Abstract
Alopecia areata (AA) is a non-scarring autoimmune disease of the hair follicle that can present at any age. Pediatric cases are commonly seen in a dermatology clinic, and management can potentially be challenging, with a small proportion of cases experiencing a chronic relapsing course marked by distressing hair loss that can bring about significant psychosocial morbidity. We review the established treatments for pediatric alopecia areata, alongside second and third line therapies that have shown to be efficacious. We also offer a treatment algorithm as a guide to the treatment of pediatric AA.
Keywords: Alopecia areata, pediatric, treatment
Introduction
Alopecia areata (AA) is a chronic inflammatory disorder, characterized by a T-cell autoimmune-mediated attack on the hair follicle, and occasionally on the nails. This leads to patches of non-scarring alopecia on the patient's scalp, face, and other hair-bearing skin of the body. The reported lifetime risk of developing AA has been estimated to be 1.7% and accounts for up to 2% of new cases in a dermatology clinic in the West.[1]
Pediatric Alopecia Areata
Pediatric alopecia areata is not uncommon. Up to one-third of newly diagnosed AA cases have been reported in patients aged 20 years and below, in both Singapore[2] and India.[3] The majority of pediatric AA patients present with localized mild disease affecting less than 50% of the scalp.[2–5] Pediatric AA has been associated with atopy, nail changes, including the twenty nail dystrophy syndrome and a positive family history. In some studies, pediatric AA has also been associated with a guarded long term prognosis, with patients having multiple relapses and progression to alopecia totalis (AT) or universalis (AU).[6,7]
Up to 50% of AA patients have spontaneous regrowth of their hair within a year without treatment,[8] thus making watchful waiting a reasonable option for young children with limited disease. For patients with more extensive or progressive disease, it would be important to discuss with the parents the various treatment options available, bearing in mind the child's ability to accept and tolerate more invasive procedures.
Treatment Options in Pediatric Alopecia Areata
A Cochrane Review of AA treatments in 2008 concluded that there is a paucity of well-designed randomized trials to guide treatment.[9] Evaluation of efficacy is also difficult in AA where spontaneous remission is common, and great disease heterogeneity exists. Significantly, there are even fewer studies on childhood AA and hence, much of the evidence pediatric is extrapolated from adult AA data. Long-term safety data is also less well-established in children, for example with the use of topical immunotherapy.
Management of pediatric AA is particularly challenging given the chronicity of the condition. It is crucial to evaluate the impact of the disease on the child's physical and emotional well-being, including issues like self-confidence, self-image, and acceptance by peers. Parental anxiety, frustration, guilt, and expectations must also be proactively managed to ensure an overall holistic management of the patient.
We review the various established treatments, as well as off label and new therapies for AA below.
Treatment options with strength of recommendation B
Topical corticosteroids (Quality of evidence III)
Local application of potent topical corticosteroids has been effective in the treatment of moderate-to-severe AA. A 12-week half-head study of 0.05% clobetasol propionate foam against placebo showed regrowth of at least 50% in 7/34 treated sites compared with 1/34 of the placebo-treated sites.[10] In patients with AT/AU, 29% (8/27) responded to 0.05% clobetasol propionate ointment under occlusion.[11]
In our center, topical steroid therapy is the first-line treatment for pediatric cases of AA, given its ease of use, convenience, and lack of pain. We advocate using a highly potent topical corticosteroid such as 0.05% clobetasol propionate lotion, and subsequently, tailing down to a lower potency corticosteroid, such as 0.1% mometasone furoate or 0.1% betamethasone valerate scalp lotion to avoid skin atrophy.
Topical minoxidil (Quality of evidence III)
Minoxidil (2, 4 dinitro-6-piperidinopyrimidine-3-oxide) is an established topical treatment for non-scarring alopecia such as AA. One study showed 3% minoxidil under occlusion led to more regrowth in extensive AA when compared to placebo.[12] In another study, comparing minoxidil at concentrations of 1% and 5% for extensive AA, patients receiving 5% minoxidil experienced more regrowth.[13] Both these studies included children, although no subgroup analysis was done for pediatric cases. In the latter study, Price included an anecdotal report of a 9-year-old girl with 100% regrowth after 48 months of monotherapy with minoxidil.[13] Since minoxidil is unlikely to have any immunomodulatory effect on the autoimmune attack of the hair follicle,[14] it likely acts synergistically with corticosteroids[15] to improve outcomes in AA.
In our center, topical 2% or 5% minoxidil is used as an adjunctive treatment, in combination with topical or intralesional steroids. Hypertrichosis on the face and neck may be more common in children, especially at higher concentrations. As such, 2% topical minoxidil may be preferred in children. Topical minoxidil may also cause irritant contact dermatitis or aggravate pre-existing seborrheic or atopic dermatitis. Serious systemic, but non-fatal, side effects such as hypotension and tachycardia were reported in a young girl who ingested 100 ml of minoxidil hair lotion.[16] As such, parents should be counseled to keep the medicines out of reach of children.
