Abstract
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is the most common hereditary vascular dementia caused by mutations in NOTCH3 gene. Pathology is manifested in small- and middle-sized arteries throughout the body, though primarily in cerebral white matter. Hemodynamics is altered in CADASIL and NOTCH3 is suggested to regulate actin filament polymerization and thereby vascular tone. We analyzed NOTCH3 expression and morphology of actin cytoskeleton in genetically genuine cultured human CADASIL vascular smooth muscle cells (VSMCs) (including a cell line homozygous for p.Arg133Cys mutation) derived from different organs, and in control VSMCs with short hairpin RNA (shRNA)-silenced NOTCH3. NOTCH3 protein level was higher in VSMCs derived from adult than newborn arteries in both CADASIL and control VSMCs. CADASIL VSMCs showed altered actin cytoskeleton including increased branching and node formation, and more numerous and smaller adhesion sites than control VSMCs. Alterations in actin cytoskeleton in shRNA-silenced VSMCs were similar as in CADASIL VSMCs. Severity of the alterations in actin filaments corresponded to NOTCH3 expression level being most severe in VSMCs derived from adult cerebral arteries. These observations suggest that hypomorphic NOTCH3 activity causes alterations in actin organization in CADASIL. Furthermore, arteries from different organs have specific characteristics, which modify the effects of the NOTCH3 mutation and which is one explanation for the exceptional susceptibility of cerebral white matter arteries.
Keywords: actin filament, adhesion site, CADASIL, NOTCH3, shRNA silencing, vascular smooth muscle cell
Introduction
Mutations in a transmembrane receptor NOTCH3 cause cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), which is the most common hereditary vascular dementia.1 The main clinical features of CADASIL are migraine with aura, ischemic attacks, cognitive impairment as well as mood disturbances and apathy.2, 3 Blood vessels are affected throughout the body, but the cerebral white matter arteries bear the brunt of the disease and the symptoms are almost exclusively neurological. The key pathogenic process is the degeneration of vascular smooth muscle cells (VSMCs) mainly in small- and middle-sized arteries. Intercellular space is widened and the affected VSMCs lose their intercellular connections and transform their shape from elongated to more rounded and irregular.4 A specific early feature of CADASIL is the accumulation of granular osmiophilic material (GOM) on or in close vicinity of the degenerating VSMCs.5, 6 The extracellular domain of NOTCH3 (N3ECD) has also been demonstrated to accumulate in the walls of the affected arteries7 and it was subsequently shown that N3ECD is a component of GOM.8 The walls of the affected vessels become thickened and fibrotic due to deposition of the ECM (extracellular matrix) proteins, for example, collagens, laminin, and fibronectin.3, 9
Vasoregulatory and circulatory disturbances are well established in CADASIL patients. Reduction of blood flow in cerebral white matter appears already around the age of 30 years and becomes more severe as the disease progresses.10 Retinal arteries have been shown to appear similarly contracted as in hypertension and retinal blood flow is somewhat decreased.11, 12 CADASIL patients have impaired endothelium-dependent vasodilation in peripheral resistant arteries13, 14 while in cerebral resistance arteries the vasoreactivity could be enhanced by systemic application of ℒ-arginine.15 Moreover, alterations in vasoregulation have also been detected in caudal arteries of transgenic mice expressing mutated (p.Arg90Cys) human NOTCH316 and in cerebral arteries in transgenic mice expressing mutated (p.Arg169Cys) rat NOTCH3.17
NOTCH3 protein is a large single pass transmembrane receptor expressed in adult human almost exclusively in VSMCs and pericytes7 and is needed for structural integrity and generation of functional arteries.18 Overexpression of the constitutively active NOTCH3 intracellular domain (N3ICD) in cultured VSMCs results in an increase of actin stress fibers and in steady-state levels of polymerized actin.18 Analogously, Notch3-/- mice manifested decreased vascular tone and impaired response to cyclic strain and shear stress in cerebral and tail resistance arteries, possibly due to decreased actin polymerization as suggested by reduced RhoA activity.19
Upregulation of contractile proteins in our proteomic analysis of cultured human CADASIL VSMCs20 prompted us to analyze in greater detail the consequences of expression of mutated NOTCH3 for the cytoskeleton of VSMCs. In the present work, we have analyzed the organization of actin cytoskeleton and adhesion complexes in cultured VSMCs derived from blood vessels of different organs from 10 CADASIL patients and from seven control individuals. All CADASIL cell lines are genetically genuine carrying either endogenous p.Arg133Cys or p.Gly528Cys mutation, and include a unique cell line homozygous for the p.Arg133Cys mutation. In addition, we investigated the changes in actin cytoskeleton in control VSMCs in which NOTCH3 is silenced by short hairpin RNA (shRNA). The findings presented here suggest that hypomorphic NOTCH3 activity results in altered organization of actin cytoskeleton in CADASIL VSMCs.
Materials and methods
Generation of Patient Cell Lines and Cell Culture
Cell lines were established from blood vessels of genetically verified CADASIL patients and control subjects as previously described.20 Nine CADASIL patients carried p.Arg133Cys mutation, eight were heterozygous and one was homozygous. In addition, one CADASIL patient carried p.Gly528Cys mutation. Vessels were obtained post partum from pregnancies, in which either of the parents was diagnosed with CADASIL (human umbilical cord arterial VSMC=HUmbVSMC and human placental VSMC=HPlaVSMC), from surgical operations (human systemic arterial VSMC=HArtVSMC) or post mortem (HArtVSMC and human cerebral arterial VSMC=HCerVSMC). The control cells were obtained from corresponding vessels of non-CADASIL individuals (Table 1). All post mortem samples were collected within 24 hours of death and all cell cultures were established immediately after obtaining the samples. The acquisition of the vessels was approved by the joint Ethical Board of the Hospital District of Varsinais-Suomi and Turku University Hospital, and the National Authority for Medicolegal Affairs of Finland.
Table 1. VSMC lines used in this study.
| Cell line | Origin | Genotypes (n) | Phenotype | Patient age |
|---|---|---|---|---|
| HUmbVSMC | Post partum, umbilical cord artery | wt (4) and p.R133C (4) | Spindle-shaped | Newborn |
| HPlaVSMC | Post partum, placental vessel | wt (4) and p.R133C (4) | Spindle-shaped | Newborn |
| HArtVSMC | Surgery and post mortem, Adult systemic artery | p.R133C (3) | Spindle-shaped | p.R133C: 61, 66, 64 |
| HCerVSMC | Post mortem, subarachnoidal branches of cerebral arteries | wt (3) p.R133C (2); p.R133C-hmz (1) p.G528C (1) | Epithelioid-shaped | WT: 61, 73, 82 p.R133C: 64, 65 p.R133C-hmz: 62 p.G528C: 68 |
Cell line designates the abbreviated names of the cell lines used in the text. Origin defines the vessel from which the cell line has been isolated. Genotype shows the NOTCH3 mutation detected in the cell lines. VSMC=vascular smooth muscle cell, wt=wild type, n=number of cell lines analyzed. Phenotype designates the observed phenotype under phase contrast light microscopy. Age designates the age of the patient from whom the cell line was established.
The cells were infected with a human papilloma virus construct E6/E7 at early passage (p1 to p3) to partially immortalize the cell lines. The infection was verified by culturing the cells in the presence of G418 (Invitrogen, Auckland, NZ, USA) (400 μg/mL) for a 10-day period. Cells were identified to be VSMCs by α-smooth muscle actin (α-SMA) staining as primary cells (passage 1) and after the viral infection (passage 2 to 5) and all VSMC lines were screened negative for mycoplasma by VenorGeM mycoplasma detection kit (Minerva Biolabs GmbH, Berlin, Germany) or with DAPI staining. Passages of the cells were matched in each experiment and were maximally at passage 10, except for the NOTCH3-silenced cells, which were at passage 18.
