Abstract
Different brain regions exhibit differing sensitivities to ischemia/excitotoxicity. Whether these differences are due to perfusion or intrinsic factors has not been established. Herein, we found no apparent association between sensitivity to ischemia/excitotoxicity and the level of expression or basal phosphorylation of calcium/calmodulin-stimulated protein kinase II (αCaMKII) or glutamate receptors. However, we demonstrated significant differences in CaMKII-mediated responses after ischemia/excitotoxic stimulation in striatum and cortex. In vivo ischemia and in vitro excitotoxic stimulation produced more rapid phosphorylation of Thr253-αCaMKII in striatum compared with cortex, but equal rates of Thr286-αCaMKII phosphorylation. Phosphorylation by CaMKII of Ser831-GluA1 and Ser1303-GluN2B occurred more rapidly in striatum than in cortex after either stimulus. The differences between brain regions in CaMKII activation and its effects were not accounted for by differences in the expression of αCaMKII, glutamate receptors, or density of synapses. These results implicate intrinsic tissue differences in Thr253-αCaMKII phosphorylation in the differential sensitivities of brain regions to ischemia/excitotoxicity.
Keywords: CaMKII, excitotoxicity, focal ischemia, protein phosphorylation
Introduction
Calcium/calmodulin-stimulated protein kinase II (CaMKII) is a multifunctional serine (Ser)/threonine (Thr) kinase, and is the most abundant calcium-activated kinase in brain. One or more members of this family are found in virtually every tissue, and mediate a diverse range of physiological responses.1, 2 The biological properties of CaMKII are controlled by multisite phosphorylation and targeting to subcellular microdomains through interactions with specific target proteins.2, 3 The roles of two phosphorylation sites, Thr286 and Thr305/6, have been well characterized. Phosphorylation at these sites has a profound effect on the activity of CaMKII, as well as targeting to various subcellular locations.2 Recently, the consequences of phosphorylation at Thr253 have begun to be elucidated. Thr253 phosphorylation occurs in vivo after a range of stimuli,4, 5 and is involved in controlling cell proliferation.3 Unlike phosphorylation at Thr286 or Thr305/6, phosphorylation at Thr253 has no direct effect on the activity of CaMKII in vitro, but has marked effects on CaMKII targeting.3, 4 For example, phosphorylation at Thr253 targets CaMKII to the postsynaptic density (PSD),4 a specialized complex of proteins adjacent to the postsynaptic membrane.
Among its many roles, CaMKII colocalizes with and regulates glutamate receptors (especially AMPA and NMDA receptors (AMPA-R and NMDA-R).6 These receptors are not only essential to normal neuronal function, but are central to the process of excitotoxic cell death, which occurs in stroke, seizures, and other conditions. Recently, a CaMKII inhibitor was shown to be neuroprotective in experimental stroke, even when administered 1 hour after ischemia.7
Ischemic stroke is the most common form of stroke, and occurs after occlusion of a cerebral blood vessel. The resultant ischemia causes widespread membrane depolarization, release of excitatory neurotransmitters, opening of ion channels, and sustained elevation of intracellular calcium, leading to eventual cell death.8 Different brain regions show varying sensitivity to ischemia in humans and animals and the reasons for this differential sensitivity remain uncertain. For example, after stroke, central (basal ganglia and deep white matter) injury progresses more rapidly than peripheral (cortical) injury in rodents and humans.9 Possible contributors to this differential sensitivity include variations in collateral circulation,10 proportion of white matter,11 and endogenous tissue differences.12 Additionally, different animal strains exhibit differing sensitivities to ischemia. For example, Spontaneously Hypertensive Rats (SHRs) and their parent Wistar Kyoto (WKY) strain exhibit dramatically different sensitivities to ischemia/excitotoxicity, unrelated to the presence of hypertension.13 These differences have been proposed to be due to poorer collateral circulation causing decreased perfusion to the ischemic cortex.14 However, Lecrux et al (2007) have shown increased expression of αCaMKII and increased basal phosphorylation of the GluA1 subunit of the AMPA-R at Ser831 in SHR striatum, and proposed that these changes may at least partly explain the enhanced vulnerability of this strain to AMPA-mediated excitotoxicity.
We have examined the expression and phosphorylation of αCaMKII and glutamate receptors in brain regions with differing sensitivities to ischemia/excitotoxicity to investigate whether this proposed model could explain the regional sensitivity to ischemia/excitotoxicity in striatum and cortex. We have confirmed Lecrux's findings in the striatum of SHRs and WKY, and extended the molecular analysis to different brain regions. Our results indicate that there are tissue-specific responses to injury, independent of perfusion factors. However, neither the expression levels nor basal phosphorylation of αCaMKII or glutamate receptors are associated with regional sensitivity to ischemia/excitotoxicity. Our data indicate that the enhanced susceptibility of brain regions to ischemia may be due to altered phosphorylation of αCaMKII at Thr253 after an excitotoxic stimulus.
Materials and methods
Materials
Primary antibodies
Rabbit antibodies to phospho-Thr253-CaMKII were as previously described (1:20,0004). Antibodies to total αCaMKII (1:5,000; Millipore, North Ryde, NSW, Australia), phospho-Thr286-CaMKII (1:1,000; Affinity BioReagents, Redfern, NSW, Australia), phospho-Thr305-CaMKII (1:1,000; Millipore), total GluN2B (1:1,000; BD Transduction Laboratories, North Ryde, NSW, Australia), phospho-Ser1303-GluN2B (1:5,000; Epitomics, Burlingame, CA, USA), total GluA1 (1:1,000; Millipore), phospho-Ser831-GluA1 (1:500; Millipore), phospho-Ser845-GluA1 (1:1,000; Millipore), total GluA2 (1:1,000; Millipore), and PSD-95 (1:1,000; Millipore) were purchased.
