Abstract
To develop a rapid detection method of Staphylococcus aureus using loop-mediated isothermal amplification (LAMP), four specific primers were designed according to six distinct sequences of the nuc gene. In addition, the specificity and sensitivity of LAMP were verified and compared with those of PCR. Results showed that the LAMP reaction was completed within 45 min at 62.5°C, and ladder bands were appeared in LAMP products analyzed by gel electrophoresis. After adding 1x SYBR Green l, the positive reaction tube showed green color and the negative reaction tube remained orange, indicating that the LAMP has high specificity. The minimal detectable concentration of LAMP was 1 × 102 CFU/mL and that of PCR was 1 × 104 CFU/mL, indicating that the LAMP was 100 times more sensitive than the PCR. The LAMP method for detection of Staphylococcus aureus has many advantages, such as simple operation, high sensitivity, high specificity, and rapid analysis. Therefore, this method is more suitable for the rapid on-site detection of Staphylococcus aureus.
1. Introduction
Cow mastitis is the most common disease in world dairy industry and has made a tremendous economic loss. It not only reduces the milk yield and the quality of milk but also endangers human health and even leads to complete loss of lactation function of dairy cows [1]. Especially the subclinical mastitis is seriously harmful to dairy industry because it has no obvious clinical symptoms and has high incidence. There are more than 20 kinds of pathogens that can cause the cow mastitis, among which the most common one is Staphylococcus aureus [2].
The microbiological assay of milk is considered as “gold standard” of the diagnosis of subclinical mastitis. The conventional method depends on the milk microbial culture and biochemical identification; this method is complicated and time consuming and it has many limitations in practice application. Along with development of molecular biology, the genome sequencings of many bacteria have been completed. In recent years, the PCR detection method based on the sequence of bacteria rRNA regions has been widely applied in clinical diagnosis of medicine and veterinary [3]. However, the PCR method still has some disadvantages such as complex operation, requiring special heating cycle equipment, and so on.
The LAMP technology was developed by Notomi et al. [4]. It is a novel DNA amplification method and can amplify target gene under isothermal conditions with high efficiency, specificity, and sensitivity. Moreover, LAMP only requires simple reaction equipment, such as water bath. At present, LAMP method has been widely applied in detection of viral, bacterial, parasitic, and other pathogens [5–8]. In this study, we designed a set of specific primers according to the conserved regions of nuc gene of Staphylococcus aureus and developed a highly sensitive and specific LAMP method for detecting clinical samples.
2. Materials and Methods
2.1. Strains and Sample Collection
Staphylococcus aureus (Strain no. ATCC 25923), Escherichia coli (Strain no. ATCC 35218), Streptococcus agalactiae (Strain no. CVCC1886), and Salmonella typhimurium (Strain no. CVCC542) were purchased from China Center of Veterinary Microbial Culture Collection (CVCC). Staphylococcus epidermidis (Strain no. CICC23664) was purchased from China Center of Industrial Culture Collection (CICC).
The milk samples were collected from dairy cows suffering from mastitis which had no treatment history. Before collection, the mammary skin of dairy cow was cleaned with warm water, the papilla was disinfected with 75% alcohol, and the collected milk from each papilla was discarded at first three times. The milk samples were collected with 10 mL sterile centrifugal tube and labeled number and date. Then the collected milk samples were frozen with ice bag and taken back to laboratory for use.
2.2. Main Reagents and Primers
Bst DNA polymerase was purchased from New England Bio-Technology Co., Ltd. (Beijing, China). Betaine (analytical grade) was purchased from Sigma-Aldrich Corp. (St. Louis, USA). Taq DNA polymerase, dNTPs, DNA extraction kits, Fluorescent dye SYBR Green l, and agarose were purchased from TaKaRa Biotechnology (Dalian) Co., Ltd. (Dalian, China).
According to the 23S rRNA sequences of Staphylococcus aureus (accession no. X68425.1) published in the GenBank, one pair of primers (P1: 5′-TCTTCAGAAGATGCGGAATA-3′; P2: 5′-TAAGTCAAACGTTAACATACG-3′) was designed using the Primer 5.0 software and then synthesized by TaKaRa Biotechnology (Dalian) Co., Ltd. (Dalian, China), and the amplified target fragments were 420 bp in size.
The nuc gene sequences of Staphylococcus aureus (accession no. EF529606) were selected from the NCBI database. After multiple sequence alignment, four primers were designed using LAMP primer design software (PrimerExplorer, http://www.Netlaboratory.com/) according to the conserved regions of nuc gene. All primers sequences for LAMP are listed in Table 1 and the binding regions of target sequences are shown in Figure 1.
