Biomolecules, including proteins, peptides and vaccines, make up a large and potent part of all new drugs and hold great promise for the future of therapeutics [1, 2]. Although oral delivery of these biotherapeutics would be desirable, there is low bioavailability of biomolecules administered by this route due to enzymatic degradation and poor absorption in the GI tract, as well as first-pass metabolism of the liver [3]. As a result, most biotherapeutics are administered by hypodermic injection, which causes pain, can lead to infection, requires trained personnel and often needs frequent, repeated injections for the patient. Consequently, there exists the need for a minimally invasive, self-administered delivery system for biomolecules.
An attractive non-invasive option is the transdermal patch, which has become well-received for the delivery of nicotine, estrogens and other drugs [4]. However, delivery across intact skin permits transport only of small, lipophilic molecules and excludes transport of biotherapeutics, due to their large size.
This study presents a novel, hybrid delivery approach to achieve the delivery efficacy of injections and the safety and patient compliance of the patch. We designed and synthesized rapidly dissolving polymer needles of micron dimensions for the painless, self-administered delivery of biomolecules. In this design, the drug is encapsulated within these polymer microneedles, and after insertion into the skin, the biocompatible polymer dissolves within minutes to release the encapsulated cargo and leave behind no biohazardous sharps or need for removal.
Previous work has shown that microscopically piercing the skin with micron-scale needles offers an effective and convenient alternative for the delivery of biomolecules, due to the efficient delivery [5, 6], lack of pain [7–9], ease of use and the expected low cost of fabrication. Microneedles have been shown to deliver proteins, DNA and vaccines in vivo using devices small enough to be integrated into a low-profile, self-administered patch [9–11].
To date, most microneedles have been made of silicon or metal [12, 13] with little work involving polymers [14–16]. There are, however, safety concerns if microneedles made of these materials were to break off in the skin, or if they were accidentally or intentionally reused. In contrast, the use of biocompatible polymers could eliminate these concerns, because the needles completely and safely dissolve within the skin, and the needle free patch backing could be safely discarded, leaving no biohazardous sharps.
Achieving this goal presents significant material challenges. The ideal polymer material would be strong enough to penetrate the skin, dissolve rapidly once in the skin, and be safely excreted by the body. Also, the fabrication process for these microneedles should take place at ambient temperatures, without organic solvents, and avoid damaging fragile biomolecules during encapsulation. No current design allows for polymer microneedles to be fabricated in this manner. Previous studies have relied on either high-temperature molding processes that risk damaging biomolecules [15, 16] or methods unsuitable for fabrication of micron-scale structures [14].
In this study, we have developed the first rapidly dissolving polymer microneedles. This advance required developing a new fabrication process to produce mechanically robust microneedles that encapsulate biomolecules under gentle processing conditions using methods suitable for inexpensive mass production. Here, we detail the new fabrication process, based on room temperature in situ polymerization, and study the mechanical, encapsulation, dissolution and delivery properties of the resulting polymer microneedles for the delivery of biomolecules to the skin.
To develop rapidly dissolving polymer microneedles, we first prepared master structures made of a polymeric photoresist epoxy (SU-8) by a photolithography method, from which we created reverse molds out of polydimethylsiloxane (PDMS) (See Methods Section). Each master structure was able to be copied into hundreds of molds, and each mold was able to be reused to produce at least a dozen microneedle arrays. PDMS was chosen as the mold material because it is flexible, lacks surface adhesion to the master structure and allows for the removal of the polymer microneedle array. These microneedle molds were then used to fabricate replicate microneedles by a new microfabrication process developed in this study, which involves the room-temperature photopolymerization of a liquid monomer within the microneedle mold. The gentle nature of this process allows for the encapsulation of biomolecules within the microneedles, and its universality allows for the formation of a multitude of polymers and copolymers as the structural material of the needles. We believe that this is the first example of an in situ polymerization of microneedles, and represents a novel approach that could be broadly applied to in situ polymerization of other microstructures as well.
