Abstract
Study design: Systematic review.
Clinical question: Do the rates and timing of adjacent segment disease (ASD) differ between cervical total disc arthroplasty (C-ADR) and anterior cervical discectomy and fusion (ACDF) in patients treated for cervical degenerative disc disease?
Methods: A systematic search of MEDLINE/PubMed and bibliographies of key articles was done to identify studies with long-term follow-up for symptomatic and/or radiographic ASD comparing C-ADR with fusion for degenerative disc disease of the cervical spine. The focus was on studies with longer follow-up (48–60 months) of primary US Food and Drug Administration trials of Prestige ST, Prodisc-C, and Bryan devices as available. Trials of other discs with a minimum of 24 months follow-up were considered for inclusion. Studies evaluating lordosis/angle changes at adjacent segments and case series were excluded.
Results: From 14 citations identified, four reports from three randomized controlled trials and four nonrandomized studies are summarized. Risk differences between C-ADR and ACF for symptomatic ASD were 1.5%–2.3% and were not significant across RCT reports. Time to development of ASD did not significantly differ between treatments. Rates of radiographic ASD were variable. No meaningful comparison of ASD rates based on disc design was possible. No statistical differences in adjacent segment range of motion were noted between treatment groups.
Conclusion: Our analysis reveals that, to date, there is no evidence that arthroplasty decreases ASD compared with ACDF; the promise of arthroplasty decreasing ASD has not been fulfilled.
Study Rationale and Context
Adjacent segment disease (ASD) following uninstrumented anterior cervical discectomy and fusion (ACDF) has been reported to have an incidence of 2.9% per year and occur in approximately 25% of cases within 10 years.1 While it is not entirely clear how much this represents an increase over the natural history of disc degeneration, one of the most compelling arguments for total disc arthroplasty has been the potential for decreasing adjacent segment degeneration. The rationale is that arthroplasty helps to preserve the biomechanics of the normal spine at the operated level and hence helps to maintain the normal biomechanical environment at the adjacent level. This article critically examines the evidence that cervical artificial disc replacement has lived up to the promise of minimizing adjacent level disease.
Clinical Questions
From comparative studies of cervical total disc arthroplasty (C-ADR) with ACDF in patients with at least 24 months follow-up:
What is the rate of ASD following C-ADR compared with the rate following fusion? (Focus on symptomatic ASD but reporting on radiographic ASD.)
Do ASD rates differ based on device design?
Is there a difference in the timing of ASD development between C-ADR and ACDF?
Methods
Study design: Systematic review.
Search: MEDLINE/PubMed and bibliographies of key articles.
Dates searched: January 2000 through October 2, 2011.
Inclusion criteria: Studies comparing C-ADR with ACDF using concurrent control group with a focus on studies with longer follow-up (48–60 months) of primary US Food and Drug Administration (FDA) trials of Prestige ST, Prodisc-C, and Bryan devices as available. Trials of other discs with a minimum of 24 months follow-up were considered for inclusion.
Exclusion criteria: Non-FDA trials on discs for which long-term data were available. Studies evaluating lordosis/angle changes at adjacent segments and case series were excluded. Four additional studies were excluded: three substantially overlapped with other studies; and one only reported results from a single site of a large multicenter clinical trial, and data on the full study were available.
Outcomes: ASD defined either radiographically or symptomatically; time to development of ASD.
Analysis: Rates of ASD were determined and risk differences calculated. Pooling of data was not done because of concerns regarding heterogeneity of treatments and populations as well as study quality.
Additional methodological and technical details are provided in the Web Appendix at www.aospine.org/ebsj.