Table 1.
Primer sequences for LAMP.
| Primer | Len | Primer sequences |
|---|---|---|
| F3 | 23 | 5′-GCATTTACGAAAAAAATGGTAGA-3′ |
| B3 | 22 | 5′-TGTTCATGTGTATTGTTAGGTT-3′ |
| FIP | 47 | 5′-GCCACGTCCATATTTATCAGTTCTAAATGCAAAGAAAATTGAAGTCG-3′ |
| BIP | 49 | 5′-TATGCTGATGGAAAAATGGTAAACGTAAACATAAGCAACTTTAGCCAAG-3′ |
Figure 1.
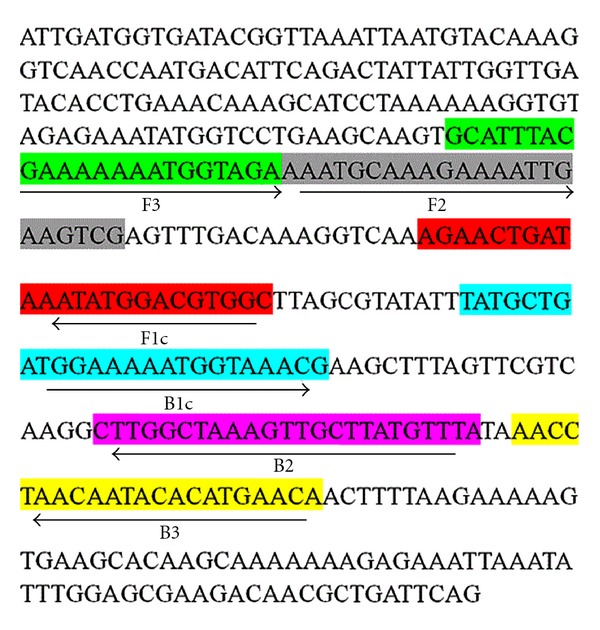
Sequences of LAMP primers in nuc gene of Staphylococcus aureus.
2.3. Preparation of DNA Template
The DNA was extracted from 1.5 mL overnight culture of organism using the DNA extraction kits according to instructions and stored at −20°C.
2.4. PCR Reaction
PCR reaction was performed in a 50-μL reaction volume containing 5.0 μL 10x PCR buffer, 3.0 μL magnesium (25 mmol/L), 4.0 μL dNTP (25 mmol/L), 0.5 μL Taq DNA polymerase (2.5 U), 1.0 μL each primer, and 2.0 μL template DNA and ddH2O was added to a final volume of 50.0 μL. The PCR temperature profiles were as follows: initial denaturation at 94.0°C for 2 min; followed by 30 cycles of denaturation at 94.0°C for 1 min, annealing at 56.0°C for 1 min, and extension at 72.0°C for 1 min; final extension at 72.0°C for 5 min. Samples (10.0 μL) of the PCR amplification products were subjected to electrophoresis on a 2% agarose gel.
2.5. LAMP Reaction
The LAMP reaction was carried out on a scale of 25.0 μL mixture containing 20.0 mmol/L Tris-HCl (pH 8.8), 10.0 mmol/L KCl, 10.0 mmol/L (NH4)2SO4, 8.0 mmol/L MgSO4, 0.1% Tween 20, 1.0 mmol/L betaine, 1.6 μmol/L each primer FIP and BIP, 0.2 μmol/L each primer F3 and B3, 1.4 mmol/L dNTP, 8 U Bst DNA polymerase, and 1.0 μL template DNA. The mixture was incubated at 63°C for 60 min and then heated at 80°C for 10 min to terminate the reaction. The negative control (sterilized ddH2O instead of template DNA) was set in each LAMP reaction.
2.6. Visualization Test
After the amplification, 1.0 μL SYBR Green l (1 : 1000) was added in each LAMP reaction tube in order to observe the color changed. Because fluorescent dye SYBR Green I can bind the double-stranded DNA and show a green fluorescence, so it is noted whether the color turned green is observed by naked eye under natural light, indicating that the reaction is positive.
After reaction, a total of 7.0 μL LAMP products were analyzed by 1.5% agarose gel electrophoresis at 80 V for 50 min. After electrophoresis, the gels were subjected to staining for 10 min by using EB and observed by using a gel imaging system.