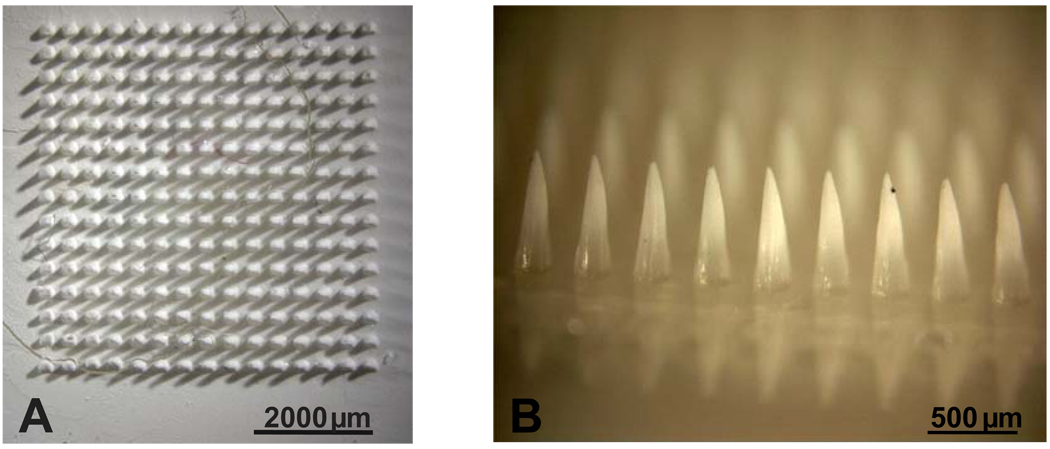
We chose to synthesize microneedles by polymerizing monomeric vinyl pyrrolidone using ultraviolet light. The resulting polyvinylpyrrolidone (PVP) microneedles are shown in Figures 1A and 1B. We used PVP as the structural material for microneedles for four reasons. First, the chemical backbone structure of the vinyl pyrrolidone monomer contains a ring, which increases intramolecular rigidity and thereby provides mechanical strength to the polymer, which is important for microneedle insertion into skin. Second, PVP has high water solubility, which facilitates rapid dissolution once inserted into the skin. Third, PVP already has a long history of clinical use as a blood plasma expander [17, 18]. Finally, the vinyl pyrrolidone monomer is liquid at ambient conditions, which facilitates processing at mild temperatures without the need for an organic solvent to fill the microneedle mold.
Figure 1. PVP polymer microneedles made by new in situ polymerization process.
(A) Overhead view and (B) side view of pure PVP microneedles. (C) Overhead view and (D) side view of PVP polymer microneedles with sulforhodamine encapsulated within microneedles, but not in the base substrate. Each microneedle measures 750 µm in height, 250 µm in base diameter and 5 µm in tip radius.
Using this approach, microneedles were produced to have a range of micron-scale feature sizes, depending on the mold geometry. For example, the conical microneedles shown in Figure 1 measure 750 µm in length, 250 µm in diameter at the base and 5 µm in radius at the tip. These microneedles represent an excellent reproduction of the geometry of the master structure and the micromolds used to prepare them (data not shown). As discussed below, this in situ micromolding approach produced similarly faithful reproduction results when creating microneedles of pyramidal geometry, microneedles using a mixture of monomers to produce a copolymer structural material, and when encapsulating model drugs within the microneedles.
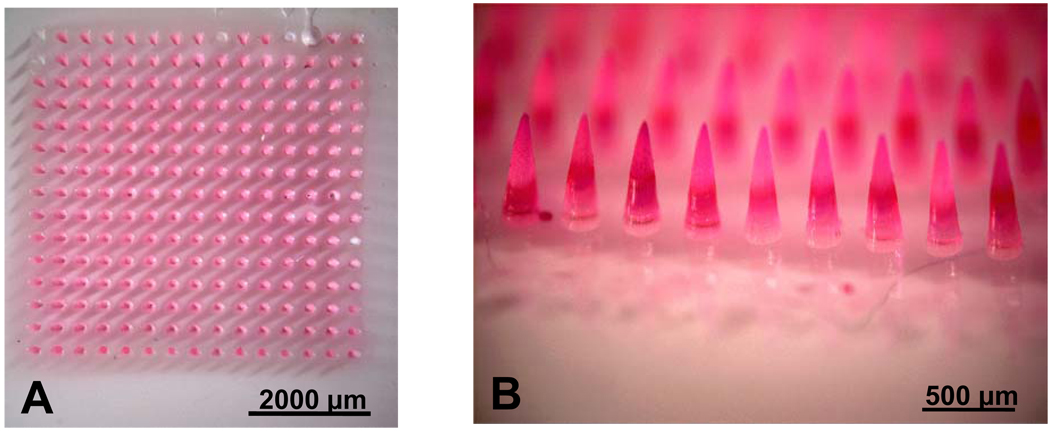
For the first generation of microneedles produced by this new fabrication process, both the microneedles and their base substrate are made of the same PVP polymer. Using this process to encapsulate a drug within the microneedles would result in the drug being distributed throughout the microneedles and the base. However, any drug encapsulated in the base would not be efficiently delivered into the skin because only the microneedles insert into the skin. Thus, an adaptation is required to encapsulate the drug exclusively within the microneedles. In this adaptation, after filling the mold with the monomer and drug mixture, all liquid on the base of the mold is carefully pipetted off, leaving liquid only in the cavities of the mold to form the microneedles. Then, a liquid monomer solution with no suspended drug is placed on the mold to form the base substrate and the setup is placed under ultraviolet light where photopolymerization takes place. This produces microneedles with drug exclusively encapsulated within the microneedles and not the base. Figures 1C and 1D show a representative PVP microneedle array with sulforhodamine encapsulated only within the microneedles, which have the same sharpness as the PVP microneedles shown in Figures 1A and 1B. This adaptation is especially important when delivering expensive biomolecules and in scenarios where precise dosing is required.