Results
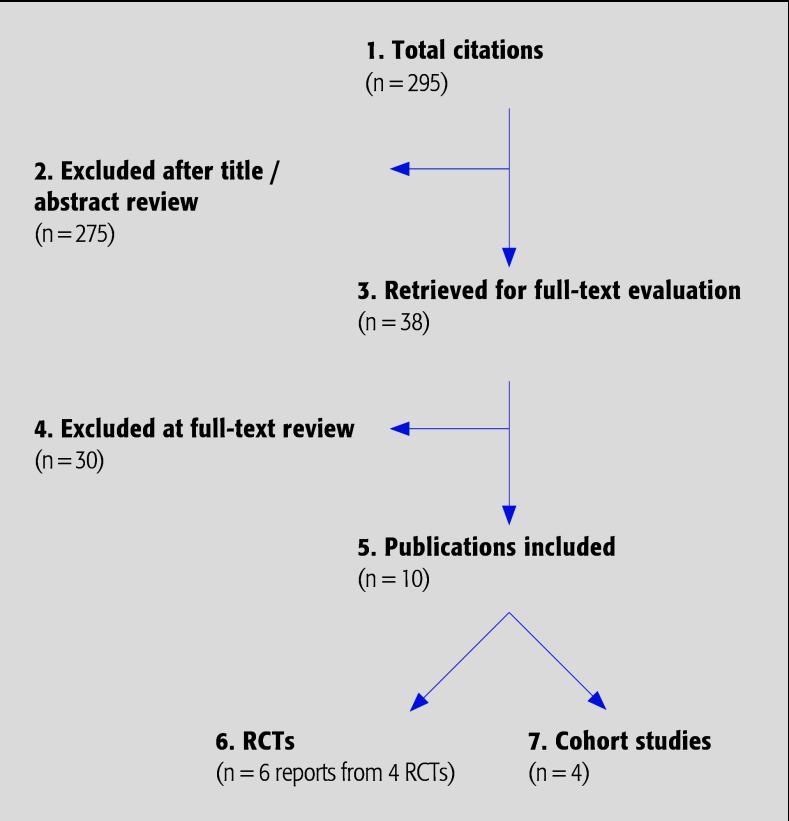
We initially identified 14 citations meeting the inclusion criteria (Fig. 1). Four reported results from two large, multicenter randomized controlled trials (RCT), CoE II, comparing cervical total disc arthroplasty (Prestige and Bryan discs) to anterior cervical discectomy and fusion (ACDF).2,3,4,5 Two reports overlapped but use different definitions of ASD;2,5 thus, only the most complete report was included for analysis.5 One additional study6 reported results from a smaller multicenter RCT (CoE II), comparing C-ADR (Kineflex|C) with ACDF, and one study,7 which reported only the results from a single clinical site of a large multicenter RCT was excluded. Two nonrandomized prospective studies (CoE III) compared C-ADR with ACDF.8,9 In addition, three cohort studies (CoE III) reported combined results of several multicenter RCTs of different C-ADR disc devices being performed at a single site,10,11 or multiple clinical sites;12 however, there was overlap between two studies11,12 and only the most complete was included for analysis.12 Thus, eight reports of comparative studies are critically summarized,3,4,5,6,8,9,10,12 four of which were from three RCTs3,4,5,6 Three reports of two trials describing adjacent segment range of motion (ROM) were identified,13,14,15 two of which were in the same patient population. The more complete published analysis was included.14
Fig. 1.
Results of literature search.
Populations were predominantly female and younger (Table 1). Different C-ADR disc devices were used including: Prestige, Bryan Discocerv/Discover, Mobi-C, Kineflex/C, and Advent. Six studies report results comparing a single disc device with ACDF, and two report results from multiple disc devices. Definitions of symptomatic ASD were all based on rate of reoperation at the adjacent level; however, definitions of radiographic ASD differed substantially between studies (Table 1).
Table 1. Summary of included studies*.
