Abstract
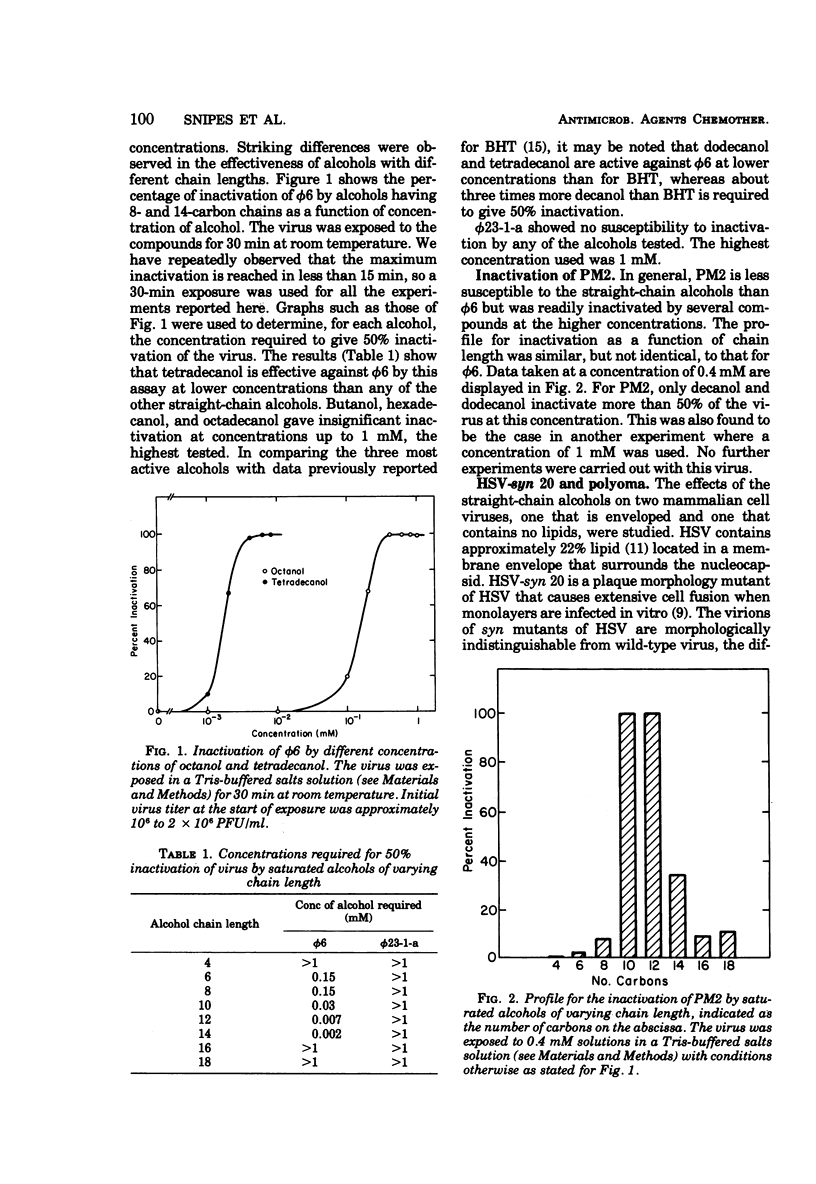
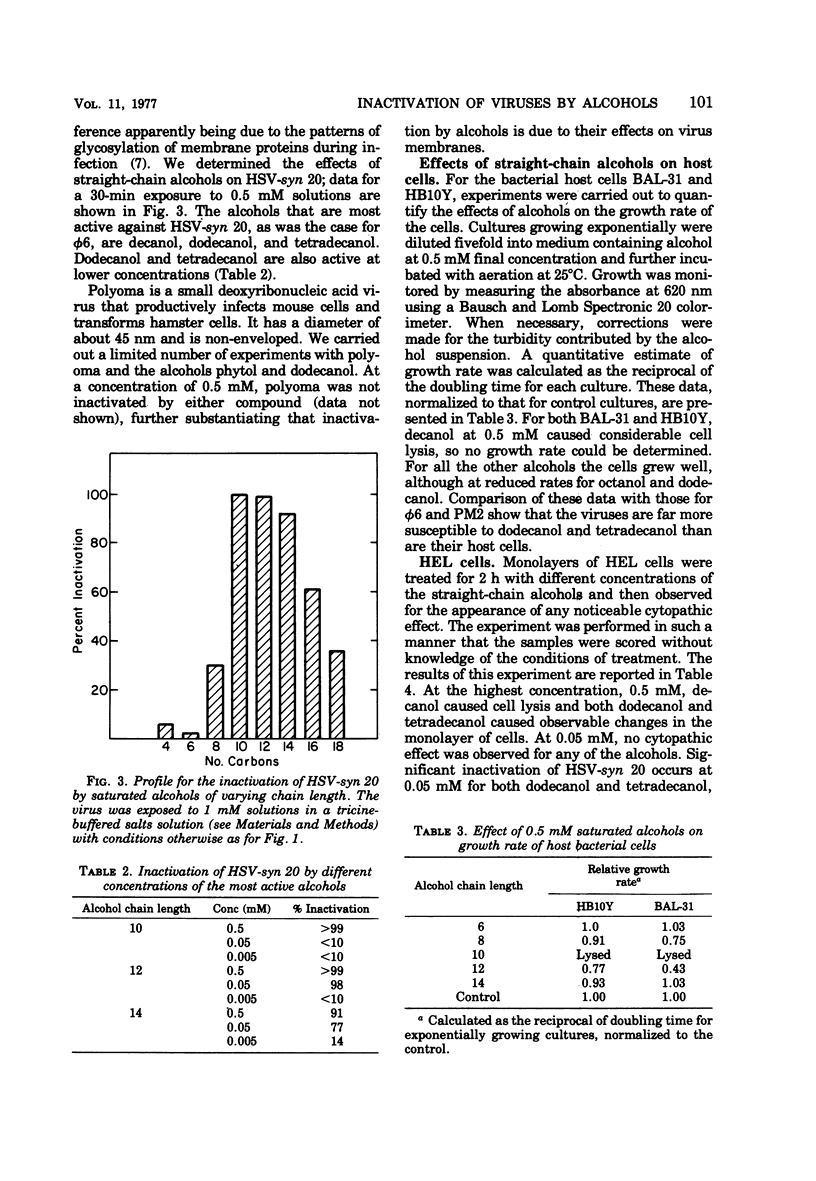
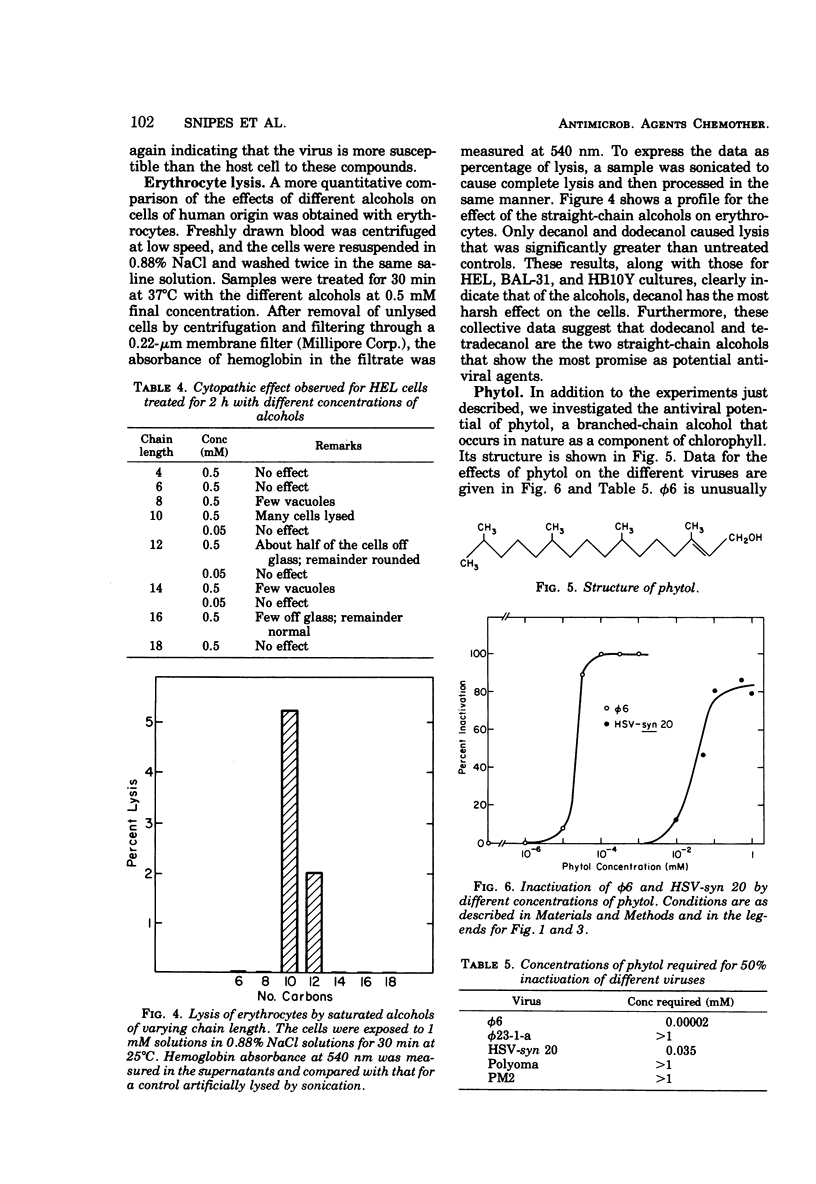
This report describes the inactivation of lipid-containing viruses by several long-chain alcohols. A striking peak in antiviral activity was found for saturated alcohols having chain lengths from 10 to 14 carbons. Viruses having different membrane structure showed different susceptibilities to alcohols having different chain lengths and structural features. Decanol, dodecanol, and tetradecanol readily inactivated herpes simplex virus and the enveloped bacterial virus φ6. The lipid-containing virus PM2 was susceptible to decanol and dodecanol but comparatively unsusceptible to tetradecanol. The branched-chain alcohol phytol, a naturally occurring component of chlorophyll, was active against φ6 and herpes simplex virus but not against PM2. Polyoma virus and the bacteriophage φ23-1-a, which do not contain lipids, were not susceptible to inactivation by any of the alcohols tested. Experiments were also carried out to determine the effects of these compounds on cells. At 0.5 mM, decanol lysed human embryonic lung cells, erythrocytes, and the bacterial hosts for φ6 and PM2. Dodecanol, tetradecanol, and phytol at this concentration were less damaging to cells. At 0.05 mM, none of the alcohols caused observable cytopathic effects on human embryonic lung cells, although several of the alcohols at this concentration were active against herpes simplex virus. Our findings suggest that