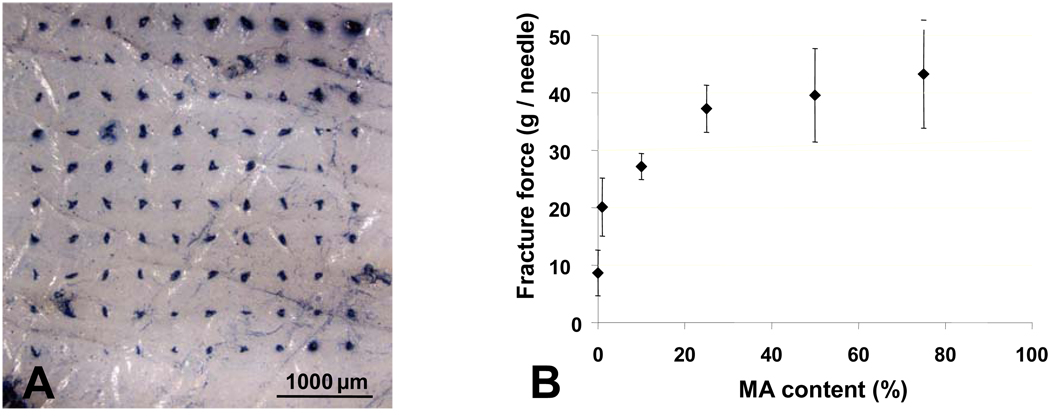
PVP microneedles are hypothesized to be sharp and strong enough to insert into the skin without breaking. We tested this hypothesis by inserting 100-microneedle arrays into porcine skin in vitro and then staining the skin after removing the microneedles to identify the sites of insertion. Figure 2A shows a representative image of the skin surface after microneedle insertion and staining. This image shows that all 100 microneedles inserted into the skin. Subsequent microscopic examination of the microneedles showed that the needles were not broken or deformed during the insertion process (data not shown.)
Figure 2. Insertion capabilities and mechanical properties of polymer microneedles.
(A) Evidence of insertion of PVP polymer microneedles into porcine cadaver skin via skin marking test (B) The mechanical strength (fracture force) of copolymer PVP-MAA microneedles increases with increasing methacrylic acid (MAA) content.
In addition, it is important to determine the microneedle dissolution kinetics in order to know the length of time the microneedles need to be left in skin prior to removal of the base. The dissolution kinetics of PVP microneedles were measured by inserting the needles into porcine skin in vitro and inspecting them after removal, which showed that the entire PVP microneedle array was dissolved in the skin within one minute (data not shown).
