Abstract
Objective
We hypothesized that aerosolized inhaled HTS given at the onset of resuscitation will decrease acute lung injury (ALI) following hemorrhagic shock by inhibiting the release of epithelial derived pro-inflammatory mediators.
Design
Animal study
Setting
Animal care facility procedure room in a medical center.
Subjects
Adult male Sprague-Dawley rats.
Interventions
Rats underwent hemorrhagic shock followed by 2 hrs of resuscitation and one hour of observation. In the study group, nebulized HTS was delivered at the end of the shock period and after 1 hr and 2 hr of resuscitation.
Measurements and Main Results
Shock provoked ALI, which was attenuated with inhaled HTS (1.56 ± 0.2 vs. 0.95 ± 0.3 mg protein/ml BALF, Shock vs. Shock +HTS, p<0.01). Nebulized HTS reduced inflammation (CINC-1 accumulation in BAL fluid 5999 ± 1267 vs. 3342 ± 859 pg/ml, Shock vs. Shock +HTS, p=0.006). Additionally, nebulized HTS inhibited MMP-13 accumulation in the BALF (1513 ± 337 pg/ml BALF vs. 230 ± 19 pg/ml, Shock vs. Shock + HTS, p=0.009) and pretreatment with an MMP-13 inhibitor was sufficient to attenuate postinjury ALI (1.42 ± 0.09 vs. 0.77 ± 0.23 mg/ml BAL protein, Shock vs. Shock + MMP-13 Inhibitor CL-82198, p=0.002).
Conclusion
Inhaled hypertonic saline attenuates postshock acute lung injury by exerting an anti-inflammatory effect on the pulmonary epithelium, suggesting a new clinical strategy to treat ALI/ARDS.
Introduction
There has been intensive investigation into the pathophysiology of the acute respiratory distress syndrome since it was first described by Ashbaugh and Petty over 40 years ago.1 It is now understood that a maladaptive immune response is central in the pathogenesis of acute lung injury (ALI) and multiple organ failure (MOF).2 The traditional view of ALI focuses on the interaction between the neutrophil (PMN) and injured endothelium; however, hemorrhagic shock also disrupts the bronchial epithelium, leading to lung leak and influx of edematous fluid. Hypertonic saline, at a cellular level, decreases alveolar macrophage activation, PMN recruitment, priming and activation, as well as cell surface adhesion molecule expression.3 Clinically, inhaled HTS is used to treat inflammation in cystic fibrosis (CF) and neonatal bronchiolitis. IL-8, a chemokine expressed by pulmonary epithelial cells and macrophages, is elevated in CF, and inhibited by hypertonic saline in vitro. 4,5,6 ,7,8,9,10,11,12,13,14 Serine proteases (i.e. neutrophil elastase) are thought to be the key mediators, however, recent evidence highlights the increasingly important role of matrix metalloproteinases (MMPs). Matrix metalloproteinases have a prominent role in the regulation of lung inflammation. Specifically, MMP-13 is increased in chronic obstructive pulmonary disease, produced by type II pneumocytes. Recent data have implicated MMP-13 in the development of sepsis-mediated ALI, while the role of MMP-13 in ALI following trauma remains unclear.15,16
Recognizing the central role of the pulmonary epithelium in ALI, nebulization has the advantage of achieving high concentrations of the therapy without producing systemic side effects. Consequently, we hypothesize that inhaled hypertonic saline modulates the pulmonary epithelial inflammatory response following hemorrhagic shock and inhibits the release of pro-inflammatory agents.
Materials and Methods
All animal experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee at the University of Colorado Denver. Sprague-Dawley rats weighing 350-400 g (Harlan Labs, Indianapolis, IN) were housed in a climate-controlled barrier facility with 12-h light/dark cycles and free access to food and water for a period of at least one week prior to experimental procedures. Pentobarbital sodium was purchased from Abbott Labs (Chicago, IL). Polyethylene tubing was acquired from Fisher Scientific (Pittsburgh, PA), and heparin was purchased from American Pharmaceutical Partner (Schaumburg, IL). Arterial blood gas and serum sodium concentrations were measured by iSTAT (Abbott Point of Care Inc, Princeton, NJ). All other reagents were purchased from Sigma-Aldrich Corp. (St. Louis, MO) unless otherwise specified. Continuous blood pressure measurement was performed using a ProPaq invasive monitoring device (Welch-Allyn, Skaneateles Falls, NY). CL-82198, a selective MMP-13 inhibitor was purchased from EMD (San Diego, CA).
Experimental model
Anesthesia was given by intraperitoneal injection of 50 mg/kg sodium pentobarbital. Local anesthesia was performed by subcutaneous injection of 1% lidocaine, and heparin (60 U/kg body weight) was added to the shed blood (SB). The femoral artery and vein were cannulated with PE 50 tubing. A separate skin incision was created to tunnel the catheters prior to closure of the groin incision. After a one-hour observation period, a 3-cm midline laparotomy was performed to mimic tissue injury. In order to normalize preshock oxygenation, the animal was placed on 40% 02 using an air-oxygen mixer (Sechrist, Anaheim, CA) at a flow rate of 2 liters per minute. Shock was induced by removing 40% of the animal’s blood volume through an arterial catheter within ten minutes of initiating the controlled hemorrhage. As a result, a mean arterial pressure of 30 mm Hg was achieved, and sustained for 45 min by withdrawing or returning SB as needed.17 Body temperature was monitored rectally and euthermia was maintained with a heat lamp. Vital signs were recorded every 10 minutes. At the start of resuscitation, animals in the nebulized HTS groups received the first dose of hypertonic saline; 2 mL of 7.5% hypertonic saline were delivered via a mixer at an air-flow rate of 2 liters per minute for 15 minutes. Animals were resuscitated by infusing 2 times the SB volume in normal saline (NS) over 30 min, followed by 1 2 SB volume over 30 min. Resuscitation was then completed using 2 times the SB volume in NS over 60 min via the femoral vein. The rats received additional doses of nebulized hypertonic saline during the second and third postshock hours. A bronchoalveolar lavage was performed using 5 mL of saline, repeating the process 2 times (15 mL total) as the animal was euthanized via a pentobarbital overdose.