Intralesional corticosteroids (Quality of evidence III)
Injection of corticosteroids into the deep dermis and upper subcutis of the affected areas is the treatment of choice for localized AA in adults, achieving adequate tissue concentrations with minimal systemic absorption.[17] It has been shown to be effective in several uncontrolled trials,[18–20] including a Singapore study that reported a significant improvement in up to 82% of patients with limited AA treated with monthly intralesional triamcinolone acetonide for 12 weeks.[21]
For scalp lesions, we use triamcinolone acetonide 10 mg/ml, injecting 0.05-0.1 ml per site, spaced 1 centimeter apart. Triamcinolone acetonide should be diluted to 2.5-5 mg/ml for the eyebrows and face. Side effects include pain, skin depressions secondary to localized atrophy, and hypopigmentation. Peri-orbital injections may be associated with complications such as glaucoma, cataracts, and retinal vascular occlusion.
Usage of intralesional corticosteroids is limited in children due to the fear of injections and pain. Some options to reduce pain include the use of smaller gauge 30 G or 32 G needles, ice or cold compresses, ethyl chloride spray, distraction therapy, and the application of topical anesthetic creams. The use of topical anesthetic creams such as EMLA (eutectic mixture of local anesthetics containing lidocaine 2.5% and prilocaine 2.5%) is an important way to decrease pain and increase patient acceptance. The maximum dose and surface area of EMLA that can be used safely in children varies according to age and can be found in Annex 2. Potential side effects of EMLA would be local irritation and rarely methaemoglobinaemia due to systemic absorption of prilocaine, especially in neonates and very young children.[22,23] An alternative is ELA-Max cream, which is prilocaine-free, which has a similar efficacy to EMLA cream and a good safety profile in children.[24]
Topical immunotherapy (Quality of evidence IIb)
Topical immunotherapy is indicated for chronic and extensive AA where prolonged topical or intralesional corticosteroid injections are impractical or ineffective. It has been shown to modulate the local autoimmune attack on the hair follicles,[25] with reported response rates ranging from 9% to 87% in adult patients. Response rates have been found to be comparable in the pediatric AA, with about one-third of patients experiencing regrowth.[26,27] Relapse rates in pediatric studies varies from 25% to 90%,[28] with cases having more extensive disease having a poorer prognosis. In our local series of Singaporean AA patients, those aged between 4 and 20 years were found to have a poorer response to topical immunotherapy.[21]
Contact sensitizers used today include diphenylcyclopropenone (DPCP) and squalene acid dibutyl ester (SADBE). The original sensitizer dintrochlorobenzene (DCNB) is no longer in use as it has been found to be potentially mutagenic in the Ames test. SADBE is a strong sensitizer but is not stable in acetone and undergoes hydrolysis in storage frequently. Hence, we favor the use of DPCP, with a protocol described by Happle et al.[29] The patient is first sensitized with 2% DPCP on a 2 centimeter bald patch on the scalp. This is left on for 24 hours and washed off. Patients are reviewed 2 weeks later. Sensitization to DPCP is successful if an eczematous reaction is noted over that patch. Subsequently, DPCP, starting at 0.0001% concentration, is applied weekly at increasing concentrations with a goal to induce a mild eczematous reaction and a tolerable itch that lasts 1-2 days. The DPCP is left on for 24 hours each time and washed off. As DPCP is degraded by light, patients are advised to shield the treated areas from light for 24 hours after application.
While topical immunotherapy has been used since 1983, with no serious long-term sequelae has been reported to date,[30,31] parents and patients should be counseled carefully regarding the common side effects of topical immunotherapy, especially irritant contact dermatitis. In severe cases, patients may have generalized eczema, regional lymphadenopathy, or urticaria. Children with atopic dermatitis or seborrheic dermatitis may be at higher risk of such side effects. Other reported cutaneous side effects include hyperpigmentation and hypopigmentation, including vitiligo. DPCP is highly sensitizing and should be applied by trained medical or nursing personnel who must be protected to avoid sensitization. Adequate precautions should be taken by family members as well.
Pulsed Systemic Corticosteroids (Quality of evidence Ib)
Pulsed systemic corticosteroid therapy is a treatment option aimed at arresting the underlying inflammatory process while attempting to avoid its long-term side effects.[32] Most clinical trials studying this treatment option are uncontrolled, or adopt different dosing protocols, making comparison and meta-analysis difficult.[33,34] Pulse intravenous methylprednisolone has been found to be beneficial in several studies,[35,36] but significant risks such as hypokalemia and cardiac arrhythmias necessitate careful patient monitoring. In children with extensive patchy AA or AT/AU, high dose pulse methylprednisolone was shown to have a poor long-term outcome, with 66% of patients having less than 30% regrowth after a median of 12 months.[37] No adverse effects were noted in this small case series.
Pulsed oral corticosteroids for AA have also been shown to be effective in children without significant side effects.[34,38] The dose tested in an uncontrolled case series of 16 young patients was equivalent to oral prednisolone 5 mg/kg/month for 3 months in patients aged 3-11 years and 300 mg/month for 3 months in patients aged 12-18 years. All patients had either extensive AA or AT, and 60% experienced excellent results, even 3 months after the end of the treatment period. The side effects reported were mild epigastric burning and transient giddiness or headache in 2 patients.
Treatment options with strength of recommendation C
Systemic corticosteroids (Quality of evidence III)
Although there are no trials examining the effects of daily systemic corticosteroids in children, it has been shown to be able to induce regrowth in adult AA.[39] However, the well-known adverse effects of long-term corticosteroid therapy, particularly growth retardation, metabolic dysregulation and reduced bone mineral density, limits its use in children. Hence, it is reserved for patients with rapid onset or rapidly progressive extensive, active AA. Oral prednisolone 0.5 mg-0.8 mg/kg/day is prescribed, and slowly tapered over 2 months, and not exceeding 3 months.
