Abstract
The rate of progression of chronic kidney disease (CKD) is difficult to predict using single measurements of serum creatinine or proteinuria. On the other hand, documented tubulointerstitial disease presages worsening CKD, but kidney biopsy is not practical for routine use and generally does not sample the tubulointerstitial compartment of the medulla. Perhaps a urine test that correlates with specific histological findings may serve as a surrogate for the kidney biopsy. Here we compared both immunoblot analysis (under non-reducing conditions) and a commercially available monomer immunoassays of Neutrophil Gelatinase Associated Lipocalin (NGAL) with pathological changes found in kidney biopsies, to determine whether specific histological characteristics associated with a specific NGAL species. We found that the urine of patients with advanced CKD contained NGAL monomers as well as higher molecular weight complexes containing NGAL, identified by MALDI-TOF/TOF mass spectroscopy. The NGAL monomer significantly correlated with glomerular filtration rate, interstitial fibrosis and tubular atrophy. Hence, specific assays of the NGAL monomer implicate histology associated with progressive, severe CKD.
Over the next decade the number of patients reaching end stage renal disease (ESRD) will double as a result of the progression of chronic diseases of the kidney (CKD)(1) but the rate of progression is difficult to predict using serum creatinine (sCr) or proteinuria measurements alone. sCr is confounded by age, muscle mass, gender, race, medications, hydration status and extrarenal clearance (2), and progression may occur even in the absence of proteinuria (3). Tubulo-interstitial disease perhaps best presages worsening CKD (3), however, kidney biopsy is not practical for routine use and generally does not sample medullary tubules. Here, we evaluated relationships between a urinary protein and biopsy findings in a CKD cohort.
Neutrophil Gelatinase Associated Lipocalin (NGAL) gene product (“monomeric” Lcn2, Siderocalin 23–26KDa protein (4)) is induced by the triggers of acute kidney injury (AKI)(4–6). NGAL is also induced by actively progressive CKD (7), advancing HIVAN (8), and end stage (9) in the absence of an acute event. It is important to correlate the specific molecular form of NGAL with kidney histology, not only to determine whether it serves as a reporter, but also to determine whether a specific form might participate in the growth of damaged tubules (9) or the defense of the urinary tract by scavenging iron (10).
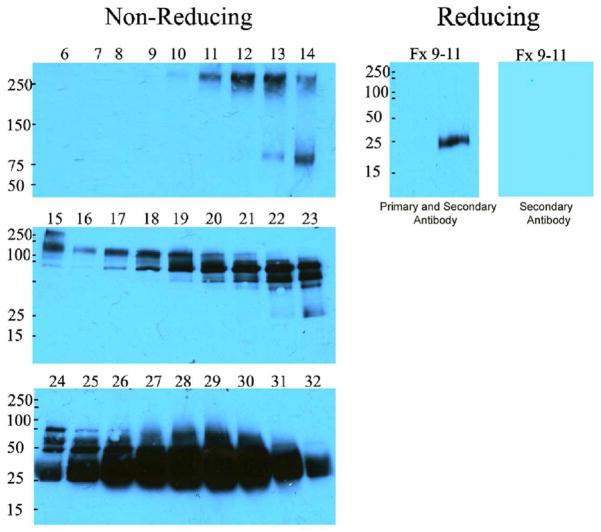
To determine the molecular form of NGAL, we analyzed the urine of 99 patients by immunoblot and found a recurrent pattern of immunoreactivities. The majority of NGAL was “monomeric”, but additional reactivities were found at >250, 125, 75KDa in non-reducing gels. The 75KDa species was potentially trimeric NGAL, and the 125KDa species was potentially NGAL-MMP-9 (11) but the >250KDa immunoreactive band was a novel complex. To identify this protein, we fractionated the urine of a Stage 5 patient using cation-exchange and gel filtration. Mass methodology identified the >250KDa complex as the secretory component of the polymeric immunoglobulin receptor (peptides: LVSLTLNLVTR; ILLNPQDKDGSFSVVITGLR; QGHFYGETAAVYVAVEER) together with α2-macroglobulin. Upon reduction, monomeric NGAL dissociated from the complex (Figure 1).