Chemicals and inhibitors
NMDA (Sigma-Aldrich, Castle Hill, NSW, Australia), glycine (Sigma-Aldrich), glutamate (Sigma-Aldrich), AMPA (Tocris, Ellisville, MI, USA), Chelerythrine chloride (Sigma-Aldrich), 2-[N-(2-hydroxyethyl)]-N-(4-methoxybenzenesulfonyl)]amino-N-(4-chlorocinnamyl)-N-methylbenzylamine) [KN-93], 2-[N-(4-methoxybenzenesulfonyl)]amino-N-(4-chlorocinnamyl)-N-methylbenzylamine, phosphate [KN-92] (Calbiochem, Kilsyth, VIC, Australia), and myristoylated-AIP [autocamtide-2-related inhibitory peptide; myristoyl-Lys-Lys-Ala-Leu-Arg-Arg-Gln-Glu-Ala-Val-Asp-Ala-Leu] (Biomol, Hamburg, Germany) were stored in stock solutions at −20°C.
Preparation of Brain Tissue
All procedures were performed approval from the University of Newcastle Animal Care and Ethics Committee, as well as in accordance with the relevant guidelines and regulations, including the NSW Animal Research Act, the NSW Animal Research Regulation, and the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes.
Micropunch homogenates
Fifteen-week-old male SHR, WKY, or Sprague-Dawley (SD) rats (390 to 515 g; n=6 per group; Animal Resource Centre, Perth, WA, Australia) were anesthetized with isoflurane for 1 hour, and then killed by decapitation. Rats were anesthetized for 1 hour so that comparisons could be made with rats that underwent stroke surgery. The brain was removed and rapidly cooled in a large volume of stirred ice-cold saline, then placed in a dissection mould. The anterior 5 mm portion of the forebrain was removed and discarded. A 6-mm coronal slice was acquired using two razor blades. Samples of striatum and sensory cortex were obtained by micropunch (2 mm diameter) from a central portion of each region and homogenized using a glass-teflon Dounce Homogenizer (20 strokes; 700 r.p.m.) in homogenization buffer (30 mmol/L Tris, pH 7.4, 1 mmol/L ethylene glycol tetraacetic acid, 4 mmol/L ethylenediaminetetraacetic acid (EDTA), 100 μmol/L ammonium molybdate, 5 mmol/L sodium pyrophosphate, 25 mmol/L sodium fluoride, 1 mmol/L sodium orthovanadate, 1 mmol/L phenylmethanesulfonylfluoride, complete protease inhibitor cocktail). Homogenates were stored at −80°C.
Microslices
Twelve–week-old male SD rats (350 to 485 g; n=8 per group; Animal Resource Centre) were anesthetized with isoflurane for 1 hour, and then killed by decapitation. The brain was removed rapidly without cooling, and dissected as described above. The central portions of the striatum and sensory cortex were removed by free-hand dissection. The brain regions were flushed with 37°C Krebs buffer (118 mmol/L sodium chloride, 4.7 mmol/L potassium chloride, 1.3 mmol/L calcium chloride, 1.2 mmol/L potassium dihydrogen phosphate, 1.2 mmol/L magnesium sulphate, 25 mmol/L sodium hydrogen carbonate, 11.7 mmol/L glucose, 0.03 mmol/L disodium EDTA, equilibrated with 95% oxygen, 5% carbon dioxide (carbogen), pH 7.4). Brain microslices (250 × 250 μm) were prepared at room temperature using a McIlwain tissue chopper.15 The microslices were washed five times with 5 ml 37°C Krebs buffer. The microslices were then equilibrated at 37°C with gentle shaking, and the Krebs buffer was changed every 15 minutes for 1 hour in total. At this time, 2 ml fresh Krebs buffer was added, and 15 to 20 microslices (150 μL) were transferred to flat-bottom polystyrene tubes. The microslices were incubated at 37°C and continually oxygenated throughout the experimental procedure.16
Excitotoxic stimulation
Microslices were stimulated with 100 μmol/L AMPA, 100 μmol/L NMDA+50 μmol/L glycine, or 2.5 mmol/L glutamate+50 μmol/L glycine (final concentration in 150 μL of Krebs buffer; control tissue received the same volume of Krebs without compounds). At various times poststimulus (0, 30, 60, 90, 120, and 300 seconds), the incubation solution was removed and replaced with 300 μL of ice-cold homogenization buffer. Microslices were then homogenized as described above.
Inhibitors
Microslices were incubated with inhibitor (final concentration in 150 μL Krebs buffer: 10 μmol/L KN-92, 10 μmol/L KN-93, 20 μmol/L Myr-AIP, 1 μmol/L chelerythrine chloride; control tissue received the same volume of Krebs without compounds) for 20 minutes at 37°C. After this time, microslices were stimulated with 100 μmol/L AMPA, 100 μmol/L NMDA+50 μmol/L glycine, or 2.5 mmol/L glutamate+50 μmol/L glycine (final concentration in 150 μL Krebs buffer), for 90 seconds, and then homogenized in homogenization buffer as described above.
Middle Cerebral Artery Occlusion
All procedures were performed in accordance with national guidelines, and with the approval of institutional Animal Ethics Committees. Male SD rats (270 to 500 g; n=6 per group) were used. Surgery for occlusion of the middle cerebral artery (MCAo) was performed under isoflurane anesthesia, according to our established method.17 Briefly, a midline neck incision was made, the carotid artery bifurcation was gently dissected and the external carotid artery was ligated. A 4-0 silicon-tipped thread was inserted ∼18 mm up the internal carotid artery via the external carotid stump, until mild resistance was felt, and a decrease in perfusion was recorded by laser Doppler perfusion monitoring of the MCA-supplied cortex, indicating successful arterial occlusion. The MCA was reperfused by retracting the occluding thread. Animals were killed by decapitation at variable times after occlusion, with or without reperfusion. The brains were removed in <2 minutes and rapidly cooled in a large volume of stirred ice-cold saline, before micropunch sampling of striatum and sensory cortex and tissue homogenization as described above.