Although PVP microneedles are strong enough to insert into skin and then rapidly dissolve within the skin, it could be important to increase microneedle mechanical strength, prolong dissolution time, or otherwise tune microneedle properties for specific needs. To achieve this control over microneedle properties, we fabricated microneedles by copolymerizing two liquid monomers – vinyl pyrollidone (VP) and methacrylic acid (MAA) – to form poly(vinylpyrrollidone-co-methacrylic acid) (PVP-MAA). We chose MAA as the second monomer because it is nontoxic, is liquid in monomeric form, has been used in the past for drug delivery purposes and has a high mechanical strength due to the rigidity of its chemical backbone [19]. In addition, a copolymer of PVP-MAA could have additional mechanical strength from hydrogen bonding between the side chains of the VP and MAA monomeric units of the polymer [20]
As shown in Figure 2B, the mechanical strength (fracture force) of the copolymer PVP-MAA containing just 1% MAA is nearly double the mechanical strength of the homopolymer PVP and increases as the methacrylic acid content is increased (ANOVA, p<0.001), such that PVP-MAA microneedles containing 25% MAA exhibit almost a four-fold increase in strength. Still greater MAA content did not significantly increase the mechanical strength (ANOVA, p>0.05). Stronger polymer microneedles could be advantageous for drug delivery to tougher tissue sites of the body where insertion is more difficult.
In addition, dissolution studies showed that the dissolution rate decreases with increasing MAA content, such that PVP-MAA microneedles containing 25% MAA dissolve after approximately 2 hours within porcine skin in vitro (data not shown). Polymer microneedles with fast dissolution rates would be attractive for rapid delivery scenarios, such as vaccinations, where microneedles can be inserted, removed, and discarded without making the patient wait. Polymer microneedles with slower, controlled dissolution rates could be desirable for situations where controlled release of a drug over time is optimal. In this scenario, a microneedle patch could be held in place on the skin with an adhesive layer, similar to ones used by conventional transdermal patches. Alternatively, these slower dissolving microneedles could be designed in the future to quickly deposit within the skin by separating the base from the microneedles, which then dissolve slowly over time within the skin.
Concerning safety, gel permeation chromatography analysis of PVP microneedle dissolution products determined that the average molecular mass of PVP is 8,970 Da with a polydispersity of 1.42. Given that PVP with molecular mass less than 20,000 Da has been shown to be safe for human use due to efficient clearance by the kidney after subcutaneous injection [17], the low measured molecular mass suggests that PVP microneedle dissolution products can be safely excreted from the body. In addition, microneedles of various designs have been shown to cause little or no pain and to be well tolerated by human subjects [7, 9]. Microneedles with geometries similar to those used in this study have also been shown to cause minimal pain[8].
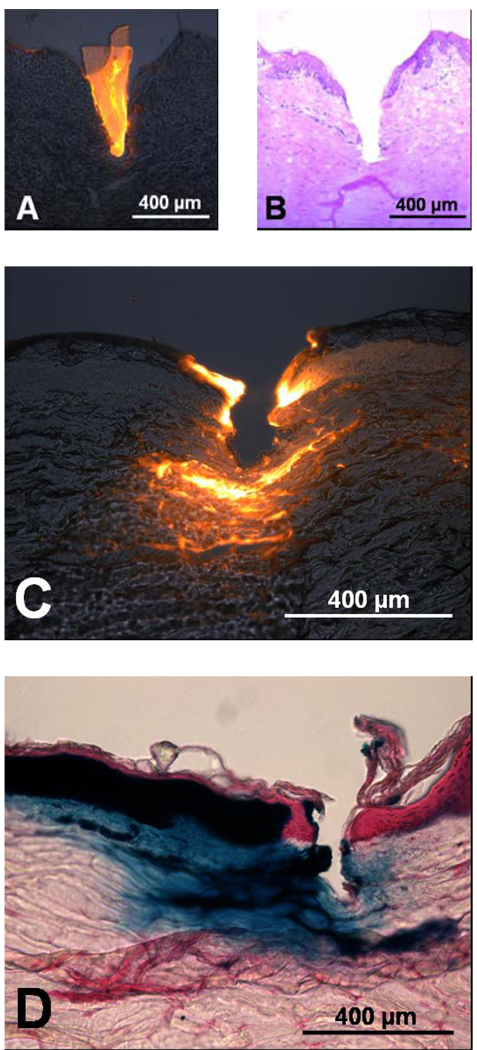
As shown in Figure 2A, PVP microneedles are sharp and strong enough to insert into the skin. However, this assay does not determine the depth of insertion. Due to the elastic nature of the skin, even microneedles which are strong and sharp enough to insert will first deform the skin surface prior to insertion. Since delivery from these polymer microneedles requires needle dissolution within the skin to release the encapsulated cargo, it is important to determine the depth of insertion. In addition, it could be beneficial to deliver drugs to specified depths within the skin, for example targeting dendritic Langerhans cells found in the epidermis for vaccination purposes [21]. To determine the depth of insertion, polymer microneedles were inserted into porcine cadaver skin in vitro, and histological sections were processed from the frozen samples. Figure 3A shows a cross section of skin after insertion of a PVP microneedle with encapsulated sulforhodamine. Figure 3B shows the same tissue sample after needle removal and H+E staining to visualize the layers of the skin and the hole left by microneedle insertion. The 750 µm-long microneedles inserted almost completely into the skin, which suggests that the entire drug encapsulated within would be efficiently delivered.