Figure 1.
Urine was fractionated by cation exchange chromatography and fractions containing immunoreactive NGAL species were then separated by filtration chromatography (Left Panels). Note that the monomer (23–26KDa, fractions 24–32) comprised the majority of immunoreactive NGAL, but additional species can be found at 75KD (fractions 19–23), 125KDa (fractions 15–18) and >250KDa (fractions 9–11). When the latter were pooled and reduced, the only immunoreactive species was the monomer (Right). These data show that in advanced CKD, a proportion of NGAL is associated with other proteins.
Because the monomeric form of NGAL has been specifically associated with epithelial stress (4, 5), we decided to quantify this species with ARCHITECT-NGAL (Abbott) assays (6) which correlated with immunoblot detection of the monomer (r =0.69, p<0.001), demonstrating the same rank order, and producing nearly identical statistical differences (ANOVA) across different diagnoses (<0.05, ARCHITECT-NGAL and <0.01, immunoblot). The ARCHITECT-NGAL demonstrated that 3.8% (SD 7.7%) of the immunoreactivity was >100KDa, but with variable contribution (0–41.9%).
Accurate quantification of the monomer permitted a comparison with clinical and pathological characteristics in the concurrent biopsy (Supplemental Table 1). Rapidly progressive glomerulonephritis (n=3), dialysis (n=10), and AKI were excluded at the time of enrollment. Six percent of our cohort had been on steroids prior to biopsy. On average, the cohort was male (70%), age 52.2 years (SD 16.8) with a GFR 57.7 mL/min (SD 34.5); hypertension was common (69.7%, mean 138.7 mmHg SD 22.0 / 81.9 mmHg SD 11.3) but diabetes was present in only 18.2%. Pathological diagnoses were diverse: Nephrotic syndromes (49.5%: membranous, focal segmental glomerulosclerosis, minimal change disease and amyloidosis), nephritic syndromes (31%: IgA and lupus nephropathies, membranoproliferative and mesangial proliferative glomerulonephridites, fibrillary and immunotactoid nephritis), diabetic nephropathy (10%), and other diagnoses (9%: nephroangiosclerosis, ESRD prior to dialysis initiation, light chain nephritis, myeloma, and isolated chronic tubulo-interstitial nephritis).
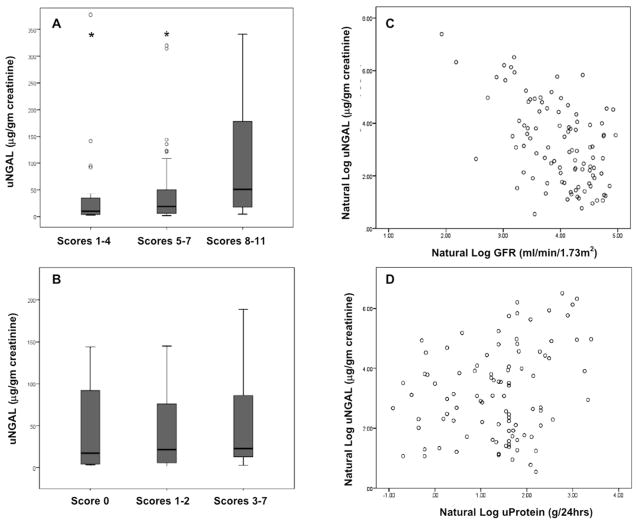
While the monomer appeared most elevated in diabetics (Supplemental Table 1), statistical significance was demonstrated by measures of disease chronicity rather than specific diagnoses. For example, the monomer correlated inversely with GFR (r=−0.53, p<0.001) and directly with chronicity indices (p=0.003) in a dose-responsive relationship (Figure 2). Additionally, the monomer specifically associated with both tubular atrophy (p=0.002) and interstitial fibrosis (p=0.006). Moderate or severe fibrosis or atrophy were associated with 3–4 fold higher levels of NGAL monomer (each p<0.01) compared to mild or absent fibrosis or atrophy (Table1, Figure 3). Similarly, fibrous crescents (p=0.001) and global glomerulosclerosis (p=0.037) were highly correlated with the monomer, both suggestive of chronic damage. For example, 96% of patients with fibrous crescents had some degree of TI disease, 35% moderate or severe. 99% of patients with global glomerulosclerosis demonstrated tubulointerstitial disease, 44% of which had moderate or severe levels. Diabetics may have somewhat higher levels of NGAL because 75% demonstrated moderate to severe interstitial fibrosis (vs. nephrotic syndrome (26%), p=0.002; vs. nephritic syndrome (9%), p=0.002) and tubular atrophy (vs. nephrotic syndrome (28%), p=0.001; vs. nephritic syndrome (16%), p=0.002) in excess of other forms of CKD, but without significant differences in GFR. The monomer also associated with proteinuria (r=0.27, p=0.008), albeit the association weakened upon adjustment for GFR (r=0.20, p=0.06).