Lung neutrophil accumulation
At the end of the experiment, lung tissue was harvested, frozen, and stored at -80°C. It was weighed, and homogenized in 10 mL of 20 mmol/L potassium phosphate buffer (PPB) and centrifuged at 40,000g for 30 minutes. The pellet was sonicated for 90 seconds in 4 mL of 50 mmol/L PPB containing 0.5 g/dL hexadecyltrimethyl ammonium bromide, incubated at 60°C and centrifuged. The supernatant (5 μL) was added to 145 μL of 50 mmol/L PPB containing 0.167 mg/mL O-dianisidine with 0.0005% hydrogen peroxide; the absorbance at 460 nm was measured with a spectrophotometer (Molecular Devices Corp, Sunnyvale, Calif) to determine myeloperoxidase (MPO) activity.18,19
Lung vascular permeability
Five ml of cold saline (NS) was injected into the trachea, aspirated a total of three times, and collected. The content of bronchoalveolar lavage fluid (BALF) collected was consistently greater than 12 mL. The BALF was then centrifuged at 400g at 4 C for 10 minutes. Bronchoalveolar lavage protein content was determined by the bicinchoninic acid assay (BCA) and absorbance read at 595 nm.
BALF Cytokines and MMPs
Due to mounting evidence that matrix MMPs are critical in the development of ALI,20 we evaluated an array of MMPs and tissue inhibitors of matrix metalloproteinases (TIMPs) using a multiplexed ELISA (RayBiotech, Norcross, GA). Cytokine-Induced Neutrophil Chemoattractant-1 (CINC-1), the murine analogue of IL-8, produced primarily by pulmonary epithelial cells and alveolar macrophages, was used as a marker of inflammation.21 CINC-1 concentration in BALF was determined using a commercial immunoassay (Quantikine ELISA Kit R&D Systems, Minneapolis, MN).
MMP-13 Inhibition
To test the effect of MMP-13 inhibition specifically, a separate group of Sprague-Dawley rats (n=5, 350-400 g) were pretreated with intratracheal instillation of an MMP-13 inhibitor (100 μM CL-82198 in 100 μl NS) before the onset of trauma (laparotomy) and hemorrhagic shock (MAP 30 mmHg × 45 min). The animals were resuscitated with a combination of normal saline and heparinized shed blood. Each animal was given a lethal dose of pentobarbital at 3 hours postinjury, at which time the bronchoalveolar lavage fluid (BALF) was collected for analysis using the colorimetric BCA protein assay.
Data Analysis
For the statistical analysis, data are reported as mean ± SEM. Student’s t-test was used to determine statistical significance.
Results
Lung vascular permeability
The primary endpoint of the study was ALI, measured by protein extravasation into the alveolar space 3 hours after injury. Trauma/hemorrhagic shock provoked marked lung permeability (1.56 ±0.2 mg protein/ml BALF; n=5), compared to sham (0.10 ±0.03 mg protein/ml BALF; n=5). This lung injury was attenuated with administration of aerosolized 7.5% saline (0.95 ±0.3 mg protein/ml BALF; p=0.018 Shock vs. Shock + HTS, n=5) [Figure 1].
Figure 1.
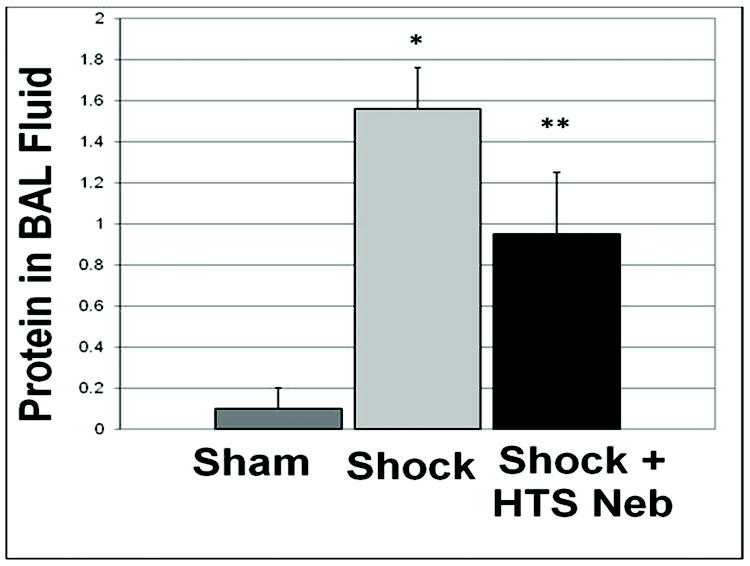
Inhaled hypertonic saline (HTS Neb) attenuated ALI following trauma/hemorrhagic shock (Shock), measured by protein extravasation into the alveolar space 3 hours after injury. * p<0.01 for Sham vs. Shock, ** p<0.01 for Shock vs. Shock + HTS; n=5 for each group.
Neutrophil accumulation in the lung
PMN accumulation, as measured by myeloperoxidase (MPO) activity, was elevated following trauma/hemorrhagic shock compared to sham (7.77 ± 1.84 U/mg vs. 2.81 ± 1.84 U/mg, p=.009 Sham vs. Shock). Nebulized HTS decreased the neutrophil accumulation in the lung, although the effect was not significant (7.77 ± 1.84 vs. U/mg 5.96 ± 1.26 U/mg, p=0.16, Shock vs. Shock + HTS groups) [Figure 2].