Genotyping
The genotype of the cell lines [presence or absence of the c.475C>T (p.Arg133Cys) or c.1582G>T (p.Gly528Cys) mutation] was verified by amplifying exon 4 (for analysis of mutation c.475C>T) or exons 9 and 10 (for analysis of mutation c.1582G>T) of NOTCH3 gene by PCR and digesting the PCR product with restriction enzymes MspA1I (c.475C>T) or BccI (c.1582G>T). Both mutations delete one restriction site producing an additional larger restriction fragment as compared with the wild-type NOTCH3 gene. In the sample from the homozygote, one restriction fragment is completely absent. Cells with the heterozygous p.Arg133Cys mutation are referred in the text as CADASIL cells, cells with homozygous p.Arg133Cys mutation as CADASIL-hmz cells, cells with p.Gly528Cys as CADASIL G528C cells, and cells without CADASIL mutation as control cells.
Silencing of NOTCH3 in Vascular Smooth Muscle Cells with Lentiviral Vectors
To generate lentiviral transduction particles, a set of five pLKO.1 vectors encoding shRNA targeting NOTCH3 gene (GenBank accession number NM_000435) and nontarget shRNA control vector (TRC1 library, Sigma-Aldrich, St Louis, MO, USA) were cotransfected into 293FT cells (Invitrogen, Carlsbad, CA, USA) along with packaging plasmids using Fugene HD (Roche Diagnostics, Mannheim, Germany). Supernatants containing infectious lentiviral particles were collected 72 hours after transfection and passed through 0.45 μm filters. Control HUmbVSMCs were seeded onto 6 cm plates (6 × 105 cells per plate) 24 hours before transduction. Viral transduction was carried out using medium containing lentiviral particles in the presence of polybrene at a final concentration of 5 μg/mL. The cells received fresh complete medium 24 hours later. The transduced control HUmbVSMCs were selected 48 hours after transduction with puromycin (Sigma-Aldrich) at a final concentration of 1 μg/mL for 5 days and were subsequently cultured without puromycin until the end of experimentation.
Validation of NOTCH3 silencing in the transduced cells was carried out by Western blot analysis 3 days after puromycin selection at passage 18. To assess the level of silencing, ImageJ analysis software (NIMH, Bethesda, MD, USA) was used to calculate the relative density of each band (N3 full lenght+N3ICD) normalized to β-actin as internal control. Stable cell lines created with three pLKO.1 shRNA vectors TRCN0000020234, TRCN0000020235, and TRCN0000020238 showed significant reduction of NOTCH3 protein expression. Reduction of NOTCH signaling activity was confirmed by Western blot analysis of HES5 expression. Detection of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used to confirm equal loading. Final experiments were performed using stable cell line generated with TRCN0000020234 or TRCN0000020235 construct (referred in text as N3-targeted shRNA1 and shRNA2, respectively).
Immunocytochemistry
Vascular smooth muscle cells were fixed with paraformaldehyde for analysis of cell surface NOTCH3 and with methanol for analysis of intracellular NOTCH3 and other intracellular proteins. Fixed cells were blocked with 3% bovine serum albumin in phosphate-buffered saline for 30 minutes and incubated with primary antibodies, followed by appropriate Alexa-fluor labeled secondary antibody (Molecular Probes, Eugene, OR, USA).
All available cell lines were analyzed for immunofluorescence for NOTCH3 and actin: HUmbVSMC: CADASIL n=4, control n=4, HPlaVSMC CADASIL n=4, control n=4, HArtVSMC: CADASIL n=3, HCerVSMC: CADASIL n=2, CADASIL-hmz n=1, control n=3. Immunofluorescence analysis of adhesion sites was performed with two CADASIL and two control VSMC lines, one derived from newborn and one from adult tissue.
Western Blot Analysis
Cultured cells at passage 5 or at 18 (shRNA-silenced cells) were washed three times with phosphate-buffered saline and lysed in RIPA-buffer with complete mini protease inhibitor cocktail (Roche, Meylan Cedex, France) on ice for 15 minutes. Cells were scraped from the culture plates and sonicated 3 × 30 seconds on water bath sonicator. Cell suspension was centrifuged at 10,000 g for 15 minutes at 4°C. Insoluble cell debris was discarded. Protein concentrations were measured by Quant kit (GE Healthcare, Amersham, UK). Western blot was performed according to the manufacturer's instructions. Antigens were detected with the primary antibodies followed by HRP-labeled secondary anti-mouse or anti-rabbit antibodies and ECL-plus substrate (GE Healthcare). Blots were scanned and visualized with a fluorescence scanner (Typhoon 9400, GE Healthcare).
Primary Antibodies
Primary antibodies used in Western blot and immunocytochemistry were NOTCH3 clone 1E4, NOTCH3 clone 5E1, both directed against N3ECD (both kind gifts from Dr A Joutel), NOTCH3 ICD (D11B8, Cell Signaling, Beverly, MA, USA), α-SMA (Sigma), HES5 (M-104, Santa Cruz Biotechnology, Heidelberg, Germany) β-Actin (Sigma), vinculin (Biohit, Helsinki, Finland), integrin β1,21 tensin (BD Transduction Laboratories, Mississauga, Canada).
Statistical Analyses
Significance of the differences of average area of adhesion complexes and the intensities in Western blots was analyzed with Student's t-test. P values <0.05 were considered significant.
Results and discussion
We have previously shown that cultured patient CADASIL HUmbVSMCs have altered expression of several proteins involved in actin polymerization and VSMC contraction, and that CADASIL VSMCs have impaired spontaneous contractility.20 In this study, we have analyzed the association of CADASIL mutations and shRNA silencing of NOTCH3 with the organization of actin cytoskeleton and adhesion complexes in cultured VSMCs derived from blood vessels of different organs from ten CADASIL patients and corresponding controls.
Growth Characteristics of the Vascular Smooth Muscle Cells
Vascular smooth muscle cell lines derived from blood vessels of different organs shared common features, but were morphologically different from each other (Table 1). HUmbVSMCs, HPlaVSMCs, and HArtVSMCs appeared as typical VSMCs.22 They were large, elongated and had prominent nucleus. In culture, they oriented themselves into parallel bundles, became spindle-shaped and have a tendency to grow in multilayered aggregates. CADASIL and control HCerVSMCs were more irregular in shape than HArtVSMCs and their appearance resembled the epithelioid-like phenotype of VSMCs isolated from human thoracic artery.23 All CADASIL VSMCs were larger in size and had slower proliferation rate than the corresponding control VSMCs, consistent with a previous study performed with HEK293 cells expressing mutant NOTCH3 (p.R133C or p.C185R).24 The proliferation rates of the VSMCs varied according to the origin of the cell line: HPlaVSMC>HArtVSMC≥HUmbVSMCs>HCerVSMC.
Expression and Cellular Localization of NOTCH3
We followed the expression of NOTCH3 by immunostaining with the 1E4 antibody, which is directed against N3ECD, and detects both N3ECD and full-length NOTCH3. Cells were fixed with paraformaldehyde for analysis of cell surface and with methanol for analysis of intracellular NOTCH3.
