Abstract
Fanconi anemia (FA) is a rare recessive DNA repair disorder that is clinically characterized by congenital malformations, progressive bone marrow failure, and increased incidence of malignancies, especially acute myeloid leukemia and squamous cell carcinomas of the head and neck (HNSCCs) and the anogenital regions. On a cellular level, typical features of the disorder are a high degree of genomic instability and an increased sensitivity to bi-functionally alkylating agents. So far, germ-line defects in 15 different FA genes have been identified. Some of these FA genes are also established as tumor susceptibility genes for familiar cancers.
In recent years, the prevention and therapy of HNSCCs in FA patients has become more important as the percentage of patients surviving into adulthood is rising. HNSCCs appear in very young FA patients without common risk factors. Since cisplatin-based chemotherapy in combination with radiotherapy, essential parts of the standard treatment approach for sporadic HNSCCs, cannot be used in FA patients due to therapy-associated toxicities and mortalities even with reduced dosing, surgery is the most important treatment option for HNSCCs, in FA patients and requires an early and efficient detection of malignant lesions. So far, no uniform treatment protocol for the management of HNSCCs in FA patients exists. Therefore, we propose that the information on affected FA patients should be collected world-wide, practical therapeutic guidelines developed and national treatment centers established.
Keywords: Fanconi anemia, HNSCC, therapy, HPV, prevention
Introduction
Fanconi anemia (FA) is an autosomal or X-chromosomal recessive chromosome instability disorder due to germ-line mutations in at least 15 DNA repair genes: FANCA/B/C/D1/D2/E/F/G/I/J/L//M/N/O/P [16,31]. Clinically, FA is characterized by congenital malformations, progressive bone marrow failure, endocrine abnormalities and a high propensity of developing malignancies early in life [35]. Although certain subtypes of FA, predominantly with biallelic mutations in FANCD1/BRCA2 and FANCN/PALB2, develop a widerange of malignancies including AML, ALL nephroblastoma, medulloblastoma, and Non-Hodgkin-Lymphoma in the first decade of life [48,52,76], heterozygous germ-line mutations in the FA genes FANCD1/BRCA2, FANCJ/BRIP1, FANCN/PALB2 and FANCO/RAD51C are a cause of hereditary susceptibility for gynecological and other tumors [18,29,50]. However, the high incidence of MDS/AML and HNSCCs in the second and third decade of life [35] in FA patients with bi-allelic mutations in classical FA genes, where heterozygous germ-line mutation carriers do not have an increased cancer incidence [50], points to the general importance of the FA/BRCA pathway for the maintenance of genome stability [16,31].
In the last 10 years, the results of treating the hematological problems in FA by allogeneic stem cell transplantation have dramatically improved, with long-term survival rates approaching 70% for matched unrelated and 90% for matched related donors [10, 21,27,40, 67,75]. This success appears to predominantly be due to the inclusion of fludarabin in the low-intensity conditioning regimens [10,21,40,67], the early detection of pre-leukemic changes and consecutively early transplantation [70], improvements in the prophylaxis of graft-versus-host disease (GvHD) and improved measures during the peritransplantation time period [40]. Independent from transplantation, androgens as an alternative treatment option for the failing hematopoietic system can improve survival at least in some patients well beyond 20 years of age and without the need for performing stem cell transplantation [58].
Therefore, with more FA patients surviving long-term and due to the fact that FA is a hereditary cancer susceptibility disorder, the spectrum of clinical problems is shifting towards the need for more efficient prophylaxis, earlier detection and also better treatment of these malignancies later in the life of FA patients. While this has partially been achieved for hematological malignancies [70], comparable strategies have still to be implemented and standardized for non-hematological tumors. In this overview, we will focus on squamous cell carcinomas of the head and neck region (HNSCC) as the second most frequent tumor entity in FA patients, partly promoted by the conditioning and immunological problems of the stem cell transplantation procedure.
HNSCC in FA
Only 6% of all human malignant tumors worldwide are squamous cell carcinomas of the head and neck region (HNSCCs) [19,51]. More than 50% of the HNSCCs have already progressed to locally advanced disease by the time of diagnosis, thus requiring aggressive multimodality approaches to achieve at least 40–50% 5-year survival [19,51]. Although the recent addition of high-dose cisplatin to surgery and local irradiation was important for lowering the rate of both local regrowth and systemic dissemination in sporadic HNSCCs, this multidisciplinary therapy has a high rate of severe adverse effects as well as an increased incidence of late effects [5,12].
In FA however, HNSCC is the most frequently diagnosed solid tumor [34,35]. In 2003, the 20-year perspective of the International FA Registry (IFAR) reported an average survival of FA patients of only <25 years [35]. HNSCCS were diagnosed in 19(3%) of 754 FA patients and half of the patients had died because of their HNSCC [34,35]. The mean tumor manifestation age, 31 years, was very young compared to the median age of diagnosis of cancer of the oral cavity and pharynx in the U.S.A. population in 2004–2008,62 years (http://seer.cancer.gov/statfacts/html/oral-cav.html). Primary tumors predominantly appeared in the oral cavity, mainly the tongue or the gingiva. Noteworthy is that approximately one third of the malignancies in the head and neck region are found in the oropharynx, nasopharynx, hypopharynx and larynx and therefore are not readily detectable by examinations of the oral cavity [2,34]. In addition, female FA patients might have a higher rate of esophageal cancers [2,34]. In 2011, a mean tumor diagnosis age of 35.5 years was reported in 12 FA patients; only 4 of these patients were tumor-free for an average of 24 months after the diagnosis of HNSCC [7].
