Abstract
Patch-stage/early mycosis fungoides (MF) is difficult to differentiate from benign dermatoses, despite several robust histologic criteria. Most studies include advanced lesions and data about early disease is limited.
Objectives:
(1) To compare the CD4:CD8 ratio in patch-stage MF versus inflammatory mimics. (2) To study patterns of CD1a expression in the epidermis and dermis in the two groups.
Materials and Methods:
Twenty cases each of early MF and inflammatory dermatoses were selected. The diagnoses were established after clinicopathologic correlation, repeat biopsies, and follow-up. The inflammatory group included pityriasis lichenoides chronica, actinic reticuloid, lichenoid purpura, and various psoriasiform dermatoses. Immunohistochemistry was done for CD4, CD8, and CD1a. Epidermal CD4, CD8 cells were quantified and CD1a was graded semi-quantitatively in the epidermis and dermis.
Results:
The average CD4:CD8 ratio was 4.2 in MF (range: 1-16.8), and 0.9 in inflammatory diseases (range: 0.43-5), which was statistically significant (P < 0.0001). None of the MF cases had a ratio <1. Four cases of pityriasis lichenoides chronica had a ratio >1. CD1a cells had a continuous or confluent epidermal pattern in almost all cases of MF, while they occurred as small or large groups in the dermis. In inflammatory dermatoses, there were either isolated or scattered CD1a+ cells in both epidermis and dermis.
Conclusions:
Elevated CD4:CD8 ratio favors MF. But there is an overlap in the lower range with pityriasis lichenoides chronica. These cases require good clinicopathologic correlation and follow-up. Patterns of CD1a expression are more reliable. Immunostains buttress morphology and are a valuable addition.
Keywords: CD4:CD8 ratio, Langerhans cells, mycosis fungoides
Introduction
What was known?
Patch-stage/early mycosis fungoides is difficult to differentiate from benign dermatoses.
Patch-stage mycosis fungoides (MF) is one of the most vexing diagnoses in dermatopathology. The often subtle, patchy, band-like lymphocytic infiltrate overlaps with an entire list of inflammatory dermatoses.[1,2] Morphology and good clinicopathologic correlation are the “gold standard” for diagnosis, for want of more consistent and conclusive tests. The most important candidates among ancillary tests include immunophenotyping and clonality for T-cell receptor (TCR) gene rearrangements.[3–5] While clonality in the correct clinical setting more or less is the clinching proof of a neoplastic nature of the infiltrate, it is positive in only about 50% of cases.[3,4] The problem of “pseudoclonality” may also arise in subtle infiltrates.[6] In a resource-poor context like our patients in India, the test is not easily accessible. Immunostains, on the other hand, are performed in most laboratories. However, there are conflicting reports of the utility of various T-cell markers. The ISCL algorithm places emphasis on loss of expression of CD3, CD5, CD7, which is difficult to assess.[7] The CD4:CD8 ratio, a seemingly more attractive and simpler proposition, has yielded conflicting results, as most inflammatory dermatoses are also CD4 predominant.[8,9] In recent years, there have been a few studies citing that Langerhans cell expression is increased in patch-stage MF.[10] We undertook this blinded study of comparing the CD4:CD8 ratio and Langerhans cell expression patterns in patch-stage MF and inflammatory dermatoses that shared a histologic overlap.
Objectives
The objectives were:
To compare the CD4:CD8 ratio in patch-stage MF versus inflammatory dermatoses and
To compare the pattern of expression of Langerhans cells in the epidermis and dermis in the two groups.
Materials and Methods
This was a retrospective study of 20 cases each of patch-stage MF and inflammatory dermatoses between 2006 and 2009. The diagnosis was established based on clinicopathologic correlation, biopsies (repeated in some cases), response to treatment, and follow-up. Clonality had been done in two cases. All cases had follow-up ranging from 6 months to 4 years. Haematoxylin and Eosin (H and E) stained slides were reviewed and sections were recut to ensure they were representative and sufficient tissue was available for immunostains. After selection, the cases were blinded.