Figure 2.
Urinary NGAL (uNGAL) monomer according to (A) chronicity and (B) activity indices of kidney biopsies concurrent with the urine samples. * P<0.01 compared to scores 8–11 in panel A. Scatter plot of uNGAL monomer according to GFR (C), r=−0.53, p<0.001, and proteinuria (D), r=0.27, p=0.008.
Table 1.
Relationship between clinical characteristics, GFR, proteinuria, NGAL-monomer and chronic histologic characteristics.
| Global Glomerulosclerosis | Atherosclerosis | Interstitial Fibrosis | Tubular Atrophy | |||||
|---|---|---|---|---|---|---|---|---|
| None (n=32) | Present (n=67) | None Mild (n=42) | Moderate Severe (n=57) | None Mild (n=68) | Moderate Severe (n=31) | None Mild (n=68) | Moderate Severe (n=31) | |
| Female (%) | 34.4 | 26.9 | 35.7 | 24.6 | 32.4 | 22.6 | 33.8 | 19.4 |
| Age (yrs) | 48.1 (17.9) | 52.4 (16.3) | 46.0 (16.9) | 55.9 (15.8) | 49.6 (17.3) | 56.2 (15.4) | 48.8 (16.7) | 58.1 (15.7) |
| HTN (%) | 50* | 74.6 | 52.4* | 77.2 | 54.4** | 93.5 | 55.9** | 90.3 |
| Diabetes (%) | 12.5 | 16.4 | 7.1 | 21.1 | 7.4** | 32.3 | 7.4** | 32.3 |
| PCreatinine (mg/dl) | 1.1 (0.5)** | 1.9 (1.3) | 1.2 (0.5)** | 2.0 (1.4) | 1.3 (0.6)** | 2.5 (1.5) | 1.3 (0.7)** | 2.4 (1.6) |
| eGFR(ml/min) | 81.7 (31.6)** | 53.2 (29.1) | 78.6 (31.5)** | 50.1 (28.2) | 73.4 (30.3)** | 38.4 (23.5) | 72.1 (31.5)** | 41.3 (24.2) |
| Proteinuria (g/24hr) | 7.0 (6.3) | 5.6 (5.8) | 5.9 (6.0) | 6.2 (6.0) | 5.5 (5.0) | 7.3 (7.8) | 5.7 (5.8) | 6.8 (6.5) |
| Mean uNGAL (μg/gr creatinine) | 53.4 (101.1)* | 105.8 (232.0) | 49.9 (80.1) | 117.8 (252.3) | 54.1 (111.0)** | 167.1 (309.8) | 54.1 (112.0)** | 163.6 (305.2) |
p<.05 compared to present
p<.01 compared to present.
Figure 3.
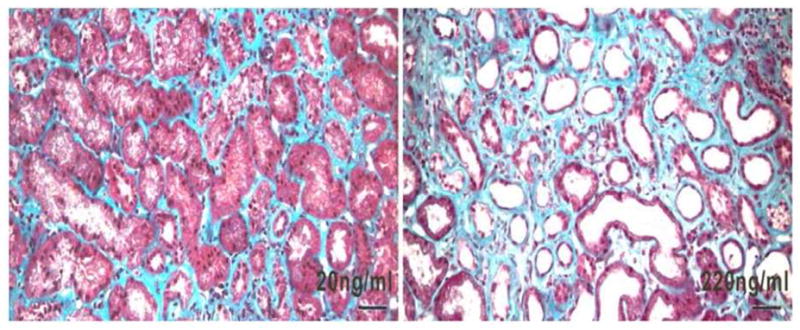
Illustrative kidney biopsies of two patients with membranous nephropathy. Urinary NGAL levels are indicated. Note the widened interstitial compartments, tubular flattening and tubular dilation in the more advanced case which expresses a ten fold higher level of NGAL. Mason trichrome stain. Bar=50μm.