Importantly, in one study [2], the cancers in the upper aerodigestive tract in a relatively high percentage of cases, 9 out of 42 patients, were diagnosed prior to the diagnosis of FA. This was probably due to hypomorphic mutations [11,25] or reversions/mosaicism in the hematopoietic system [3]. As FA cells are hypersensitive to standard genotoxic treatment approaches including the mono- and bifunctional alkylators such as cisplatin, mitomycin C and cyclophosphamide as well as irradiation, undiagnosed FA patients will experience severe complications under standard HNSCC therapy and develop lethal treatment-associated toxicities [7,11,25,69].
Based on a comparison between a North-American Survey group of nontransplanted (n = 145) and the Saint Louis Hospital-derived group of transplanted FA patients (n=117), it was calculated that approximately 50% of the nontransplanted FA patients will develop HNSCC by 45 years of age [56]. In contrast, the cumulative incidence of HNSCC was estimated at approximately 100% for transplanted FA patients at the same age [56]. Major transplant-associated risk factors that influence the manifestation of HNSCC and also other epithelial tumors are the conditioning regimen and the occurrence of acute and chronic GvHD [55]. Of 13 FA patients with HNSCC who were transplanted at the Saint Louis Hospital between 1976 and 2007, all patients had received irradiation-based conditioning and all had developed extensive chronic GvHD [42]. In this study, patients developed HNSCCs on average 10 years after transplantation, predominantly in the oral cavity. At the last reported follow-up, only 2 patients were alive 9 and 23 months after HNSCC diagnosis despite the use of surgery, radiation and/or chemotherapy [42].
In summary, the cumulative incidence to develop HNSCC in FA patients increases at a greater-than-linear rate, approaching 4.4% per year by age 40 for nontransplanted FA patients and 10.1% per year >15 years after transplantation [56]. Thus, FA patients have a more than 700-fold higher HNSCC incidence than the normal population [55,56]. Based on reports in the literature, it was estimated that FA-patients also have a 2000-fold higher risk of developing esophageal cancer [2]. Although more FA patients are now surviving the first two decades of life, over-all survival of FA patients with HNSCC is poor due to a high percentage of tumor progress under therapy and also early relapses in combination with severe toxicities of the usually reduced irradiation and/or chemotherapy [2,7,34,63].
Behavioral risk factors for HNSCC
Behavioral consumption of alcohol and smoking/tobacco use are well established risk factors for the development of HNSCC [44,54]. Estimates suggest that in sporadic HNSCC, heavy smoking increases the tumor risk 20-fold and heavy alcohol consumption 5-fold [54]. In combination, both factors lead to a 50-fold elevated risk for the development of HNSCC [54] and also negatively impact survival [44]. Additional factors that promote the development of HNSCC are eating habits with low proportions of fruits and vegetables [39], bad dental hygiene [57], and chronic exposure to betel or areca nuts [44,54].
In contrast to the normal population, the majority of FA patients experience HNSCC frequently at a very young age and often without exposure to any of these risk factors. Only 3 out of 19 patients with HNSCC registered in the IFAR [35] and only one third (4 out of 12) of FA patients in a follow-up study indicated the consumption of nicotine and/or alcohol [7].
HPV/p53
In 1983, infection with oncogenic human papilloma viruses (HPVs) was first described in the context of sporadic HNSCC[68]. Today, local infections with oncogenic HPV are well established to play a major role in the pathogenesis of sporadic HNSCC [19,51,71] and other human cancers [47,66]. From more than 120 different HPV subtypes that have been isolated, infections with the high-risk strains HPV 16 (approximately 90% of cases) and HPV 18 are prevalent in patients with epithelial cancers world-wide [19,47,51]. The transforming activity of these high-risk serotypes is predominantly the consequence of two viral oncogenes, E6 and E7, that are expressed in HPV-infected cells (reviewed in [46]). E6 binds to the DNA-binding region of human P53 and therefore inhibits its tumor suppressive functions, including induction of apoptosis and cell cycle arrest. E7 mediates poly-ubiquitination and thereby degradation of the tumor suppressor protein RB [46]. E7 also inhibits the tumor suppressor p21 (CIP1) and indirectly leads to reduced cell cycle arrest and thus to increased cell proliferation. Both viral oncogenes are also involved in the activation of the WNT signaling pathway, at least in HPV16 positive gynecological SCCs [46].
Multiple studies have demonstrated that HPV infections in the normal population are predominantly transmitted through sexual activities [13,14,19,30]. Especially certain sexual behaviors such as open-mouthed kissing, oral sex, oral-anal contacts and a high number of sexual partners have been strongly associated with an elevated risk of developing an HPV positive tumor [13,14,30]. However, it is important to note that it is completely unknown how and when HPV infection occurs in FA patients. Infection with HPV16 virus activates the FA pathway in normal cells and increases the genomic instability in FA cells [64,65]. A genetic cross showed that Fancd2 deficient mice transgenic for the HPV16 E7 oncogene have a higher incidence of chemically induced HNSCC compared to the Fancd2 deficient control animals[49]. In FA patients however, there is conflicting data on the impact of HPV for the pathogenesis of SCCs. Kutler et al. detected HPV DNA in 84% of SCC samples from 25 FA patients, compared to only 36% of specimens from their nonFA control tumor group [34]. 15 of the 18 HNSCCs and 6 of the 7 of the anogential SCC were positive in their FA patient group [34]. Since the HPV E6 protein inhibits p53 [59,60], they also sequenced p53 in tumor tissues from both groups. No mutations in p53 were detected in samples from FA patients compared to 36% of tumors in their control group [36]. These observations in FA patients and the findings in mice [49] strongly support the hypothesis that defects in the FA/BRCA pathway are associated with increased susceptibility to HPV infections and therefore a higher propensity for developing HPV-triggered SCCs [36].