| Author (year) | Disc | % Follow-up | Demographics | Definitions |
|---|---|---|---|---|
| RCTs | ||||
| Sasso et al 5(2011)† n = 463 |
Bryan | 24 mo: Bryan: 95% (230/242) ACDF: 88% (194/221) | Bryan disc: n = 242 Female: 54% Mean age: 44.4 y ACDF: n = 221 Female: 49% Mean age: 44.7 y |
Symptomatic ASD: description of criteria for determination not reported; authors report number of secondary procedures‡ |
| 48 mo: Bryan: 75% (181/242) ACDF: 62% (138/221) | ||||
| Mummaneni et al 4,$(2007) Burkus et al 3,$(2010) N = 541 |
Prestige | 24 mo: Prestige: 80% (223/276) ACDF: 75% (198/265) |
Prestige disc: n = 276 Female: 54% Mean age: 43.3 y ACDF: n = 265 Female: 54% Mean age: 43.9 y |
Symptomatic ASD: description of criteria for determination not reported; authors report number of secondary surgeries at adjacent level |
| 60 mo: Prestige: 52% (144/276) ACDF: 48% (127/2651) | ||||
| Coric et al 6(2011) N = 269 |
Kineflex/C | 24 mo: Kineflex/C: 88% (119/136) ACDF: 87% (115/133) |
Total sample: Female: 59.1% Mean age: 43.8 y |
Symptomatic ASD: description of criteria for determination not reported; authors report rate of reoperation Radiographic: assessment of disc height, extent of osteophyte formation, degree of end plate sclerosis with grading as none, mild, moderate, or severe adjacent level degeneration; for those with preexisting moderate adjacent-level degeneration, a 1-grade change was required to classify the case as ASD; for others a 2-grade increase was classified as ASD |
| Cohort | ||||
| Coric et al 10(2010) N = 90 |
Bryan Kineflex/C Discover | Overall mean follow-up: 38 mo Follow-up of total sample: 92% (90/98) |
Total sample: Female: 61% Mean age: 46 y |
Symptomatic ASD: Clinically symptomatic adjacent-level disease ultimately determined by the rate of reoperation at the level directly adjacent to the treated level |
| Maldonado et al 8(2011) N = 190 |
Discocerv/Discover | 36 mo: Total sample: 91% (190/208) |
Discocerv/Discover: Female: 64% Mean age: 46.9 y ACDF: Female: 57% Mean age: 46.5 y |
Radiographic ASD: determined by new anterior osteophyte formation or enlargement of existing osteophytes, increased or new narrowing of a disc space (>30%), new or increased calcification of the anterior longitudinal ligament and the formation of radial osteophytes |
| Park et al 9(2011) N = 33 |
Mobi-C | Overall mean follow-up: Mobi-C: 28 mo ACDF: 30 mo |
Total sample: Male: 61% Mean age: 39 y |
Radiographic ASD: Development of new spondylotic changes in the adjacent vertebral bodies or a decrease of more than 10% in the height of adjacent discs |
| Nunley et al 12(2011) N = 182 |
Mixed devices∥ | Overall median follow-up: 38 mo Follow-up of total sample: 94% (170/180) C-ADR: 94% (112/120) ACDF: 92% (57/62) |
Total sample: Female: 55% Mean age: 45 y | Symptomatic/radiographic ASD: Determined by clinical and radiological (via Hillebrand criteria) evidence of ASD, and receipt of active intervention (subsequent surgery or medical management (pain medication, physical therapy, or steroid injection) for management of symptoms |
| Adjacent segment range of motion–RCT data | ||||
| Sasso et al 5 (2008) Total sample N = 463 Preoperative radiographs: Cephalad Bryan n =234 ACDF n = 209 Caudal Bryan n =148 ACDF n = 111 |
Bryan | Follow-up: 24 mo Follow-up: radiographs available at 24 mo Cephalad Bryan: 82% (192/234) ACDF: 77% (161/209) Caudal Bryan: 89% (132/148) ACDF: 86% (95/111) |
Bryan disc: n = 242 Female: 54% Mean age: 44.4 y ACDF: n = 221 Female: 49% Mean age: 44.7 y |
Angular ROM: determined on flexion and extension radiographs; a line was drawn along superior end plate of the cranial vertebrae; the difference between the two radiographs at each level was ROM; radiographs independently evaluated |
| Kelly et al 14 (2011) N = 209 Preoperative radiographs ProDisc n =100 ACDF n = 99 |
ProDisc | Follow-up: 24 mo Follow-up %: NR |
ProDisc: n = 100 Female: 56% Mean age: 42.1 y ACDF: n = 99 Female: 54% Mean age: 43.5 y |
ROM: Based on series of three later view radiographs in neutral, maximum active flexion, and maximum active extension; ROM from flexion through extension calculated using pattern recognition software; radiographs independently evaluated |
ACDF indicates anterior cervical discectomy and fusion; ASD, adjacent segment disease; RCT, randomized controlled trials; ROM, range of motion; NR, not reported or with respect to follow-up loss to follow-up not reported or could not be determined from data presented by authors.
Parent study is Anderson et al;2 this study was excluded because the definition of symptomatic ASD differed from Sasso et al, and Sasso et al5 reported 24-month data.
Does not include secondary procedures which involved both index and adjacent levels.
Mummaneni et al 4 is the original report of the FDA IDE study, Burkus et al 3 report the long-term follow-up.