Immunohistochemistry was performed on 5 μm paraffin sections for antibodies to CD4 (clone 145, Biogenex, heat retrieval, USA), CD8 (clone 4812, Biogenex, heat retrieval, USA), and CD1a (clone 010, Biogenex, heat retrieval, USA). The stains were developed using diaminobenzidine (DAB).
CD4 and CD8 positive cells were counted in the epidermis in a minimum of ten 40× microscopic fields (on Nikon E200 microscope) and expressed as a ratio. CD1a positive Langerhans cells in the epidermis were graded as isolated, scattered, numerous continuous and confluent aggregates. In the dermis, they were graded as scattered, small groups or large groups. All parameters were assessed by two pathologists independently and an average was taken in case of numeric values.
Z-test was used to determine the statistical significance of the parameters between the two groups using SPSS software for Windows (P < 0.05).
Results
The CD4:CD8 ratio in MF ranged from 1 to 16.8, with a mean of 4.2. Twelve cases scored >2 and none scored <1 [Figures 1a,c,d]. In the inflammatory group, the ratio ranged from 0.43 to 5 (mean 0.9), with only one case of PLC scoring >2 (i.e. score of 5) and 15 cases scoring <1 [Table 1 and Figures 2a,c,d]. The cases that scored between 1 and 2 included pityriasis lichenoides chronica (PLC) and lichenoid purpura. The difference in CD4:CD8 ratios between the two groups was statistically significant (P < 0.0001).
Figure 1.
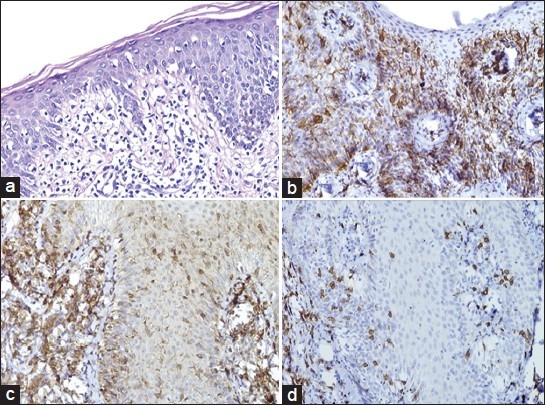
(a) Patch-stage MF showing disproportionate, basilar epidermotropism, lymphocyte atypia, and extensive wiry dermal collagen (H and E, ×200); (b) Confluent aggregates of Langerhans cells in the epidermis (CD1a, ×200); (c) Predominance of CD4+ T-cells (CD4, ×200); (d) Scattered CD8 lymphocytes (CD8, ×200)
Table 1.
Comparison of CD4:CD8 ratio in patch-stage MF and inflammatory dermatoses
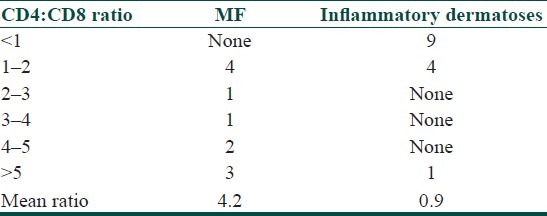
Figure 2.