In sharp contrast to this, van Zeeburg et al. could not detect HPV DNA in 4 HNSCC cell lines from FA patients or in 7 HNSCC cell lines from patients with sporadic tumors [73]. In addition, p53 mutations were detected in all FA HNSCC cell lines and in 4 of the 7 cell lines from sporadic HNSCC patients [72,73]. In a follow-up publication, van Zeeburg et al. again did not detect HPV DNA in 16 HNSCC specimens from FA patients and indirect HPV analysis by p16 immunostaining only showed positive staining in tumors from 2 of the 13 analyzed FA patients [74]. P53 mutations were detected in the majority (8 out of 13) of analyzed patients [74].
These completely opposite findings obtained from FA patients in the U.S.A. and in Europe currently makes it impossible to finally judge the impact of HPV infections on the highly increased incidence of SSCs in FA patients. However, it appears worth mentioning that the incidence of HPV infection and p53 mutations in nonFA patients reported by Kutler et al. in their control group of sporadic SCCs [34] is similar to what has been reported by others in independent studies [19,20]. In addition, other authors have also reported high percentages of HPV infection in their FA patients [15,23]. Our own findings revealed WV infection in only one out of 30 sporadic HNSCC cell lines, whereas analysis of 123 tumor specimens detected HPV infection in 37 cases (30%) [4,24].
Therapy of HNSCC
The current state-of-the-art treatment of HNSCC is a multidisciplinary approach, combining surgery, chemo- and radiotherapy options [6,61]. Primary surgical removal of the cancers with clear tumor cell-free margins followed by local radiotherapy and concurrent delivery of high-dose chemotherapy [17] has improved the 5-year survival rate in the majority of HNSCC patients significantly 16,37,61]. The backbone of the chemotherapy regimen is the crosslinking alkylating agent cisplatin which is usually given in intermediate high-doses of 50–100 mg/m2 [6,61]. Addition of other drugs such as 5-fluorouracil, methotrexate, and paclitaxel may increase the prognosis in some instances, but also have been associated with increased toxicities that limited their clinical utility [43].
Unfortunately, all cells of FA patients are exquisitely sensitive to DNA cross-linking agents [16,31]. Therefore, systemic cisplatin treatment cannot be used in this patient population due to the very high toxicities and organ failures induced even by reduced dosing leading to fatal outcome [7,63]. Consequently, radiotherapy can be used as a localized cancer treatment approach in FA [26], but it also seems to be associated with severe complications in FA HNSCC patients [7,41,69]. A recent larger study confirmed that radiotherapy is problematic in FA patients, as one third of FA patients (4 out of 12) died during the course of therapy. Pancytopenia was observed in half of the patients and most of them suffered from partly severe mucositis and dysphagia [7]. In 2002, the outcome for 14 FA patients with SCCs who received radiotherapy was summarized from the literature [1]. Cancers in this cohort included 10 HN, 3 esophageal and 1 vaginal SSCs. Although the numbers are small, it appears as if local irradiation >34 Gray was associated with severe toxicities, mucositis, edema, ulceration or local bleeding in the 10 HNSCC patients, and only two patients in the literature were alive 3 and 10 months after the initial diagnosis of the cancer [1]. Unfortunately, these severe clinical complications of the toxic radiotherapy are not necessarily predictable by in vitro sensitivity testing of lymphoid cells of FA patients [41]. As a consequence of the high iatrogenic morbidity and toxicity, individualized therapy approaches were suggested for FA patients with extended courses at lower daily doses (150–180 cGray per fraction) and intensified monitoring of the hematological and organ toxicities during treatment [7].
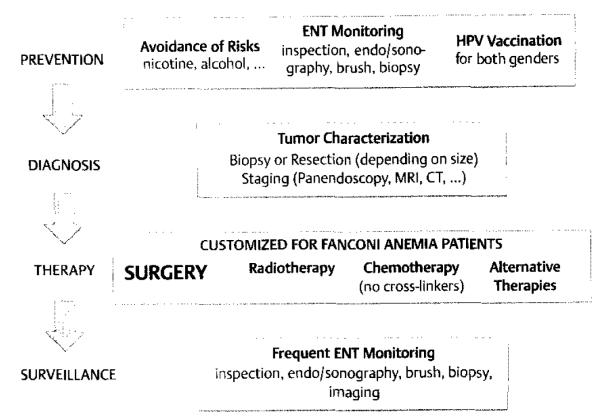
Based on these experiences, it is obvious that the most important therapeutic option in FA patients with HNSCC is the complete surgical resection of the cancer at an early stage (Fig. 1). This should be possible in the majority of FA tumor patients, as two thirds of tumors appear to be located in the oral cavity [35]. Surgery can either be performed conventionally or in certain cases by laser resection (Scheckenbach et al. unpublished). Neck dissections should be added if any lymph node metastasis appears even remotely possible, as loco-regional tumor control by other means might be more difficult to achieve [7].
Fig. 1.
Algorithm for prophylaxis and treatment in HNSCCs of FA patients (For details, see text).