Figure 3. Microneedle insertion and protein delivery into skin.
(A) Fluorescence microscopy image of a PVP polymer microneedle with encapsulated sulforhodamine inserted into porcine skin. (B) Brightfield microscopy image of the same skin section after microneedle removal showing the depth of microneedle insertion, stained with hemotoxilin and eosin. (C) Fluorescence microscopy image showing delivery of fluorescently labeled bovine serum albumin by PVP polymer microneedles to porcine skin. (D) Brightfield microscopy image of delivery of enzymatically active β-galactosidase via PVP polymer microneedles to porcine skin. The blue color represents the enzymatic conversion of X-gal by the delivered β-galactosidase
The ultimate goal of this study was to produce polymer microneedles that can successfully encapsulate and deliver active biomolecules. To assess this objective, red-fluorescent bovine serum albumin was encapsulated within PVP polymer microneedles and delivered to porcine skin. Figure 3C shows a histological section prepared 15 min after microneedle insertion. The fluorescent protein has been delivered to both the dermis and epidermis and it has diffused a short distance away from the insertion site. This demonstrates the ability of the new polymer microneedles to deliver a biomolecule to the skin.
To assess if biomolecules can retain activity after encapsulation within polymer microneedles, we encapsulated another model protein, β-galactosidase, in PVP microneedles; dissolved them in PBS; and measured enzymatic activity of the resulting solution. The normalized activity of β-galactosidase after encapsulation and release from polymer microneedles was 0.99±0.01, (n=5) which was statistically indistinguishable from (i) a solution of β-galactosidase in PBS (1.00±0.00) and (ii) a solution of β-galactosidase in PBS containing dissolved PVP from empty microneedles (0.99±0.01). This demonstrates that the in situ polymerization fabrication and microneedle dissolution processes are gentle enough to retain the activity of an encapsulated biomolecule. As further validation of this result, Figure 3D shows a histological section of porcine skin after delivery of β-galactosidase from PVP microneedles and exposure to X-gal. The enzymatic conversion of the X-gal substrate by β-galactosidase to its blue-colored product demonstrates that the β-galactosidase delivered into the skin is enzymatically active.
These findings suggest that rapidly dissolving polymer microneedles offer an exciting new drug delivery alternative to the hypodermic needle. They combine the painless, self-administrative abilities of the transdermal patch with the ability to deliver biotherapeutics, which is only possible in current clinical practices using hypodermic needles in most cases. The polymer microneedles created by the new in situ polymerization fabrication process developed in this study dissolve within the skin within a minute, thereby delivering the encapsulated cargo and leaving behind no biohazardous sharps associated with dirty needles.
The gentle nature of this new fabrication process allows for the encapsulation of fragile biomolecules and its universality allows for the use of many different copolymer systems, which could lead to the creation of other molded drug delivery devices. In addition, this process allows tuning of the mechanical strength and dissolution rate of the structural polymer material depending on the delivery site and the time course for the molecule to be delivered. These polymer microneedles were shown to successfully insert into the skin and deliver an encapsulated active protein. This new drug delivery platform shows future promise for the delivery of a range of biomolecules, including vaccines, proteins, peptides and nucleotides.