Cell surface NOTCH3
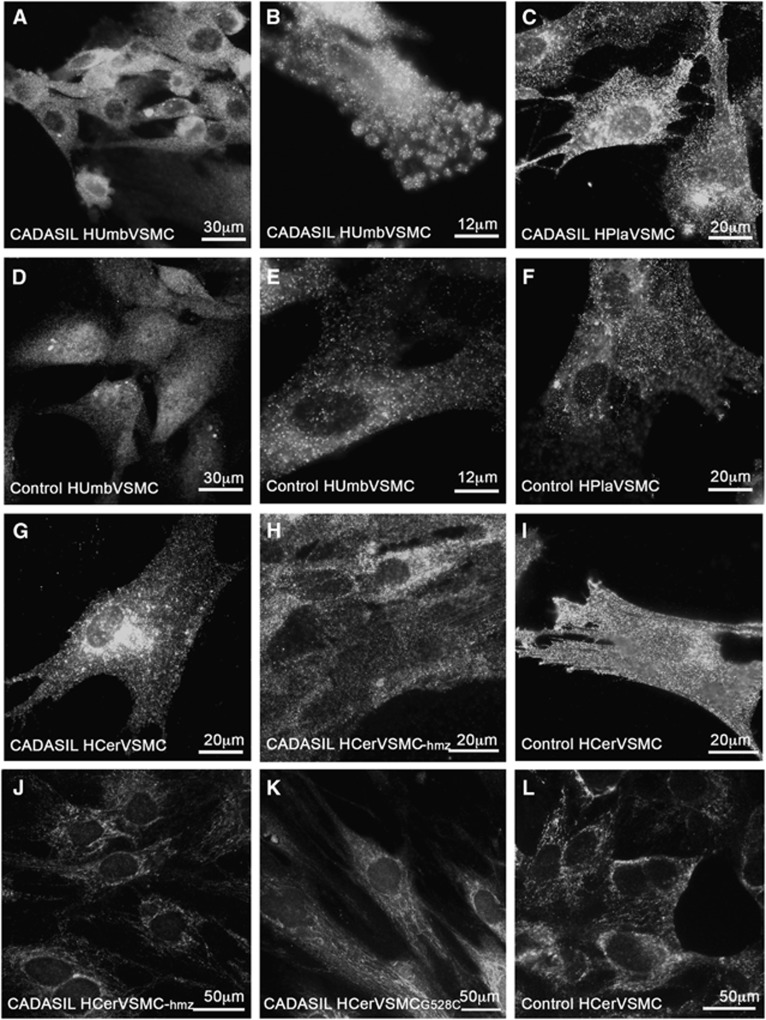
N3ECD immunoreactivity on the cell surface was clustered in aggregates in CADASIL VSMCs, whereas control VSMCs were uniformly stained (Figures 1A–I). After binding of ligands wild-type N3ECD is transendocytosed and degraded, whereas in CADASIL the mutated N3ECD accumulates, which in vivo appears as deposits of N3ECD incorporated into GOM on arterial VSMCs.4, 6, 7, 8 The observed aggregation may be caused by an unpaired cysteine residue in mutated NOTCH3, which increases interreceptor disulphide bridges.25, 26 However, some other conformational changes in N3ECD might also contribute to the aggregation. This may result in formation of homo- and hetero-oligomers,25 which in turn may lead to accumulation of N3ECD on VSMCs.
Figure 1.
Subcellular localization of NOTCH3 in CADASIL vascular smooth muscle cells (VSMCs). For detection of cell surface NOTCH3, VSMCs were fixed with 3.5% phosphate-buffered paraformaldehyde. NOTCH3 forms more aggregates on the cell surface in CADASIL than in control VSMCs (A–I). For intracellular NOTCH3 detection, VSMCs were fixed with methanol. Permeabilized VSMCs show that NOTCH3 does not aggregate inside the cell in either CADASIL or control VSMCs (J–L). Immunostainings were performed with antibody (1E4) directed against N3ECD recognizing full-length NOTCH3 and N3ECD. Number of cell lines analyzed: HUmbVSMC: CADASIL n=4, control n=4, HPlaVSMC CADASIL n=4, control n=4, HArtVSMC: CADASIL n=3, HCerVSMC: CADASIL n=2, CADASIL-hmz n=1, control n=3.
Intracellular NOTCH3
In our study, intracellular NOTCH3 appeared to be localized in membranous structures, most likely located within Golgi complex and endoplasmic reticulum (Figures 1J–L). Only a few intracellular NOTCH3 positive aggregates were detected with similar frequency in CADASIL and control VSMC lines. Similar aggregates have been reported in two studies on cultured transfected HEK293 cells.27, 28 In the first study, a higher frequency of aggregates was reported in HEK cells overexpressing mutated murine NOTCH3 than in those expressing wild-type NOTCH3.27 In the second study, the number of aggregates was similar in HEK cells irrespective of whether they expressed mutated or wild-type human NOTCH3.28
Total cellular NOTCH3
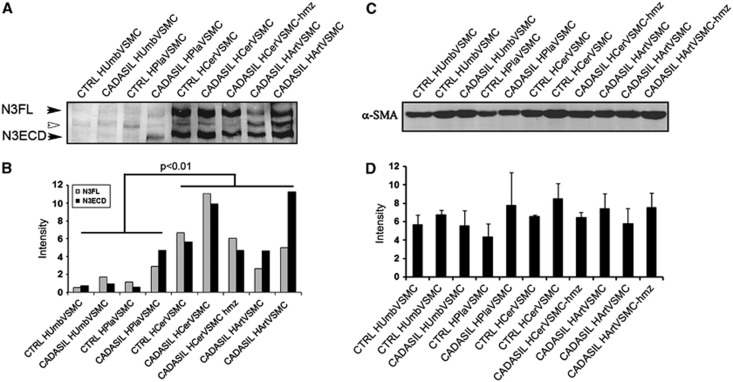
Although immunostaining showed more N3ECD aggregates on the surface of CADASIL than control VSMCs, we were unable to detect statistically significant difference in the quantities of full-length NOTCH3 or N3ECD between any CADASIL versus control VSMCs (Figures 2A and 2B).
Figure 2.
Total NOTCH3 and α-SMA expression in CADASIL and control vascular smooth muscle cells (VSMCs). (A) Western blot analysis of the expression of NOTCH3 detected with 5E1 directed against N3ECD in CADASIL and control VSMCs. In all, 40 μg of cell lysate was loaded on each lane. The 280-kDa full-length NOTCH3 (N3FL) and 220 kDa N3ECD are indicated with arrowheads. The 245-kDa band in between (white arrowhead) is unknown. (B) The expression of NOTCH3 is significantly higher in HCerVSMCs and HArtVSMCs than in HUmbVSMCs or HPlaVSMCs. There are no statistically significant differences in the total expression level of NOTCH3 or N3FL/N3ECD ratio between CADASIL versus the corresponding control or between control versus heterozygous or homozygous VSMCs. (C) Western blot analysis of α-SMA expression in CADASIL and control VSMCs. In all, 20 μg of cell lysate was loaded on each lane. (D) Even though, CADASIL cells have altered actin filament organization, the total amount of α-SMA in CADASIL VSMCs is not significantly different from that in corresponding control VSMCs. The bar graphs show the average intensities and ±s.e.m. from two independent experiments. Both experiments were repeated two times with cells at passage 5.
Several different experimental models using transfected cells have been applied to analyze the processing of mutated NOTCH3. These have shown impaired or protracted S1 cleavage27, 29 or have indicated that mutated NOTCH3 is correctly processed.28, 30 Because in our Western blot analysis, there was no statistically significant difference in the full-length NOTCH3/N3ECD-ratio between CADASIL versus control VSMC lines, the S1 cleavage of mutated NOTCH3 appears to occur normally in CADASIL VSMCs. The data suggest that in human VSMCs with endogenous NOTCH3 expression, the microenvironment in Golgi and endoplasmic reticulum is able to prevent intracellular interreceptor crosslinking and aggregation of the mutated receptor. Thus, the aggregation does not occur until the receptor reaches the cell surface and therefore the accumulation of N3ECD on VSMCs in CADASIL is most likely due to its local aggregation and/or its impaired clearance.