Dithranol (Quality of evidence IV)
Topical dithranol has been reported to be mildly effective in several small studies[40,41] and has been postulated to act by suppressing the local Th1 immune reaction at the hair follicle. It is available in 0.2% to 0.5% cream or ointment base and needs to be used for at least 3 months for clinical effect. Contact dermatitis is common, and it can stain the patient's clothes. It may be useful in children who have failed first- or second-line therapies.
Phototherapy (Quality of evidence IIb)
Topical PUVA is a widely used modality with reported success rates of 50-70%.[42,43] However, most studies were uncontrolled, and a retrospective analysis suggest that is may be no better than the natural course of AA,[44] except for the beard area.[45] Like dithranol, it may have a role when combined with corticosteroids for extensive AA.[46] In recalcitrant cases of adult AA, combination therapy with oral prednisolone at 20 mg/day and twice-weekly oral PUVA resulted in good regrowth of terminal hairs for some patients. No equivalent studies have been conducted in children, but due to uncertain response rates and potential side effects from cumulative UVA radiation including photo-aging, eye damage, and increased skin cancer risk, PUVA should only be considered as third- or fourth-line treatment. Narrow-band UVB (NBUVB) therapy has not been shown to be effective in extensive AA. Only 20% of patients achieved excellent responses with NBUVB in a recent uncontrolled study, but these patients also received systemic corticosteroids concurrently.[47] To our knowledge, there have been no reports on the efficacy of UVA1 therapy on childhood AA.
Laser therapy (Quality of evidence III)
The 308-nm Excimer laser showed some efficacy in treating AA in several small case series, mainly as an adjuvant treatment in recalcitrant cases.[48,49] There has been a case report of the use of fractional photothermolysis laser to effectively treat AA in a patient who previously failed topical minoxidil, topical steroids, and intralesional steroid injections.[50]
Immunosuppressive agents (Quality of evidence III)
Corticosteroid-sparing immunosuppressive agents such as methotrexate, azathioprine, and cyclosporine have been reported in several cases series to be effective in AA.
Methotrexate has been reported to produce 64% recovery when used together with daily oral prednisolone for adult patients with AT/AU.[51] A retrospective uncontrolled study of children with severe AA treated with methotrexate for a mean of 14.2 months had only 38.4% response.[52]
A pilot study of 20 patients (age range 8-57) treated with azathioprine 2 mg/kg/day showed a significant regrowth of 52.3% after a mean of 3 months.[53] Cyclosporine has been postulated to be an ideal drug for the treatment of AA due to its ability to suppress T-helper cells, suppress interferon-gamma (IFN-γ) production, with an additional side effect of hypertrichosis. Reported efficacy of oral cyclosporine range from 25% when combined with low-dose oral prednisolone[54] to 88.4% when combined with weekly pulsed methylprednisolone.[55] In general, use of these agents is limited in children because they require frequent blood tests to monitor for potential side effects.
Topical calcineurin inhibitors (Quality of evidence Ib)
Topical calcineurin inhibitors (TCIs) have been used in pediatric inflammatory conditions such as atopic dermatitis, psoriasis, and vitiligo with success.[56] The main advantage of TCIs lie in their steroid-sparing effects and may be an option for facial or eyebrow involvement in young children. However, studies supporting their efficacy are lacking. A recent randomized trial comparing intralesional corticosteroids, topical corticosteroids, and TCIs in 78 adult patients with localized AA showed topical tacrolimus to be the least efficacious of the three.[57] Furthermore, one case report reported no response in AA with topical pimecrolimus.[58]
Topical retinoids (Quality of evidence IIb)
There are limited published studies for topical retinoids in the treatment of AA and none in children. An uncontrolled, non-randomized 3-month trial of 80 patients with limited AA showed a 70% response rate with topical steroids, 55% with topical tretinoin 0.05%, and 20% in controls.[59] Topical bexarotene, a retinoid X receptor agonist, was studied in single-blinded, half-head study involving 42 patients, which showed some efficacy.[60]
Treatment options with strength of recommendation D
Prostaglandin analogues (Quality of evidence Ib)
A new and emerging treatment for non-scarring hair loss is the use of prostaglandin F2α analogues, such as latanoprost and bimatoprost, which have been shown to cause eyelash hypertrichosis. It is postulated to work by enhancing conversion of hairs from telogen to anagen stage.[61] However, topical latanoprost and bimatoprost were both found to be ineffective in stimulating eyelash regrowth in several randomized controlled studies of AA patients with eyelash loss.[62,63] Although the safety of prostaglandin analogues in the treatment of pediatric glaucoma is well-established,[64] this novel off-label treatment for AA has not been validated in children.
Biologics (Quality of evidence IIb)
Although tumor necrosis factor (TNF)-α has been found to be significantly elevated in the sera of AA patients,[65] anti-TNF-α therapies have found to be ineffective in AA.[66–68] As such, biologics cannot be recommended for treatment of AA at this juncture.