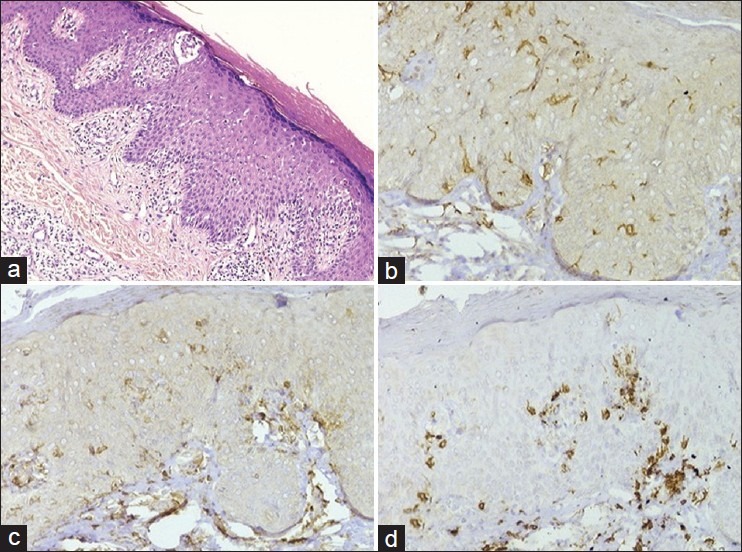
(a) Actinic reticuloid showing epidermotropic lymphocytes, spongiosis, and patchy dermal perivascular and lichenoid infiltrate (H and E, ×200); (b) Scattered Langerhans cells (CD1a, ×200); (c and d) Preponderance of CD8+ cells (d) in comparison with (c) (CD4, ×200 and CD8, ×200)
The expression patterns of Langerhans cells in the epidermis and dermis are depicted in Figures 3 and 4. They were either isolated or scattered in all 20 cases of inflammatory dermatoses [Figure 2b], while they occurred as continuous or confluent aggregates in 18 of the 20 MF cases [Figure 1c]. The same was also reflected in the dermal CD1a patterns, present as small or large groups in 19/20 MF cases. 16/20 inflammatory dermatoses had only scattered dermal CD1a cells.
Figure 3.
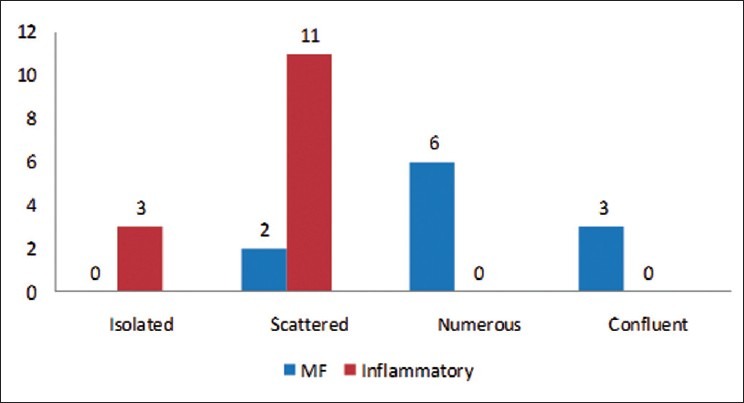
Expression patterns of epidermal CD1a+ Langerhans cells in MF and inflammatory dermatoses
Figure 4.

Comparison of dermal CD1a expression in MF and inflammatory dermatoses
Of the 12 cases (8 MF + 4 inflammatory) that had a ratio between 1 and 2, all 8 cases of MF showed increased CD1a+ cells in comparison to only 1 case of PLC among the inflammatory diseases that showed this pattern.
Discussion
MF is as a T-cell lymphoma, generally of the CD4 T-helper phenotype. In early/patch-stage MF, the infiltrate is band-like and mild comprising chiefly neoplastic and some reactive T-cells. When progression to plaque/tumor stage occurs, tumor cells increase remarkably and simplify diagnosis. It is the early/“suspicious” infiltrates that require the support of ancillary tests. MF, like most T-cell lymphomas, is characterized by an aberrant phenotype, showing loss of pan-T cell antigens CD2, CD3, CD5, and CD7.[7] Epidermal/dermal discordance in the expression of these markers is said to be important in diagnosis.[7] Stevens et al., in their study of 138 cases of MF, did not find loss of T-cell antigens to be discriminatory.[11] Loss of markers is difficult to assess on immunostains.