NOTCH3 in different vascular beds
The expression of NOTCH3 was different in VSMCs derived from different vascular beds, being significantly higher (P<0.01; Figure 2B) in adult (HCerVSMCs and HArtVSMCs) than in newborn (HUmbVSMCs or HPlaVSMCs) VSMCs and furthermore, appears higher in HCerVSMCs than in HArtVSMCs (no statistical significance). Given the notion that NOTCH3 is expressed only at low levels in newborn (perinatal) VSMCs suggests that NOTCH3 is primarily needed after birth in the postnatal period18 when the blood pressure rises and the blood circulation becomes dependent on the newborn's own heart.31 These results suggest that the expression of NOTCH3 is induced during postnatal period and is needed in human adults, possibly to prime the blood vessels for increased pressure and maintain arterial integrity.18 NOTCH3 may also have an important and specific, yet unknown, role in VSMC function in those areas, where its expression is high (e.g., cerebral arteries).
Actin Filaments
Experimental studies have provided evidence of the role of NOTCH3 in actin filament polymerization in vitro18 and in vasoregulation in vivo.16, 17, 19, 32 Similar vasoregulatory results have been obtained from human studies showing impaired endothelium-dependent vasodilation in peripheral resistant arteries in CADASIL patients.13, 14 These data are in agreement with the notion that NOTCH3 promotes actin polymerization and may be involved in a sensory system that provides VSMCs with ability to rearrange actin in response to mechanical strain. To study the effects of mutated NOTCH3 on actin cytoskeleton, we analyzed the α-SMA immunopositive actin filaments in CADASIL and control VSMCs.
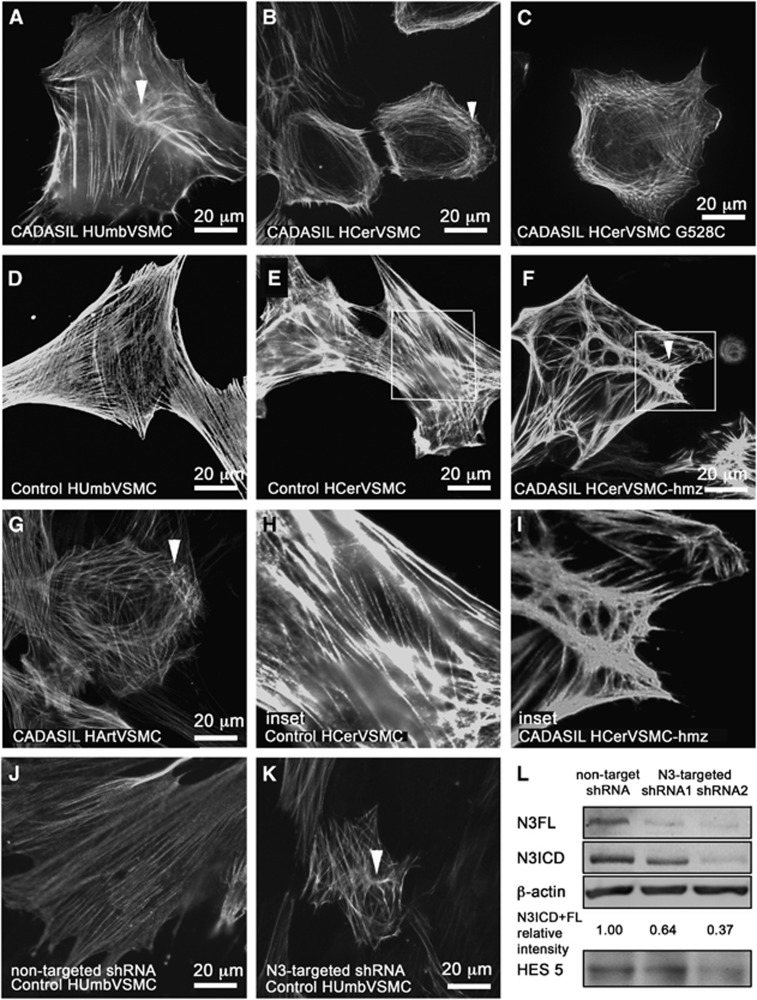
The staining revealed altered organization of actin filaments in CADASIL VSMCs as compared with control VSMCs (Figure 3). In all CADASIL VSMC lines isolated from the newborn (HUmbVSMCs and HPlaVSMCs; Figure 3A: CADASIL HUmbVSMC, D control HumbVSMC), the abnormalities in actin filaments were only minor: many of the cells appeared as control cells and about 39% had minor abnormalities in the organization of the actin cytoskeleton (representative images shown). Abnormalities in the organization of actin filaments were most severe in CADASIL HCerVSMCs: on average 72% of the HCerVSMCs had major abnormalities in the organization of the actin cytoskeleton (representative images shown) (Figures 3B, C, inset I, control E, inset H). In 64% of HCerVSMCs from a heterozygous p.Arg133Cys patient (Figure 3B), actin filaments circled the cell and there were few nodes. In 69% of HCerVSMCs derived from a CADASIL patient with heterozygous p.Gly528Cys mutation showed similar nodes and circular orientation of filaments (Figure 3C). In 82% of HCerVSMCs-hmz (Figure 3F, inset I), actin filaments were in robust bundles and they formed several nodes with clusters of haphazardly oriented filaments (representative image shown). Such substantial deviations were not detected in any of the heterozygous CADASIL VSMC lines. In all, 60% of CADASIL HArtVSMCs also exhibited prominent abnormalities: fewer and shorter actin filaments, some with abnormal nodes and increased branching (Figure 3G). Similar correlation of altered actin filament organization was observed between HCerVSMCs and HArtVSMCs cell lines established from same patient (p.R133C; age 64).
Figure 3.
Actin cytoskeleton in CADASIL vascular smooth muscle cells (VSMCs). VSMCs fixed with methanol were immunostained for smooth muscle cell α-actin (α-SMA). α-SMA filament have altered organization in CADASIL VSMCs. In all CADASIL VSMCs the α-SMA positive actin filaments are short, poorly organized and form nodes (clusters of short haphazardly oriented filaments, selected five marked with arrowheads) (A: HUmbVSMC, B: HCerVSMC, C: HCerVSMC-G528C, F: HCerVSMC-hmz, and G: HArtVSMC). Actin filament nodes in CADASIL VSMCs are shown in higher magnification in an inset (I: HCerVSMC-hmz). Control VSMCs have robust cell spanning actin filaments (D: HUmbVSMC and E: HCerVSMC and an inset H: HCerVSMC). Number of cell lines analyzed: HUmbVSMC: CADASIL n=4, control n=4, HPlaVSMC CADASIL n=4, control n=4, HArtVSMC: CADASIL n=3, HCerVSMC: CADASIL n=2, CADASIL-hmz n=1, control n=3. Control HUmbVSMCs were transduced with nontarget short hairpin RNA (shRNA) (J) or NOTCH3-targeted shRNA2 (K). Silencing of NOTCH3 expression induced similar alterations to organization of actin filaments as observed in CADASIL VSMCs. Silencing of NOTCH3 expression was verified by Western blot (L). NOTCH3-targeted shRNA1 and shRNA2 reduced relative intensity of NOTCH3 expression by 36 and 63% when compared with nontarget shRNA. NOTCH3 signaling activity is also reduced as indicated by lower expression level of NOTCH3 target gene HES5 (shRNA1 5% and shRNA2 30%). Relative intensity is calculated from an average of combined N3FL and N3ICD intensities. Intensities are averages from two independent experiments normalized to β-actin. Average intensities are shown in proportion to nontarget shRNA sample (relative intensity). N3FL and N3ICD were detected by antibody directed against N3ICD.