In sporadic HNSCC, targeted therapies have been a major focus of efforts to increase the dismal prognosis for advanced stages in the last years [43]. The most developed approach here is based on the fact that the Epidermal Growth Factor Receptor (EGFR) is often overexpressed and activated in sporadic HNSCC [9]. As the EGFR is a proto-oncogene, an increased activity promotes tumor progression and invasion, inhibition of apoptosis, angiogenesis and metastasis [9]. Importantly, although the EGFR targeting antibody Cetuximab is FDA-approved in combination with radiotherapy for advanced HNSCC [9], therapy with EGFR antibodies is not a sufficient alternative to radiation or chemotherapy with cisplatin in sporadic HNSCC [62]. In addition, treatment with Cetuximab is associated with a wide spectrum of adverse reactions [38] and instances of using Cetuximab in FA patients have not been reported so far.
Recently, photodynamic therapy (PDT) based on an interaction between oxygen, light and a photosensitizer to induce apoptosis/necrosis has been introduced for local tumor control [28]. Experiences in FA - neither in FA cells in vitro nor in patients in vivo - have not been reported. Finally, based on the fact that p53 is either mutated or inactivated by HPV infections in the majority of HNSCCs, clinical trials have been conducted with adenoviruses specifically targeting cells with nonfunctional p53 [38]. None of these approaches has received FDA approval yet and would thus be readily available for FA patients with HNSCCs.
Therefore, due to our very limited treatment options for HNSCC in FA patients, it appears mandatory to systematically collect and analyze the treatments and outcomes that have been observed in FA patients worldwide. As most cases of HNSCCs in FA patients have never been published, a feasible approach might be to ask the national FA family support groups for access to FA patients and their families and thereby expand our knowledge for what has been tried and achieved in FA patient clinical care so far on an individualized basis.
Screening and prevention of HNSCC
The extremely high incidence of HNSCC and the uttermost importance of early surgical interventions to achieve cure for HNSCC in FA patients emphasizes the need for regular and rigorous surveillance measures. In addition, as only two thirds of all HNSCCs in FA patients are located in the oral cavity [35], surveillance should ideally be performed by a specialist (e.g. ENT or oral surgeon) and should also include the naso-, oro- and hypopharynx as well as the larynx and possibly the esophagus, especially in older patients and if there are any signs of reflux or dysphagia. Semiannual examinations might already be indicated as early as 10–12 years of age, particularly if the patient had undergone stem cell transplantation [42,53]. Without prior transplantation, screening could start later at the age of 15 years, however extensive examinations of the upper aerodigestive tract every 6–8 weeks appears necessary in FA patients with leukoplakia and recurrent oral lesions [34]. To thoroughly access all the different areas where HNSCC can develop, the use of a flexible endoscope is inevitable (Fig. 1).
Early detection of pre-malignant lesions or HNSCC can be sought by a variety of screening methods [45]. Different light-based screening aids are in development based on the fact that abnormal metabolic and structural changes lead to differences in absorbance and reflectance properties [32]. Analogous to the detection of cervical cancer, brush biopsies can be performed as a milder alternative to scalpel biopsies [33]. Since no tissue specimen but only single cells are available in brush biopsies, these probes are limited to cytological investigations; however, additional DNA ploidy analysis can complement these cytological investigations [33]. Several other test methods are in development and studied in different centers [45].
Increased surveillance should be performed for any dysplastic lesions. In general, even mildly dysplastic areas should be removed if feasible (Fig. 1). Finally, essential for the prevention of HNSCC is avoidance of additional risk factors such as nicotine and alcohol, maintenance of a healthy lifestyle and careful oral hygiene. In a transplant setting, it appears to be very important to avoid acute or chronic GvHD≥II, especially in combination with irradiation for conditioning.
Finally, although the impact of HPV for the development of SCC in FA patients has not been clarified yet, standard HPV vaccination for girls and women can be considered a safe procedure, based on experiences with more than 40 million doses of vaccines distributed in the U.S.A. alone (Center for Disease Control and Prevention (CDC); October 25, 2011: http://www.cdc.gov/media/releases/2011/t1025_hpv_12yroldvaccine.html). Vaccination seems to have the greatest impact on antibody titer and protection when given between 11–12 years of age and before any exposure to HPV. There are two different vaccines available, the quadrivalent Gardasil® from Merck (http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000703/human_ med_000805.sjsp&jsenabled=false) and the bivalent Cervarix® from GlaxoSmithKline (http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000721/human_med_000694.sjsp&jsenabled=false), both using recombinant virus-like particles without any viral DNA. While Gardasil is effective against the oncogenic HPV16 and −18 and also the low risk HPV6 and −11 strains associated genital warts, Cervarix only includes HPV16 and −18, but may induce higher antibody titers after standard vaccination [8,22]. In 2009, the quadrivalent HPV vaccine was also approved by the FDA for usage in boys and men and is thought to prevent genital warts and anal cancer, which in more than 80% are associated with HPV. In October 2011, the Advisory Committee on Immunization Practices (ACIP) in the U.S.A. recommended to also vaccinate all 11- and 12-year-old males against HPV using the quadrivalent vaccine. Although this will be associated with significant costs, the expert panel considered the vaccination an important opportunity to reduce the spread of HPV from males to females and to decrease the burden of HPV-related diseases in both genders (http://www.cdc.gov/media/releases/2011/t1025_hpv_12yroldvaccine.html).
