Abstract
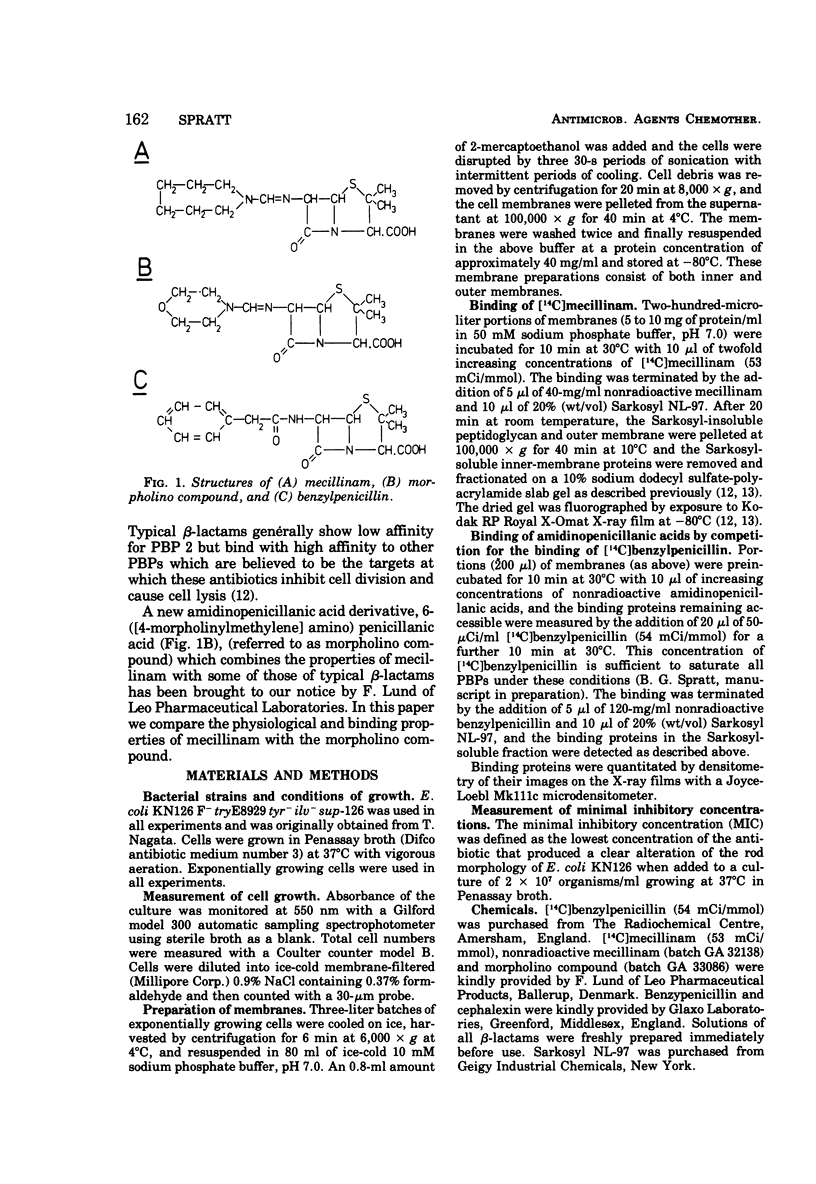
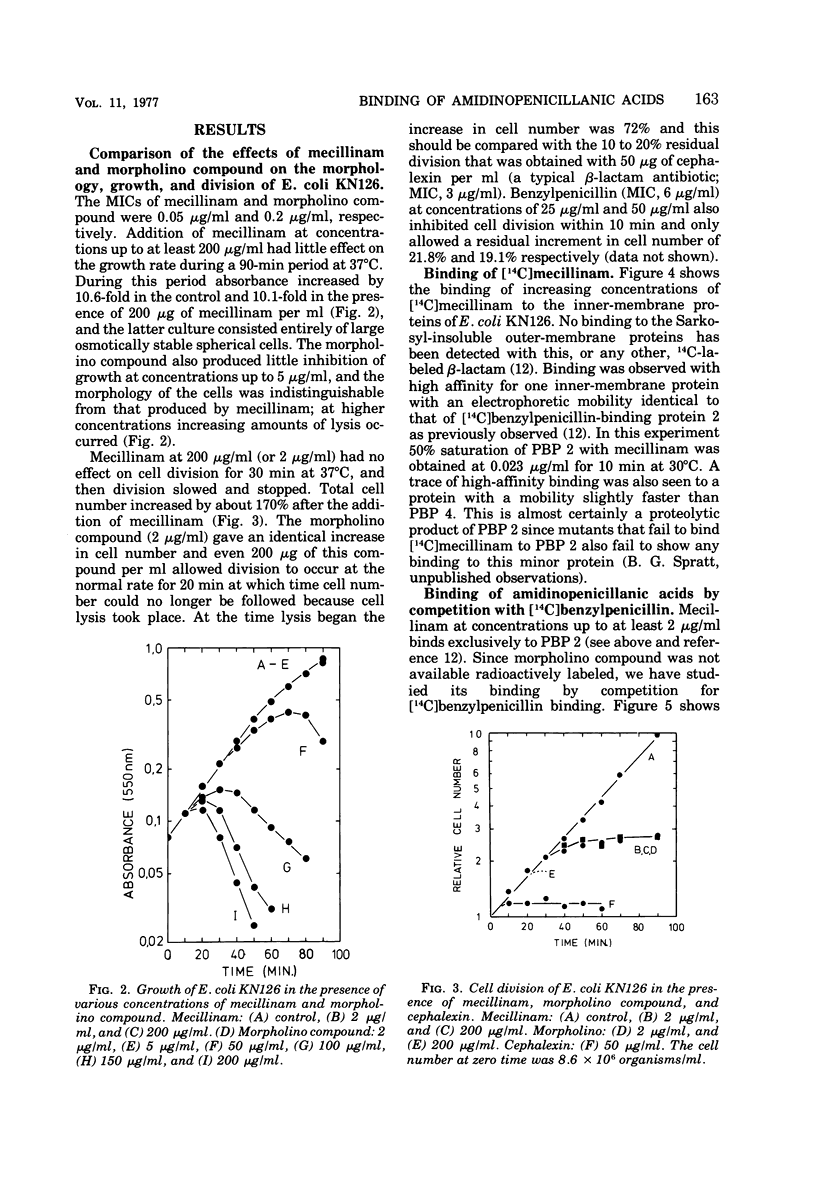
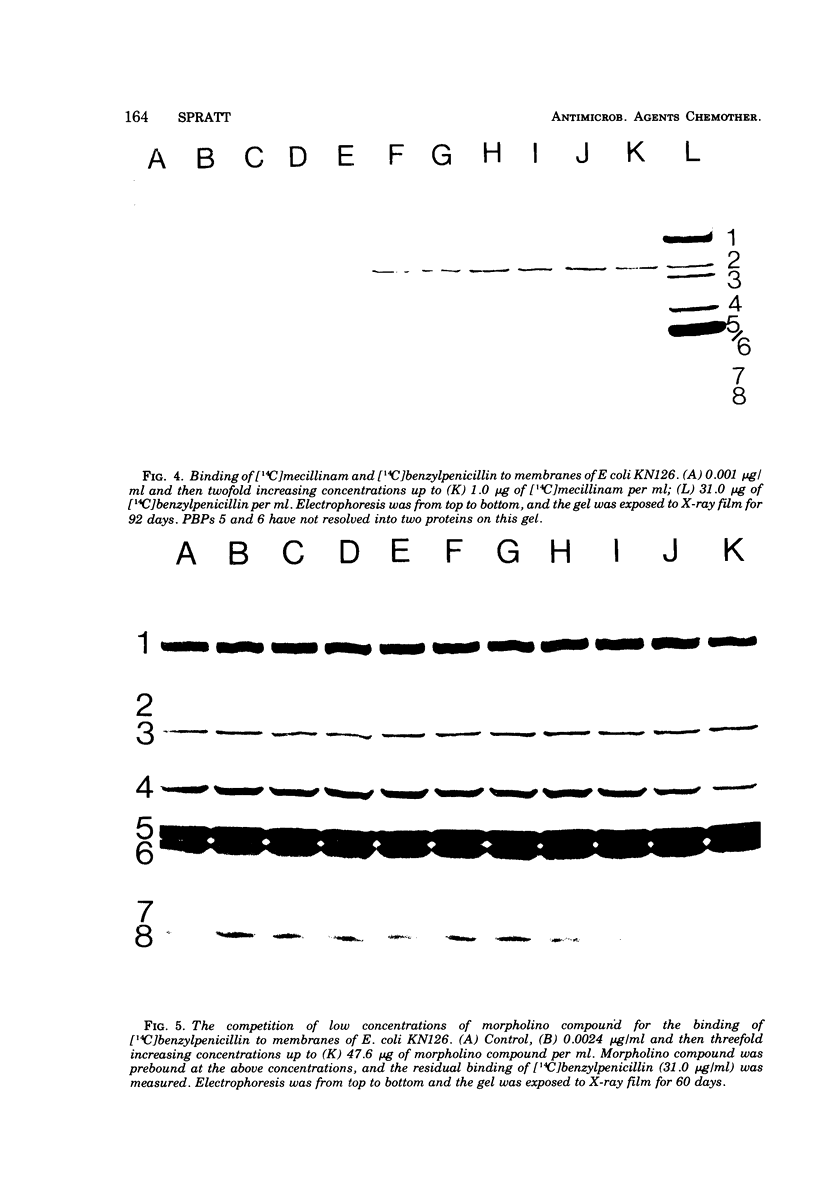
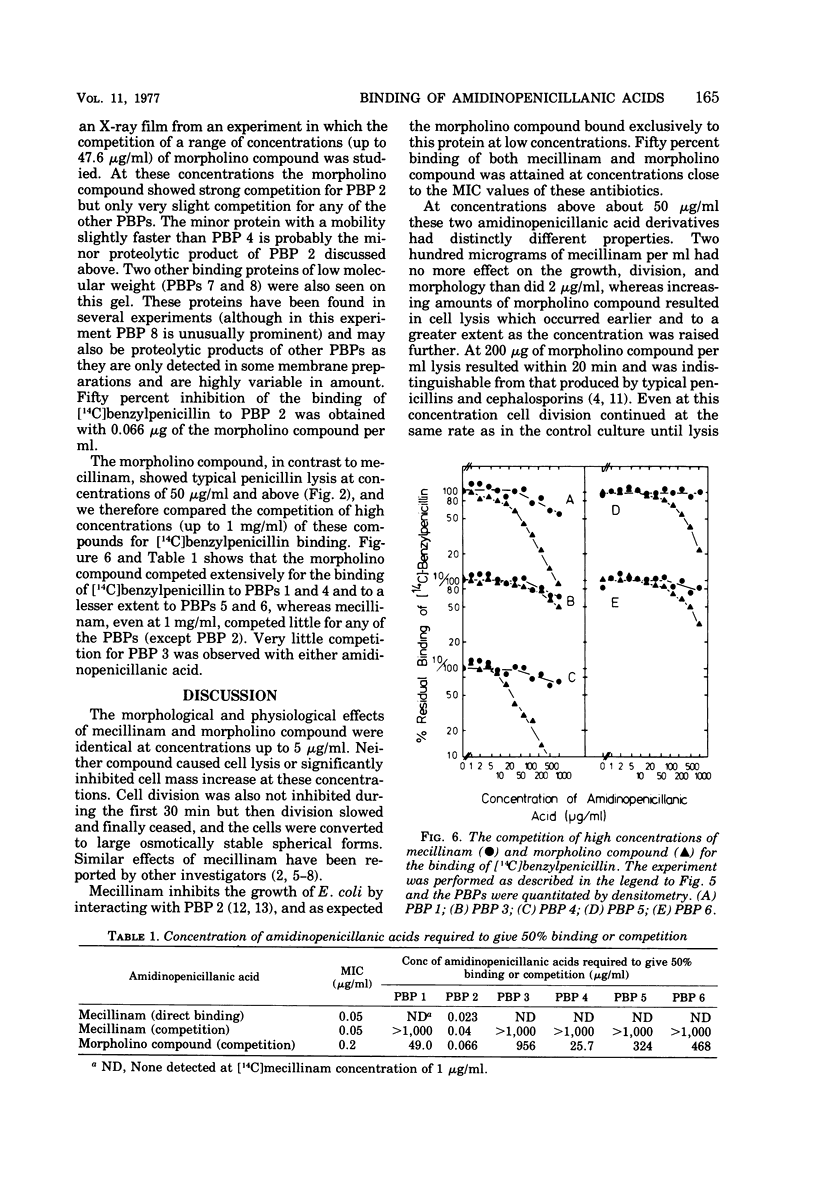
The 6-β-amidinopenicillanic acid derivative, mecillinam, was highly specific in its action on the growth of Escherichia coli. Concentrations from the minimal inhibitory concentration (0.05 μg/ml) up to at least 200 μg/ml resulted in the conversion of E. coli rods into osmotically stable spherical cells without significantly inhibiting cell growth or causing cell lysis. A second amidinopenicillanic acid derivative [6-([4-morpholinylmethylene] amino) penicillanic acid] showed identical effects on cell growth at concentrations from its minimal inhibitory concentration (0.2 μg/ml) up to at least 5 μg/ml but, at higher concentrations, increasing amounts of lysis occurred. Neither of these compounds showed the immediate inhibition of cell division that is observed with typical β-lactam antibiotics. We have compared the binding of these two amidinopenicillanic acids to the individual penicillin-binding proteins of E. coli. Both compounds showed a high specificity of binding to penicillin-binding protein 2 at low concentrations. At higher concentrations mecillinam still maintained its high specificity for protein 2 and very little binding of mecillinam to any of the other binding proteins was detected with