Western Blotting
Tissue homogenates (10 to 20 μg) were separated using 10% SDS-PAGE, and then transferred onto PVDF membranes as described previously.3 So that comparison could be made across experiments performed on separate days, a standard curve of naïve hippocampus (5 to 30 μg) was included on each blot. This hippocampal standard was only freeze-thawed once, and it was verified that the standard did not change over time. Only immunoblots whose standard curves were linear were included. Primary antibody binding was detected by incubation with donkey anti-rabbit or sheep anti-mouse horseradish peroxidase-linked secondary antibody, and the ECL Plus Immunoblotting Detection System (GE Healthcare, Castle Hill, NSW, Australia). Blots were scanned with a Fujifilm LAS-3000 Imaging System (Brookvale, NSW, Australia) and analyzed with MultiGauge Software (Fujifilm).
Data Analysis
All statistical analyses were conducted using GraphPad Prism software V4.0 (GraphPad, San Diego, CA, USA). Comparisons between ischemic and contralateral hemisphere values, brain regions, and stimulated and nonstimulated brain slices were made using a paired Student's two-tailed t-test. Comparisons between rat strains were made using one-way analysis of variance. All data are presented as the mean±standard error of the mean (s.e.m.) for the number of replicates (n).
Results
Differences in Level of Expression or Basal Phosphorylation of αCaMKII and Glutamate Receptors Do Not Account for Regional Sensitivity to Excitotoxicity or Ischemia
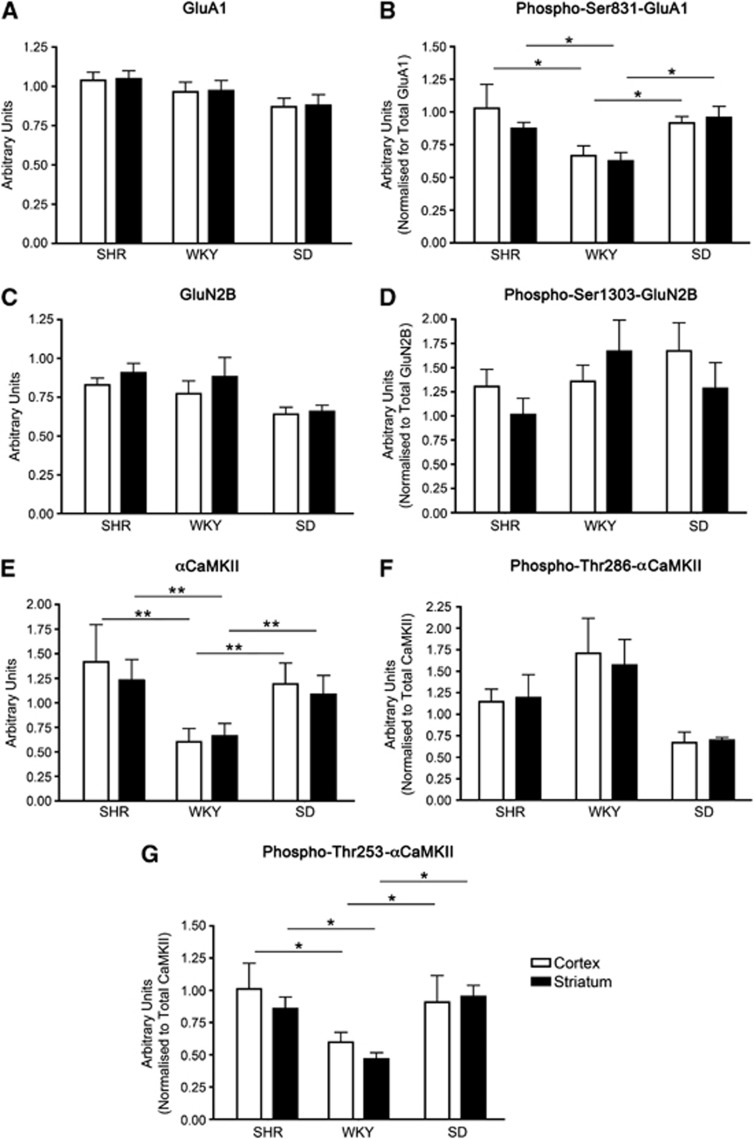
Different brain regions within the one animal exhibit differing sensitivities to excitotoxicity/ischemia. However, the mechanisms governing this enhanced vulnerability have not been identified. To test whether increased regional sensitivity to ischemia/excitotoxicity is due to variations in αCaMKII and glutamate receptor expression and/or basal phosphorylation, we examined expression in two brain regions (striatum and cortex) in three rat strains (SHRs, SDs, and WKYs). In each strain, striatum progresses to infarction more rapidly than cortex. Therefore, if the mechanism proposed by Lecrux et al (2007) to account for the species difference in sensitivity to ischemia/excitotoxicity is also responsible for the regional difference in sensitivity we predicted that there would be higher levels of αCaMKII expression and basal phosphorylation of Ser831-GluA1 in the striatum when compared with cortex. However, the expression of αCaMKII (Figure 1E), GluA1 (Figure 1A), and basal phosphorylation of Ser831-GluA1 (Figure 1B) did not differ between striatum and cortex in any strain. Additionally, no difference was observed between brain regions for basal Thr286-αCaMKII (Figure 1F) or Thr253-αCaMKII (Figure 1G) phosphorylation.
Figure 1.
