Abstract
Leukemia cutis is the infiltration of neoplastic leukocytes or their precursors into the epidermis, the dermis, or the subcutis, resulting in clinically identifiable cutaneous lesions. Leukemia cutis may follow, precede or occur concomitantly with the diagnosis of systemic leukemia. A 50-year-old woman presented with asymptomatic multiple cutaneous nodules all over the body of 4 months duration. Cutaneous examination showed multiple hyperpigmented nodules and plaques involving face, trunk, and extremities. Peripheral smear showed abnormally elevated leucocyte count (TLC-70,000) with abnormal cells: myeloblasts 40%, promyelocytes 8% and myelocytes 39%. Auer rods were present in few myeloblasts. Bone marrow aspiration showed increased cellularity, erythroid hyperplasia with megaloblastic change, increased myeloblasts with maturation arrest. Immunohistochemistry showed strongly positive myeloperoxidase infiltrating cells and negative for CD20 and CD3 consistent with the diagnosis of AML–M 2 with leukemia cutis. This case is reported for its rarity.
Keywords: Auer rods, leukemia cutis, myeloperoxidase
Introduction
What was known?
Acute myeloid leukemia presenting as leukemia cutis was rare with reported incidence ranging between 2.9 and 3.7% probably due to paucity of diagnostic facilities and lack awareness among dermatologists and physician.
Leukemia cutis is the infiltration of neoplastic leukocytes or their precursors into the epidermis, the dermis, or the subcutis, resulting in clinically identifiable cutaneous lesions. The dermatologist is often instrumental in the diagnosis of leukemia cutis. Accurate diagnosis has tremendous prognostic significance and may establish a diagnosis in cases in which leukemia cutis is the harbinger of a systemic leukemic process. A diagnosis of leukemia cutis generally portends a poor prognosis and strongly correlates with additional sites of extramedullary involvement. This can alter the appropriate treatment regimen for a patient.[1]
Case Report
A 50-year-old woman presented with asymptomatic multiple cutaneous nodules all over the body of 4 months duration and make associated with high grade fever and dyspnoea. There was no history of epistaxis or glove and stocking anesthesia. General examination revealed pallor and lymphadenopathy. Cutaneous examination showed multiple hyperpigmented nodules and plaques involving face, trunk, and extremities [Figures 1 and 2]. Nodules ranged between 1 and 2 cms and plaques ranged between 2 and 3 cms in diameter. The papules and nodules were soft to firm in consistency and were not tender. There was no sensory deficit and no nerve thickening. Oral cavity showed hypertrophy of gums. There was no hepatospleenomegaly. With these clinical features a provisional diagnosis of Mycosis fungoides was entertained. Lepromatous leprosy, Scleromyxoedema, post kala-azar dermal lieshmaniasis and leukemia cutis were considered in the differential diagnosis. She was investigated and C.B.P and counts were normal. Blood chemistry including liver function tests were found to be within normal limits. X-ray chest and ultrasonography of abdomen was normal. Serum proteins and immunoglobulin profile were normal. Slit skin smear was negative for AFB. Imprint smear for leishmania was negative. However, hemogram was repeated after 2 weeks and at that time the peripheral smear showed abnormally elevated leucocyte count (TLC-70,000) with abnormal cells: myeloblasts 40%, promyelocytes 8%, myelocytes 39%. Auer rods were present in few myeloblasts [Figure 3]. Bone marrow aspiration showed increased cellularity, erythroid hyperplasia with megaloblastic change, increased myeloblasts with maturation arrest. Megakaryocytes and platelets were grossly decreased. Lymphocytes were also decreased. Fine Needle Aspiration Cytology and biopsy of cervical lymph node showed nonspecific lymphadenitis. With bone marrow and peripheral smear findings, the patient was diagnosed as a case of acute myeloid leukemia (AML-M2) as per French-American-British (FAB) classification. Histopathological examination (HPE) of the skin nodule taken from back of trunk showed normal epidermis with subepidermal Grenz zone [Figure 4]. Dermis showed sheet like infiltrate of round to ovoid cells with ill defined cytoplasm, vesicular nuclei with single prominent nucleoli. Mitosis was also seen, suggestive of non-Hodgkin's lymphoma [Figure 5]. Immunohistochemistry showed strongly positive myeloperoxidase infiltrating cells [Figure 6] and negative for CD20 and CD3. Fite Faracco stain for AFB was negative. Morphology and immune profile were consistent with the involvement by extra medullary granulocytic tumor. The patient suddenly died probably due to blast crisis before treatment could be instituted.