In contrast, the monomer was not associated with activity indices of glomerular disease (p=0.847, Figure 2) including glomerular leukocyte infiltrates (p=0.34), mesangial proliferation (p=0.616), cellular crescents (p=0.569), fibrinoid necrosis (p=0.168), nor strongly with monocellular-predominant interstitial infiltrates (p=0.056), while there was an association with mesangial matrix expansion (p=0.004) implying that the monomer was better associated with chronic rather than acute cellular changes.
Neutrophils and macrophages may be present in the interstitium and contribute to NGAL but our pathological analysis failed to associate monomer with interstitial cellular infiltrates including the dominant lympho-monocytic interstitial infiltrate. Additionally, iso-electric point analysis of DTT reduced samples demonstrated similar iso-electric points for monomeric NGAL in patients with AKI or CKD (pI 7.1, 8.2, 8.5, 8.8–9.2), whereas neutrophil NGAL demonstrated a different pattern of alkaline shifted species (pI 8.5–9.2). Urinary myeloperoxidase, a protein released from neutrophils, also did not correlate with NGAL monomer in the current (R2=0.087) or in a previously identified cohort (R2=0.0092) whereas NGAL and myeloperoxidase were highly correlated in isolated blood neutrophils (R2=0.99).
In summary, while the monomer of NGAL was the predominant, if not the only form expressed in AKI, known and novel complexes formed by disulfide linkages were found in CKD urine. These complexes might form in Thick Ascending Limb or in the urinary space because both the extracellular secretory component of polymeric IgR (68KDa) and NGAL are secreted from the luminal membrane of the TAL (12). α-2-macroglobulin can also be found in CKD urine (13) whereas its receptor LRP1/CD91 is found in the tubulo-interstitial compartment where it may concentrate and endocytose α2-macroglobulin (14). Perhaps these transport proteins provide a clearance mechanism, rather than directly depict epithelial damage. In this light, the site of complex formation might clarify their clinical significance, as recently demonstrated by the localization of dimer to neutrophils, and monomer to epithelia (15).
Most importantly, this study showed that monomer reflects the severity of tubulo-interstitial, rather than glomerular specific pathologies (3). Tubulointerstitial genes (Scara5, Col6a1-3, Nfix, Acvrl1, and myogenic proteins; Yang, Schmidt-Ott, Barasch, Unpublished) induced by NGAL in rat kidney mesenchyme are compatible with this hypothesis. These data are novel because they suggest that the monomer is expressed in many common forms of advanced CKD, whereas previously characterized patterns of NGAL expression were disease restricted such as the HIVAN microcyst (8) and the TAL and α-intercalated cell in AKI (5). Consequently, we propose that the association of NGAL with kidney disease may be better appreciated by specific measurements of monomer, since NGAL complexes >100KDa contribute variably to total immunoreactivity and potentially involve proteins from non-renal cell types. The same type of molecular analysis applies to other urinary ‘biomarkers’ as well.
