/p38 and inhibiting IkappaBalpha kinase-NFkappaB. The Journal of biological chemistry. 2009;284:3804–3813. doi: 10.1074/jbc.M807191200. [DOI] [PubMed] [Google Scholar]
- 69.Ling CQ, Li B, Zhang C, Zhu DZ, Huang XQ, Gu W, et al. Inhibitory effect of recombinant adenovirus carrying melittin gene on hepatocellular carcinoma. Ann Oncol. 2005;16:109–115. doi: 10.1093/annonc/mdi019. [DOI] [PubMed] [Google Scholar]
- 70.Wu J, Zhao W, Zhong L, Han Z, Li B, Ma W, et al. Self-complementary recombinant adeno-associated viral vectors: packaging capacity and the role of rep proteins in vector purity. Human gene therapy. 2007;18:171–182. doi: 10.1089/hum.2006.088. [DOI] [PubMed] [Google Scholar]
- 71.Kube DM, Srivastava A. Quantitative DNA slot blot analysis: inhibition of DNA binding to membranes by magnesium ions. Nucleic acids research. 1997;25:3375–3376. doi: 10.1093/nar/25.16.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]