Authors did not report which devices were used.
Longer-term data (48–60 months) for the Prestige5 and Bryan3 discs were available. Data from 24 months for these discs were summarized for comparison. Data at 24 months from the FDA investigational device exemption (IDE) trial of Kineflex/C discs were available.6
Further details on the class of evidence (Table 2) ratings and study characteristics for these studies can be found in the Web Appendix at www.aospine.org/ebsj.
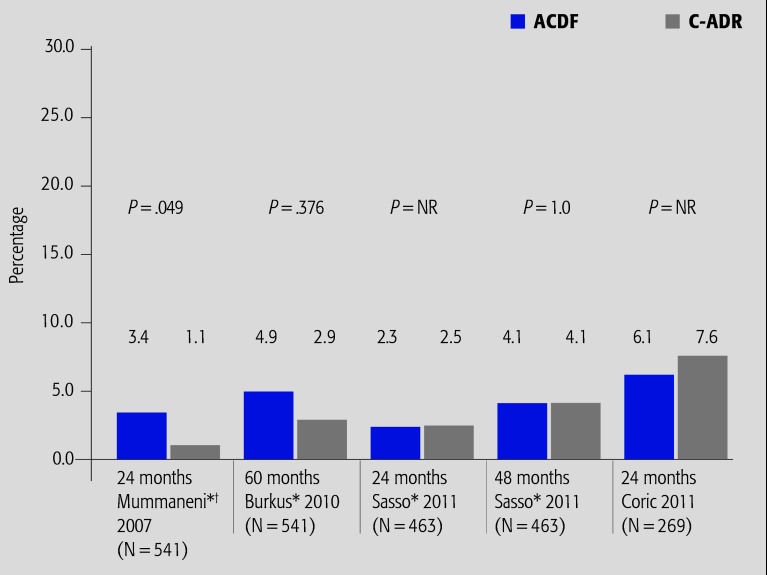
Symptomatic ASD (Fig. 2)
Fig. 2.
Summary of results from FDA IDE randomized controlled trials (CoE II) reporting rates of symptomatic ASD. NR indicates not reported; ACDF, anterior cervical discectomy and fusion; and C-ADR, cervical total disc arthroplasty.
* Percentages reported at 24 and 48 or 60 months were calculated using the full cohort (n); therefore, the denominator included participants who had either dropped out or had not yet completed follow-up.
† The article by Mummaneni et al 4 is the original report of the FDA IDE study; Burkus et al 3 report the long-term follow-up.
Data from FDA IDE trials provide the focus for this summary.
The difference in risks of symptomatic ASD were similar across studies (range of risk difference between treatment arms: 1.5%–2.3%), and was only marginally significant for one study at 24 months.4
At 24 months, one RCT reported a marginally significant decreased risk of symptomatic ASD in the Prestige disc treatment group;4 however; two additional FDA RCTs using other devices reported no differences in the rates of symptomatic ASD between treatment arms at 24 months.5,6
In studies with longer follow-up, rates of symptomatic ASD increased in both treatment groups over time; however, there were no statistically significant differences in rates of symptomatic ASD between treatment groups at either 48 or 60 months.3,5 Low follow-up rates in these reports should be considered when interpreting these results.