Therefore, based on the exquisite susceptibility of FA cells to HPV infections [49,64,65], the excellent safety profiles of the available vaccine(s) and the general recommendation for vaccination of all 10-to 11-year-old children against HPV in the USA, we consider it important to now include both genders of FA patients in the vaccination approaches for HPV16 and −18 and perhaps also HPV6 and −11 worldwide. If future studies reveal that FA patients have a greatly increased susceptibility, possibly in combination with an immunological defect to clear HPV, it might even be appropriate to vaccinate FA patients for HPV immediately after diagnosis of FA.
Conclusion
HNSCCs are a growing problem in the clinical care of FA patients, due to both, the high incidence in transplanted but also in non-transplanted patients and the very limited therapeutic options for manifest malignancies. Early detection and surgical removal are paramount to overcome the poor outcome of HNSCCs in FA patients. Prospectively, vaccination for oncogenic HPV may reduce the occurrence of HNSCC and new conservative therapeutic strategies that are not based on cross-linking chemotherapeutics and irradiation such as targeted therapies may improve survival of FA patients with HNSCC. Nevertheless, ongoing and future efforts should also include standardized diagnostic and therapeutic treatments for all FA patients and an intensified exchange of medical and genetic information between different clinics and institutions worldwide. Since FA patients with HNSCC are such a small and difficult-to-achieve-cure collective, it is absolutely necessary to share experiences and systematically develop and standardize new prevention and treatment approaches. This can only be achieved by a unified approach.
Acknowledgements
We would like to thank all patients and their families for the support and the confidence that we have experienced over the years. We gratefully acknowledge our colleagues that were not included as authors, for the long-term clinical care of the FA patients. We also would like to apologize to all colleagues whose publications we could not include due to space limitations. This work was supported by the HHU Forschungsfoerderung Fund (to K.S.), the BMBF networks of inherited bone marrow failure syndromes (to H.H.) and Foamyvirus-mediated genetic therapy for FANCA (FoneFA, to H.H.) and the NIH R0Is CA138237-01 and CA155294-01 (to H.H.). This work was also supported by grants from the German Family Support Group “Aktionskreis Fanconi-Anämie e. V.”, the “Fanconi-Anämie-Stiftung” and “Kinderstern e.V.” to H.H. All authors contributed to writing the manuscript.
Footnotes
Conflict of interest: Three of the authors, K.S., M.F., and H.H., may receive royalties based on a license agreement with Myriad Genetics, Salt Lake City, U.S.A. on the use of RAD51C as a cancer susceptibility gene for diagnostic and therapeutic approaches. The others authors have nothing to declare.
References
- 1.Alter BP. Radiosensitivity in Fanconi’s anemia patients. Radiother Oncol. 2002;62:345–347. doi: 10.1016/s0167-8140(01)00474-1. [DOI] [PubMed] [Google Scholar]
- 2.Alter BP. Fanconi’s anemia, transplantation, and cancer. Pediatr Transplant. 2005;9(Suppl 7):81–86. doi: 10.1111/j.1399-3046.2005.00440.x. [DOI] [PubMed] [Google Scholar]
- 3.Alter BP, Joenje H, Oostra AB, et al. Fanconi anemia: adult head and neck cancer and hematopoictic mosaicism. Arch Otolaryngol Head Neck Surg. 2005;131:635–639. doi: 10.1001/archotol.131.7.635. [DOI] [PubMed] [Google Scholar]
- 4.Balz V, Scheckenbach K, Gotte K, et al. Is the p53 inactivation frequency in squamous cell carcinomas of the head and neck underestimated? Analysis of p53 exons 2-11 and human papillomavirus 16/18 E6 transcripts in 123 unselected tumor specimens. Cancer Res. 2003;63:1188–1191. [PubMed] [Google Scholar]
- 5.Bernier J, Domenge C, Ozsanin M, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350:1945–1952. doi: 10.1056/NEJMoa032641. [DOI] [PubMed] [Google Scholar]
- 6.Bernier J, Pfister DC, Cooper JS. Adjuvant chemo- and radiotherapy for poor prognosis head and neck squamous cell carcinomas. Crit Rev Oncol Hematol. 2005;56:353–364. doi: 10.1016/j.critrevonc.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Birkeland AC, Auerbach AD, Sanborn E, et al. Postoperative clinical radiosensitivity in patients with fanconi anemia and head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2011;137:930–934. doi: 10.1001/archoto.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Block SL, Nolan T, Sattler C, et al. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6,11,16, and 18) L1 virus-like particle vaccine in male and female adolescents and young adult women. Pediatrics. 2006;118:2135–2145. doi: 10.1542/peds.2006-0461. [DOI] [PubMed] [Google Scholar]
- 9.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 10.Chaudhury S, Auerbach AD, Kernan NA, et al. Fludarabine-based cytoreductive regimen and 4-cell-depleted grafts from alternative donors for the treatment of high-risk patients with Fanconi anaemia. Br J Haematol. 2008;140:644–655. doi: 10.1111/j.1365-2141.2007.06975.x. [DOI] [PubMed] [Google Scholar]