Holistic management of pediatric AA patients
Management of AA should also include an assessment of the child's psychosocial well-being, emotional response, and coping mechanisms to their disease, as well as the expectations of the caregivers. AA has been associated with adjustment disorder, anxiety, and depression in adults and children.[69] Adolescents are particularly prone to body image disorders due to their need for peer acceptance and establishment of their identity. A small sample of 14 children and adolescent patients with AA found a high rate of depression (50%) and obsessive-compulsive disorder (35.7%).[70] In addition, both adult and pediatric AA patients have been found to have heightened stress perception, causing them to handle stressful life events less effectively than healthy subjects.[71] This is important as AA has been reported to be triggered by stressful events in the patient's life,[68] and poor coping mechanisms may perpetuate disease activity.
Patients with significant psychiatric co-morbidities should be co-managed together with a psychiatrist.[72] Anti-depressants such as paroxetine, a selective serotonin re-uptake inhibitor,[73] and hypnotherapy[74] have been shown in small trials to help hair regrowth over placebo in adults.[75] AA support groups are important resource groups for patients and their caregivers to gain further insight, emotional support, and acceptance of their condition.[76] We have found that cosmetic camouflage with wigs or “scalp prostheses” have been very helpful in patients and should be proactively discussed.
Conclusions
Alopecia areata is a common yet challenging condition to manage in the Pediatric Dermatology clinic. While many patients with localized AA will respond well to first-line treatment with topical or intralesional corticosteroids, some patients will require more aggressive or second-line therapy. Pediatric age of onset, more extensive disease (scalp involvement more than 50%, ophiasis, or AT/AU), and recalcitrance to initial therapies[7] may highlight patients who will prove to be challenging to manage. Some of these patients may benefit from a cocktail of established therapies, whereby the synergy between two or more established therapies proves to be better than monotherapy.[41] Future studies focusing on such combination therapies, as well as novel new treatments not mentioned in this review because of lack of evidence (non-TNF-α biologics, drugs directed against nerves like capsaicin, and low-level light therapy devices), will expand the choices available to dermatologists, patients and their parents in the treatment of pediatric AA.
We have included a treatment algorithm for pediatric cases of AA (Annex 3) as a guide, but treatment will need to be individualized and based on discussion with the child and their parents.
Annex 1
Levels of evidence
Ia Evidence obtained from meta-analysis of randomized controlled trials.
Ib Evidence obtained from at least one randomized controlled trial.
IIa Evidence obtained from at least one well-designed controlled study without randomization.
IIb Evidence obtained from at least one other type of well-designed quasi-experimental study.
III Opinions of respected authorities based on clinical experience, descriptive studies or reports of expert committees
IV Evidence inadequate due to problems of methodology (e.g. sample size or length of comprehensiveness of follow-up or conflicts of evidence).
Grades of recommendation
-
A
There is good evidence to support the use of the procedure
-
B
There is fair evidence to support the use of the procedure
-
C
There is poor evidence to support the use of the procedure
-
D
There is fair evidence to support the rejection of the use of the procedure
-
E
There is good evidence to support the rejection of the use of the procedure
Annex 2
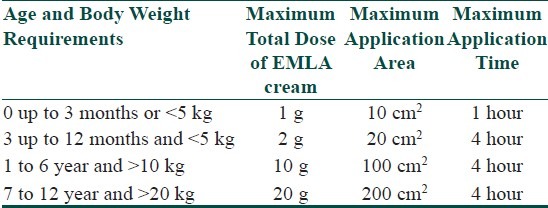
Maximum doses of EMLA according to age, for use on intact skin (From EMLA Online Data Sheet, AstraZeneca, Auckland, NZ).
Annex 3: Summary of Recommendations for Treatment of AA in the Pediatric Age Group
For limited patchy AA
First-line treatment:
< 10 years old: Topical steroids +/- topical Minoxidil lotion
>10 years old: Intralesional steroids +/- Minoxidil lotion
Second-line treatment:
Topical immunotherapy (DPCP)
Third-line treatment:
Dithranol
Fourth-line treatment:
Topical PUVA therapy
Excimer laser therapy
Other miscellaneous treatment
For rapidly progressive, extensive AA