Vascular smooth muscle cell lines from adult arteries (HCerVSMCs and HArtVSMCs) produced greater amount of NOTCH3 than the VSMCs from newborn blood vessels (HUmbVSMCs and HPlaVSMCs) (Figure 2). Therefore, the degree of abnormalities in actin filaments appeared to correspond to the NOTCH3 expression level. In addition, the actin abnormalities where more prominent in homozygous cell line compared with heterozygous cell lines (82% versus 69% and 64%) (Figure 3), supporting the role of NOTCH3 in organization of actin cytoskeleton network. To see if the NOTCH3 mutation affects the whole actin cytoskeleton and not just the α-SMA organization, we performed total actin filament staining with phalloidin (actin α, β, and γ). Phalloidin staining showed similar irregular filaments as α-SMA immunostaining (not shown), which suggests that the whole actin filament cytoskeleton is disorganized in CADASIL cells. Our results suggest that homozygousity gives rise to more severe phenotype in VSMCs, although patient studies have not shown major difference between homozygous and heterozygous patients.33 This may reflect the effects of genetic modifiers, life choice factors, and complexity of intact tissue compared with cell culture.
We have demonstrated that PDGFR-β is a direct NOTCH3 target gene and that the induction of PDGFR-β expression in response to activation of NOTCH by ligand is blunted in cultured patient CADASIL VSMCs.34 Because PDGFR-β has been shown to be involved in actin polymerization, it is possible that a mutation in NOTCH3 affects PDGFR-β signaling/expression and thereby causes alterations in actin dynamics, providing a direct link between NOTCH3 and the actin cytoskeleton organization.35
Although the organization of actin filaments showed variable degrees of abnormalities, Western blot analyses disclosed no statistically significant quantitative alteration of α-SMA levels between CADASIL and control VSMC cell lines (Figures 2C and 2D).
Recently, a weak hypomorphic NOTCH3 allele was linked to increased stroke susceptibility in aged transgenic mice.36 Therefore, we analyzed if inhibiting NOTCH3 signaling by shRNA can induce aberrations in actin filaments in control VSMCs expressing wild-type NOTCH3. Transduction of HUmbVSMCs with NOTCH3-targeted shRNA1 and shRNA2 reduced NOTCH3 expression levels by ca 36% and 63% compared with control cells transduced with nontarget shRNA (Figure 3L). Reduced levels of NOTCH3 target gene HES5 expression in shRNA1 (5%) and shRNA2 (30%) cell lines confirmed that also the signaling activity of NOTCH3 was reduced compared with control cells (nontarget shRNA) (Figure 3L). Inhibition of NOTCH3 expression and signaling activity by shRNA1 and shRNA2 indeed induced similar actin abnormalities in control VSMCs as in CADASIL cells (Figures 3J and 3K).
In vitro and in vivo studies have demonstrated that mutated receptors are functional and able to rescue Notch3−/− phenotype.2, 29, 30 However, even a weak hypomorphic NOTCH3 allele was linked to increased stroke susceptibility in aged transgenic mice.36 Given the dosage sensitivity of NOTCH signaling in the development, the weak hypomorphic activity might, in addition to actin organization abnormalities, contribute to the CADASIL pathology over the years by dysdifferentiation of VSMCs (synthetic versus contractile phenotype) leading to increased production of ECM proteins and reduced ability of vasoregulation. Hypomorphic activity could also cause a failure in differentiation of vascular wall precursor cell to VSMCs and therefore lead to unbalanced turnover of arterial VSMCs. The hypomorphic receptor activity does not rule out the neomorphic functions of the mutated receptor e.g. receptor multimerization, formation of GOM and possible toxicity of the aggregates; in fact, these mechanisms might be tightly linked to each other.
Our results showed that CADASIL patient's VSMCs derived from blood vessels of different vascular beds are differentially affected by the disease. Furthermore, it has been demonstrated in Notch3−/− mice that the contractile activity of isolated aorta is different from that of cerebral arteries.37 Within the brain the most severely affected blood vessels in CADASIL patients are small penetrating arteries in cerebral white matter, where also the majority of lacunar infarcts occur. The pathological changes—fibrotic thickening of arteriolar walls and luminal stenosis—are markedly less severe in cerebral cortical arteries, and consequently the cerebral cortex is relatively spared,3, 9 which suggests that there are significant structural, metabolic, and functional differences between arteries even within the same organ (brain).
Adhesion Sites in Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy Cells
Cell adhesion is regulated by dynamic assemblies of structural and signaling proteins that link the filamentous actin cytoskeleton to the ECM. To assess the major CADASIL-related differences in VSMC adhesion sites, we analyzed the distribution of adhesion molecules, which are associated with the organization of actin filaments, in two CADASIL (HUmbVSMC and HArtVSMC) and two control (HUmbVSMC and HCerVSMC) VSMC lines.
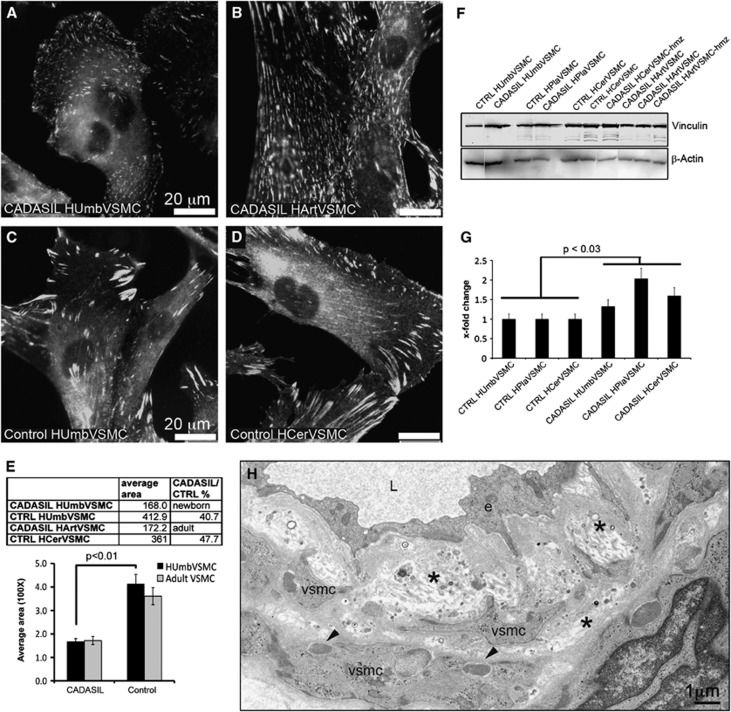
Arterial abnormalities in CADASIL include widening of the subendothelial space, thickening of the basement membrane and detachment of VSMCs from each other (Figure 4H).38 Vinculin is a key molecule at adhesion sites (focal complexes and focal adhesions) anchoring actin filaments to the membrane bound integrins. Immunostaining of VSMCs for vinculin showed a markedly greater number of adhesion sites in CADASIL than in control VSMCs derived from newborn tissue (HUmbVSMC) (Figures 4A and 4C). These adhesion sites were spread widely over the basal surface of the CADASIL VSMCs, whereas in the control VSMCs they were mostly at cellular extensions. The sizes of individual adhesion sites were smaller in CADASIL than in control HUmbVSMCs (E): in CADASIL HUmbVSMCs the average size of individual adhesion sites was 40.7% (±3.5%) of that in control HUmbVSMCs (P<0.01). A CADASIL cell line derived from adult tissue (HArtVSMC) showed similar pattern of small adhesion sites spread all over basal surface of the cell compared with control cell line derived from adult tissue (HCerVSMC) (Figures 4B). In addition, in Western blot analysis the amount of vinculin was greater (P<0.03) in all CADASIL VSMCs than in corresponding control VSMC lines (Figures 4F and 4G). Immunostaining for two other adapter molecules, talin and focal adhesion kinase, followed the same distribution pattern as seen with vinculin in all VSMCs analyzed. Immunostaining for cortactin, an enhancer of cortical actin polymerization, and phosphotyrosine, reflecting the level of tyrosine phosphorylation, did not show differences between CADASIL and control VSMCs analyzed (not shown).