- 11.Compostella A, Toffolutti T, Soloni P, et al. Multiple synchronous tumors in a child with Fanconi anemia. J Pediatr Surg. 2010;45:e5–e8. doi: 10.1016/j.jpedsurg.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Cooper JS, Pajak TF, Forastiere M, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 13.D’Souza G, Agrawal Y, Halpern J, et al. Oral sexual behaviors associated with prevalent oral human papillomavirus infection. J Infect Dis. 2009;199:1263–1269. doi: 10.1086/597755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 15.de Araujo M, Rubira-Bullen I, Santos C, et al. High prevalence of oral human papillomavirus infection in Fanconi’s anemia patients. Oral Dis. 2011;17:572–576. doi: 10.1111/j.1601-0825.2011.01803.x. [DOI] [PubMed] [Google Scholar]
- 16.Deans AJ, West SC. DNA interstrand crosslink repair and cancer. Nat Rev Cancer. 2011;11:467–480. doi: 10.1038/nrc3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dewit L. Combined treatment of radiation and cisdiamminedichloroplatinum (II): a review of experimental and clinical data. Int J Radiat Oncol Biol Phys. 1987;13:403–426. doi: 10.1016/0360-3016(87)90015-0. [DOI] [PubMed] [Google Scholar]
- 18.Erkko H, Xia B, Nikkila J, et al. A recurrent mutation in PALB2 in Finnish cancer families. Nature. 2007;446:316–319. doi: 10.1038/nature05609. [DOI] [PubMed] [Google Scholar]
- 19.Fakhry C, Gillison ML. Clinical implications of human papilloinavirus in head and neck cancers. J Clin Oncol. 2006;24:2606–2611. doi: 10.1200/JCO.2006.06.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human pa pillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 21.Furzin A, Davies SM, Smith FO, et al. Matched sibling donor haematopoietic stem cell transplantation in Fanconi anaemia: an update of the Cincinnati Children’s experience. Br J Haematol. 2007;136:633–640. doi: 10.1111/j.1365-2141.2006.06460.x. [DOI] [PubMed] [Google Scholar]
- 22.Giannini SI, Hanon E, Moris P, et al. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (A504) compared to aluminium salt only. Vaccine. 2006;24:5937–5949. doi: 10.1016/j.vaccine.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Flan TJ, Lee CH, Yoe CW, et al. Synchronous multifocal HPV-related neoplasm involving both the genital tract and the head-and-neck area: a case report of Fanconi anemia. Radiother Oncol. 2009;92:138–141. doi: 10.1016/j.radonc.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmann TK, Sonkoly F, Hauser U, et al. Alterations ill the p53 pathway and their association with radio- and chemosensitivity in head and neck squamous cell carcinoma. Oral Oncol. 2008;44:1100–1109. doi: 10.1016/j.oraloncology.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Hosoya V, Lefor A, Hiroshima Y, et al. Successful treatment of esophageal squamous cell carcinoma in a patient with Fanconi anemia. Jpn J Clin Oncol. 2010;40:805–810. doi: 10.1093/jjco/hyq049. [DOI] [PubMed] [Google Scholar]
- 26.Huck K, Hanenberg H, Gudowius S, et al. Delayed diagnosis and complications of Fanconi anaemia at advanced age - a paradigm. Br J Haematol. 2006;133:188–197. doi: 10.1111/j.1365-2141.2006.05998.x. [DOI] [PubMed] [Google Scholar]
- 27.Huck K, Hanenberg H, Nürnberger W, et al. Favourable long -term outcome after matched sibling transplantation for Fanconi anemia (PA) and in vivo T-cell depletion. Kiln Padiatr. 2008;220:147–152. doi: 10.1055/s-2008-1065326. [DOI] [PubMed] [Google Scholar]
- 28.Jerjes W, Upile T, Hamdoon Z, et al. Photodynamic therapy outcome for T1/T2 No oral squamous cell carcinoma. Lasers Surg Med. 2011;43:463–469. doi: 10.1002/lsm.21071. [DOI] [PubMed] [Google Scholar]
- 29.Jones S, Hruban RH, Kamiyama M, et al. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science. 2009;324:217. doi: 10.1126/science.1171202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kero K, Rautava J, Syrjanen K, et al. Human papillomavirus genotypes in male genitalia and their concordance among pregnant spouses participating in the Finnish Family HPV study. J Sex Med. 2011;8:2522–2531. doi: 10.1111/j.1743-6109.2011.02378.x. [DOI] [PubMed] [Google Scholar]
- 31.Kitao H, Takata M. Fanconi anemia: a disorder defective in the DNA damage response. Int J Hematol. 2011;93:417–424. doi: 10.1007/s12185-011-0777-z. [DOI] [PubMed] [Google Scholar]
- 32.Koch FP, Kaemmerer PW, Biesterfeld S, et al. Effectiveness of autofluorescence to identify suspicious oral lesions - a prospective, blinded clinical trial. Clin Oral Investig. 2011;15:975–982. doi: 10.1007/s00784-010-0455-1. [DOI] [PubMed] [Google Scholar]
- 33.Koch FP, Kunkel M, Biesterfeld S, et al. Diagnostic efficiency of differentiating small cancerous and precancerous lesions using mucosal brush smears of the oral cavity - a prospective and blinded study. Clin Oral Investig. 2011;15:763–769. doi: 10.1007/s00784-010-0434-6. [DOI] [PubMed] [Google Scholar]
- 34.Kutler DI, Auerbach AD, Satagopan J, et al. High incidence of head and neck squamous cell carcinoma in patients with Fanconi anemia. Arch Otolaryngol Head Neck Surg. 2003;129:106–112. doi: 10.1001/archotol.129.1.106. [DOI] [PubMed] [Google Scholar]