Oral prednisolone starting at 0.5-0.8 mg/kg/day and tailed off over 2 months
-
+/- topical steroids +/- topical Minoxidil lotion
or
Pulsed systemic corticosteroids
If hair loss still extensive, proceed to treatment for chronic extensive AA
Chronic extensive AA
First-line treatment:
Topical steroids with topical Minoxidil lotion
Second-line treatment:
Topical immunotherapy (DPCP)
Third-line treatment:
Pulsed systemic corticosteroids
Topical PUVA therapy
Azathioprine
Other miscellaneous therapies
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Safavi KH, Muller SA, Suman VJ, Moshell AN, Melton LJ., 3rd Incidence of alopecia areata in Olmsted County, Minnesota, 1975 through 1989. Mayo Clin Proc. 1995;70:628–33. doi: 10.4065/70.7.628. [DOI] [PubMed] [Google Scholar]
- 2.Tan E, Tay YK, GIam YC. A clinical study of childhood alopecia areata in Singapore. Pediatr Dermatol. 2002;19:298–301. doi: 10.1046/j.1525-1470.2002.00088.x. [DOI] [PubMed] [Google Scholar]
- 3.Sharma VK, Dawn G, Kumar B. Profile of alopecia areata in Northern India. Int J Dermatol. 1996;35:22–7. doi: 10.1111/j.1365-4362.1996.tb01610.x. [DOI] [PubMed] [Google Scholar]
- 4.Nanda A, Al-Fouzan AS, Al-Hasawi F. Alopecia areata in children: A clinical profile. Pediatr Dermatol. 2002;19:482–5. doi: 10.1046/j.1525-1470.2002.00215.x. [DOI] [PubMed] [Google Scholar]
- 5.Xiao FL, Yang S, Liu JB, He PP, Yang J, Cui Y, et al. The epidemiology of childhood alopecia areata in China: A study of 226 patients. Pediatr Dermatol. 2006;23:13–8. doi: 10.1111/j.1525-1470.2006.00161.x. [DOI] [PubMed] [Google Scholar]
- 6.De Waard-van der Spek FB, Oranje AP, De Raeymaecker DM, Peereboom-Wynia JD. Juvenile versus maturity-onset al.opecia areata - a comparative retrospective clinical study. Clin Exp Dermatol. 1989;14:429–33. doi: 10.1111/j.1365-2230.1989.tb02604.x. [DOI] [PubMed] [Google Scholar]
- 7.Tosti A, Bellavista S, Iorizzo M. Alopecia areata: A long term follow-up study of 191 patients. J Am Acad Dermatol. 2006;55:438–41. doi: 10.1016/j.jaad.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 8.MacDonald Hull SP, Wood ML, Hutchinson PE, Sladden M, Messenger AG. Guidelines for the management of alopecia areata. Br J Dermatol. 2003;149:692–9. doi: 10.1046/j.1365-2133.2003.05535.x. [DOI] [PubMed] [Google Scholar]
- 9.Delamere FM, Sladden MM, Dobbins HM, Leonardi-Bee J. Interventions for alopecia areata. Cochrane Database Syst Rev. 2008;2:CD004413. doi: 10.1002/14651858.CD004413.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Tosti A, Iorizzo M, Botta GL, Milani M. Efficacy and safety of a new clobetasol propionate 0.05% foam in alopecia areata: A randomized, double-blind placebo-controlled trial. J Eur Acad Dermatol Venereol. 2006;20:1243–7. doi: 10.1111/j.1468-3083.2006.01781.x. [DOI] [PubMed] [Google Scholar]
- 11.Tosti A, Piraccini BM, Pazzaglia M, Vincenzi C. Clobetasol propionate 0.05% under occlusion in the treatment of alopecia totalis/universalis. J Am Acad Dermatol. 2003;49:96–8. doi: 10.1067/mjd.2003.423. [DOI] [PubMed] [Google Scholar]
- 12.Price VH. Double-blind, placebo-controlled evaluation of topical Minoxidil in extensive alopecia araeta. J Am Acad Dermatol. 1987;16:730–6. doi: 10.1016/s0190-9622(87)70095-4. [DOI] [PubMed] [Google Scholar]
- 13.Fiedler-Weiss VC. Topical Minoxidil solution (1% and 5%) in the treatment of alopecia areata. J AmAcad Dermatol. 1987;16:745–8. doi: 10.1016/s0190-9622(87)80003-8. [DOI] [PubMed] [Google Scholar]
- 14.Khoury EL, Price VH, Abdel-Salam MM, Stern M, Greenspan JS. Topical Minoxidil in alopecia areata: No effect on the perifollicular lymphoid infiltration. J Invest Dermatol. 1992;99:40–7. doi: 10.1111/1523-1747.ep12611409. [DOI] [PubMed] [Google Scholar]
- 15.Olsen EA, Carson SC, Turney EA. Systemic steroids with or without 2% topical Minoxidil in the treatment of alopecia areata. Arch Dermatol. 1992;128:1467–73. [PubMed] [Google Scholar]
- 16.Aprahamian A, Escoda S, Patteau G, Merckx A, Chéron G. Minoxidil intoxication, the pharmacological agent of a hair lotion. Arch Pediatr. 2011;18:1302–4. doi: 10.1016/j.arcped.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 17.Firooz A, Tehranchi-Nia Z, Ahmed AR. Benefits and risks of intralesional corticosteroids in the treatment of dermatological disease. Clin Exp Dermatol. 1995;20:363–70. doi: 10.1111/j.1365-2230.1995.tb01351.x. [DOI] [PubMed] [Google Scholar]
- 18.Kubeyinje EP. Intralesional triamcinolone acetonide in alopecia areata amongst 62 Saudi Arabs. East Afr Med J. 1994;71:674–5. [PubMed] [Google Scholar]