2.7. Specificity Test
A total of 40 milk samples were collected from dairy cow suffering from mastitis, and then they were detected by using the developed LAMP. In addition, the DNA of Escherichia coli, Streptococcus agalactiae, Salmonella typhimurium, and Staphylococcus epidermidis were extracted and the specificity test was carried out according to the developed LAMP. The specificity of LAMP was determined by gel electrophoresis according to the specific ladder bands. The result was compared with the specificity of PCR. The samples of LAMP positive reaction were identified by biochemical tests after cultured.
2.8. Sensitivity Test
Taking Staphylococcus aureus as test object, the bacterial liquids were diluted to 1 × 106, 1 × 105, 1 × 104, 1000, 100, and 10 CFU/mL, and then the DNA extraction were carried out. The 10-fold serial diluents of DNA were used as template for LAMP and PCR amplification, respectively. The detection rate and the minimal detectable concentration of LAMP were compared with those of PCR, respectively.
3. Results
3.1. Visualization of LAMP
A lot of repeat sequences containing target DNA will be amplified when the target DNA is existing in the LAMP reaction system. Thus, the positive control tube and the sample tubes turned green after adding SYBR Green l due to the presence of LAMP products (target DNA), while the negative control tube remained orange (Figures 2(a) and 2(b)). The LAMP assay produced specific ladder bands on the gel (Figures 3 and 4(a)).
Figure 2.
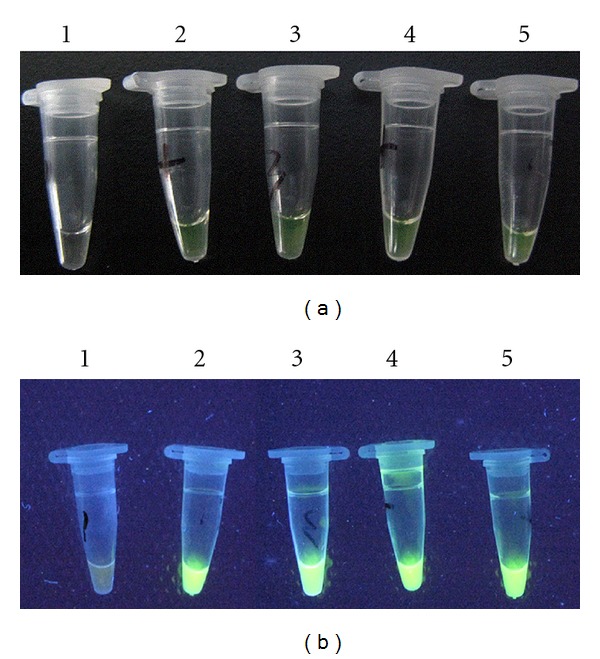
(a) Visualization of LAMP with SYBR Green l (reaction observed by naked eyes). 1: negative control; 2: positive control; 3–5: samples. (b) Visualization of LAMP with SYBR Green l (reaction observed by UV lamp). 1: negative control; 2: positive control; 3–5: samples.
Figure 3.
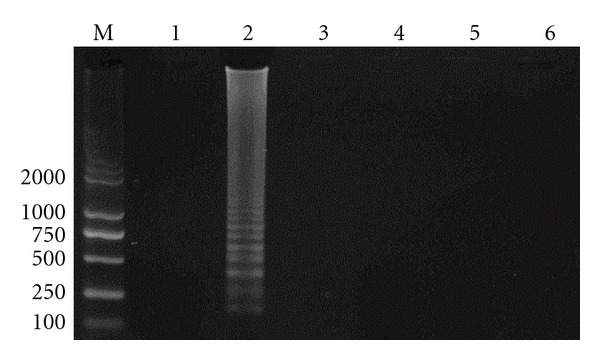
Specificity of LAMP. M: DL2000 DNA marker; 1: negative control; 2: Staphylococcus aureus; 3: Escherichia coli; 4: Streptococcus agalactiae; 5: Salmonella typhimurium; 6: Staphylococcus epidermidis.
Figure 4.