- 35.Cutler DI, Singh B, Satagopon J, et al. A 20-year perspective on the International Fanconi Anemia Registry (WAR) Blood. 2003;101:1249–1256. doi: 10.1182/blood-2002-07-2170. [DOI] [PubMed] [Google Scholar]
- 36.Kutler DI, Wreesmann VB, Goberdhan A, et al. Human papillomavirus DNA and p53 polymorphisms in squamous cell carcinomas from Fanconi anemia patients. J Natl Cancer Inst. 2003;95:1718–1721. doi: 10.1093/jnci/djg091. [DOI] [PubMed] [Google Scholar]
- 37.Lavaf A, Genden EM, Cesaretti JA, et al. Adjuvant radiotherapy improves overall survival for patients with lymph node-positive head and neck squamous cell carcinoma. Cancer. 2008;112:535–543. doi: 10.1002/cncr.23206. [DOI] [PubMed] [Google Scholar]
- 38.Lee J, Moon C. Current status of experimental therapeutics for head and neck cancer. Exp Biol Med. 2011;236:375–389. doi: 10.1258/ebm.2010.010354. [DOI] [PubMed] [Google Scholar]
- 39.Macfarlane GJ, Zheng T, Marshall JR, et al. Alcohol, tobacco, diet and the risk of oral cancer: a pooled analysis of three case-control studies. Eur J Cancer B Oral Oncol. 1995;31B:181–187. doi: 10.1016/0964-1955(95)00005-3. [DOI] [PubMed] [Google Scholar]
- 40.MacMillan ML, Hughes MR, Agarwal S, et al. Cellular therapy for fanconi anemia: the past, present, and future. Biol Blood Marrow Transplant. 2011;17:5109–5114. doi: 10.1016/j.bbmt.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 41.Marcou Y, D’Andrea A, Jeggo PA, et al. Normal cellular radiosensitivity in an adult Fanconi anaemia patient with marked clinical radiosensitivity. Radiother Oncol. 2001;60:75–79. doi: 10.1016/s0167-8140(01)00370-x. [DOI] [PubMed] [Google Scholar]
- 42.Masserot C, Peffault de Latour R, Rocha V, et al. Head and neck squamous cell carcinoma in 13 patients with Fanconi anemia after hematopoietic stem cell transplantation. Cancer. 2008;113:3315–3322. doi: 10.1002/cncr.23954. [DOI] [PubMed] [Google Scholar]
- 43.Matta A, Ralhan R. Overview of current and future biologically based targeted therapies in head and neck squarnous cell carcinoma. Head Neck Oncol. 2009;1:6. doi: 10.1186/1758-3284-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mayne ST, Cartmel B, Kirsh V, et al. Alcohol and tobacco use prediagnosis and postdiagnosis, and survival in a cohort of patients with early stage cancers of the oral cavity, pharynx, and larynx. Cancer Epidemiol Biomarkers Prev. 2009;18:3368–3374. doi: 10.1158/1055-9965.EPI-09-0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mehrotra R, Gupta DK. Exciting new advances in oral cancer diagnosis: avenues to early detection. Head Neck Oncol. 2011;3:33. doi: 10.1186/1758-3284-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moody CA, Laimins LA. Human papillomavirus oncoproteins: pathways to transformation. Nat Rev Cancer. 2010;10:550–560. doi: 10.1038/nrc2886. [DOI] [PubMed] [Google Scholar]
- 47.Munoz N, Basch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 48.Offit K, Levran O, Mullaney B, et al. Shared genetic susceptibility to breast cancer, brain tumors, and Fanconi anemia. J Natl Cancer Inst. 2003;95:1548–1551. doi: 10.1093/jnci/djg072. [DOI] [PubMed] [Google Scholar]
- 49.Park JW, Pitot HC, Strati K, et al. Deficiencies in the Fanconi anemia DNA damage response pathway increase sensitivity to HPV-associated head and neck cancer. Cancer Res. 2010;70:9959–9968. doi: 10.1158/0008-5472.CAN-10-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pennington KP, Swisher EM. Hereditary ovarian cancer: Beyond the usual suspects. Gynecol Oncol. 2012;124:347–353. doi: 10.1016/j.ygyno.2011.12.415. [DOI] [PubMed] [Google Scholar]
- 51.Psyrri A, Boutati E, Karageorgopoulou S. Human papillomavirus in head and neck cancers: biology, prognosis, hope of treatment, and vaccines. Anticancer Drugs. 2011;22:586–590. doi: 10.1097/CAD.0b013e328344ec44. [DOI] [PubMed] [Google Scholar]
- 52.Reid S, Renwick A, Seal S, et al. Biallelic BRCA2 mutations are associated with multiple malignancies in childhood including familial Wilms tumour. J Med Genet. 2005;42:147–151. doi: 10.1136/jmg.2004.022673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reinhard H, Peters I, Gottschling S, et al. Squamous Cell Carcinoma of the Tongue in a 13-year-old Girl With Fanconi Anemia. J Pediatr Hematol Oncol. 2007;29:488–491. doi: 10.1097/MPH.0b013e318063ef14. [DOI] [PubMed] [Google Scholar]
- 54.Rodriguez T, Altieri A, Chatenoud L, et al. Risk factors for oral and pharyngeal cancer in young adults. Oral Oncol. 2004;40:207–213. doi: 10.1016/j.oraloncology.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 55.Rosenberg PS, Greene MH, Alter BP. Cancer incidence in persons with Fanconi anemia. Blood. 2003;101:822–826. doi: 10.1182/blood-2002-05-1498. [DOI] [PubMed] [Google Scholar]
- 56.Rosenberg PS, Socie G, Alter BP, et al. Risk of head and neck squamous cell cancer and death in patients with Fanconi anemia who did and did not receive transplants. Blood. 2005;105:67–73. doi: 10.1182/blood-2004-04-1652. [DOI] [PubMed] [Google Scholar]