Methods
Polymer microneedle fabrication
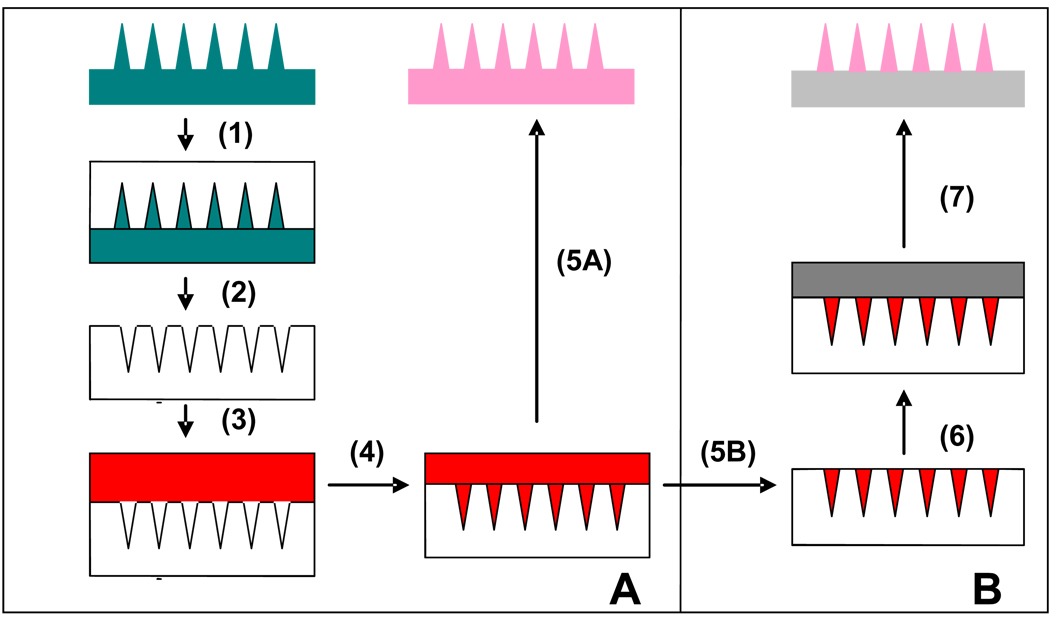
As described previously [16], microneedle master structures were created using a lens-based technique to produce microneedles made of SU-8 epoxy photoresist (Figure 4A). Arrays of 225 (15×15) conical microneedles were made with the following geometry: 250-µm base diameter, 5-µm tip radius, and 750-µm height. Additionally, master structure arrays of 100 (10×10) pyramidal microneedles were fabricated to have a 300-µm base width, 5-µm tip radius, and 650-µm height. Next, microneedle molds were made of polydimethylsiloxane (PDMS, 10 mm thick, Dow Corning 182 Sylgard, Midland MI) to exactly inverse-replicate the master structures. This was done by pouring PDMS over the microneedle master structure and allowing the polymer to cure overnight.
Figure 4. New in situ fabrication process for polymer microneedles.
(A) (1) PDMS is poured onto microneedle master structure. (2) PDMS microneedle mold is cured and peeled off. (3) Liquid monomer and drug are pipetted onto the mold. (4) Vacuum is applied to pull the solution into the microneedle mold. (5A) System is placed under a UV lamp to polymerize microneedles, which are subsequently peeled out of the reusable mold. (B) (5B) Excess solution is removed from the surface. (6) A liquid monomer solution with no drug is applied to the surface. (7) System is placed under UV lamp to polymerize the microneedles, which are then peeled off.
Polymer microneedles were created using the following new photopolymerization process. A mixture of the liquid monomer, vinyl pyrrolidone (200 µL, Sigma Aldrich, St Louis MO), and free-radical initiator, AIBN (1.5 wt%, Sigma Aldrich), was applied to the PDMS mold, covering the entire array. The system was placed inside a vacuum oven (VWR, Cornelius OR) under −30 mm Hg vacuum for 2 min at room temperature (22 – 23 °C) to fill the liquid mixture into the microneedle mold. Next, the system was placed under a UV lamp (100 W, 300 nm, BLAK RAY, Upland CA) for 30 min at room temperature to induce photopolymerization. Finally, the polymer microneedle array was gently peeled out of the mold. Microneedles made of the copolymer poly(vinylpyrrolidone-methacrylic acid) were created in a similar manner, where a mixture of the liquid monomers, vinyl pyrrolidone and methacrylic acid, was used.