Figure 1.
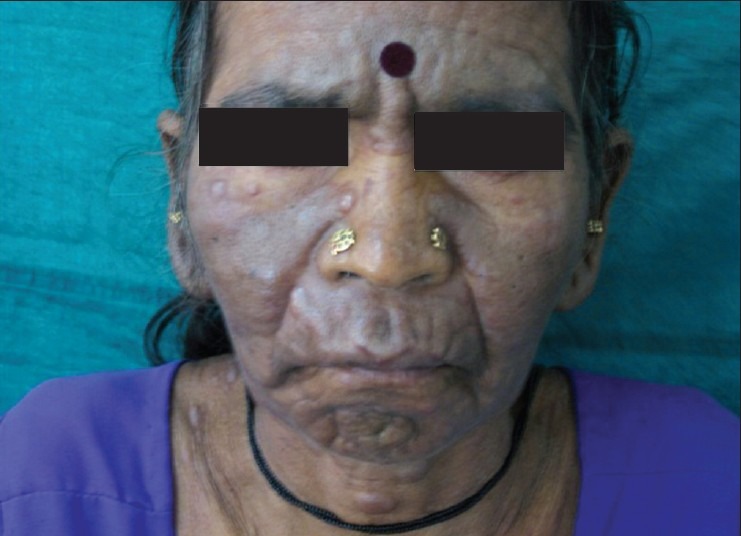
Multiple nodules and plaques on face
Figure 2.
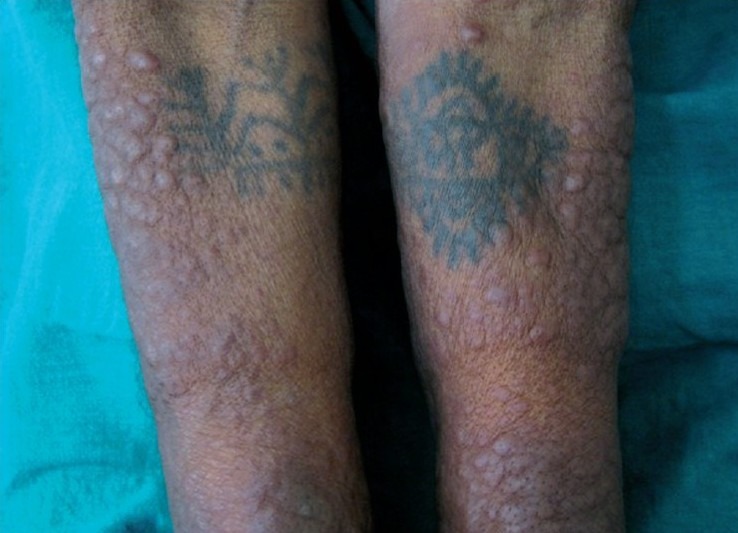
Multiple nodules and plaques on flexor aspect of forearms
Figure 3.
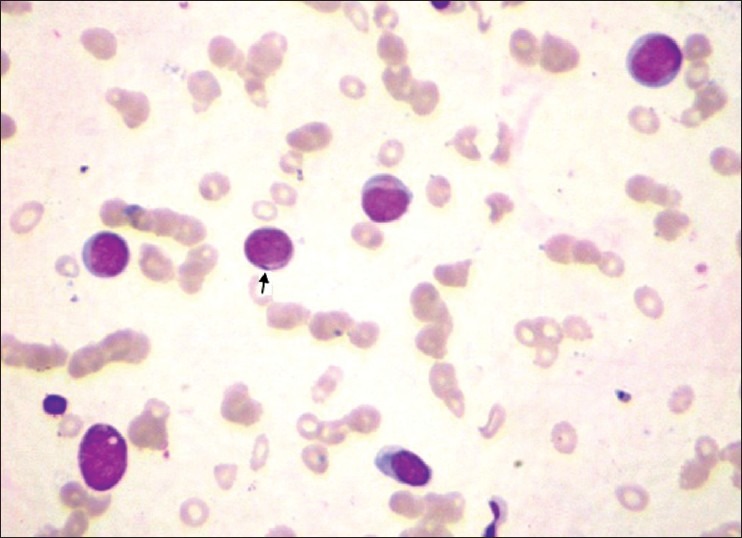
×100 Lieschman stained peripheral smear showing Auer rods in myeloblasts
Figure 4.