METHODS
The University of Parma and Columbia approved the study; informed consent was obtained. Patients (1/2005 – 4/2008) were >18 yrs. Urine was centrifuged (12,000rpm × 10min), stored (between 2 months-3 years at −80°C) and analyzed in batch with non-reducing 4% to 15% polyacrylamide gels (Bio-Rad Laboratories, Hercules, CA) and monoclonals (1:1000; AntibodyShop, Gentofte, Denmark) using standards (0.3 to 3ng) of human NGAL by workers blinded to biopsy data. ARCHITECT-NGAL assays were performed at Cincinnati Children’s Hospital. Immunoreactivity was evaluated by filtering the urine (Microcon Ultracel YM-100, 100kDa filter; Millipore Corporation), or by MiniS (Mes 20mM, pH6.0 + gradient of NaCl, 0.5M) and Superdex200 (PBS) chromatography. NGAL containing complexes were digested with trypsin, batch fractionated on a Poros 50 R2 RP micro-tip, and the resulting peptide pools analyzed by MALDI-reTOF, using a BRUKER UltraFlex TOF/TOF instrument (Bruker Daltonics; Bremen, Germany) (16). Selected experimental masses (m/z) were taken to search human non-redundant protein database (NCBI) utilizing the Mascot Peptide Mass Fingerprint (17), version 2.3.01, with a mass accuracy restriction better than 35 ppm, and maximum allowed one cleavage site missed per peptide. To confirm PMF results with scores <40, mass spectrometric sequencing of selected peptides was done by MALDI-TOF/TOF (MS/MS) re-analysis, using the UltraFlex instrument in ‘LIFT’ mode. Fragment ion spectra were taken to search NR using Mascot MS/MS Ion Search program (17). Iso-electric focusing (Kendrick Labs, Madison, WI; (18) utilized PVDF immunoblotting.
Kidney biopsies (obtained as part of routine care in all subjects at the time of urine sampling) were formalin-fixed, paraffin embedded, and sections stained with hematoxylin/eosin, Masson’s trichrome stain, silver methenamine and periodic-acid Schiff. IgG, IgM, IgA, C3 and fibrinogen deposition were detected by immunoflourescence. Biopsies were graded by two independent pathologists blinded to both diagnosis and NGAL level. Glomerular, tubular, interstitial and vascular lesions were scored as 0, absent; 1, mild; 2, moderate; 3, severe.
The sensitivity of myeloperoxidase ELISA (Alpco. Co) was determined using serial dilutions of neutrophils isolated from citrated blood (19). While the ELISA was sensitive to a 0.01X dilution, immunoblot detection of NGAL was consistent only to 0.1X, indicating that detection of myeloperoxidase compared favorably with NGAL. GFR was estimated by MDRD formula (20), uCr measured by QuantiChrom Creatinine Assay Kit (BioAssay Systems), sCr by Jaffe, uProtein by nephelometry.
Statistical analysis (SPSS v16.0, Chicago)
Continuous variables were log-transformed and compared by Student’s t-test for unequal variances or ANOVA. Categorical variables were compared by χ2. The null hypothesis was rejected at p<0.05. Data were represented as the mean ± standard deviation (SD).
Supplementary Material
Mean values and cohort baseline characteristics by etiology of CKD, including urinary NGAL monomer.
Acknowledgments
We thank Lynne Lacomis for help with mass spectrometry and sample preparation. Supported by the Emerald Foundation, the March of Dimes, the Doris Duke Foundation, the National Center for R esearch Resources, a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research and the NCI Cancer Center Support Grant P30 CA08748. The contribution of Gabriella Fanti in managing the sera and urine bank at Parma University is gratefully acknowledged.
Footnotes
DISCLOSURES
The funding sources had no role in study design, data collection, analysis, interpretation or presentation. College of Physicians and Surgeons of Columbia University, New York, New York and Cincinnati Children’s Hospital, Cincinnati, Ohio have licensed NGAL to Abbott Labs and to Biosite-Alere. Dr. Devarajan has received lecture honoraria from Abbott and Biosite-Alere. Dr. Nickolas has a consultation agreement with Abbott.