Polymer microneedles with encapsulated sulforhodamine and other compounds
A modified process was developed to encapsulate model drugs within the microneedles, but not within the base substrate (Fig. 4B). Sulforhodamine (Molecular Probes, Eugene OR) was added at a concentration of 10−4 M to vinyl pyrrolidone monomer containing 1.5 wt% AIBN initiator. This mixture was applied to the mold and a vacuum was applied to pull the monomer drug mixture into the microneedle holes. Next, the solution that remained puddled on the surface of the mold was gently removed with a micropipette and returned to the stock solution for re-use. Then, 200 µl of a solution of the vinyl pyrrolidone monomer and AIBN initiator was applied to the PDMS microneedle mold to make up the base substrate. Finally, the system was placed under the UV lamp where photopolymerization occurred, resulting in a polymer microneedle array with needles made of PVP and encapsulated sulforhodamine and a base substrate made only of PVP. The same fabrication process was used to preferentially encapsulate fluorescently labeled bovine serum albumin (Molecular Probes) and the enzyme β-galactosidase (Sigma-Aldrich) in the microneedles.
Fracture Force Test
The mechanical strength of the copolymer PVP-MAA microneedles was tested for the following molar monomeric ratios (VP/MAA): 100/0, 99/1, 90/10, 75/25, 50/50, 25/75. In this experiment, the failure force under axial load was measured using a displacement force test station (Model 921A, Tricor Systems, Elgin, IL) following established protocols [16, 22]. Force versus displacement curves were generated by pressing the array of microneedles (20–25 needles per array) against a hard metal surface at a rate of 0.5 mm/sec. The average microneedle fracture force was calculated by dividing the maximum fracture force by the number of needles.
Imaging microneedle insertion
To identify sites of microneedle insertion, a PVP microneedle array was inserted into pig skin in vitro with approval from the Georgia Tech IACUC. The array was removed and a hydrophobic dye (0.4% Trypan blue solution, Sigma Aldrich) was placed on the skin for 5 min to stain the sites of microneedle penetration. The skin was then washed thoroughly under water to remove excess dye from the surface. Finally, the skin was imaged by stereomicroscopy (Olympus SZX9, Japan) to identify the microneedle insertion locations.
To determine depth of microneedle insertion, a polymer microneedle array was applied to pig skin in vitro. Next, the microneedles embedded in the skin were flash frozen in situ in liquid nitrogen. The sample was cut into 10-µm sections using a microcryostat (MICROM HM560, Waldorf Germany) and these sections were stained using hematoxylin and eosin. Finally, the histological sections were examined by stereomicroscopy to determine the depth of insertion.
Delivery of fluorescent-labeled protein
Polymer microneedles were made of PVP with 0.2 wt% Texas-Red bovine serum albumin preferentially encapsulated within the microneedles. The base of the microneedle array was made of PVP. The microneedle array was inserted into pig skin and left for 1 min. The array was then removed and inspected by bright field and fluorescence microscopy to validate that the microneedles had completely dissolved within the skin and only the base remained. After 15 min, the skin was flash frozen using liquid nitrogen and cut using the microcryostat into sections of 10 µm. The sections were examined by fluorescence microscopy (Nikon E600W, Japan) to image the extent and distribution of protein delivery.
Enzyme encapsulation and activity assay
The activity of β-galactosidase was measured after encapsulation within polymer microneedles to determine if the fabrication and dissolution processes damage the enzyme. A “dose” of 1.0 mg of β-galactosidase was encapsulated within an array of PVP microneedles. The microneedles were then dissolved in cold (4° C) PBS, and the activity of the released enzyme was measured, using the manufacturer’s protocol [23]. This involves creating an enzymatic reaction with nitrophenol and monitoring the product by absorbance at 410 nm. Positive controls were tested, containing β-galactosidase in cold PBS, and β-galactosidase in PBS containing previously dissolved, placebo PVP microneedles.
Delivery of enzymatically active β-galactosidase was also studied in pig skin in vitro. In this case, an array of PVP microneedles containing 50 µg β-galactosidase was inserted into pig skin and then removed after 18 h. The skin was flash frozen and 10 µm sections were taken using the microcryostat. These sections were then fixed in cold formalin and stained with an X-Gal solution overnight at 37 °C and counterstained with nuclear fast red. X-Gal binds to active β-galactosidase and produces a blue product that is visualized using a stereomicroscope.
Figure 5.
Acknowledgements
We thank Harvinder Gill, Michael Heffernan, Jung-Hwan Park and Genggeng Qi for helpful discussions. M.R.P. is the Emerson-Lewis Faculty Fellow. This work was carried out at the Institute for Bioengineering and Bioscience and the Center for Drug Design, Development and Delivery at Georgia Tech. This work was supported in part by the Southeast Regional Center of Excellence for Emerging Infections and Biodefense and the National Institutes of Health.