- 19.Porter D, Burton JL. A comparison of intralesional triamcinolone hexacetonide and triamcinolone acetonide in alopecia areata. Br J Dermatol. 1971;85:272–3. doi: 10.1111/j.1365-2133.1971.tb07230.x. [DOI] [PubMed] [Google Scholar]
- 20.Chang KH, Rojhirunsakool S, Goldberg LJ. Treatment of severe alopecia areata with intralesional steroid injections. J Drugs Dermatol. 2009;8:909–12. [PubMed] [Google Scholar]
- 21.Tan E, Tay YK, Goh CL, Chin Giam Y. The pattern and profile of alopecia areata in Singapore - a study of 219 Asians. Int J Dermatol. 2002;41:748–53. doi: 10.1046/j.1365-4362.2002.01357.x. [DOI] [PubMed] [Google Scholar]
- 22.Frayling IM, Addison GM, Chattergee K, Meakin G. Methaemoglobinaemia in children treated with prilocaine-lignocaine cream. Br Med J. 1990;301:153–4. doi: 10.1136/bmj.301.6744.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Touma S, Jackson JB. Lidocaine and prilocaine toxicity in a patient receiving treatment for mollusca contagiosa. J Am Acad Dermatol. 2001;44:399–400. doi: 10.1067/mjd.2001.111898. [DOI] [PubMed] [Google Scholar]
- 24.Eichenfield LF, Funk A, Fallon-Freidlander S, Cunningham BB. A clinical study to evaluate the efficacy of ELA-Max as compared to EMLA cream for pain reduction of venipuncture in children. Pediatrics. 2002;109:1093–9. doi: 10.1542/peds.109.6.1093. [DOI] [PubMed] [Google Scholar]
- 25.Rokhsar CK, Shupack JL, Vafai JJ, Washenik K. Efficacy of topical sensitizers in the treatment of alopecia areata. J Am Acad Dermatol. 1998;39:751–61. doi: 10.1016/s0190-9622(98)70048-9. [DOI] [PubMed] [Google Scholar]
- 26.MacDonald Hull SP, Pepall L, Cunliffe WJ. Alopecia areata in children: Response to treatment with diphencyprone. Br J Dermatol. 1991;125:164–8. doi: 10.1111/j.1365-2133.1991.tb06064.x. [DOI] [PubMed] [Google Scholar]
- 27.Schuttelaar ML, Hamstra JJ, Plinck EP, Peereboom-Wynia JD, Vuzevski VD, Mulder PG, et al. Alopecia areata in children: Treatment with diphencyprone. Br J Dermatol. 1996;135:581–5. [PubMed] [Google Scholar]
- 28.Tosti A, Guidetti MS, Bardazzi F, Misciali C. Long-term results of topical immunotherapy in children with alopecia totalis or alopecia universalis. J Am Acad Dermatol. 1996;35:199–201. doi: 10.1016/s0190-9622(96)90323-0. [DOI] [PubMed] [Google Scholar]
- 29.Happle R, Hausen BM, Wiesner-Menzel L. Diphencyprone in the treatment of alopecia areata. Acta Derm Venereol (Stockh) 1983;63:49–52. [PubMed] [Google Scholar]
- 30.Bolduc C, Shapiro J. DPCP for the treatment of alopecia areata. Skin Therapy Lett. 2000;5:3–4. [PubMed] [Google Scholar]
- 31.Alkhalifah A, Alsantali A, Wang E, McElwee KJ, Shapiro J. Alopecia areata update: Part II Treatment. J Am Acad Dermatol. 2010;62:191–202. doi: 10.1016/j.jaad.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 32.Ramam M. Dexamethasone pulse therapy in dermatology. Indian J Dermatol Venereol Leprol. 2003;69:319–22. [PubMed] [Google Scholar]
- 33.Sharma VK. Pulsed administration of corticosteroids in the treatment of alopecia areata. Int J Dermatol. 1996;35:133–6. doi: 10.1111/j.1365-4362.1996.tb03281.x. [DOI] [PubMed] [Google Scholar]
- 34.Sharma VK, Muralidhar S. Treatment of widespread alopecia areata in young patients with monthly oral corticosteroid pulse. Pediatr Dermatol. 1998;15:313–7. doi: 10.1046/j.1525-1470.1998.1998015313.x. [DOI] [PubMed] [Google Scholar]
- 35.Friedli A, Labarthe MP, Engelhardt E, Feldmann R, Salomon D, Saurat JH. Pulse methylprednisolone therapy for severe alopecia areata: An open prospective study of 45 patients. J Am Acad Dermatol. 1998;39:597–602. doi: 10.1016/s0190-9622(98)70009-x. [DOI] [PubMed] [Google Scholar]
- 36.Im M, Lee SS, Lee Y, Kim CD, Seo YJ, Lee JH, et al. Prognostic factors in methylprednisolone pulse therapy for alopecia areata. J Dermatol. 2011;38:767–72. doi: 10.1111/j.1346-8138.2010.01135.x. [DOI] [PubMed] [Google Scholar]
- 37.Hubiche T, Léauteé-Labrèze, Taïeb A, Boralevi F. Poor long term outcome of severe alopecia areata in children treated with high dose pulse corticosteroid therapy. Br J Dermatol. 2008;158:1136–7. doi: 10.1111/j.1365-2133.2008.08458.x. [DOI] [PubMed] [Google Scholar]
- 38.Sharma VK, Gupta S. Twice weekly 5mg dexamethasone oral pulse in the treatment of extensive alopecia areata. J Dermatol. 1999;26:562–5. doi: 10.1111/j.1346-8138.1999.tb02049.x. [DOI] [PubMed] [Google Scholar]
- 39.Fiedler-Weiss VC, Buys CM. Evaluation of anthralin in the treatment of alopecia areata. Arch Dermatol. 1987;123:1491–3. [PubMed] [Google Scholar]