Figure 4.
Vinculin positive adhesion sites in CADASIL vascular smooth muscle cells (VSMCs). Methanol fixed cells immunostained for vinculin show markedly greater number of small-sized adhesion sites in (A, B) CADASIL (HUmbVSMC and HArtVSMC) than (C, D) control VSMCs (HUmbVSMCs and HCerVSMC). (E) The areas of adhesion sites were measured from CADASIL and control VSMC lines immunostained for vinculin using ImageJ (NIH, Bethesda, MD, USA) software. From each cell line, at least 100 adhesion sites were measured. The average area of individual adhesion sites in CADASIL cell line derived from newborn tissue (HUmbVSMC) is 40.7% of that in control VSMCs (HUmbVSMCs). (C) The same pattern of small and numerous adhesion sites was detected also in CADASIL cell line derived from adult tissue (HArtVSMCs) whereas (D) control adult cell line (HCerVSMCs) showed large and elongated adhesion sites similarly to control HUmbVSMCs. Bar graph shows average size of adhesion sites in two CADASIL and two control cell lines. Significance is calculated only for the tissue matched cell lines. Total vinculin amount in the cell lysates at passage 5. (F) Western blot analysis shows a greater amount of total vinculin protein detected by vinculin antibody in CADASIL VSMCs than in corresponding control cells. In all, 20 μg of cell lysate was loaded on each lane and equal loading was verified by redetection of the blot with β-actin antibody. (G) Bar graph shows x-fold change of average vinculin intensities in comparison to control from three replicates. Intensities are normalized to β-actin intensity. (H) Electron micrograph of a dermal artery of CADASIL patient double stained with uranyl acetate and lead citrate. Note widening of the subendothelial space, thickening of the basal lamina and detachment of VSMCs from each other. Two arrow heads point granular osmiophilic material (GOM) in the indentations of VSMCs. e=endothelial cell, L=arterial lumen, *=subendothelial space and basal lamina.
Adhesion sites are large multimolecular complexes that interconnect cells to neighboring cells or to the surrounding ECM. Adhesion is mediated by transmembrane integrins and associated cytosolic adapter proteins, which provide a direct link to the actin filaments. In VSMCs integrins (mainly αvβ3 and α5β1) are considered to be involved for example in the regulation of contractile function and myogenic behavior.39 Adhesion sites are dynamic structures, which are transformed by continuous association and dissociation of proteins according to received signals and thus their molecular composition is heterogeneous. In culture, the adhesion sites are transformed (matured) from (1) early state focal complexes, through (2) classic elongated focal adhesions to (3) fibrillar adhesions driven by actomyosin contraction.40
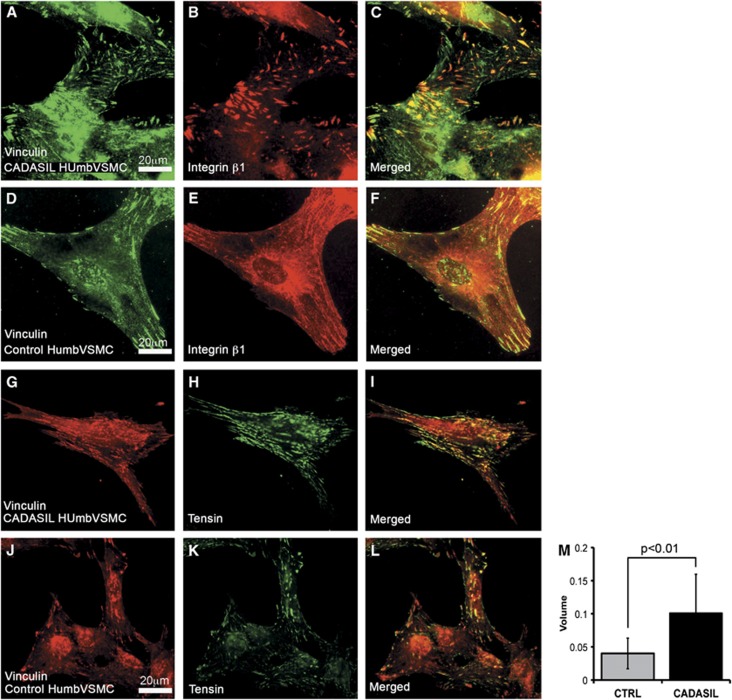
To assess the maturation of the adhesion sites, we analyzed the distribution of β1 and β3 integrins and the adapter protein tensin. Integrin β1 appeared as ribbon-like structures, which were more frequent in CADASIL than in control VSMCs. In both CADASIL and control VSMCs (Figures 5A–5F), integrin β1 positive ribbon-like structures were either positive or negative for vinculin, the former representing focal complexes or focal adhesions and the latter mature fibrillar adhesions.40 Integrin β3 localized to the same structures as vinculin and did not show substantial difference between CADASIL and control VSMCs (data not shown). Remarkably, a greater number of adhesion sites were positive for tensin in CADASIL than in control VSMCs. (Figures 5G–5L) and the average volume of tensin immunoreactivity was significantly higher in CADASIL cells compared with control cells (P<0.01) (Figure 5M).
Figure 5.
Integrin β1 and adapter protein tensin in adhesion sites. Methanol fixed vascular smooth muscle cells (VSMCs) were immunostained with integrin β1 or tensin antibody. (A–F) Integrin β1 forms thin ribbon-like structures in the center of the cells, parts of which are vinculin positive. In the two CADASIL (only HUmbVSMC shown) VSMC lines analyzed the β1 positive ribbons were more frequent than in the two control VSMC lines (only HUmbVSMC shown). (G–L) Tensin colocalizes partly to the same adhesion structures as vinculin in both CADASIL VSMC lines analyzed and control cell lines (only HUmbVSMC shown). (M) Bar graph shows the average volume (area × intensity) of tensin immunoreactivity in CADASIL and control VSMCs (P<0.01). From each cell line, at least 500 adhesion sites were measured. Tensin localization indicates maturation of adhesion sites to the stages of late focal adhesions and fibrillar adhesions and the change from a structural adhesion to an adhesion that interacts with signaling.
Both talin and tensin are adapter proteins that bind to the same region in the tail of β integrins.41 Talin is usually found in early adhesion (focal complexes and focal adhesions) and it functions mainly as a structural adaptor, whereas tensin is usually found in more mature adhesion sites (late focal adhesions and fibrillar adhesions) and it appears to assemble a signaling complex.40 Thus, the switch of talin to tensin in binding to integrins determines the change from a structural adhesion to an adhesion that interacts with signaling.