Levels of protein expression and basal protein phosphorylation of αCaMKII and glutamate receptor expression in the cortex and striatum of Spontaneously Hypertensive (SHR), Wistar Kyoto (WKY), and Sprague-Dawley (SD) rats. (A) Total GluA1, (B) phospho-Ser831-GluA1, (C) total GluN2B, (D) phospho-Ser1303-GluN2B, (E) total αCaMKII, (F) phospho-Thr286-αCaMKII, and (G) phospho-Thr253-αCaMKII expression in the striatum and cortex of SHR, WKY, and SD rats. The striatum and sensory cortex were dissected from male 15-week-old rats (n=6), homogenized, and examined for GluA1, GluN2B, and αCaMKII expression/basal phosphorylation by western blotting. Total protein expression is shown per mg total tissue protein, whereas phosphorylation levels are normalized to total protein expression (i.e., phosphorylation/total expression). * denotes statistical significant difference from the same region compared with SHRs as determined by one-way analysis of variance (ANOVA) (*P<0.05; **P<0.01). CaMKII, calcium/calmodulin-stimulated protein kinase II.
We next investigated other variables that may contribute to differences in susceptibility to excitotoxicity/ischemia, including the expression and basal phosphorylation of the GluN2B subunit of the NMDA-R. We found no difference in the level of expression of total GluN2B (Figure 1C) or basal phosphorylation of Ser1303-GluN2B (Figure 1D) between these brain regions. Furthermore, we found no difference in the expression of the GluA2 subunit of the AMPA-R between SD cortex and striatum (2.72±0.45 and 2.48±0.39 arbitrary units/mg protein, respectively). In addition, as previously described,18 a similar level of PSD-95 (a postsynaptic protein used as a measure of excitatory synapse numbers19) was observed in both the cortex and striatum of SDs (1.437±0.083 and 1.222±0.065 arbitrary units/mg protein, respectively). This indicates that a difference in the density of synapses is also an unlikely explanation for the difference in regional susceptibility to excitotoxicity/ischemia.
As these three rat strains exhibit differing sensitivities to excitotoxicity/ischemia (after a given period of focal ischemia, SHRs have the largest infarcts, followed by SDs and then WKYs20), we hypothesized that SHRs would exhibit the highest αCaMKII and glutamate receptor expression and basal phosphorylation, WKYs would possess the lowest, and SDs expression/basal phosphorylation would be of an intermediate level. We found that αCaMKII expression (Figure 1E) and basal phosphorylation of Ser831-GluA1 (Figure 1B) were significantly higher in SHRs than in WKYs, without any difference in the level of expression of GluA1 (Figure 1A), confirming the findings of Lecrux et al (2007). However, αCaMKII expression (Figure 1E) and basal phosphorylation of Ser831-GluA1 (Figure 1B) in SDs were not significantly different from SHRs though they were significantly higher than in WKY; the same pattern was observed for basal Thr253-αCaMKII phosphorylation (Figure 1G). Additionally, no significant difference was observed between rat strains for basal Thr286-αCaMKII phosphorylation (Figure 1F). Therefore, while differences in expression and basal phosphorylation of αCaMKII or glutamate receptors may be a major contributor to the differences in sensitivity to ischemia/excitotoxicity between SHR and WKY, additional factors are likely to be important in other rat strains.
Striatum and Cortex Respond Differently to Excitotoxic Stimulation In Vitro
Since variations in the level of expression and basal phosphorylation of αCaMKII and glutamate receptors did not appear to be associated with susceptibility of different brain regions to ischemia/excitotoxicity, we determined whether the phosphorylation of αCaMKII or glutamate receptors in the striatum and cortex would differ in response to an excitotoxic stimulus. Brain microslices derived from the striatum and cortex of SDs were stimulated with AMPA, NMDA+glycine, or glutamate+glycine. These three stimuli were chosen as they allowed the selective activation of the two main excitotoxic pathways (AMPA and NMDA-R mediated), as well as a stimulus that activated multiple excitotoxic pathways (glutamate+glycine stimulation activates all ionotropic and metabotropic glutamate receptors). SDs were used for these experiments as they are an outbred strain, and they are of intermediate susceptibility to stroke/ischemia when compared with SHRs and WKYs.20 Phosphorylation of αCaMKII, sites on glutamate receptors known to be phosphorylated by CaMKII, and a control site that is phosphorylated by another protein kinase were examined.
To establish time courses for each phosphorylation site, preliminary experiments were performed over 5 minutes for each stimulus. A series of 5 time points, ranging from 30 seconds to 5 minutes were examined. There was considerable variation among the phosphorylation sites in the rates of phosphorylation (Supplementary Figures 1 to 3). The optimum poststimulus time at which to compare the responses was found to be 90 seconds as this was on the linear portion of the time course for the phosphorylation sites examined (except Thr305-αCaMKII) and the differences in phosphorylation at this time best reflected the pattern of overall differences between cortex and striatum. Therefore, the results in Figures 2, 3 and 4 are all quantitative comparisons at 90 seconds after stimulus.