Figure 2.
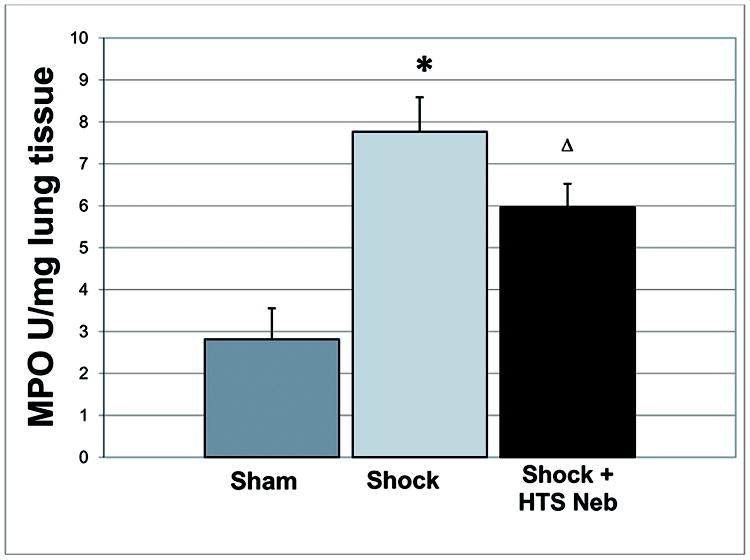
PMN accumulation, as measured by myeloperoxidase (MPO) activity, was elevated following trauma/hemorrhagic shock compared to sham, and decreased following nebulized HTS treatment, although the effect did not reach statistical significance (7.77 ± 1.84 U/mg 5.96 ± 1.26 U/mg, p=0.16, Shock vs. Shock + HTS groups). * p<0.01 for Sham vs. Shock, Δ p>0.05 Shock vs. Shock + HTS; n=5 for each group.
Histology
Formalin-fixed lung sections stained with hematoxylin and eosin were evaluated by a pathologist in a blinded fashion. Lung sections show disruption of distal airspaces caused by trauma and hemorrhagic shock (Figure 3B). Nebulized HTS administration protected the integrity of the basement membrane (Figure 3C), giving it an appearance resembling normal rat lung parenchyma (Figure 3A). Additionally, MMP-13 was detectable in normal rat lung epithelium lining the alveoli, and following T/HS in the injured lung there was prominent MMP-13 fluorescence in the epithelium lining the injured alveolus by epitope staining [Figure 4].
Figure 3. Nebulized hypertonic saline preserves alveolar structure.
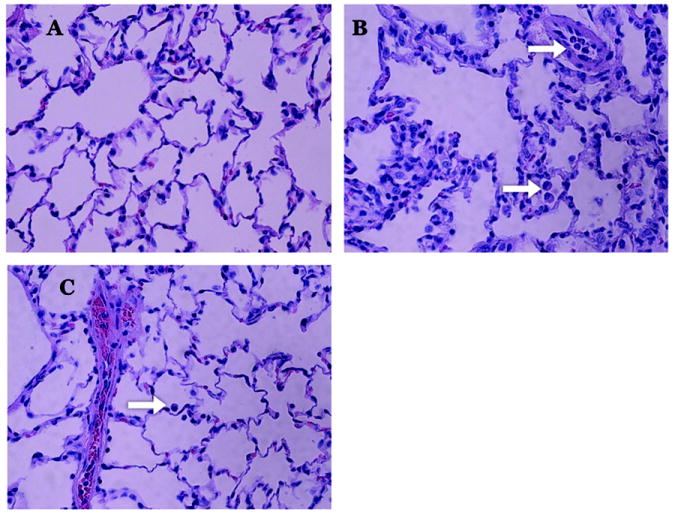
Hemorrhagic shock causes inflammatory changes including arcuate PMN-mediated inflammation, edema formation, and diffuse alveolar damage with obliteration of distal airspaces (B) compared to normal rat lung (A). While PMN accumulation in the vessels and lung parenchyma following hemorrhagic shock is unchanged (white arrows B,C), the pulmonary epithelium is substantially less congested with preservation of alveoli in the group receiving nebulized HTS and decreased arcuate inflammation, edema formation, and alveolar damage (C).
Figure 4. MMP-13 is present in the lung following injury.
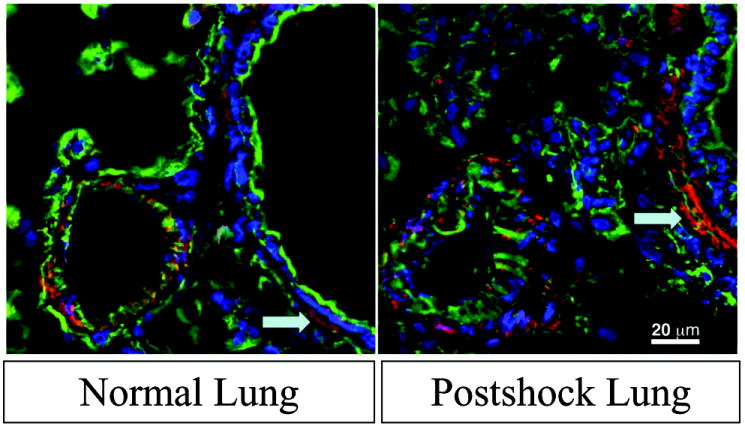
On immunofluorescence, MMP-13 (red) in lung tissue increases as a result of hemorrhagic shock. WGA (green), a glycoprotein stain, and DAP-I (blue), a nuclear stain are used as background.