Structure, subcellular distribution, and molecular composition of adhesion sites in CADASIL VSMCs suggests a switch in the balance of adhesion sites from focal complexes towards more mature and stable focal and fibrillar adhesions in CADASIL VSMCs. This change in adhesion site maturation together with altered actin cytoskeleton might affect the ability of the cells to respond to different stimuli, for example to narrowing or dilating the arterial lumen. Interestingly, also VSMCs of transgenic mice expressing human NOTCH3 with a mutation p.Arg90Cys exhibit cytoskeletal changes with the presence of more numerous and larger cytoplasmic dense bodies as well as more frequent and thicker dense plaques (tissue equivalent to adhesion sites in cultivated cells).42 The observed enlargement of subendothelial space of the arteries and the loss of intercellular connections of VSMCs in CADASIL patients (Figure 4H)4, 5 and in mouse expressing mutated NOTCH342 might be caused by alterations in actin filament structures, deviant formation of the adhesion sites or changed integrin affinity/avidity, which all could be initiated by abnormal NOTCH3 function.
Conclusions
Cultured genuine patient derived CADASIL VSMCs expressing endogenous mutated NOTCH3 with two different mutations do not show impaired processing or intracellular aggregation of NOTCH3 but form aggregates on the cell surface. In accordance with the abnormal contractile characteristics of CADASIL patient's arteries, CADASIL VSMCs have altered organization of actin cytoskeleton. Similar alterations could be induced in control VSMCs when NOTCH3 expression was inhibited by shRNA. Furthermore, in CADASIL VSMCs, actin cytoskeleton is more severely affected in adult than in newborn VSMCs corresponding directly to the NOTCH3 expression levels. The results of the present study support the view that VSMCs in different vascular beds are dissimilar and consequently the pathogenic effects of mutated NOTCH3 are also dissimilar in different arteries.9, 37 We detected alterations also in the adhesion sites of CADASIL VSMCs where actin filaments are attached to the plasma membrane and VSMCs to the surrounding tissue. This may alter the ability of VSMCs to attach to the surrounding connective tissue and affect the ability of VSMCs to contract the arterial wall. Thus, NOTCH3 appears to play an important role in the regulation of actin organization, VSMC adhesion complexes and therefore arterial hemodynamics.
The authors declare no conflict of interest.
Footnotes
This work was financially supported by the Sigrid Jusélius foundation, the Päivikki and Sakari Sohlberg Foundation, the Finnish Academy, EVO research funds of the Helsinki and Turku University Hospitals and Turku City Hospital, Swedish Research Council and Regional Operative Program (ROP) Calabria ESF 2007/2013 - IV Axis Human Capital—Operative Objective M2-Action D.5: Codice CUP H25E10000320002.
References
- Joutel A, Vahedi K, Corpechot C, Troesch A, Chabriat H, Vayssiere C, et al. Strong clustering and stereotyped nature of Notch3 mutations in CADASIL patients. Lancet. 1997;350:1511–1515. doi: 10.1016/S0140-6736(97)08083-5. [DOI] [PubMed] [Google Scholar]
- Monet-Lepretre M, Bardot B, Lemaire B, Domenga V, Godin O, Dichgans M, et al. Distinct phenotypic and functional features of CADASIL mutations in the Notch3 ligand binding domain. Brain. 2009;132:1601–1612. doi: 10.1093/brain/awp049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalimo H, Miao Q, Tikka S, Mykkanen K, Junna M, Roine S, et al. CADASIL: the most common hereditary subcortical vascular dementia. Future Neurol. 2008;3:683–704. [Google Scholar]
- Ruchoux MM, Guerouaou D, Vandenhaute B, Pruvo JP, Vermersch P, Leys D. Systemic vascular smooth muscle cell impairment in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Acta Neuropathol (Berl) 1995;89:500–512. doi: 10.1007/BF00571504. [DOI] [PubMed] [Google Scholar]
- Ruchoux MM, Chabriat H, Bousser MG, Baudrimont M, Tournier-Lasserve E. Presence of ultrastructural arterial lesions in muscle and skin vessels of patients with CADASIL. Stroke. 1994;25:2291–2292. doi: 10.1161/01.str.25.11.2291. [DOI] [PubMed] [Google Scholar]
- Tikka S, Mykkanen K, Ruchoux MM, Bergholm R, Junna M, Poyhonen M, et al. Congruence between NOTCH3 mutations and GOM in 131 CADASIL patients. Brain. 2009;132:933–939. doi: 10.1093/brain/awn364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joutel A, Andreux F, Gaulis S, Domenga V, Cecillon M, Battail N, et al. The ectodomain of the Notch3 receptor accumulates within the cerebrovasculature of CADASIL patients. J Clin Invest. 2000;105:597–605. doi: 10.1172/JCI8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiko A, Shimizu A, Nagata E, Takahashi K, Tabira T, Suzuki N. Notch3 ectodomain is a major component of granular osmiophilic material (GOM) in CADASIL. Acta Neuropathol (Berl) 2006;112:333–339. doi: 10.1007/s00401-006-0116-2. [DOI] [PubMed] [Google Scholar]
- Miao Q, Paloneva T, Tuominen S, Poyhonen M, Tuisku S, Viitanen M, et al. Fibrosis and stenosis of the long penetrating cerebral arteries: the cause of the white matter pathology in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Brain Pathol. 2004;14:358–364. doi: 10.1111/j.1750-3639.2004.tb00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen S, Miao Q, Kurki T, Tuisku S, Poyhonen M, Kalimo H, et al. Positron emission tomography examination of cerebral blood flow and glucose metabolism in young CADASIL patients. Stroke. 2004;35:1063–1067. doi: 10.1161/01.STR.0000124124.69842.2d. [DOI] [PubMed] [Google Scholar]
- Roine S, Harju M, Kivela TT, Poyhonen M, Nikoskelainen E, Tuisku S, et al. Ophthalmologic findings in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy: a cross-sectional study. Ophthalmology. 2006;113:1411–1417. doi: 10.1016/j.ophtha.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Harju M, Tuominen S, Summanen P, Viitanen M, Poyhonen M, Nikoskelainen E, et al. Scanning laser Doppler flowmetry shows reduced retinal capillary blood flow in CADASIL. Stroke. 2004;35:2449–2452. doi: 10.1161/01.STR.0000145048.94499.b9. [DOI] [PubMed] [Google Scholar]
- Stenborg A, Kalimo H, Viitanen M, Terent A, Lind L. Impaired endothelial function of forearm resistance arteries in CADASIL patients. Stroke. 2007;38:2692–2697. doi: 10.1161/STROKEAHA.107.490029. [DOI] [PubMed] [Google Scholar]
- Gobron C, Vahedi K, Vicaut E, Stucker O, Laemmel E, Baudry N, et al. Characteristic features of in vivo skin microvascular reactivity in CADASIL. J Cereb Blood Flow Metab. 2007;27:250–257. doi: 10.1038/sj.jcbfm.9600356. [DOI] [PubMed] [Google Scholar]
- Peters N, Freilinger T, Opherk C, Pfefferkorn T, Dichgans M. Enhanced L-arginine-induced vasoreactivity suggests endothelial dysfunction in CADASIL. J Neurol. 2008;255:1203–1208. doi: 10.1007/s00415-008-0876-9. [DOI] [PubMed] [Google Scholar]