References
- 1.Walsh G. Nat Biotechnol. 2006;24:769. doi: 10.1038/nbt0706-769. [DOI] [PubMed] [Google Scholar]
- 2.Datamonitor. 2004:1. (Ed: D. USA) [Google Scholar]
- 3.Langer R. Nature. 1998;392:5. [PubMed] [Google Scholar]
- 4.Prausnitz MR, Mitragotri S, Langer R. Nat Rev Drug Discov. 2004;3:115. doi: 10.1038/nrd1304. [DOI] [PubMed] [Google Scholar]
- 5.MR Prausnitz JM, Raeder-Devens J. In: Percutaneous Penetration Enhancers. Maibach ESaH., editor. Boca Raton: CRC Press; 2006. [Google Scholar]
- 6.Prausnitz MR. Adv Drug Deliv Rev. 2004;56:581. doi: 10.1016/j.addr.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 7.Kaushik S, Hord AH, Denson DD, McAllister DV, Smitra S, Allen MG, Prausnitz MR. Anesth Analg. 2001;92:502. doi: 10.1097/00000539-200102000-00041. [DOI] [PubMed] [Google Scholar]
- 8.Harvinder S Gill MRP. Journal of Diabetes Science and Technology. 2007;1:725. doi: 10.1177/193229680700100517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mikszta JA, Alarcon JB, Brittingham JM, Sutter DE, Pettis RJ, Harvey NG. Nat Med. 2002;8:415. doi: 10.1038/nm0402-415. [DOI] [PubMed] [Google Scholar]
- 10.Lin W, Cormier M, Samiee A, Griffin A, Johnson B, Teng CL, Hardee GE, Daddona PE. Pharm Res. 2001;18:1789. doi: 10.1023/a:1013395102049. [DOI] [PubMed] [Google Scholar]
- 11.Martanto W, Davis SP, Holiday NR, Wang J, Gill HS, Prausnitz MR. Pharm Res. 2004;21:947. doi: 10.1023/b:pham.0000029282.44140.2e. [DOI] [PubMed] [Google Scholar]
- 12.Reed ML, Lye WK. Proceedings of the Ieee. 2004;92:56. [Google Scholar]
- 13.McAllister DV, Wang PM, Davis SP, Park JH, Canatella PJ, Allen MG, Prausnitz MR. Proc Natl Acad Sci U S A. 2003;100:13755. doi: 10.1073/pnas.2331316100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito Y, Hagiwara E, Saeki A, Sugioka N, Takada K. Eur J Pharm Sci. 2006;29:82. doi: 10.1016/j.ejps.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Miyano T, Tobinaga Y, Kanno T, Matsuzaki Y, Takeda H, Wakui M, Hanada K. Biomed Microdevices. 2005;7:185. doi: 10.1007/s10544-005-3024-7. [DOI] [PubMed] [Google Scholar]
- 16.Park JH, Allen MG, Prausnitz MR. J Control Release. 2005;104:51. doi: 10.1016/j.jconrel.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Robinson BV. PVP : a critical review of the kinetics and toxicology of polyvinylprrolidone (povidone) Chelsea, MI: Lewis Publishers; 1990. [Google Scholar]
- 18.Rogero SO, Malmonge SM, Lugao AB, Ikeda TI, Miyamaru L, Cruz AS. Artif Organs. 2003;27:424. doi: 10.1046/j.1525-1594.2003.07249.x. [DOI] [PubMed] [Google Scholar]
- 19.Victor SP, Sharma CP. J Biomater Appl. 2002;17:125. doi: 10.1106/088532802028583. [DOI] [PubMed] [Google Scholar]
- 20.Barbu E, Sarvaiya I, Green KL, Nevell TG, Tsibouklis J. J Biomed Mater Res A. 2005;74:598. doi: 10.1002/jbm.a.30329. [DOI] [PubMed] [Google Scholar]
- 21.Mitragotri S. Nat Rev Immunol. 2005;5:905. doi: 10.1038/nri1728. [DOI] [PubMed] [Google Scholar]
- 22.Davis SP, Landis BJ, Adams ZH, Allen MG, Prausnitz MR. J Biomech. 2004;37:1155. doi: 10.1016/j.jbiomech.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Sigma-Aldrich. Enzymatic Assay of Beta-Galactosidase. 1999 [Google Scholar]