CINC-1 in BAL fluid
Pulmonary cytokine activity was quantified in the BALF. CINC-1, the murine analogue of human IL-8, was elevated substantially following trauma/hemorrhagic shock. CINC-1 accumulation in the BALF decreased by nearly 50% following nebulized HTS administration (CINC-1: 5999 ± 1267 pg/ml vs. 3342 ± 859 pg/ml, Shock vs. Shock + HTS groups, p=0.03) [Figure 5].
Figure 5. Nebulized HTS decreases chemokine accumulation in BALF by 44%.
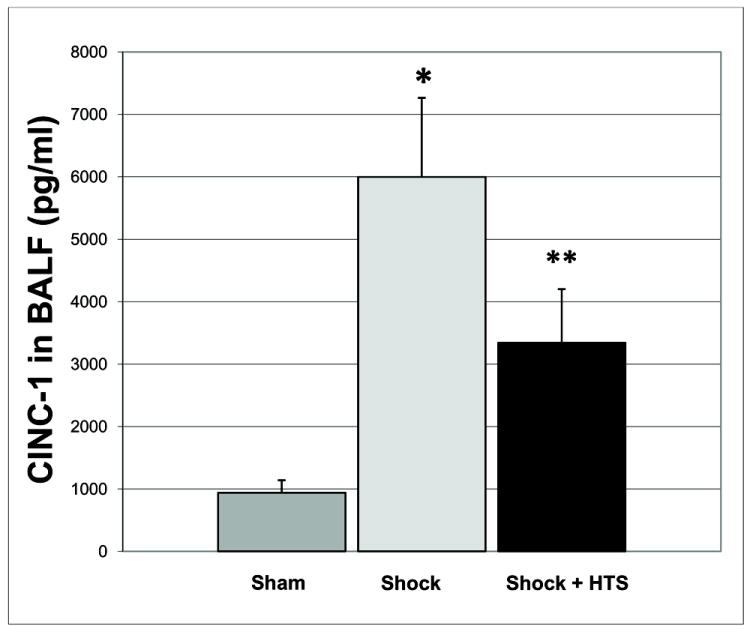
Cytokine induced neutrophil chemoattractant-1 (CINC-1), the murine analogue to human IL-8, was elevated substantially following trauma/hemorrhagic shock. CINC-1 accumulation in the BALF was blunted following nebulized HTS administration. * p<0.05 for Sham vs. Shock, ** p<0.05 for Shock vs. Shock + HTS; n=5 for each group.
Matrix metalloproteinases
MMP-13, although present in the normal lung tissue, was virtually undetectable in normal BAL fluid (n=5, value below the limit of detection < 43.6 pg/ml), increasing dramatically following trauma/hemorrhagic shock to 1513 ± 337 pg/ml. Nebulized HTS inhibited MMP-13 accumulation in the BAL fluid to 230 ± 19 pg/ml, Shock vs. Shock + HTS, p=0.009, n=5 in both groups [Figure 6]. MMP 8 produced by PMNs and epithelium was elevated but unchanged. TIMP-4 was detected, but not significantly altered by HTS. TIMP-1 levels were decreased 5.1-fold by nebulized HTS (17.6 ± 9.9 pg/ml BALF vs. 2.4 ± 1.4 pg/ml, Shock vs. Shock + HTS groups, p=0.0575, n=5 in both groups, data are summarized in [Figure 7]). It has been suggested that dysfunction of the constitutive inhibitors of MMPs (TIMPs) are the driving factor for acute lung inflammation in asthma exacerbation rather than increased activity of the MMPs themselves.22 In rodents, MMP-13 performs the role of human MMP-1.23 We therefore measured TIMP-1, the inhibitor of rodent MMP-13 to explore this hypothesis, and found that, although nebulized HTS decreased TIMP-1 levels in BALF by 5-fold, the levels were inadequate for the production necessary for TIMP-1 to provide a 1:1 stoichiometric inhibition of MMP-13. Thus loss of TIMP-1 is unlikely to be a significant mechanism of nebulized HTS, compared to the massive increase in MMP13 induced by shock and ameliorated by HTS.
Figure 6. Nebulized HTS inhibits MMP-13 accumulation in the BAL fluid.
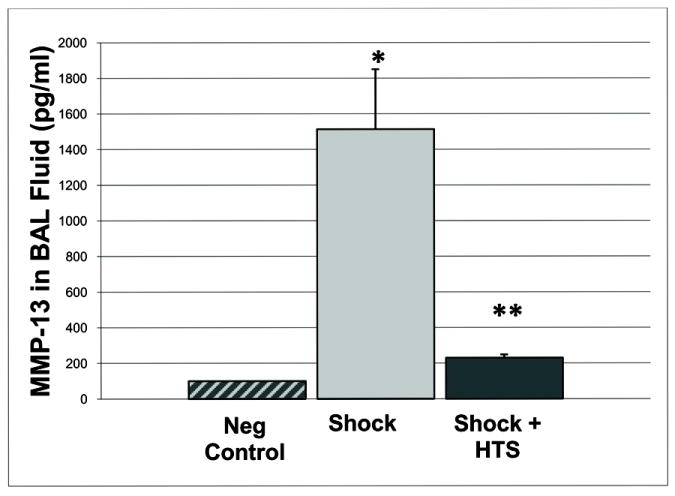
* p<0.05 for Sham vs. Shock, ** p<0.05 for Shock vs. Shock + HTS; n=5 for each group.
Figure 7. Nebulized HTS acts primarily on MMP-13.