Earlier studies have differing views on the utility of CD4:CD8 ratio. Nuckols et al. studied 35 cases of MF, where most had ratios >2.[9] Florell et al. reported a ratio of 3.5 in MF and 1-1.6 in benign/suspicious infiltrates.[12] High ratios, they concluded, are specific for MF, but show poor sensitivity. They also evaluated other markers like MIB-1 and CD30, which were higher in MF but not useful.[12] Cotta et al. found a CD4:CD8 ratio of 4 in MF.[8] Our findings validate these studies. Elevated CD4:CD8 ratios certainly favor MF, especially when greater than 2. It is important to note that eight cases of MF and four inflammatory dermatoses (PLC and lichenoid purpura) scored between 1 and 2, which is a significant overlap. In this range, it is neither sensitive nor specific. There was one PLC that scored 5, and this patient showed complete resolution of lesions with conservative therapy. It should not be forgotten that CD4 also stains other cells, notably Langerhans cells, which also contribute to the number.[13] CD4 also contributed to diagnosis by highlighting the pattern of basilar epidermotropism or “tagging” of lymphocytes. It should also be remembered that not all MF are of the CD4 phenotype. CD8 phenotype is also noted, especially in hypopigmented MF, which is commoner in Asian population.[14] It is believed that MF arises in a background of chronic antigenic stimulation. There is an increase in CD1a+ epidermal Langerhans cells and dermal dendritic cells in early stages of the disease and these are lost in advanced stages.[10] Some models postulate that the CD4+ cells are infected with a retrovirus on their interaction with Langerhans cells and transform into neoplastic cells. This increase is also seen in other T-cell proliferations, like lymphomatoid papulosis, but not in B-cell lymphomas.[15] Goteri et al. showed that an increased dermal (but not epidermal) CD1a+ cells correlated with the density of the neoplastic infiltrate.[10]
In our study, CD1a expression emerged as an important discriminator between MF and inflammatory dermatoses. It was remarkably increased in MF in both the epidermal and dermal compartments. Even in those cases where CD4:CD8 ratio overlapped, the difference in CD1a pattern was maintained, except for a single case of PLC. The continuous or confluent pattern was never seen in inflammatory lesions. Therefore, we opine that CD1a expression patterns are very useful in this context.
There was one case that had a CD4:CD8 ratio of 5 and increased CD1a expression. This was deemed to be PLC based on morphology and complete resolution of lesions on conservative treatment, but it is quite likely that this may have been early MF. We will have to wait and watch if this patient returns with new lesions.
The major drawback of our study is the relatively small number of cases studied, as we have included only patch- stage lesions. It is not clear from some of the previous studies on CD4:CD8 ratio with a larger number of cases whether only patch-stage lesions were included.[9,12] Clonality for TCR gene rearrangements was done only in two cases, one each of MF (clone positive) and contact dermatitis (clone negative). In summary, an elevated CD4:CD8 ratio, together with an increase in the expression of CD1a+Langerhans/dermal dendritic cells is useful in distinguishing patch-stage MF from inflammatory dermatoses. In India, where clonality is not easily available, this finding has important implications in supporting the pathologist's suspicion and is easily interpreted in the context of morphology. We hope that the findings of this pilot study will be validated by others, particularly in this geographic region. But it should never be forgotten that clinicopathologic correlation still remains the reference standard for diagnosis in MF and any additional tests should be read in the correct clinical circumstances.
What is new?