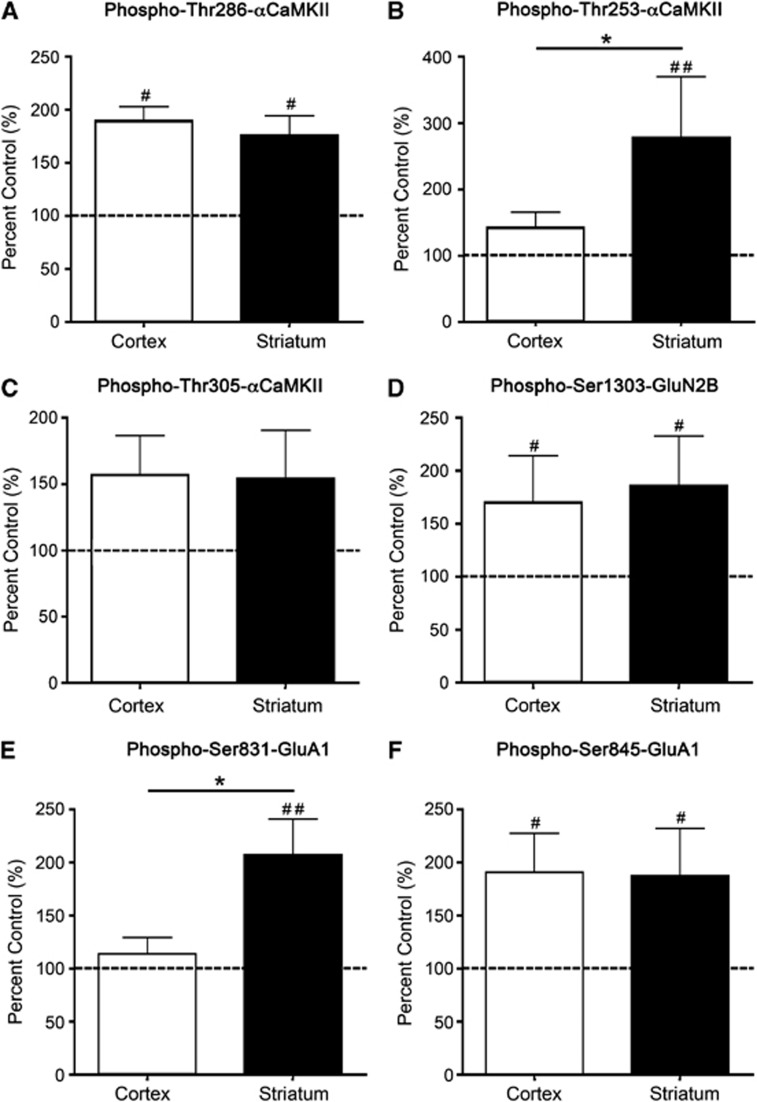
Figure 2.
αCaMKII, GluA1, and GluN2B phosphorylation after stimulation with 100 μmol/L AMPA for 90 seconds. (A) Thr286-αCaMKII, (B) Thr253-αCaMKII, (C) Thr305-αCaMKII, (D) Ser1303-GluN2B, (E) Ser831-GluA1, and (F) Ser845-GluA1 phosphorylation in the striatum and cortex of Sprague-Dawley (SD) rats after 90 seconds stimulation with 100 μmol/L AMPA. Microslices were generated from the striatum and sensory cortex of male 12-week-old rats (n=20), stimulated with AMPA, homogenized, and examined for αCaMKII/GluA1/GluN2B phosphorylation by western blotting. Phosphorylation levels are normalized to total protein expression (i.e., phosphorylation/total expression), and are expressed as percent nonstimulated control. #Denotes statistical significance from nonstimulated control (#P<0.05, ##P<0.01); *denotes statistical significance between the two brain regions (P<0.05). CaMKII, calcium/calmodulin-stimulated protein kinase II.
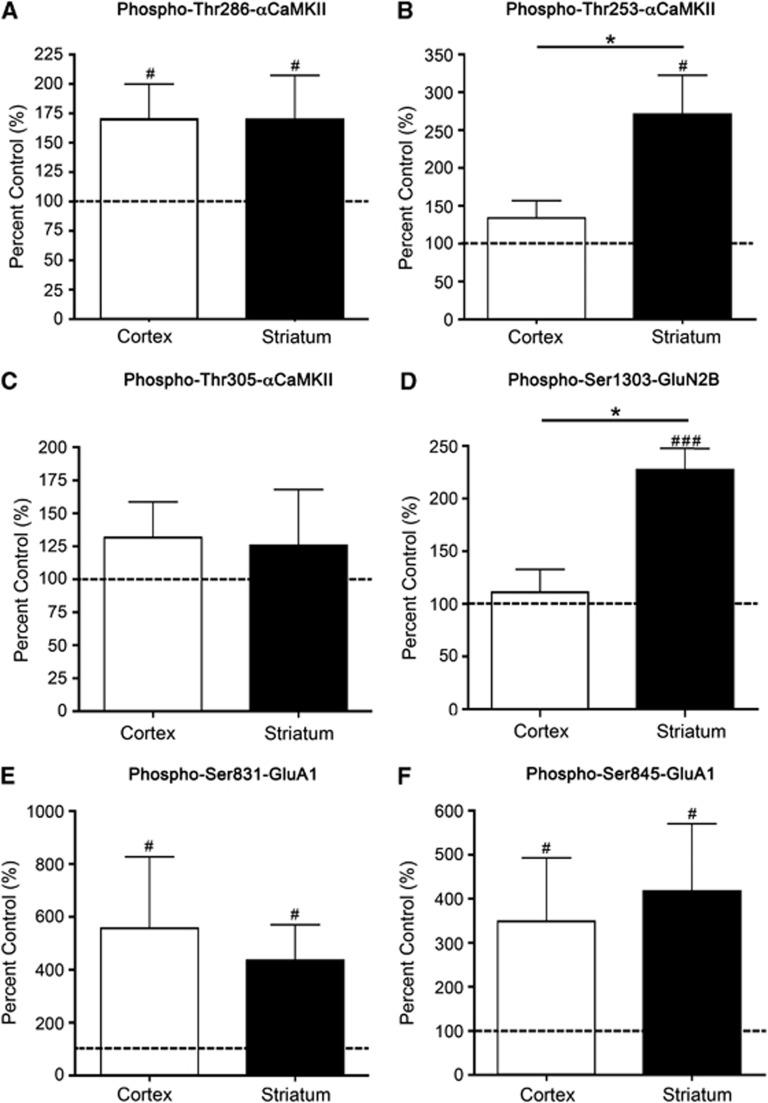
Figure 3.