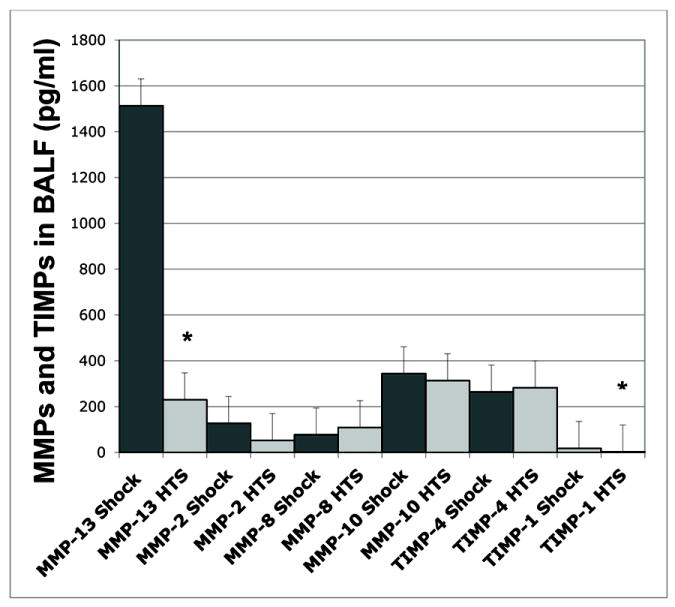
In order to confirm a lack of disruption of the extracellular matrix equilibrium, a multiplex assay was performed. Out of the array of MMPs and their inhibitors, TIMPs, MMP-13 was the only MMP significantly regulated by the effects of nebulized HTS. * p < 0.05 for Shock vs. Shock + HTS.
MMP-13 Inhibition
Recognizing that epithelial MMP-13 may be an important mediator of pulmonary inflammation via its collagenase and gelatinase activities, in a separate experiment, animals were treated with an MMP-13 inhibitor. In these animals, hemorrhagic shock caused marked lung permeability, which was attenuated with MMP-13 inhibition. Pretreatment with an MMP-13 inhibitor was sufficient to attenuate postinjury ALI as reflected by BAL protein (1.42 ± 0.09 vs. 0.77 ± 0.23 mg/ml protein in BALF, Shock vs. Shock + MMP-13 Inhibitor, n=5, p=0.002) [Figure 8]. These data implicate MMP-13 and the epithelium in the development of ALI.
Figure 8. MMP-13 Inhibition Attenuates ALI.
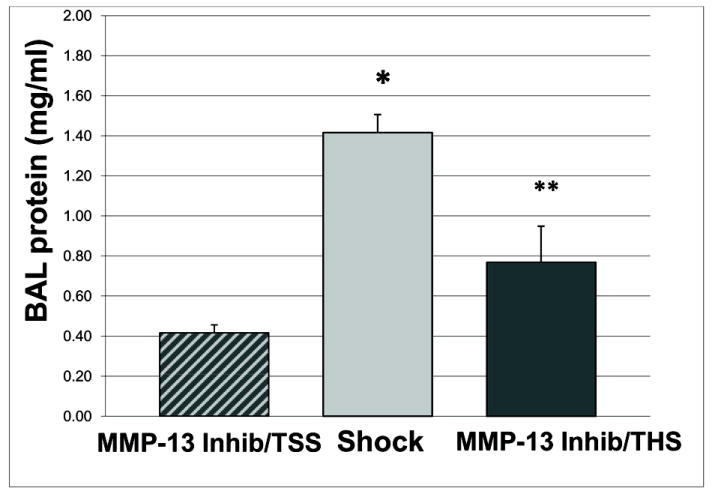
Pretreatment with the MMP-13 inhibitor CL-82198 was sufficient to attenuate postinjury ALI (1.42 + 0.09 vs. 0.77 + 0.23 mg/ml protein in BALF, Shock vs. Shock + MMP-13 Inhibitor. * p<0.05 for MMP-13 inhibitor pretreatment + trauma/sham shock (TSS) vs. Shock, ** p<0.05 for Shock vs. MMP-13 inhibitor pretreatment + trauma/hemorrhagic shock (T/HS)); n=5 for each group.
Serum Sodium
To determine if nebulized HTS was acting locally and not via systemic hypertonicity, serum sodium levels at baseline and at the end of resuscitation were measured and unchanged between the two groups (Shock group: 139 ±4.5 mEq/L baseline vs. 143 ± 0.2 mEq/L at end of resuscitation; Shock + HTS group: baseline 140 ± 0.2 mEq/L vs. 145 ± 1 mEq/L at end of resuscitation. Serum Na Baseline: Shock vs. Shock + HTS groups, p=0.39, Serum Na at end of resuscitation: Shock vs. Shock + HTS groups, p=0.15). Osmolarity of the BAL fluid following HTS neb administration was measured using the Advanced Micro Osmomometer 3000 (Norwood, MA), and unchanged compared to the control group (Shock group 288 ± 0.54 vs. HTS Shock group 311 ± 8.0 mOsm/ml, p=0.11).
Discussion
Our data indicate that nebulized hypertonic saline attenuates lung injury following experimental trauma/hemorrhagic shock. Nebulized HTS has already shown clinical benefit for patients with CF and neonatal bronchiolitis, and our study supports it as a novel treatment for ALI/ARDS.