- 57.Rosenquist K, Wennerberg J, Schildt EB, et al. Oral status, oral infections and some lifestyle factors as risk factors for oral and oropharyngeal squamous cell carcinoma. A population-based case-control study in southern Sweden. Acta Otolaryngol. 2005;125:1327–1336. doi: 10.1080/00016480510012273. [DOI] [PubMed] [Google Scholar]
- 58.Scheckenbach K, Morgan M, Filger-Brillinger J, et al. Treatment of the bone marrow failure in Vanconi anemia patients with danazol. Blood Cells Mol Dis. 2012;48:128–131. doi: 10.1016/j.bcmd.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 59.Scheffner M, Huibregtse JM, Vierstra RD, et al. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993;75:495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- 60.Schaffner M, Werness BA, Huibregtse JM, et al. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 61.Seiwert TY, Cohen EE. State-of-the-art management of locally advanced head and neck cancer. Br J Cancer. 2005;92:1341–1348. doi: 10.1038/sj.bjc.6602510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soulieres D, Senzer NN, Vokes EE, et al. Multicenter phase II study of erlotinib, an oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with recurrent or metastatic squamous cell cancer of the head and neck. J Clin Oncol. 2004;22:77–85. doi: 10.1200/JCO.2004.06.075. [DOI] [PubMed] [Google Scholar]
- 63.Spanier G, Pohl F, Giese T, et al. Fatal course of tonsillar squamous cell carcinoma associated with Fanconi anaemia: A mini review. J Craniomaxillofac Surg. 2011 doi: 10.1016/j.jcms.2011.08.013. online Sept. 17. [DOI] [PubMed] [Google Scholar]
- 64.Spardy N, Duensing A, Charles D, et al. The human papillomavirus type 16 E7 oncoprotein activates the Fanconi anemia (FA) pathway and causes accelerated chromosomal instability in FA cells. J Virol. 2007;81:13265–13270. doi: 10.1128/JVI.01121-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spardy N, Duensing A, Hoskins EE, et al. HPV-16 E7 reveals a link between DNA replication stress, fanconi anemia D2 protein, and alternative lengthening of telomere-associated promyelocytic leukemia bodies. Cancer Res. 2008;68:9954–9963. doi: 10.1158/0008-5472.CAN-08-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.St Guily JL, Jacquard AC, Pretet JL, et al. Human papillomavirus genotype distribution in oropharynx and oral cavity cancer in France – The EDITH VI study. J Clin Virol. 2011;51:100–104. doi: 10.1016/j.jcv.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 67.Stepensky P, Shapira MY, Balashov D, et al. Bone marrow transplantation for Fanconi anemia using fludarabine-based conditioning. Biol Blood Marrow Transplant. 2011;17:1282–1288. doi: 10.1016/j.bbmt.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 68.Syrjanen K, Syrjanen S, Lemberg M, et al. Morphological and immunohistochemical evidence suggesting human papillomavirus (HPV) involvement in oral squamous cell carcinogenesis. Int J Oral Surg. 1983;12:418–424. doi: 10.1016/s0300-9785(83)80033-7. [DOI] [PubMed] [Google Scholar]
- 69.Tan IB, Cutcutache I, Zang ZJ, et al. Fanconi’s anemia in adulthood: chemoradiation-induced bone marrow failure and a novel FANCA mutation identified by targeted deep sequencing. J Clin Oncol. 2011;29:e591–e594. doi: 10.1200/JCO.2011.35.1064. [DOI] [PubMed] [Google Scholar]
- 70.Tönnies H, Huber S, Kühl JS, et al. Clonal chromosomal aberrations in bone marrow cells of Fanconi anemia patients: gains of the chromosomal segment 3q26q29 as an adverse risk factor. Blood. 2003;101:3872–3874. doi: 10.1182/blood-2002-10-3243. [DOI] [PubMed] [Google Scholar]
- 71.Tata JE, Chevarie-Davis M, Richardson LA, et al. Epidemiology and burden of HPV infection and related diseases: implications for prevention strategies. Prev Med. 2011;53(Suppl 1):S12–S21. doi: 10.1016/j.ypmed.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 72.van Zeeburg HJ, Snijders PJ, Joenje H, et al. Re: Human papillomavirus DNA and p53 polymorphisms in squamous cell carcinomas from Fanconi anemia patients. J Natl Cancer Inst. 2004;96:968. doi: 10.1093/jnci/djh178. author reply 968–969. [DOI] [PubMed] [Google Scholar]
- 73.van Zeeburg NJ, Snijders PJ, Pals G, et al. Generation and molecular characterization of head and neck squamous cell lines of fanconi anemia patients. Cancer Res. 2005;65:1271–1276. doi: 10.1158/0008-5472.CAN-04-3665. [DOI] [PubMed] [Google Scholar]
- 74.van Zeeburg NJ, Snijders PJ, Wu T, et al. Clinical and molecular characteristics of squamous cell carcinomas from Fanconi anemia patients. J Natl Cancer Inst. 2008;100:1649–1653. doi: 10.1093/jnci/djn366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wagmer JR, Eapen M, Macmillan MI, et al. Unrelated donor bone marrow transplantation for the treatment of Fanconi anemia. Blood. 2007;109:2256–2262. doi: 10.1182/blood-2006-07-036657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wagner JE, Tolar J, Levran O, et al. Germline mutations in BRCA2: shared genetic susceptibility to breast cancer, early onset leukemia and Fanconi anemia. Blood. 2004;103:3226–3229. doi: 10.1182/blood-2003-09-3138. [DOI] [PubMed] [Google Scholar]