- 40.Deshpande D, Dhurat R, Saraogi P, Mishra S, Nayak C. Extensive alopecia areata: Not necessarily recalcitrant to therapy. Int J Trichology. 2011;3:80–3. doi: 10.4103/0974-7753.90807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kamel MM, Salem SA, Attia HH. Successful treatment of resistant alopecia areata with a phototoxic dose of ultraviolet A after topical 8-methoxypsoralen application. Photodermatol Photoimmunol Photomed. 2011;27:45–50. doi: 10.1111/j.1600-0781.2010.00564.x. [DOI] [PubMed] [Google Scholar]
- 42.Broniarczyk-Dyla G, Wawrzycka-Kaflik A, Dubla-Berner M, Prusinska-Bratos M. Effects of psoralen-UV-A-Turban in alopecia areata. Skinmed. 2006;5:64–8. doi: 10.1111/j.1540-9740.2006.04240.x. [DOI] [PubMed] [Google Scholar]
- 43.Healy E, Rogers S. PUVA treatment for alopecia areata-does it work? A retrospective review of 102 cases. Br J Dermatol. 1993;129:42–4. doi: 10.1111/j.1365-2133.1993.tb03309.x. [DOI] [PubMed] [Google Scholar]
- 44.Fernández-Guarino M, Harto A, García-Morales I, Pérez-García B, Arrazola JM, Jaén P. Failure to treat alopecia areata with photodynamic therapy. Clin Exp Dermatol. 2008;33:585–7. doi: 10.1111/j.1365-2230.2008.02712.x. [DOI] [PubMed] [Google Scholar]
- 45.Ito T, Aoshima M, Ito N, Uchiyama I, Sakamoto K, Kawamura T, et al. Combination therapy with oral PUVA and corticosteroid for recalcitrant alopecia areata. Arch Dermatol Res. 2009;301:373–80. doi: 10.1007/s00403-009-0936-8. [DOI] [PubMed] [Google Scholar]
- 46.Bayramgürler D, Demirsoy EO, Aktürk AŞ, Kıran R. Narrowband ultraviolet B phototherapy for alopecia areata. Photodermatol Photoimmunol Photomed. 2011;27:325–7. doi: 10.1111/j.1600-0781.2011.00612.x. [DOI] [PubMed] [Google Scholar]
- 47.Al-Mutairi N. 308-nm excimer laser for the treatment of alopecia areata in children. Pediatr Dermatol. 2009;26:547–50. doi: 10.1111/j.1525-1470.2009.00980.x. [DOI] [PubMed] [Google Scholar]
- 48.Ohtsuki A, Hasegawa T, Ikeda S. Treatment of alopecia areata with 308-nm excimer lamp. J Dermatol. 2010;37:1032–5. doi: 10.1111/j.1346-8138.2010.00942.x. [DOI] [PubMed] [Google Scholar]
- 49.Yoo KH, Kim MN, Kim BJ, Kim CW. Treatment of alopecia areata with fractional photothermolysis laser. Int J Dermatol. 2010;49:845–7. doi: 10.1111/j.1365-4632.2009.04230.x. [DOI] [PubMed] [Google Scholar]
- 50.Joly P. The use of methotrexate alone or in combination with low doses of oral corticosteroids in the treatment of alopecia totalis or universalis. J Am Acad Dermatol. 2006;55:632–6. doi: 10.1016/j.jaad.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 51.Royer M, Bodemer C, Vabres P, Pajot C, Barbarot S, Paul C, et al. Efficacy and tolerability of methotrexate in severe childhood alopecia areata. Br J Dermatol. 2011;165:407–10. doi: 10.1111/j.1365-2133.2011.10383.x. [DOI] [PubMed] [Google Scholar]
- 52.Farshi S, Mansouri P, Safar F, Khiabanloo SR. Could azathioprine be considered as a therapeutic alternative in the treatment of alopecia areata? Int J Dermatol. 2010;49:1188–93. doi: 10.1111/j.1365-4632.2010.04576.x. [DOI] [PubMed] [Google Scholar]
- 53.Shapiro J, Lui H, Tron V, Ho V. Systemic cyclosporine and low dose prednisone in the treatment of chronic severe alopecia areata: A clinical and immunopathologic evaluation. J Am Acad Dermatol. 1997;36:114–7. doi: 10.1016/s0190-9622(97)70342-6. [DOI] [PubMed] [Google Scholar]
- 54.Kim BJ, Min SU, Park KY, Choi JW, Park SW, Youn SW, et al. Combination therapy of cyclosporine and methylprednisolone on severe alopecia areata. J Dermatolog Treat. 2008;19:216–20. doi: 10.1080/09546630701846095. [DOI] [PubMed] [Google Scholar]
- 55.Wollina U. The role of topical calcineurin inhibitors for skin diseases other than atopic dermatitis. Am J Clin Dermatol. 2007;8:157–73. doi: 10.2165/00128071-200708030-00003. [DOI] [PubMed] [Google Scholar]
- 56.Kuldeep C, Singhal H, Khare AK, Mittal A, Gupta LK, Garg A. Randomised comparison of topical betamethesome valerate foam, intralesional triamcinolone acetonide and tacrolimus ointment in management of localized alopecia areata. Int J Trichol. 2011;3:20–4. doi: 10.4103/0974-7753.82123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rigopoulos D, Gregoriou S, Korfitis C, Gintzou C, Vergou T, Katrinaki A, et al. Lack of response of alopecia areata to pimecrolimus cream. Clin Exp Dermatol. 2007;32:456–7. doi: 10.1111/j.1365-2230.2007.02367.x. [DOI] [PubMed] [Google Scholar]