We are merely proposing a novel method of delivery; the use of hypertonic saline in trauma is well established. HTS has optimal qualities for trauma resuscitation by reducing intracranial pressure via its higher osmotic load and attenuating the body’s pro-inflammatory response to reduce the incidence of ARDS and MOF.24 Amidst a growing body of in vitro, in vivo, and preclinical studies, in 2008 a large clinical trial comparing HTS with conventional NS resuscitation in the field showed that HTS increased early mortality and was no better than standard of care for overall mortality. 25 One of the proposed mechanisms for increased mortality in patients receiving hypertonic saline was a higher rate of early hemorrhage, because HTS impairs enzymatic clotting function when replacing 10% or more of the blood volume.26
While hypertonicity acts systemically on the endothelium, nebulized HTS acts locally, delivering very small quantities of sodium to the systemic circulation.27 In our model, 2 mL of 7.5% hypertonic saline were nebulized into a breathing mixer with high air-flow (2L/min) for 15 minutes. Assuming a particle deposition of approximately 10% over the entire 15 minute session, suggests pulmonary addition of about 750ug or 13umoles of NaCl and 10ul of H2O per session.28 Therefore, it is unlikely that nebulized HTS exerted its activity at the endothelial-PMN interface. In our study, nebulized HTS exerted a minimal effect on neutrophil accumulation in the lung, while attenuating accumulation of the chemokine CINC-1 in the BALF. These data suggest that the inhaled hypertonic saline is acting locally on the epithelium in the lung to modulate inflammation rather than mediating the interaction between the PMN and the vascular endothelium.
In the clinical setting, Reeves et al. have also touted the anti-inflammatory effects of nebulized HTS in patients with CF.29 In their study, aerosolized HTS led to degradation of IL-8 and decreased neutrophil efficiency. Total IL-8 levels decreased by 33% in CF patients following treatment with neb HTS, which was comparable to our study, in which neb HTS decreased CINC-1 levels (murine analogue of IL-8) in the BALF by 44%. In CF patients, the data show a reduction in neutrophil chemotaxis as a result of chemokine degradation and disruption of IL-9:glycosaminoglycan complexes, suggesting that the nebulized HTS may be facilitating resolution of inflammation via a similar mechanism post-injury.
Additionally, our data show that hemorrhagic shock-induced lung permeability was attenuated with MMP-13 inhibition, further implicating the epithelium in the development of ALI. MMPs are a family of metalloendopeptidases, and collectively, are able to completely degrade the extracellular matrix. While MMPs are not normally expressed in healthy tissues, they are integral in maintaining the extracellular matrix and tissue remodeling during repair processes.30 MMP-13 is increased in Type II pneumocytes during chronic inflammation, and recent data have implicated MMP-13 in the development of sepsis-mediated acute lung injury.15,16 Recognizing that epithelial MMP-13 may be an important mediator of pulmonary inflammation via its collagenase and gelatinase activities, we show that pretreatment with an MMP-13 inhibitor prior to trauma/hemorrhagic shock attenuates ALI.
Although we did not measure it directly, our study suggests that when the epithelial Na channels are impaired during ALI/ARDS following, nebulized HTS may augment alveolar fluid clearance in a similar way as it has been shown to do in CF. In addition to its anti-inflammatory effects, inhaled HTS may facilitate the resolution of alveolar edema by augmenting active ion transport across the pulmonary epithelium.31 The ability of nebulized HTS to increase the airway surface volume, and improve mucous clearance in CF patients has been the basis for giving this therapy.32 In ARDS, the epithelial sodium channels become overwhelmed, and impaired alveolar fluid clearance leads to pulmonary edema formation.33 The observation that inhaled HTS alters airway ion permeability may partly explain its recent success in treating bronchiolitis. 34
We acknowledge that choosing a model that is relevant to the human disease is critical for successful translational research.35 Because no single animal model can fully reproduce the pathologic findings of human ALI/ARDS, our model focuses on inducing the pathologic “ALI triad” as described by Matthay and Matute-Bello.36,37 Over 45 years of clinical experience in caring for trauma patients form the basis for the clinically relevant model of early acute lung injury used in this study. In fact, the first patient with ARDS successfully treated by Ashbaugh and Petty was a 15 year old trauma patient in hemorrhagic shock resulting from a motor vehicle crash.38
To our knowledge, this study is the first to propose nebulized hypertonic saline as a therapy for ALI/ARDS. A Phase II clinical trial to evaluate the role of nebulized HTS is currently underway in the Surgical Intensive Care Unit at Denver Health Medical Center, a location 3 miles from the historic Colorado General Hospital, where Ashbaugh and Petty made their original contribution.
This study has several potential limitations. Neutrophil accumulation in lung tissue was decreased, although not significantly. This could be due to the small sample size or due to the early time course of the study may have led to the small absolute reduction in neutrophils in the lung tissue. Additionally, our model incorporates tissue injury (laparotomy) and hemorrhagic shock. We did not account for lung injury from direct trauma to the chest as can occur clinically, however, nebulized HTS may be beneficial for these patients as well, via enhanced clearance of edema fluid. Although we looked at TIMPs as endogenous inhibitors of MMPs, recent work has shown that non-specific protease inhibitors (i.e. alpha-2 macroglobulin) may also have an important role in regulation of MMPs.39 Additionally, the choice of resuscitation fluid alone can affect inflammation.40,41 Other studies, however, have shown that NS and LR resuscitation fluids have equivalent effects on inflammatory markers, and that even large volume normal saline resuscitation has been shown to have an insignificant effect on oxygenation compared to LR.42,43 Although NS may contribute to a hyperchloremic metabolic acidosis, the calcium content in LR can impair coagulation. Therefore, in our model of trauma and hemorrhagic shock, we prefer to use normal saline.
In summary, nebulized hypertonic saline reduces epithelial cytokine and MMP-13 accumulation in BAL fluid, thereby attenuating acute lung injury and preserving basement membrane integrity in the distal airways without altering PMN accumulation in the lung. These data further support the role of epithelial damage in the pathogenesis of acute lung injury. These results suggest that nebulized HTS attenuates ALI by suppressing epithelial inflammation, supporting further research to its use as a novel strategy to treat ALI/ARDS.