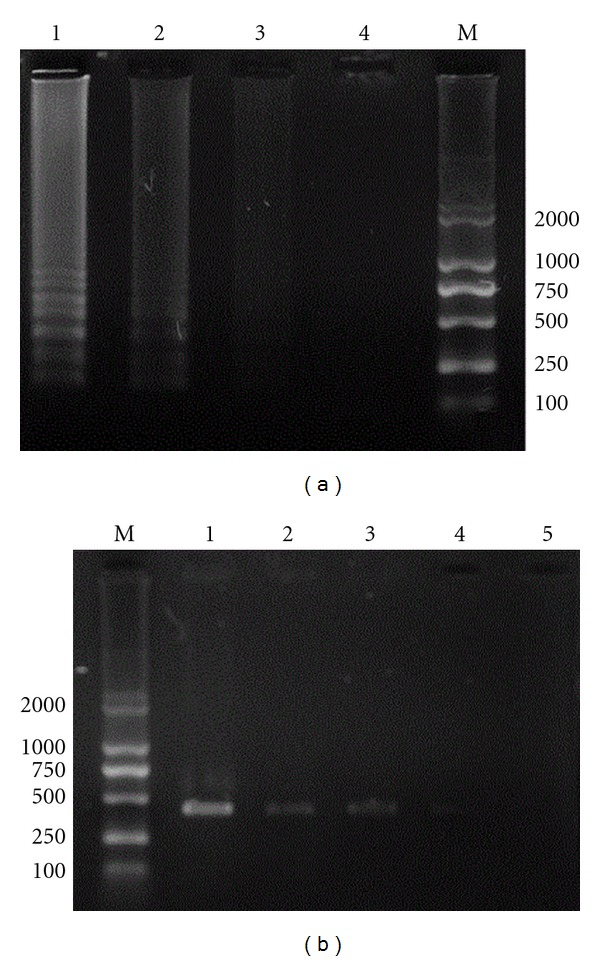
(a) Comparative sensitivity of LAMP and PCR (sensitivity of LAMP). M: DL2000 DNA marker; 1: 103 CFU/mL; 2: 102 CFU/mL; 3: 10 CFU/mL; 4: negative control. (b) Comparative sensitivity of LAMP and PCR (sensitivity of PCR). M: DL2000 DNA marker; 1: 106 CFU/mL; 2: 105 CFU/mL; 3: 104 CFU/mL; 4: 103 CFU/mL; 5: 102 CFU/mL.
3.2. Specificity of LAMP
The Staphylococcus aureus, Escherichia coli, Streptococcus agalactiae, Salmonella typhimurium, and Staphylococcus epidermidis were detected by using the developed LAMP, respectively. The results showed that the amplification product was positive only when Staphylococcus aureus was detected. Although the time of isothermal amplification was increased to 1 h, the DNA of other strains could not be detected. These demonstrated that the LAMP method had high specificity for detecting Staphylococcus aureus (Figure 3).
3.3. Sensitivity of LAMP
The 10-fold serial diluents of DNA extracted from Staphylococcus aureus was used as template for LAMP and PCR amplification, respectively. As shown in Figures 4(a) and 4(b), after gel electrophoresis, the LAMP products produced a ladder band while the PCR products produced one band at 420 bp. The minimal detectable concentration of LAMP was 1 × 102 CFU/mL and that of PCR was 1 × 104 CFU/mL.
3.4. Evaluation of LAMP
A total of 40 milk samples were collected from dairy cow suffering from mastitis, and the Staphylococcus aureus was detected by using the developed LAMP method and conventional PCR method, respectively. Results showed that the detection rate of LAMP was 100% and that of PCR was 85%; the minimal detectable concentration of LAMP was 1 × 102 CFU/mL and that of PCR was 1 × 104 CFU/mL. The sensitivity of the LAMP was 100 times higher than that of PCR. At the same time, the results of LAMP reaction were consistent with the results of biochemical tests.
4. Discussion
Staphylococcus aureus is the main pathogens causing mastitis in dairy cows. It is also one of the main pathogens causing food contamination and thereby leading to food poisoning. At present, the food poisoning caused by Staphylococcus aureus has increasingly become a global public health problem and posed a serious threat to human safety and health [9–11]. Currently, there are many methods for detecting Staphylococcus aureus such as the conventional isolation, culture and biochemical identification, immunological detection, and molecular detection. The conventional method is complicated and time consuming and its sensitivity is low. The immunological method has low specificity and sensitivity. The PCR method is sensitive, accurate, and rapid but requires expensive equipment and tedious electrophoresis. Therefore, it does not meet the needs of detection in field and grass-roots sectors and thus limiting the application of PCR method.