References
- 1.Gilbertson DT, Liu J, Xue JL, Louis TA, et al. Projecting the number of patients with end-stage renal disease in the United States to the year 2015. J Am Soc Nephrol. 2005;16:3736–3741. doi: 10.1681/ASN.2005010112. [DOI] [PubMed] [Google Scholar]
- 2.Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38:1933–1953. [PubMed] [Google Scholar]
- 3.Remuzzi G, Ruggenenti P, Benigni A. Understanding the nature of renal disease progression. Kidney Int. 1997;51:2–15. doi: 10.1038/ki.1997.2. [DOI] [PubMed] [Google Scholar]
- 4.Nickolas TL, O’Rourke MJ, Yang J, Sise ME, et al. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med. 2008;148:810–819. doi: 10.7326/0003-4819-148-11-200806030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paragas N, Qiu A, Zhang Q, Samstein B, et al. The Ngal reporter mouse detects the response of the kidney to injury in real time. Nat Med. 17:216–222. doi: 10.1038/nm.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nickolas TLKS-O, Canetta P, Forster C, Singer E, Sise M, Elger A, Maarouf O, Valle D Sola-Del, O’Rourke M, Sherman E, Lee P, Geara A, Imus P, Guddati A, Polland A, Rahman W, Elitok S, Malik N, Giglio J, El-Sayegh S, Devarajan P, Hebbar S, Saggi SJ, Hahn B, Kettritz R, Lutz FC, Barasch J. Diagnostic and Prognostic Stratification in the Emergency Department Using Urinary Biomarkers of Nephron Damage: A Multicenter Prospective Cohort Study. Journal of the American College of Cardiology. 2012:59. doi: 10.1016/j.jacc.2011.10.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolignano D, Lacquaniti A, Coppolino G, Donato V, et al. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:337–344. doi: 10.2215/CJN.03530708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paragas N, Nickolas TL, Wyatt C, Forster CS, et al. Urinary NGAL marks cystic disease in HIV-associated nephropathy. J Am Soc Nephrol. 2009;20:1687–1692. doi: 10.1681/ASN.2009010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viau A, El Karoui K, Laouari D, Burtin M, et al. Lipocalin 2 is essential for chronic kidney disease progression in mice and humans. J Clin Invest. 120:4065–4076. doi: 10.1172/JCI42004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goetz DH, Holmes MA, Borregaard N, Bluhm ME, et al. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002;10:1033–1043. doi: 10.1016/s1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]
- 11.Yan L, Borregaard N, Kjeldsen L, Moses MA. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J Biol Chem. 2001;276:37258–37265. doi: 10.1074/jbc.M106089200. [DOI] [PubMed] [Google Scholar]
- 12.Rice JC, Spence JS, Megyesi J, Goldblum RM, et al. Expression of the polymeric immunoglobulin receptor and excretion of secretory IgA in the postischemic kidney. Am J Physiol. 1999;276:F666–673. doi: 10.1152/ajprenal.1999.276.5.F666. [DOI] [PubMed] [Google Scholar]
- 13.van Goor H, Diamond JR, Ding G, Kaysen G. Alpha macroglobulins and the low-density-lipoprotein-related protein/alpha-2-macroglobulin receptor in experimental renal fibrosis. Exp Nephrol. 1999;7:35–43. doi: 10.1159/000020582. [DOI] [PubMed] [Google Scholar]
- 14.Lorent K, Overbergh L, Delabie J, Van Leuven F, et al. Distribution of mRNA coding for alpha-2-macroglobulin, the murinoglobulins, the alpha-2-macroglobulin receptor and the alpha-2-macroglobulin receptor associated protein during mouse embryogenesis and in adult tissues. Differentiation. 1994;55:213–223. doi: 10.1046/j.1432-0436.1994.5530213.x. [DOI] [PubMed] [Google Scholar]
- 15.Cai L, Rubin J, Han W, Venge P, et al. The origin of multiple molecular forms in urine of HNL/NGAL. Clin J Am Soc Nephrol. 5:2229–2235. doi: 10.2215/CJN.00980110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sebastiaan Winkler G, Lacomis L, Philip J, Erdjument-Bromage H, et al. Isolation and mass spectrometry of transcription factor complexes. Methods. 2002;26:260–269. doi: 10.1016/S1046-2023(02)00030-0. [DOI] [PubMed] [Google Scholar]
- 17.Perkins DN, Pappin DJ, Creasy DM, Cottrell JS. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999;20:3551–3567. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 18.O’Farrell PH. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- 19.Kjeldsen L, Bainton DF, Sengelov H, Borregaard N. Identification of neutrophil gelatinase-associated lipocalin as a novel matrix protein of specific granules in human neutrophils. Blood. 1994;83:799–807. [PubMed] [Google Scholar]
- 20.Levey AS, Bosch JP, Lewis JB, Greene T, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Mean values and cohort baseline characteristics by etiology of CKD, including urinary NGAL monomer.