- Dubroca C, Lacombe P, Domenga V, Maciazek J, Levy B, Tournier-Lasserve E, et al. Impaired vascular mechanotransduction in a transgenic mouse model of CADASIL arteriopathy. Stroke. 2005;36:113–117. doi: 10.1161/01.STR.0000149949.92854.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joutel A, Monet-Lepretre M, Gosele C, Baron-Menguy C, Hammes A, Schmidt S, et al. Cerebrovascular dysfunction and microcirculation rarefaction precede white matter lesions in a mouse genetic model of cerebral ischemic small vessel disease. J Clin Invest. 2010;120:433–445. doi: 10.1172/JCI39733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenga V, Fardoux P, Lacombe P, Monet M, Maciazek J, Krebs LT, et al. Notch3 is required for arterial identity and maturation of vascular smooth muscle cells. Genes Dev. 2004;18:2730–2735. doi: 10.1101/gad.308904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Chantemele EJ, Retailleau K, Pinaud F, Vessieres E, Bocquet A, Guihot AL, et al. Notch3 is a major regulator of vascular tone in cerebral and tail resistance arteries. Arterioscler Thromb Vasc Biol. 2008;28:2216–2224. doi: 10.1161/ATVBAHA.108.171751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihalainen S, Soliymani R, Iivanainen E, Mykkanen K, Sainio A, Poyhonen M, et al. Proteome analysis of cultivated vascular smooth muscle cells from a CADASIL patient. Mol Med. 2007;13:305–314. doi: 10.2119/2006-00069.Ihalainen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylanne J, Virtanen I. The Mr 140,000 fibronectin receptor complex in normal and virus-transformed human fibroblasts and in fibrosarcoma cells: identical localization and function. Int J Cancer. 1989;43:1126–1136. doi: 10.1002/ijc.2910430628. [DOI] [PubMed] [Google Scholar]
- Gimbrone MA, Cotran RS. Human vascular smooth muscle in culture. Growth and ultrastructure. Lab Invest. 1975;33:16–27. [PubMed] [Google Scholar]
- Li S, Fan YS, Chow LH, Van Den Diepstraten C, van Der Veer E, Sims SM, et al. Innate diversity of adult human arterial smooth muscle cells: cloning of distinct subtypes from the internal thoracic artery. Circ Res. 2001;89:517–525. doi: 10.1161/hh1801.097165. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Adachi K, Yoshizaki K, Kunimoto S, Kalaria RN, Watanabe A. Mutations in NOTCH3 cause the formation and retention of aggregates in the endoplasmic reticulum, leading to impaired cell proliferation. Hum Mol Genet. 2010;19:79–89. doi: 10.1093/hmg/ddp468. [DOI] [PubMed] [Google Scholar]
- Opherk C, Duering M, Peters N, Karpinska A, Rosner S, Schneider E, et al. CADASIL mutations enhance spontaneous multimerization of NOTCH3. Hum Mol Genet. 2009;18:2761–2767. doi: 10.1093/hmg/ddp211. [DOI] [PubMed] [Google Scholar]
- Duering M, Karpinska A, Rosner S, Hopfner F, Zechmeister M, Peters N, et al. Co-aggregate formation of CADASIL-mutant NOTCH3: a single-particle analysis. Hum Mol Genet. 2011;20:3256–3265. doi: 10.1093/hmg/ddr237. [DOI] [PubMed] [Google Scholar]
- Karlstrom H, Beatus P, Dannaeus K, Chapman G, Lendahl U, Lundkvist JA. CADASIL-mutated Notch 3 receptor exhibits impaired intracellular trafficking and maturation but normal ligand-induced signaling. Proc Natl Acad Sci USA. 2002;99:17119–17124. doi: 10.1073/pnas.252624099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low WC, Santa Y, Takahashi K, Tabira T, Kalaria RN. CADASIL-causing mutations do not alter Notch3 receptor processing and activation. Neuroreport. 2006;17:945–949. doi: 10.1097/01.wnr.0000223394.66951.48. [DOI] [PubMed] [Google Scholar]
- Peters N, Opherk C, Zacherle S, Capell A, Gempel P, Dichgans M. CADASIL-associated Notch3 mutations have differential effects both on ligand binding and ligand-induced Notch3 receptor signaling through RBP-Jk. Exp Cell Res. 2004;299:454–464. doi: 10.1016/j.yexcr.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Joutel A, Monet M, Domenga V, Riant F, Tournier-Lasserve E. Pathogenic mutations associated with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy differently affect Jagged1 binding and Notch3 activity via the RBP/JK signaling Pathway. Am J Hum Genet. 2004;74:338–347. doi: 10.1086/381506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubrow AB, Hulman S, Kushner H, Falkner B. Determinants of blood pressure in infants admitted to neonatal intensive care units: a prospective multicenter study. Philadelphia Neonatal Blood Pressure Study Group. J Perinatol. 1995;15:470–479. [PubMed] [Google Scholar]
- Lacombe P, Oligo C, Domenga V, Tournier-Lasserve E, Joutel A. Impaired cerebral vasoreactivity in a transgenic mouse model of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy arteriopathy. Stroke. 2005;36:1053–1058. doi: 10.1161/01.STR.0000163080.82766.eb. [DOI] [PubMed] [Google Scholar]
- Tuominen S, Juvonen V, Amberla K, Jolma T, Rinne JO, Tuisku S, et al. Phenotype of a homozygous CADASIL patient in comparison to 9 age-matched heterozygous patients with the same R133C Notch3 mutation. Stroke. 2001;32:1767–1774. doi: 10.1161/01.str.32.8.1767. [DOI] [PubMed] [Google Scholar]
- Jin S, Hansson EM, Tikka S, Lanner F, Sahlgren C, Farnebo F, et al. Notch signaling regulates platelet-derived growth factor receptor-beta expression in vascular smooth muscle cells. Circ Res. 2008;102:1483–1491. doi: 10.1161/CIRCRESAHA.107.167965. [DOI] [PubMed] [Google Scholar]
- Andrae J, Gallini R, Betsholtz C. Role of platelet-derived growth factors in physiology and medicine. Genes Dev. 2008;22:1276–1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arboleda-Velasquez JF, Manent J, Lee JH, Tikka S, Ospina C, Vanderburg CR, et al. PNAS Plus: hypomorphic Notch 3 alleles link Notch signaling to ischemic cerebral small-vessel disease. Proc Natl Acad Sci USA. 2011;108:E128–E135. doi: 10.1073/pnas.1101964108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arboleda-Velasquez JF, Zhou Z, Shin HK, Louvi A, Kim HH, Savitz SI, et al. Linking Notch signaling to ischemic stroke. Proc Natl Acad Sci USA. 2008;105:4856–4861. doi: 10.1073/pnas.0709867105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruchoux MM, Maurage CA. CADASIL: Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. J Neuropathol Exp Neurol. 1997;56:947–964. [PubMed] [Google Scholar]
- Martinez-Lemus LA, Sun Z, Trache A, Trzciakowski JP, Meininger GA. Integrins and regulation of the microcirculation: from arterioles to molecular studies using atomic force microscopy. Microcirculation. 2005;12:99–112. doi: 10.1080/10739680590896054. [DOI] [PubMed] [Google Scholar]
- Zaidel-Bar R, Cohen M, Addadi L, Geiger B. Hierarchical assembly of cell-matrix adhesion complexes. Biochem Soc Trans. 2004;32:416–420. doi: 10.1042/BST0320416. [DOI] [PubMed] [Google Scholar]
- Legate KR, Fassler R. Mechanisms that regulate adaptor binding to beta-integrin cytoplasmic tails. J Cell Sci. 2009;122:187–198. doi: 10.1242/jcs.041624. [DOI] [PubMed] [Google Scholar]
- Ruchoux MM, Domenga V, Brulin P, Maciazek J, Limol S, Tournier-Lasserve E, et al. Transgenic mice expressing mutant Notch3 develop vascular alterations characteristic of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Am J Pathol. 2003;162:329–342. doi: 10.1016/S0002-9440(10)63824-2. [DOI] [PMC free article] [PubMed] [Google Scholar]