- 58.Das S, Ghorami RC, Chatterjee T, Banerjee G. Comparative assessment of topical steroids, topical tretinoin (0.05%) and dithranol paste in alopecia areata. Indian J Dermatol. 2010;55:148–9. doi: 10.4103/0019-5154.62747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Talpur R, Vu J, Bassett R, Stevens V, Duvic M. Phase I/II randomized bilateral half-head comparison of topical bexarotene 1% gel for alopecia areata. J Am Acad Dermatol. 2009;61:592.e1–9. doi: 10.1016/j.jaad.2009.02.037. [DOI] [PubMed] [Google Scholar]
- 60.Sasaki S, Hozumi Y, Kondo S. Influence of prostaglandin F2alpha and its analogues on hair regrowth and follicular melanogenesis in a murine model. Exp Dermatol. 2005;14:323–8. doi: 10.1111/j.0906-6705.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- 61.Roseborough I, Lee H, Chwalek J, Stamper RL, Price VH. Lack of efficacy of topical latanoprost and bimatoprost ophthalmic solutions in promoting eyelash growth in patients with alopecia areata. J Am Acad Dermatol. 2009;60:705–6. doi: 10.1016/j.jaad.2008.08.029. [DOI] [PubMed] [Google Scholar]
- 62.Ross EK, Bolduc C, Lui H, Shapiro J. Lack of efficacy of topical latanoprost in the treatment of eyebrow alopecia areata. J Am Acad Dermatol. 2005;53:1095–6. doi: 10.1016/j.jaad.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 63.Black AC, Jones S, Yanovitch TL, Enyedi LB, Stinnett SS, Freedman SF. Latanoprost in pediatric glaucoma – pediatric exposure over a decade. J AAPOS. 2009;13:558–62. doi: 10.1016/j.jaapos.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 64.Kasumagic-Halilovic E, Prohic A, Cavaljuga S. Tumor necrosis factor-alpha in patients with alopecia areata. Indian J Dermatol. 2011;56:494–6. doi: 10.4103/0019-5154.87124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tosti A, Pazzaglia M, Starace M, Bellavista S, Vincenzi C, Tonelli G. Alopecia areata during treatment with biologic agents. Arch Dermatol. 2006;142:1653–4. doi: 10.1001/archderm.142.12.1653. [DOI] [PubMed] [Google Scholar]
- 66.Strober BE, Siu K, Alexis AF, Kim G, Washenik K, Sinha A, et al. Etanercept does not effectively treat moderate to severe alopecia areata: An open-label study. J Am Acad Dermatol. 2005;52:1082–4. doi: 10.1016/j.jaad.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 67.Price VH, Hordinsky MK, Olsen EA, Roberts JL, Siegfried EC, Rafal ES, et al. Subcutaneous efalizumab is not effective in the treatment of alopecia areata. J Am Acad Dermatol. 2008;58:395–402. doi: 10.1016/j.jaad.2007.10.645. [DOI] [PubMed] [Google Scholar]
- 68.Ruiz-Doblado S, Carrizosa A, Garcia-Hernandez MJ. Alopecia areata: Psychiatric comorbidity and adjustment to illness. Int J Dermatol. 2003;42:434–7. doi: 10.1046/j.1365-4362.2003.01340.x. [DOI] [PubMed] [Google Scholar]
- 69.Ghanizadeh A. Comorbidity of psychiatric disorders in children and adolescents with alopecia areata in a child and adolescent psychiatry clinical sample. Int J Dermatol. 2008;47:1118–20. doi: 10.1111/j.1365-4632.2008.03743.x. [DOI] [PubMed] [Google Scholar]
- 70.Díaz-Atienza F, Gurpequi M. Environmental stress but not subjective distress in children and adolescents with alopecia areata. J Psychosom Res. 2011;71:102–7. doi: 10.1016/j.jpsychores.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 71.Devar JV. Is alopecia areata psychosomatic? Indian J Psychiatry. 1983;25:140–3. [PMC free article] [PubMed] [Google Scholar]
- 72.Cipriani R, Perini GI, Rampinelli S. Paroxetine in alopecia areata. Int J Dermatol. 2001;40:600–1. doi: 10.1046/j.1365-4362.2001.01261-3.x. [DOI] [PubMed] [Google Scholar]
- 73.Willemsen R, Vanderlinden J, Deconinck A, Roseeuw D. Hypnotherapeutic management of alopecia areata. J Am Acad Dermatol. 2006;55:233–7. doi: 10.1016/j.jaad.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 74.Ricciardi A, Ruberto A, García-Hernández MJ, Kotzalidis GD, Trevisi M, Persechino S, et al. Alopecia areata with comorbid depression: Early resolution with combined paroxetine-triamcinolone treatment. J Eur Acad Dermatol Venereol. 2006;20:1000–1. doi: 10.1111/j.1468-3083.2006.01592.x. [DOI] [PubMed] [Google Scholar]
- 75.Goh C, Lane AT, Bruckner AL. Support groups for children and their families in pediatric dermatology. Paediatr Dermatol. 2007;24:302–5. doi: 10.1111/j.1525-1470.2007.00407.x. [DOI] [PubMed] [Google Scholar]
- 76.Ehsani AH, Toosi S, Seirafi H, Akhyani M, Hosseini M, Azadi R, et al. Capsaicin vs clobetasol for the treatment of localized alopecia areata. JEADV. 2009;23:1451–2. doi: 10.1111/j.1468-3083.2009.03243.x. [DOI] [PubMed] [Google Scholar]