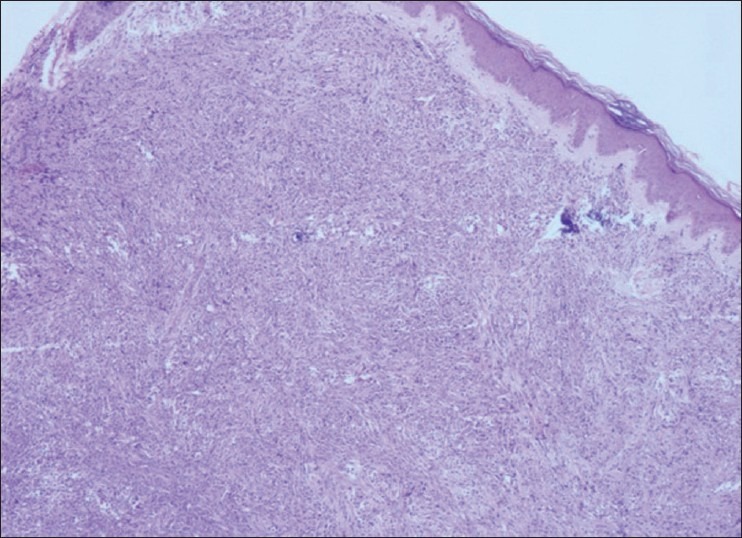
×10 HPE of the nodule H and E stain showing subepidermal Grenz zone
Figure 5.
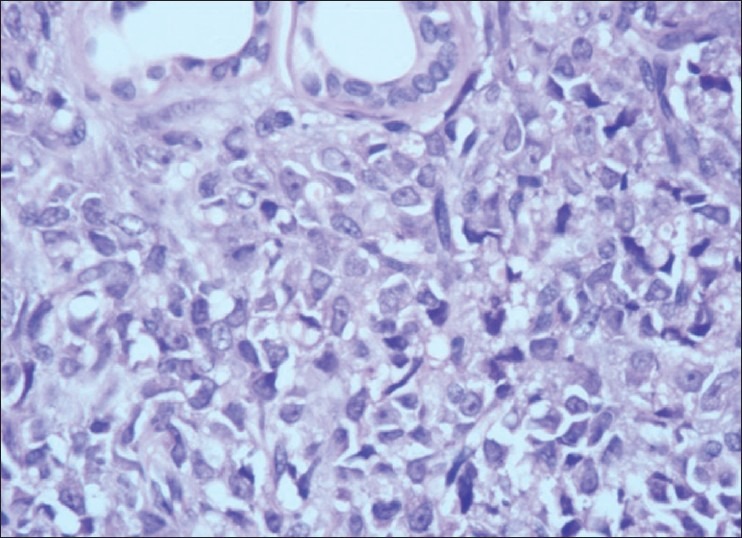
×40 HPE of the nodule H and E stain. Dermis shows sheet like infiltration of round to ovoid cells with ill defined cytoplasm, vesicular nucleus with single prominent nucleoli. Mitosis is also seen
Figure 6.