In a secondary analysis of RCT data from two sites (disc types not reported), ASD was defined as clinico-radiographic evidence of ASD and use of active intervention for management (surgery or conservative treatment). At last follow-up (median, 38 months), 16.8% of C-ADR and 14% of patients with ACDF were considered to have ASD.12
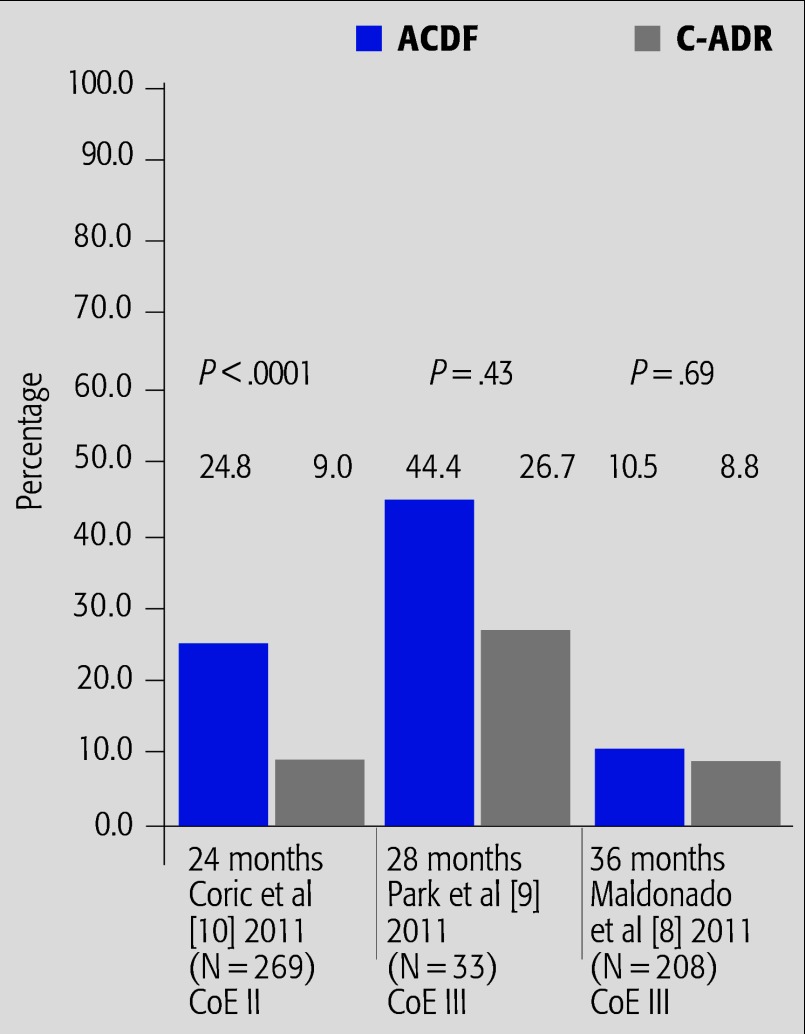
Radiographic ASD (Fig. 3)
Fig. 3.
Summary of results from studies reporting rates of radiographic ASD*.
* Definitions of radiographic ASD differ between studies. See Table 1.
The primary evidence for this outcome is from cohort studies (CoE III).
Rates of radiographic ASD differed dramatically across studies (8%–27% for C-ADR and 10%–44% for ACDF), likely due to differences in definitions of radiographic ASD. Rates were generally higher in the ACDF treatment group.
At 24 months, one RCT reported a significant decreased risk of radiographic ASD in the Kineflex/C treatment group.6 This same study reported, however, similar rates of symptomatic ASD between groups.
In cohort studies with longer follow-up,8,9 there were no differences in rates of radiographic ASD between treatment arms at 28 or 36 months (range of risk differences between treatment arms: 1.7%–17.7%).
ROM at adjacent segments
In one RCT,15 there were no statistical differences in angular ROM at the cranial and caudal segments between patients receiving the Bryan disc and those who had ACDF either preoperatively or at 24 months. The authors report no consistent correlation between angular ROM at adjacent segments with NDI, neck or arm pain in either treatment group. Loss to follow-up (>15%) with respect to cephalad motion evaluation was noted and may lead to potential selection bias.
In a post-hoc analysis of the ProDisc IDE trial,14 there were no statistical differences in adjacent segment motion between treatment groups. Within groups, time from surgery was a significant predictor of change in adjacent segment motion.
Timing of ASD development
Data from cohort analyses (CoE III) were available.
-
With regard to the timing of ASD development, one cohort study8 and one secondary analysis of RCT data12 report no statistical differences between C-ADR and ACDF.
In a secondary analysis of RCT data from two sites (disc types not reported), there was no significant difference in mean time free from clinico-radiographic ASD between treatment arms (C-ADR: 48.7 ± 1.04 months; ACDF: 46.04 ± 0.6 months; P > .05).12
In another cohort, time to radiographic ASD was not different between C-ADR and ACDF (data not reported; P = .072).8
Clinical Guidelines
No clinical guidelines specific to the focus of this topic were found. General clinical guidelines related to cervical C-ADR are summarized elsewhere in this issue.