1. Elevated CD4:CD8 ratio favors MF, but there is an overlap in the lower range with pityriasis lichenoides chronica.
2. Patterns of CD1a expression are more reliable.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Smoller BR, Bishop K, Glusac E, Kim YH, Hendrickson M. Reassessment of histologic parameters in the diagnosis of mycosis fungoides. Am J Surg Pathol. 1995;19:1423–30. doi: 10.1097/00000478-199512000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Cho-Vega JH, Tschen JA, Duvic M, Vega F. Early-stage mycosis fungoides variants: Case-based review. Ann Diagn Pathol. 2010;14:369–85. doi: 10.1016/j.anndiagpath.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Bakels V, van Oostveen JW, van der Putte SC, Meijer CJ, Willemze R. Immunophenotyping and gene rearrangement analysis provide additional criteria to differentiate between cutaneous T-cell lymphomas and pseudo-T-cell lymphomas. Am J Pathol. 1997;150:1941–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Bergman R, Faclieru D, Sahar D, Sander CA, Kerner H, Ben-Aryeh Y, et al. Immunophenotyping and T-cell receptor gamma gene rearrangement analysis as an adjunct to the histopathologic diagnosis of mycosis fungoides. J Am Acad Dermatol. 1998;39:554–9. doi: 10.1016/s0190-9622(98)70003-9. [DOI] [PubMed] [Google Scholar]
- 5.Kandolf Sekulović L, Cikota B, Stojadinović O, Basanović J, Skiljević D, Medenica LJ, et al. TCRgamma gene rearrangement analysis in skin samples and peripheral blood of mycosis fungoides patients. Acta Dermatovenerol Alp Panonica Adriat. 2007;16:149–55. [PubMed] [Google Scholar]
- 6.Böer A, Tirumalae R, Bresch M, Falk TM. Pseudoclonality in cutaneous pseudolymphomas - A pitfall in interpretation of rearrangement studies. British J of Dermatol. 2008;159:394–402. doi: 10.1111/j.1365-2133.2008.08670.x. [DOI] [PubMed] [Google Scholar]
- 7.Pimpinelli N, Olsen EA, Santucci M, Vonderheid E, Haeffner AC, Stevens S, et al. Defining early Mycosis Fungoides. JAAD. 2005;53:1053–63. doi: 10.1016/j.jaad.2005.08.057. [DOI] [PubMed] [Google Scholar]
- 8.Cotta AC, Cintra ML, de Souza EM, Magna LA, Vassallo J. Reassessment of diagnostic criteria in cutaneous lymphocytic infiltrates. Sao Paulo Med J. 2004;122:161–5. doi: 10.1590/S1516-31802004000400006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nuckols JD, Shea CR, Horenstein MG, Burchette JL, Prieto VG. Quantitation of intraepidermal T-cell subsets in formalin-fixed, paraffin-embedded tissue helps in the diagnosis of mycosis fungoides. J Cutan Pathol. 1999;26:169–75. doi: 10.1111/j.1600-0560.1999.tb01824.x. [DOI] [PubMed] [Google Scholar]
- 10.Goteri G, Filosa A, Mannello B, Stramazzotti D, Rupoli S, Leoni P, et al. Density of neoplastic lymphoid infiltrate, CD8+T cells, and CD1a+ dendritic cells in mycosis fungoides. J Clin Pathol. 2003;56:453–9. doi: 10.1136/jcp.56.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stevens SR, Ke MS, Birol A. A simple clinical scoring system to improve the sensitivity and standardization of the diagnosis of mycosis fungoides type cutaneous T-cell lymphoma: Logistic regression of clinical and laboratory data. Br J Dermatol. 2003;149:513–22. doi: 10.1046/j.1365-2133.2003.05458.x. [DOI] [PubMed] [Google Scholar]
- 12.Florell S, Cessna M, Lundell RB, Boucher KM, Bowen GM, Harris RM, et al. Usefulness (or Lack There of) of Immunophenotyping in atypical cutaneous T-Cell infiltrates. Am J Clin Pathol. 2006;125:727–36. doi: 10.1309/3JK2-H6Y9-88NU-AY37. [DOI] [PubMed] [Google Scholar]
- 13.Ortonne N, Buyukbabani N, Delfau-Larue MH, Bagot M, Wechsler J. Value of the CD8-CD3 ratio for the diagnosis of mycosis fungoides. Mod Pathol. 2003;16:857–62. doi: 10.1097/01.MP.0000084112.81779.BB. [DOI] [PubMed] [Google Scholar]
- 14.El-Shabrawi-Caelen L, Cerroni L, Medeiros LJ, McCalmont TH. Hypopigmented mycosis fungoides: Frequent expression of a CD8+ T-cell phenotype. Am J Surg Pathol. 2002;26:450–7. doi: 10.1097/00000478-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Pigozzi B, Bordignon M, Fortina AB, Michelotto G, Alaibac M. Expression of the CD1a molecule in B- and T-lymphoproliferative skin conditions. Oncology Reports. 2006;15:347–51. [PubMed] [Google Scholar]