αCaMKII, GluA1, and GluN2B phosphorylation after stimulation with 100 μmol/L NMDA+50 μmol/L glycine for 90 seconds. (A) Thr286-αCaMKII, (B) Thr253-αCaMKII, (C) Thr305-αCaMKII, (D) Ser1303-GluN2B, (E) Ser831-GluA1, and (F) Ser845-GluA1 phosphorylation in the striatum and cortex of Sprague-Dawley (SD) rats after 90 seconds stimulation with 100 μmol/L NMDA+50 μmol/L glycine. Microslices were generated from the striatum and sensory cortex of male 12-week-old rats (n=16), stimulated with NMDA+glycine, homogenized, and examined for αCaMKII/GluA1/GluN2B phosphorylation by western blotting. Phosphorylation levels are normalized to total protein expression (i.e., phosphorylation/total expression), and are expressed as percent nonstimulated control. #Denotes statistical significance from nonstimulated control (#P<0.05, ###P<0.001); *denotes statistical significance between the two brain regions (P<0.05). CaMKII, calcium/calmodulin-stimulated protein kinase II.
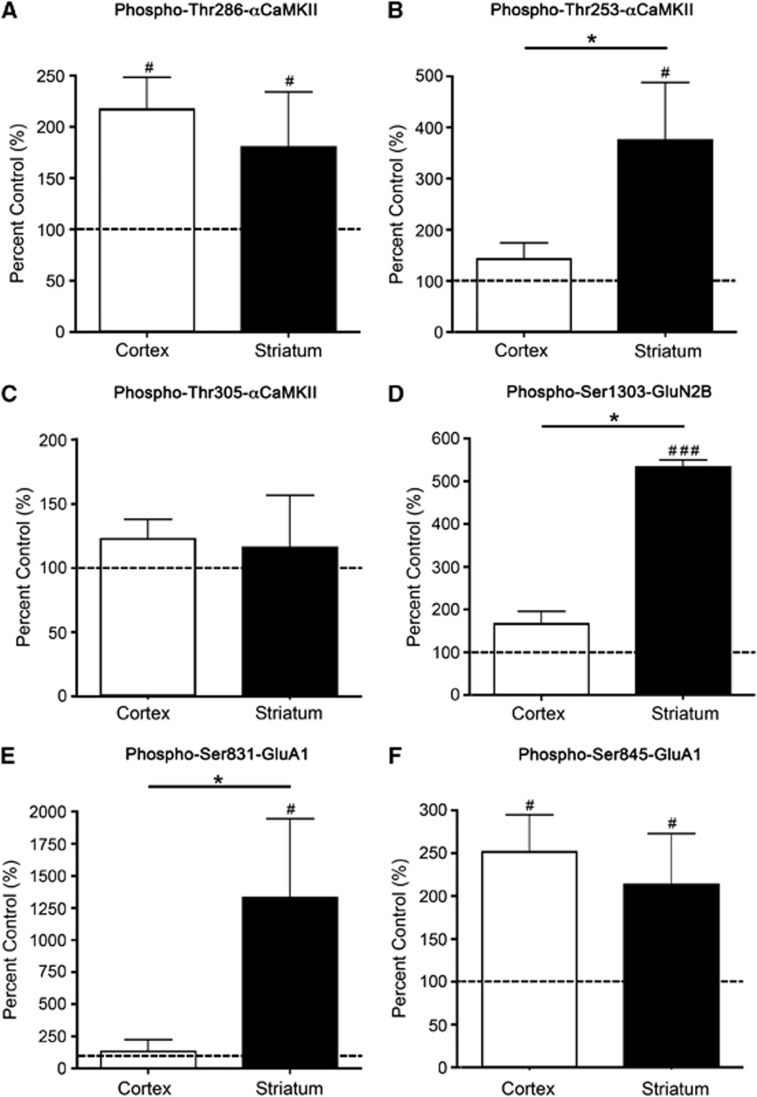
Figure 4.
αCaMKII, GluA1, and GluN2B phosphorylation after stimulation with 2.5 mmol/L glutamate+50 μmol/L glycine for 90 seconds. (A) Thr286-αCaMKII, (B) Thr253-αCaMKII, (C) Thr305-αCaMKII, (D) Ser1303-GluN2B, (E) Ser831-GluA1, and (F) Ser845-GluA1 phosphorylation in the striatum and cortex of Sprague-Dawley (SD) rats after 90 seconds stimulation with 2.5 mmol/L glutamate+50 μmol/L glycine. Microslices were generated from the striatum and sensory cortex of male 12-week-old rats (n=16), stimulated with glutamate+glycine, homogenized, and examined for αCaMKII/GluA1/GluN2B phosphorylation by western blotting. Phosphorylation levels are normalized to total protein expression (i.e., phosphorylation/ total expression), and are expressed as percent nonstimulated control. #Denotes statistical significance from nonstimulated control (#P<0.05, ###P<0.001); *denotes statistical significance between the two brain regions (P<0.05). CaMKII, calcium/calmodulin-stimulated protein kinase II.
In both cortex and striatum, stimulation of microslices with AMPA produced a significant elevation in the phosphorylation of Thr286-αCaMKII (Figure 2A; Supplementary Figure 4). However, the AMPA-induced phosphorylation of Thr253-αCaMKII was very different in the two tissues: striatum showed an almost threefold increase in phosphorylation compared with nonstimulated control tissue after 90 seconds, whereas the response in cortex was not significantly different from control at this time (Figure 2B). At 5 minutes after stimulation, Thr253-αCaMKII phosphorylation was increased in both the cortex (203% control; P=0.043) and striatum (348% control; P=0.009; Supplementary Figure 1B) compared with the control, and remained significantly elevated in the striatum compared with cortex (P=0.03). Phosphorylation of αCaMKII at Thr305, which is a secondary event that requires prior phosphorylation of Thr286 and the dissociation of calcium/calmodulin, was not significantly greater than control in either tissue until 5 minutes after stimulation (cortex: 187% control, striatum: 178% control; P=0.04 for each; Supplementary Figure 1C) and was not significantly different between the two regions at this time.