dodecanol, tetradecanol, and phytol may warrant further studies as potential antiviral agents, particularly for topical application to virus-infected areas of the skin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braunstein S. N., Franklin R. M. Structure and synthesis of a lipid-containing bacteriophage. V. Phospholipids of the host BAL-31 and of the bacteriophage PM2. Virology. 1971 Mar;43(3):685–695. doi: 10.1016/0042-6822(71)90292-3. [DOI] [PubMed] [Google Scholar]
- Cupp J., Wanda P., Keith A., Snipes W. Inactivation of the lipid-containing bacteriophage PM2 by butylate hydroxytoluene. Antimicrob Agents Chemother. 1975 Dec;8(6):698–706. doi: 10.1128/aac.8.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S. Properties of bacteriophage PM2: a lipid-containing bacterial virus. Virology. 1968 Apr;34(4):738–747. doi: 10.1016/0042-6822(68)90094-9. [DOI] [PubMed] [Google Scholar]
- Franklin R. M. Structure and synthesis of bacteriophage PM2, with particular emphasis on the viral lipid bilayer. Curr Top Microbiol Immunol. 1974;(68):107–159. doi: 10.1007/978-3-642-66044-3_5. [DOI] [PubMed] [Google Scholar]
- Harrison S. C., Caspar D. L., Camerini-Otero R. D., Franklin R. M. Lipid and protein arrangement in bacteriophage PM2. Nat New Biol. 1971 Feb 17;229(7):197–201. doi: 10.1038/newbio229197a0. [DOI] [PubMed] [Google Scholar]