Acknowledgments
Funding: NIH Grants T32-GM008315 and P50-GM49222
Footnotes
The authors have not disclosed any potential conflicts of interest
Impact: Nebulized hypertonic saline (HTS) has been used successfully to treat lung inflammation in cystic fibrosis and bronchiolitis. This study demonstrates that nebulized HTS attenuates acute lung injury following experimental trauma/hemorrhagic shock via inhibition of MMP-13. These findings highlight the importance of the pulmonary epithelium in inflammation, and may have important implications in the treatment of acute lung injury/ARDS.
Conception and design: CS, DF, EM, AB, FA, JH, MW; Analysis and interpretation: CS, FG, EM, AB, JH, MF, FW, FG, JH, MW; Drafting the manuscript for important intellectual content: CS, EM, AB, MW.
References
- 1.Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967 Aug 12;2(7511):319–23. doi: 10.1016/s0140-6736(67)90168-7. [DOI] [PubMed] [Google Scholar]
- 2.Ciesla DJ, Moore EE, Johnson JL, et al. The role of the lung in postinjury multiple organ failure. Surgery. 2005;138:749–57. doi: 10.1016/j.surg.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 3.Pascual JL, Ferri LE, Seely AJ, et al. Hypertonic saline resuscitation of hemorrhagic shock diminishes neutrophil rolling and adherence to endothelium and reduces in vivo vascular leakage. Ann Surg. 2002;236:634–636. doi: 10.1097/00000658-200211000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elkins MR, Robinson M, Rose BR, et al. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med. 2006;354:229–240. doi: 10.1056/NEJMoa043900. [DOI] [PubMed] [Google Scholar]
- 5.Cuschieri J, Gourlay D, Garcia I, et al. Hypertonic preconditioning inhibits macrophage responsiveness to endotoxin. J Immunol. 2002;168:1389–96. doi: 10.4049/jimmunol.168.3.1389. [DOI] [PubMed] [Google Scholar]
- 6.Angle N, Hoyt DB, Coimbra R, et al. Hypertonic saline resuscitation diminishes lung injury by suppressing neutrophil activation after hemorrhagic shock. Shock. 1998;9:164–170. doi: 10.1097/00024382-199803000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Zallen G, Moore EE, Tamura DY, et al. Hypertonic saline resuscitation abrogates neutrophil priming by mesenteric lymph. J Trauma. 2000;48:45–48. doi: 10.1097/00005373-200001000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez RJ, Moore EE, Ciesla DJ, et al. Hyperosmolality abrogates neutrophil cytotoxicity provoked by post-shock mesenteric lymph. Shock. 2002;18:29–32. doi: 10.1097/00024382-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Deitch EA, Shi HP, Feketeova E, et al. Hypertonic saline resuscitation limits neutrophil activation after trauma-hemorrhagic shock. Shock. 2003;19:328–33. doi: 10.1097/00024382-200304000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Kuzik BA, Al-Oadhi SA, Kent S, et al. Nebulized hypertonic saline in the treatment of viral bronchiolitis in infants. J Pediatr. 2007;151:266–270. doi: 10.1016/j.jpeds.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, Mendoza-Sassi RA, Wainwright C, et al. Nebulized hypertonic saline solution for acute bronchiolitis in infants. Cochrane Database Syst Rev. 2008;8 doi: 10.1002/14651858.CD006458.pub2. CD006458. [DOI] [PubMed] [Google Scholar]
- 12.Khan TZ, Wagener JS, Bost T, et al. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med. 1995;151:1075–1082. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
- 13.Powers KA, Woo J, Khadaroo RG, et al. Hypertonic resuscitation of hemorrhagic shock upregulates the anti-inflammatory response by alveolar macrophages. Surgery. 2003;134:312–8. doi: 10.1067/msy.2003.246. [DOI] [PubMed] [Google Scholar]
- 14.Hatanaka E, Shimomi FM, Curi R, et al. Sodium chloride inhibits cytokine production by lipopolysaccharide-stimulated human neutrophils and mononuclear cells. Shock. 2007;27:32–5. doi: 10.1097/01.shk.0000238061.69579.a5. [DOI] [PubMed] [Google Scholar]
- 15.Lee EJ, In KH, Kim JH, et al. Proteomic analysis in lung tissue of smokers and COPD patients. Chest. 2009;135:344–52. doi: 10.1378/chest.08-1583. [DOI] [PubMed] [Google Scholar]
- 16.Yamakawa N, Uchida T, Matthay MA, et al. Proteolytic Release of the Receptor for Advanced Glycation End-Products from in vitro and in situ Alveolar Epithelial Cells. Am J Physiol Lung Cell Mol Physiol. 2011;300:L516–25. doi: 10.1152/ajplung.00118.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peltz ED, Moore EE, Zurawel AA, et al. Proteome and system ontology of hemorrhagic shock: exploring early constitutive changes in postshock mesenteric lymph. Surgery. 2009;146:347–57. doi: 10.1016/j.surg.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradley P, Priebat D, Christensen R, et al. Measurement of cutaneous inflammation: estimation of neutrophil content with and enzyme marker. J Invest Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 19.Masuno T, Moore EE, Cheng AM, et al. Prehospital hemoglobin-based oxygen carrier resuscitation attenuates postinjury acute lung injury. Surgery. 2005;138:335–41. doi: 10.1016/j.surg.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 20.Fligiel SEG, Standiford T, Fligiel HM, et al. Matrix metalloproteinases and matrix metalloproteinase inhibitors in acute lung injury. Hum Pathol. 2006;37:422–30. doi: 10.1016/j.humpath.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 21.Himi T, Yoshioka I, Kataura A. Production and gene expression of IL-8-like cytokine GRO/CINC-1 in rat nasal mucosa. Acta Otolaryngol. 1997;117:123–127. doi: 10.3109/00016489709118003. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka H, Miyazaki N, Oashi K, et al. Sputum matrix metalloproteinase-9: tissue inhibitor of metalloproteinase-1 ratio in acute asthma. J Allergy Clin Immunol. 2000;105:900–905. doi: 10.1067/mai.2000.105316. [DOI] [PubMed] [Google Scholar]