To determine whether certain glutamate receptor sites are phosphorylated to a greater extent in the striatum compared with the cortex after an excitotoxic stimulus, we examined Ser1303-GluN2B phosphorylation (phosphorylated by CaMKII or protein kinase C (PKC)21, 22), and GluA1 phosphorylation at Ser831 (phosphorylated by CaMKII or PKC23, 24) and Ser845 (phosphorylated by PKA but not CaMKII or PKC25) in these brain regions at various times poststimulus. AMPA-induced stimulation produced a significant increase in Ser1303-GluN2B (Figure 2D) and Ser845-GluA1 (Figure 2F) phosphorylation in both regions. By contrast, the AMPA-induced stimulation in Ser831-GluA1 phosphorylation was different in the two tissues: after 90 seconds the phosphorylation had doubled in striatum (Figure 2E) whereas no significant increase above control was detectable in cortex, although by 5 minutes there was a significant increase (146% control, P=0.02; Supplementary Figure 1E). Ser831-GluA1 phosphorylation was also increased in the striatum at this time (262% control, P=0.008; Supplementary Figure 1E), and was significantly elevated in the striatum compared with the cortex (P=0.03).
Stimulation of microslices by NMDA+glycine (an obligatory coagonist for NMDA-R activation) produced a significant elevation in the phosphorylation of Thr286-αCaMKII (Figure 3A; Supplementary Figure 4), Ser831-GluA1 (Figure 3E), and Ser845-GluA1 (Figure 3F) at 90 seconds that was similar in cortex and striatum. The phosphorylation of Thr305-αCaMKII was not significantly increased in either tissue after 90 seconds (Figure 3C), but was significantly elevated after 5 minutes (cortex: 595% control, striatum: 486% control; P=0.03 for both; Supplementary Figure 2C). By contrast, NMDA-induced stimulation more than doubled the phosphorylation of Thr253-αCaMKII (Figure 3B) and Ser1303-GluN2B (Figure 3D) in striatum after 90 seconds whereas the increase in cortex was not statistically significant at this time. Thr253-αCaMKII phosphorylation was significantly greater than the control after 2 minutes in both the cortex (202% control; P=0.04) and striatum (325% control, P=0.03; Supplementary Figure 2B). Similarly, Ser1303-GluN2B phosphorylation was significantly elevated compared with control after 5 minutes in both cortex (360% control, P=0.02) and striatum (814% control, P=0.02; Supplementary Figure 2D).
To provide a more physiological stimulus, glutamate+glycine was used. The overall pattern of phosphorylation resembled a combination of those observed in response to AMPA and NMDA+glycine (Figures 2 and 3). Adding glutamate+glycine produced an increase in Thr286-αCaMKII (Figure 4A; Supplementary Figure 4) and Ser845-GluA1 (Figure 4F) phosphorylation that did not significantly differ between cortex and striatum. The glutamate+glycine-induced phosphorylation of Thr305-αCaMKII was not significantly increased compared with the control at 90 seconds (Figure 4B), but was significantly elevated at 5 minutes after stimulation (cortex: 198% control, striatum: 182% control, P=0.04 for both regions; Supplementary Figure 3C). The glutamate+glycine-induced increase in Thr253-αCaMKII phosphorylation was also comparable to those observed after stimulation with either AMPA or NMDA+glycine: an elevation in striatum (Figure 4B) with no significant increase in cortex after 90 seconds. The level of Thr253-αCaMKII phosphorylation was significantly higher than the control in both the cortex (214% control, P=0.04) and striatum (424% control, P=0.03; Supplementary Figure 3B) after 2 minutes. Stimulating the microslices with glutamate+glycine produced a similar selective increase in Ser1303-GluN2B phosphorylation in the striatum to that observed after NMDA stimulation and a similar selective increase in Ser831-GluA1 phosphorylation in the striatum to that observed after AMPA stimulation (Figures 2E, 3D, 4D and 4E). There was no significant increase in the phosphorylation of either receptor at these sites in cortex at 90 seconds (Figures 4D and 4E) although phosphorylation was significantly increased at 5 minutes for both receptors in cortex (Ser1303-GluN2B: 321% control, P=0.03 and Ser831-GluA1: 284% control, P=0.04) and striatum (Ser1303-GluN2B: 834% control, P=0.03 and Ser831-GluA1: 802% control, P=0.03; Supplementary Figures 3D and 3E).