- Knowles R. W., Person S. Effects of 2-deoxyglucose, glucosamine, and mannose on cell fusion and the glycoproteins of herpes simplex virus. J Virol. 1976 May;18(2):644–651. doi: 10.1128/jvi.18.2.644-651.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maugh T. H., 2nd Chemotherapy: antiviral agents come of age. Science. 1976 Apr 9;192(4235):128–132. doi: 10.1126/science.192.4235.128. [DOI] [PubMed] [Google Scholar]
- Person S., Knowles R. W., Read G. S., Warner S. C., Bond V. C. Kinetics of cell fusion induced by a syncytia-producing mutant of herpes simplex virus type I. J Virol. 1975 Jan;17(1):183–190. doi: 10.1128/jvi.17.1.183-190.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands J. A., Cupp J., Keith A., Snipes W. Temperature sensitivity of the assembly process of the enveloped bacteriophage phi6. Biochim Biophys Acta. 1974 Dec 10;373(2):277–285. doi: 10.1016/0005-2736(74)90151-5. [DOI] [PubMed] [Google Scholar]
- Snipes W., Cupp J., Sands J. A., Keith A., Davis A. Calcium requirement for assemby of the lipid-containing bacteriophage PM2. Biochim Biophys Acta. 1974 Mar 29;339(3):311–322. doi: 10.1016/0005-2736(74)90158-8. [DOI] [PubMed] [Google Scholar]
- Snipes W., Person S., Keith A., Cupp J. Butylated hydroxytoluene inactivated lipid-containing viruses. Science. 1975 Apr 4;188(4183):64–66. doi: 10.1126/science.163494. [DOI] [PubMed] [Google Scholar]
- Wanda P., Cupp J., Snipes W., Deith A., Rucinsky T., Polish L., Sands J. Inactivation of the enveloped bacteriophage phi6 by butylated hydroxytoluene and butylated hydroxyanisole. Antimicrob Agents Chemother. 1976 Jul;10(1):96–101. doi: 10.1128/aac.10.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]