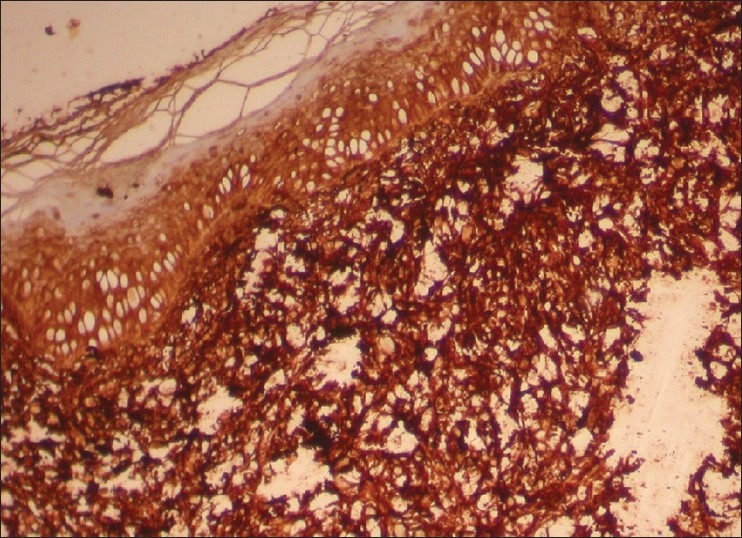
×10 HPE of the nodule Myeloperoxidase stain showing Myeloperoxidase positive infiltrating cells
Discussion
In general, international incidence of leukemia cutis is thought to be between 2.9 and 3.7% for acute myeloid leukemia (AML). Seven percent of cases present as aleukemic leukemia cutis.[2] AML shows the second highest rates of leukemia cutis.[3] Leukemia cutis may follow, precede or occur concomitantly with the diagnosis of systemic leukemia. The majority of leukemia cutis occurs at presentation with systemic leukemia (23-44%) or in the setting of an established leukemia (44-77%). Rarely, leukemia cutis can predate development of detectable leukemia in the peripheral blood and/or bone marrow.[4] There are some gene abnormalities associated with leukemia cutis, including numerical abnormalities of chromosome 8, translocation (8; 21) (q22; q22), and inversion (16) (p13; q22).[2,5] The pathophysiology of the specific migration of leukemic cells to the skin is not clear. It has been speculated that the chemokine integrin and other adhesion molecules may play a role in skin specific homing of T and B leukemic cells.
Leukemia cutis appears to be associated with higher percentage of extra medullary leukemic involvement at other sites. The central nervous system localization of AML occurs more frequently in patients with leukemia cutis (17%).[6]
Cutaneous manifestations of leukemia are described as specific (infiltrates of leukemic cells, or leukemia cutis) or nonspecific (inflammatory, paraneoplastic, or secondary to marrow failure). Leukemia cutis is a specific sign of systemic leukemia and is the result of dissemination of leukemic cells to the skin. The clinical appearance of leukemia cutis is variable with erythematous to violaceous papules or nodules being the most frequent lesions (60%), followed by infiltrated plaques, generalised cutaneous eruption and erythroderma. They are frequently asymptomatic.[7] The nodules are typically firm or rubbery in consistency, and can become purpuric if the patient is thrombocytopenic. The case under study also presented with asymptomatic generalized infiltrated plaques and nodules. Unusual presentations include those resembling figurate erythemas, guttate psoriasis, stasis dermatitis and ulcers. There are also reports of leukemia cutis localizing to sites of herpetic lesions, trauma, intravenous catheters and recent surgeries.
Nonspecific cutaneous signs of leukemia are much more common than leukemia cutis, occurring in up to 30-40% of patients with leukemia. The most common nonspecific signs are hemorrhagic, including petechiae, purpura and ecchymosis. Other signs related to marrow failure include those associated with infections, such as those associated with varicella-zoster virus, herpes simplex virus, or cutaneous mycoses. The list of reactive or paraneoplastic lesions is long and includes generalized pruritus, Sweet's syndrome, pyoderma gangrenosum, exfoliative dermatitis, urticaria erythema multiforme, erythema nodosum, ichthyosis, and vasculitis. There is also an increased incidence of drug eruptions in patients with leukemia.
Incidence of acute myeloblastic leukemia is higher in men. However, the reported case is a female. Heredity, radiation, chemical and occupational exposure and drugs have been implicated in the development of AML. No such history was forth coming in the reported case.