Discussion
Conclusions from this review are limited by the following methodological concerns:
Mummaneni et al 4, Burkus et al 3 (60-month follow-up), and Sasso et al (48-month follow-up) 5: the denominator used to calculate the proportions of participants with ASD in each treatment arm in these studies is based on the full-sample size, not on the number of patients available at the time of follow-up; therefore, the denominator included participants who had either dropped out or had not yet completed follow-up. Thus, the rates of ASD in these studies may be artificially low.
Some studies 3,5 had substantial loss to follow-up. This may bias results if participants with symptoms were more likely to return for radiographic work-up than participants without symptoms, and would artificially increase ASD rates. In addition, loss to follow-up differed by treatment arm in at least one study 5, which may make rates of ASD artificially elevated in the treatment arm with more follow-up (especially if the denominator used to calculate ASD rates does not account for loss to follow-up). Furthermore, in the study by Burkus et al 3, the 60-month evaluations were ongoing (at the time of publication) and thus, loss to follow-up was primarily related to participants not having reached their 60-month time point in the study.
It is difficult to compare rates of radiographic ASD across studies because authors use substantially different definitions for radiographic ASD. The study 9 reporting the highest rates used a definition with the lowest threshold for change in the height of adjacent discs (10%). Nunley et al 12 used a definition that included both radiographic and symptomatic elements. (See the Table for complete descriptions of definitions).
The relationship between radiographic and symptomatic ASD is not clear. Radiographic ASD may be an intermediate/indirect outcome. No direct relationship between radiographic and symptomatic ASD was evaluated.
An association between adjacent segment motion and function or clinically relevant outcomes is not evident.
Conclusions regarding rates of symptomatic ASD are challenging given the variations in reporting of symptomatic ASD based on surgical intervention at adjacent levels following the index procedure. In one study 5, participants who had undergone reoperation at the index and adjacent site were not included as symptomatic ASD cases; however, in other studies 3,4, participants who had undergone reoperation at the index and adjacent sites were included as symptomatic ASD. Other studies do not report cases of surgical intervention at both the index and adjacent site 6,10. It should be noted that reoperation was at the discretion of the surgeon and the patient but the surgeon can heavily influence the patient’s decision. Therefore, it is possible that the surgeon may have had subconscious or conscious bias against reoperating on a disc patient. If true, the reoperation rates for the arthroplasty patients may have been artificially low.
Based on the above, there is inconclusive evidence to support the role of arthroplasty as a means of decreasing adjacent level disease. Part of the problem is that there is no uniform definition of adjacent level disease. But even if we limit our definition to those cases that required operation at the adjacent level, the evidence for arthroplasty being superior to ACDF is lacking. Some of the lack of evidence is due to inadequate follow-up and there are unpublished but nationally presented reports that with the Prodisc-C, there is a statistically significant difference in reoperation rates at 5 years. Until the peer-review process is completed and the article published, however, we cannot comment on the validity of their conclusions.
Conclusions
Our review suggests that, to date, the promise of arthroplasty decreasing the risk of ASD compared with ACDF remains unfulfilled. Perhaps some of the longer-term follow-up data, which is currently in the peer-review process, or data from other arthroplasty trials will reveal differences. Until such time, arthroplasty’s main advantage appears to be motion preservation and not adjacent level preservation.
Evidence Summary
Table 2. Adjacent segment disease.
| Outcomes | Strength of evidence | Conclusions/comments |
|---|---|---|
| 1. ASD – symptomatic |
|
|
| 2. ASD – radiographic |
|
|
| 3. Timing of ASD |
|
|
| 4. Range of motion at adjacent segments |
|
Acknowledgment
The authors wish to thank Ellen VanAlstyne for assistance with literature search, evaluation and abstraction and Daniel Hadidi for assistance with data abstraction.
Footnotes
Royalties from Medtronic for posterior cervical fusion system, Biomet for anterior cervical plate, Osprey Allografts Stock ownership in Spinal kinetics, Amedica, Nexgen, Paradigm spine, PSD, Spineology.