concentrations up to 1 mg/ml. The morpholino compound, however, showed extensive binding to proteins 1 and 4, and slight binding to proteins 5 and 6 at high concentrations. The morpholino compound therefore combined both the physiological properties and the binding properties of mecillinam with some of those of typical penicillins and cephalosporins. Lysis probably occurs at high concentrations of morpholino compound because it binds to penicillin-binding protein 1, since this is believed to be the target with which β-lactams interact to inhibit cell elongation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore R. S., Klein J. O., Wilcox C., Finland M. Synergy of mecillinam (FL1060) with penicillins and cephalosporins against Proteus and Klebsiella, with observations on combinations with other antibiotics and against other bacterial species. Antimicrob Agents Chemother. 1976 Apr;9(4):701–705. doi: 10.1128/aac.9.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIAK J., HAHN F. E. Penicillin-induced lysis of Escherichia coli. Science. 1957 Jan 18;125(3238):119–120. doi: 10.1126/science.125.3238.119. [DOI] [PubMed] [Google Scholar]
- Greenwood D., O'Grady F. FL 1060: a new beta-lactam antibiotic with novel properties. J Clin Pathol. 1973 Jan;26(1):1–6. doi: 10.1136/jcp.26.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunberg E., Cleeland R., Beskid G., DeLorenzo W. F. In vivo synergy between 6 beta-amidinopenicillanic acid derivatives and other antibiotics. Antimicrob Agents Chemother. 1976 Apr;9(4):589–594. doi: 10.1128/aac.9.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James R., Haga J. Y., Pardee A. B. Inhibition of an early event in the cell division cycle of Escherichia coli by FL1060, an amidinopenicillanic acid. J Bacteriol. 1975 Jun;122(3):1283–1292. doi: 10.1128/jb.122.3.1283-1292.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund F., Tybring L. 6 -amidinopenicillanic acids--a new group of antibiotics. Nat New Biol. 1972 Apr 5;236(66):135–137. doi: 10.1038/newbio236135a0. [DOI] [PubMed] [Google Scholar]
- Matsuhashi S., Kamiryo T., Blumberg P. M., Linnett P., Willoughby E., Strominger J. L. Mechanism of action and development of resistance to a new amidino penicillin. J Bacteriol. 1974 Feb;117(2):578–587. doi: 10.1128/jb.117.2.578-587.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior N. H., Blom J., Tybring L., Birch-Andersen A. Light and electron microscopy of the early response of Escherichia coli to a 6beta-amidinopenicillanic acid (FL 1060). Acta Pathol Microbiol Scand B Microbiol Immunol. 1973 Aug;81(4):393–407. doi: 10.1111/j.1699-0463.1973.tb02222.x. [DOI] [PubMed] [Google Scholar]