The FAB classification divides AML into 8 main subtypes M0 to M7, based on the morphology and the state of differentiation of the leukemic cells.[3] Acute myelomonocytic leukemia (AML-M4) and (AML-M5) have the highest rates of skin involvement of all the subtypes and are reported to be as high as 30%. Most patients of AML present with nonspecific symptoms. Significant haemorrhage occurs in acute promyelocytic leukemia. Infiltration of gingivae, skin or meninges is characteristic of monocytic subtypes (FAB-M4 and M5). Gingival hyperplasia is observed in 42% of AML-M5 and 55% of AML-M4.[7] However, the reported case had gingival hyperplasia, which is unusual in AML-M2. Detailed examination of the peripheral blood and bone marrow is crucial for the diagnosis of the specific type of leukemia, allowing correct treatment of these patients. The diagnosis of AML is established by presence of >20% myeloblasts in blood or bone marrow. In most cases, immunohistochemical study is necessary to characterize immunophenotype of tumor cells. Lysozyme is a sensitive marker of myeloid lineage. Lactic acid dehyydrogenase seems to be higher in patients with leukemia cutis and may be an additional sign of poor prognosis.[2]
Current treatment involves chemotherapy. The various antineoplastic agents used in the treatment of AML are Daunorubicin, Idaurubicin, Cytarabin, Tretinoin, Gemtuzimab, Etoposide and methotrexate. Radiotherapy is considered to be beneficial in widespread skin involvement; a higher incidence of skin relapses suggest that skin acts as a sanctuary for leukemic cells.[6] The use of whole-body electron-beam radiation seems to be a valuable alternative to conventional irradiation, avoiding systemic toxicity.
The prognosis is poor, with many patients having other extramedullary disease and poor survival rates. Most patients die within months of diagnosis. The survival rate is 30% at 2 years in patients with AML without skin lesions as compared to 6% in patients with skin lesions indicating grave prognosis of leukemia cutis.[3]
What is new?
Acute myeloid leukemia is the second most common cause of leukemia cutis. In 23 to 44% of patients leukemia cutis manifest at the time of presentation with systemic leukemia. Dermatologist plays pivotal role in the diagnosis of leukemia, but unfortunately prognosis is grave by then.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
References
- 1.Cho-Vega JH, Medeiros LJ, Prieto VG, Vega F. Leukemia cutis. Am J Clin Pathol. 2008;129:130–42. doi: 10.1309/WYACYWF6NGM3WBRT. [DOI] [PubMed] [Google Scholar]
- 2.Agis H, Weltermann A, Fonatsch C, Haas O, Mitterbauer G, Müllauer L, et al. A comparative study on demographic, haematological and cytogeentic findings and prognosis in acute myeloid leukaemia with or without leukemia cutis. Ann Hematol. 2002;81:90–5. doi: 10.1007/s00277-001-0412-9. [DOI] [PubMed] [Google Scholar]
- 3.Ramnarayanan J, Singhal S. Leukemia cutis. E medicine dermatology [Internet] [Last cited in 2010 Jan 1]. Web MD [updated on 2005 Jun 7]. Available from: http://emedicine.medscape.com/article/1097702-overview .
- 4.Yones SS, Khorasgani MG, Iqbal T, Arrifin N, Mehta R, Skibinska G, et al. Aleukemic leukemia cutis. Egypt Dermatol Online J. 2009;5:1–8. [Google Scholar]
- 5.Kubonishi I, Seto M, Murata N, Kamioka M, Taguchi H, Miyoshi I. Translocation (10;11) (p13;q13) and MLL rearrangement in a case of AML (M5a) with aggressive leukemia cutis. Cancer Genet Cytogenet. 1998;104(Suppl 1):28–31. doi: 10.1016/s0165-4608(97)00414-7. [DOI] [PubMed] [Google Scholar]
- 6.Zweegman S, Vermeer MH, Bekkink MW, van Der Valk P, Nanayakkara P, Ossenkoppele GJ. Leukemia cutis: Clinical features and treatment strategies. Haematologica. 2002;87:ECR13. [PubMed] [Google Scholar]
- 7.Koizumi H, Kumakiri M, Ishizuka M, Ohkawara A, Okabe S. Leukemia cutis in acute myelomonocytic leukemia: Infiltration to minor traumas and scars. J Dermatol. 1991;18:281–5. doi: 10.1111/j.1346-8138.1991.tb03083.x. [DOI] [PubMed] [Google Scholar]