The LAMP is a new DNA amplification technology developed by Notomi et al. [4]. This method relies on four specific primers and Bst DNA polymerase that has helicase function, and then the target sequences can be amplified with high efficiency, rapidity, and specificity under isothermal conditions. Four specific primers can recognize the six conserved regions of target gene, ensuring the high specificity and sensitivity of LAMP. The target DNA fragments can be amplified 109 to 1010 times within 1 h under isothermal conditions, indicating that the LAMP is rapid, stable, and efficient [12]. Although there are prone to produce the cross-reaction in LAMP assay, the cross-reaction may be reduced through standardizing operations and some special treatment such as strict experimental partition, adding Dutp and uracil-N-glycosylase (UDG) in reaction system, and disinfecting experimental equipments with alcohol or ultraviolet.
In this study, the LAMP for detecting Staphylococcus aureus is more sensitive than PCR. The positive result can be observed by naked eyes and determined according to the green fluorescence. Therefore, the LAMP is a simple, rapid, specific, sensitive, practical, and visualized detection method of Staphylococcus aureus and it is especially suitable for detection in field and grass-roots sectors.
Conflict of Interests
The authors declare that they have no conflict of interests to this work.
Acknowledgments
This study was financially supported by the Ministry of Science and Technology of China (no. 2011BAD34B02). The authors claim that none of the material in the paper has been published or is under consideration for publication elsewhere.
References
- 1.Jurgen SH, Barbel K, Wilfried W, Michael Z, Axel S, Klaus F. The epidemiology of Staphylococcus aureus infections from subclinical mastitis in dairy cows during a control programme. Veterinary Microbiology. 2003;96(1):91–102. doi: 10.1016/s0378-1135(03)00204-9. [DOI] [PubMed] [Google Scholar]
- 2.Yao HC. Veterinary Microbiology Experiment Guidance. Beijing, China: China Agriculture Press; 2002. [Google Scholar]
- 3.Leblond-Bourget N, Philippe H, Mangin I, Decaris B. 16S rRNA and 16S to 23S internal transcribed spacer sequence analyses reveal inter- and intraspecific Bifidobacterium phylogeny. International Journal of Systematic Bacteriology. 1996;46(1):102–111. doi: 10.1099/00207713-46-1-102. [DOI] [PubMed] [Google Scholar]
- 4.Notomi T, Okayama H, Masubuchi H, et al. Loop-mediated isothermal amplification of DNA. Nucleic Acids Research. 2000;28(12, article E63) doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hara-Kudo Y, Nemoto J, Ohtsuka K, et al. Sensitive and rapid detection of Vero toxin-producing Escherichia coli using loop-mediated isothermal amplification. Journal of Medical Microbiology. 2007;56(3):398–406. doi: 10.1099/jmm.0.46819-0. [DOI] [PubMed] [Google Scholar]
- 6.Hara-Kudo Y, Yoshino M, Kojima T, Ikedo M. Loop-mediated isothermal amplification for the rapid detection of Salmonella. FEMS Microbiology Letters. 2005;253(1):155–161. doi: 10.1016/j.femsle.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 7.Ohtsuki R, Kawamoto K, Kato Y, Shah MM, Ezaki T, Makino SI. Rapid detection of Brucella spp. by the loop-mediated isothermal amplification method. Journal of Applied Microbiology. 2008;104(6):1815–1823. doi: 10.1111/j.1365-2672.2008.03732.x. [DOI] [PubMed] [Google Scholar]
- 8.Seki M, Yamashita Y, Torigoe H, Tsuda H, Sato S, Maeno M. Loop-mediated isothermal amplification method targeting the lytA gene for detection of Streptococcus pneumoniae. Journal of Clinical Microbiology. 2005;43(4):1581–1586. doi: 10.1128/JCM.43.4.1581-1586.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.le Loir Y, Baron F, Gautier M. Staphylococcus aureus and food poisoning. Genetics and Molecular Research. 2003;2(1):63–76. [PubMed] [Google Scholar]
- 10.Martín MC, Fueyo JM, González-Hevia MA, Mendoza MC. Genetic procedures for identification of enterotoxigenic strains of Staphylococcus aureus from three food poisoning outbreaks. International Journal of Food Microbiology. 2004;94(3):279–286. doi: 10.1016/j.ijfoodmicro.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Shriver-Lake LC, Shubin YS, Ligler FS. Detection of staphylococcal enterotoxin B in spiked food samples. Journal of Food Protection. 2003;66(10):1851–1856. doi: 10.4315/0362-028x-66.10.1851. [DOI] [PubMed] [Google Scholar]
- 12.Mori Y, Nagamine K, Tomita N, Notomi T. Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochemical and Biophysical Research Communications. 2001;289(1):150–154. doi: 10.1006/bbrc.2001.5921. [DOI] [PubMed] [Google Scholar]