- Neu H. C. Mecillinam, a novel penicillanic acid derivative with unusual activity against gram-negative bacteria. Antimicrob Agents Chemother. 1976 May;9(5):793–799. doi: 10.1128/aac.9.5.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. T., Burman L. FL-1060: a new penicillin with a unique mode of action. Biochem Biophys Res Commun. 1973 Apr 16;51(4):863–868. doi: 10.1016/0006-291x(73)90006-5. [DOI] [PubMed] [Google Scholar]
- Schwarz U., Asmus A., Frank H. Autolytic enzymes and cell division of Escherichia coli. J Mol Biol. 1969 May 14;41(3):419–429. doi: 10.1016/0022-2836(69)90285-x. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G., Pardee A. B. Penicillin-binding proteins and cell shape in E. coli. Nature. 1975 Apr 10;254(5500):516–517. doi: 10.1038/254516a0. [DOI] [PubMed] [Google Scholar]
- Stárka J., Moravová J. Cellular division of penicillin-induced filaments of Escherichia coli. Folia Microbiol (Praha) 1967;12(3):240–247. doi: 10.1007/BF02868738. [DOI] [PubMed] [Google Scholar]
- Tybring L. Mecillinam (FL 1060), a 6beta-amidinopenicillanic acid derivative: in vitro evaluation. Antimicrob Agents Chemother. 1975 Sep;8(3):266–270. doi: 10.1128/aac.8.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tybring L., Melchior N. H. Mecillinam (FL 1060), a 6beta-amidinopenicillanic acid derivative: bactericidal action and synergy in vitro. Antimicrob Agents Chemother. 1975 Sep;8(3):271–276. doi: 10.1128/aac.8.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]