- 23.Gibbs DF, Warner RL, Weiss SJ, et al. Characterization of matrix metalloproteinases produced by rat alveolar macrophages. Am J Respir Cell Mol Biol. 1999;20:1136–1144. doi: 10.1165/ajrcmb.20.6.3483. [DOI] [PubMed] [Google Scholar]
- 24.Bulger EM, Jurkovich GJ, Nathens AB, et al. Hypertonic resuscitation of hypovolemic shock after blunt trauma: a randomized controlled trial. Arch Surg. 2008;143:139–48. doi: 10.1001/archsurg.2007.41. discussion 149. [DOI] [PubMed] [Google Scholar]
- 25.Bulger EM, May S, Kerby JD, et al. Out-of-hospital hypertonic resuscitation after traumatic hypovolemic shock: a randomized, placebo controlled trial. Ann Surg. 2011 Mar;253:431–41. doi: 10.1097/SLA.0b013e3181fcdb22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reed RL, 2nd, Johnston TD, et al. Hypertonic saline alters plasma clotting times and platelet aggregation. J Trauma. 1991 Jan;31(1):8–14. doi: 10.1097/00005373-199101000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Pascual JL, Khwaja KA, Ferri LE, et al. Hypertonic saline resuscitation attenuates neutrophil lung sequestration and transmigration by diminishing leukocyte-endothelial interactions in a two-hit model of hemorrhagic shock and infection. J Trauma. 2003;54:121–30. doi: 10.1097/00005373-200301000-00015. [DOI] [PubMed] [Google Scholar]
- 28.Middleton PG, Pollard KA, Wheatley JR. Hypertonic saline alters ion transport across the human airway epithelium. Eur Respir J. 2001;17:195–9. doi: 10.1183/09031936.01.17201950. [DOI] [PubMed] [Google Scholar]
- 29.Reeves EP, Williamson M, O’Neill SJ, et al. Nebulized hypertonic saline decreases IL-8 in sputum of patients with cystic fibrosis. Am J Respir Crit Care Med. 2011;183:1517–23. doi: 10.1164/rccm.201101-0072OC. [DOI] [PubMed] [Google Scholar]
- 30.Crosby LM, Waters CM. Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol. 2010;298:715–31. doi: 10.1152/ajplung.00361.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthay MA, Folkesson HG, Verkman AS. Salt and water transport across alveolar and distal airway epithelia in the adult lung. Am J Physiol. 1996;270:487–503. doi: 10.1152/ajplung.1996.270.4.L487. [DOI] [PubMed] [Google Scholar]
- 32.Donaldson SH, Bennett WD, Zeman KL, et al. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N Engl J Med. 2006;354:241–50. doi: 10.1056/NEJMoa043891. [DOI] [PubMed] [Google Scholar]
- 33.Lecuona E, Saldias F, Comellas A, et al. Ventilator-associated lung injury decreases lung ability to clear edema in rats. Am J Respir Crit Care Med. 1999;159:603–609. doi: 10.1164/ajrccm.159.2.9805050. [DOI] [PubMed] [Google Scholar]
- 34.Middleton PG, Pollard KA, Wheatley JR. Hypertonic saline alters ion transport across the human airway epithelium. Eur Respir J. 2000;17:195–199. doi: 10.1183/09031936.01.17201950. [DOI] [PubMed] [Google Scholar]
- 35.Deitch EA. Animal models of sepsis and shock: a review and lessons learned. Shock. 1998 Jan;9(1):1–11. doi: 10.1097/00024382-199801000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2008 Sep;295(3):L379–99. doi: 10.1152/ajplung.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.http://www.thoracic.org/clinical/critical-care/critical-care-research/animal-models-of-acute-lung-injury.php
- 38.Petty TL. In the cards was ARDS: how we discovered the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:602–3. doi: 10.1164/ajrccm.163.3.16331. [DOI] [PubMed] [Google Scholar]
- 39.Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol. 2003;200:448–464. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
- 40.Kellum JA, Song M, Venkataraman R. Effects of hyperchloremic acidosis on arterial pressure and circulating inflammatory molecules in experimental sepsis. Chest. 2004;125:243–248. doi: 10.1378/chest.125.1.243. [DOI] [PubMed] [Google Scholar]
- 41.Kaplan LJ, Kellum JA. Fluids, pH, ions and electrolytes. Curr Opin Crit Care. 2010;16:323–31. doi: 10.1097/MCC.0b013e32833c0957. [DOI] [PubMed] [Google Scholar]
- 42.Watters JM, Brundage SI, Todd SR, et al. Resuscitation with lactated ringer’s does not increase inflammatory response in a Swine model of uncontrolled hemorrhagic shock. Shock. 2004;22:283–7. doi: 10.1097/01.shk.0000135288.54535.8a. [DOI] [PubMed] [Google Scholar]
- 43.Phillips CR, Vinecore K, Hagg DS, et al. Resuscitation of haemorrhagic shock with normal saline vs. lactated Ringer’s: effects on oxygenation, extravascular lung water and haemodynamics. Crit Care. 2009;13:R30. doi: 10.1186/cc7736. [DOI] [PMC free article] [PubMed] [Google Scholar]