References
- 1.Hilibrand A S, Carlson G D, Palumbo M A. et al. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg. 1999;81(4):519–528. doi: 10.2106/00004623-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Anderson P A, Sasso R C, Riew D. Comparison of adverse events between the Bryan artificial cervical disc and anterior cervical arthrodesis. Spine (Phila Pa 1976) 2008;33(12):1305–1312. doi: 10.1097/BRS.0b013e31817329a1. [DOI] [PubMed] [Google Scholar]
- 3.Burkus J K, Haid R W, Traynelis V C. et al. Long-term clinical and radiographic outcomes of cervical disc replacement with the Prestige disc: results from a prospective randomized controlled clinical trial. J Neurosurg Spine. 2010;13(3):308–318. doi: 10.3171/2010.3.SPINE09513. [DOI] [PubMed] [Google Scholar]
- 4.Mummaneni P V, Burkus J K, Haid R W. et al. Clinical and radiographic analysis of cervical disc arthroplasty compared with allograft fusion: a randomized controlled trial. J Neurosurg Spine. 2007;6(3):198–209. doi: 10.3171/spi.2007.6.3.198. [DOI] [PubMed] [Google Scholar]
- 5.Sasso R C, Anderson P A, Riew D. et al. Results of cervical arthroplasty compared with anterior discectomy and fusion: four-year clinical outcomes in a prospective, randomized controlled trial. J Bone Joint Surg. 2011;93(18):1684–1692. doi: 10.2106/JBJS.J.00476. [DOI] [PubMed] [Google Scholar]
- 6.Coric D, Nunley P D, Guyer R D. et al. Prospective, randomized, multicenter study of cervical arthroplasty: 269 patients from the Kineflex|C artificial disc investigational device exemption study with a minimum 2-year follow-up: clinical article. J Neurosurg Spine. 2011;15(4):348–358. doi: 10.3171/2011.5.SPINE10769. [DOI] [PubMed] [Google Scholar]
- 7.Garrido B J, Taha T A, Sasso R C. Clinical outcomes of Bryan cervical disc arthroplasty: a prospective, randomized, controlled, single site trial with 48-month follow-up. J Spinal Disord Tech. 2010;23(6):367–371. doi: 10.1097/BSD.0b013e3181bb8568. [DOI] [PubMed] [Google Scholar]
- 8.Maldonado C V, Paz R D, Martin C B. Adjacent-level degeneration after cervical disc arthroplasty versus fusion. Eur Spine J. 2011;20 03:403–407. doi: 10.1007/s00586-011-1916-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park S B Jahng T A Chung C K Remodeling of adjacent spinal alignments following cervical arthroplasty and anterior discectomy and fusion Eur Spine J 2011. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coric D, Cassis J, Carew J D. et al. Prospective study of cervical arthroplasty in 98 patients involved in 1 of 3 separate investigational device exemption studies from a single investigational site with a minimum 2-year follow-up: clinical article. J Neurosurg Spine. 2010;13(6):715–721. doi: 10.3171/2010.5.SPINE09852. [DOI] [PubMed] [Google Scholar]
- 11.Jawahar A, Cavanaugh D A, Kerr E J. et al. Total disc arthroplasty does not affect the incidence of adjacent segment degeneration in cervical spine: results of 93 patients in three prospective randomized clinical trials. Spine J. 2010;10(12):1043–1048. doi: 10.1016/j.spinee.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 12.Nunley P D Jawahar A Kerr E J et al. Factors affecting the incidence of symptomatic adjacent level disease in cervical spine after total disc arthroplasty: 2–4 years follow-up of 3 prospective randomized trials Spine (Phila Pa 1976) 2011. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.Auerbach J D, Anakwenze O A, Milby A H. et al. Segmental contribution towards total cervical range of motion: a comparison of cervical disc arthroplasty and fusion. Spine (Phila Pa 1976) 2011;36(25):E1593–1599. doi: 10.1097/BRS.0b013e31821cfd47. [DOI] [PubMed] [Google Scholar]
- 14.Kelly M P, Mok J M, Frisch R F. et al. Adjacent segment motion after anterior cervical discectomy and fusion versus Prodisc-c cervical total disk arthroplasty: analysis from a randomized, controlled trial. Spine (Phila Pa 1976) 2011;36(15):1171–1179. doi: 10.1097/BRS.0b013e3181ec5c7d. [DOI] [PubMed] [Google Scholar]
- 15.Sasso R C, Best N M, Metcalf N H. et al. Motion analysis of bryan cervical disc arthroplasty versus anterior discectomy and fusion: results from a prospective, randomized, multicenter, clinical trial. J Spinal Disord Tech. 2008;21:393–399. doi: 10.1097/BSD.0b013e318150d121. [DOI] [PubMed] [Google Scholar]