CaMKII is Responsible for the Observed Glutamate Receptor Phosphorylation
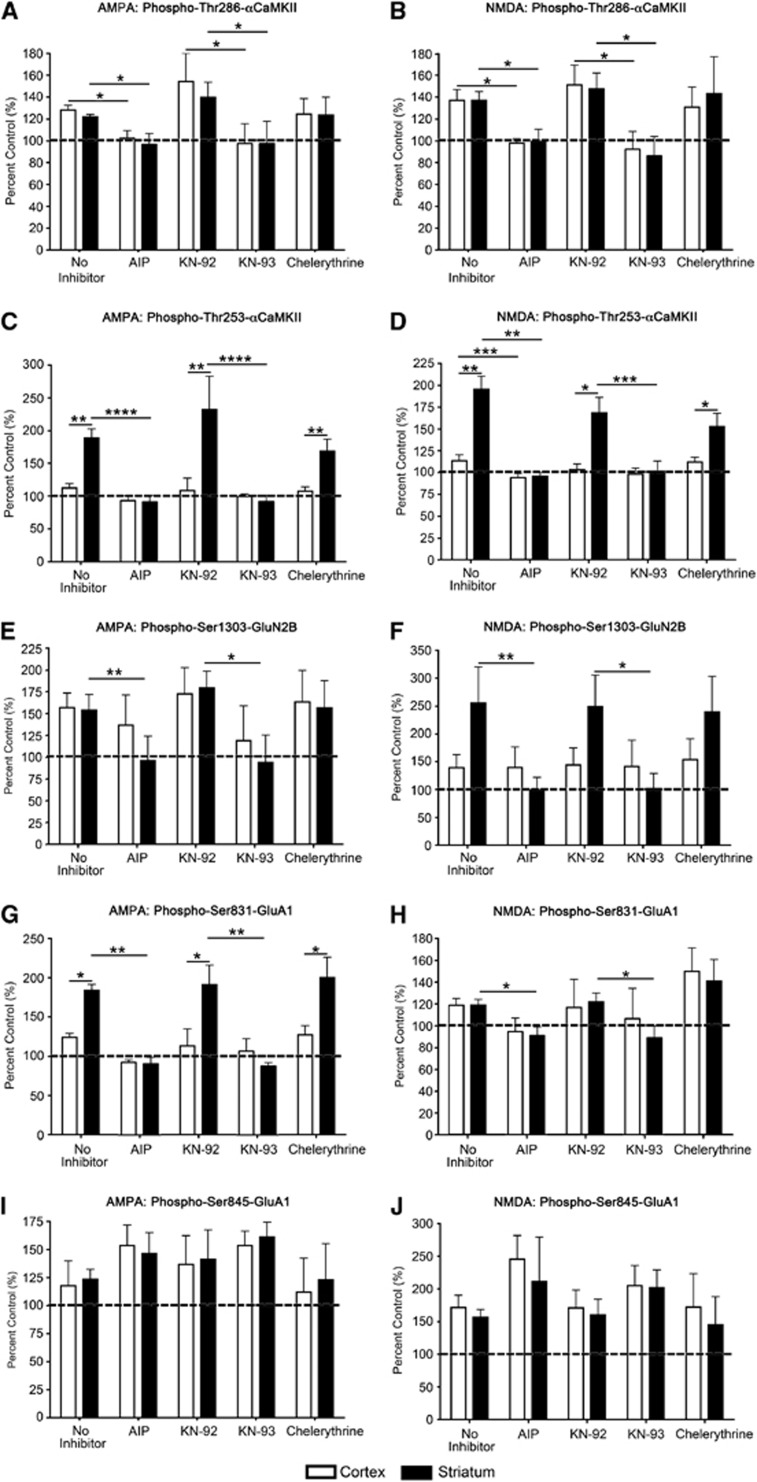
The data presented in Figures 2, 3 and 4 suggest a potential relationship between Thr253-αCaMKII phosphorylation and glutamate receptor phosphorylation at Ser831-GluA1 and Ser1303-GluN2B. However, both PKC and CaMKII can phosphorylate these sites. To ascertain whether CaMKII or PKC is responsible for the enhanced phosphorylation of Ser831-GluA1 or Ser1303-GluN2B in the striatum after an excitotoxic stimulus, brain microslices were treated with inhibitors of either CaMKII (myr-AIP, KN-93, and the inactive analog, KN-92) or PKC (chelerythrine chloride). The two CaMKII inhibitors were used at concentrations that are relatively specific for CaMKII,1 and have no effect on PKC activity.
Our results show that preincubation of microslices with myr-AIP and KN-93, but not KN-92 or chelerythrine, blocked the AMPA- and NMDA-induced increases in Thr286-αCaMKII (Figures 5A and 5B) and Thr253-αCaMKII (Figures 5C and 5D) phosphorylation. No change in total αCaMKII expression after AMPA or NMDA stimulation was observed (data not shown). Both CaMKII inhibitors (myr-AIP and KN-93) significantly blocked the stimulus-induced increase in the phosphorylation of Ser831-GluA1 and Ser1303-GluN2B after AMPA or NMDA stimulation in the striatum (Figures 5E, 5F, 5I and 5J). The PKC inhibitor chelerythrine had no significant effect on the stimulus-induced phosphorylation of any site (Figure 5). No change was observed in total GluN2B or GluA1 expression after AMPA or NMDA stimulation (data not shown). These results show that, although PKC is potentially capable of phosphorylating both Ser831-GluA1 and Ser1303-GluN2B, in these microslices under the conditions used, CaMKII activity appears to be responsible for the observed phosphorylation of these receptors at these sites. However, a constitutively active form of death-associated protein kinase 1 can phosphorylate Ser1303-GluN2B,26 so a role for death-associated protein kinase 1 in regulating this site after an excitotoxic stimulus cannot be ruled out.
Figure 5.
αCaMKII and glutamate receptor phosphorylation after inhibition of CaMKII and protein kinase C (PKC) activation, and stimulation with 100 μmol/L AMPA or 100 μmol/L NMDA+50 μmol/L glycine for 90 seconds. (A, B) Thr286-αCaMKII, (C, D) Thr253-αCaMKII, (E, F) Ser1303-GluN2B, (G, H) Ser831-GluA1, and (I, J) Ser845-GluA1 phosphorylation in the striatum and cortex of Sprague-Dawley (SD) rats after 20 minutes preincubation with myr-AIP, KN-92, KN-93, chelerythrine chloride, or no inhibitor, and then 90 seconds stimulation with (A, C, E, G, I) 100 μmol/L AMPA or (B, D, F, H, J) 100 μmol/L NMDA+50 μmol/L glycine. Microslices were generated from the striatum and sensory cortex of male 12-week-old rats (n=8), stimulated with AMPA or NMDA±inhibitor, homogenized, and examined for αCaMKII and glutamate receptor phosphorylation by western blotting. Phosphorylation levels are normalized to total protein expression (i.e., phosphorylation/total expression), and are expressed as percent nonstimulated control. *Denotes statistical significance (*P<0.05; **P<0.01; ***P<0.001; ****P<0.0001). CaMKII, calcium/calmodulin-stimulated protein kinase II.
The Time Course of αCaMKII Phosphorylation is Different in Striatum and Cortexafter Experimental Stroke In Vivo
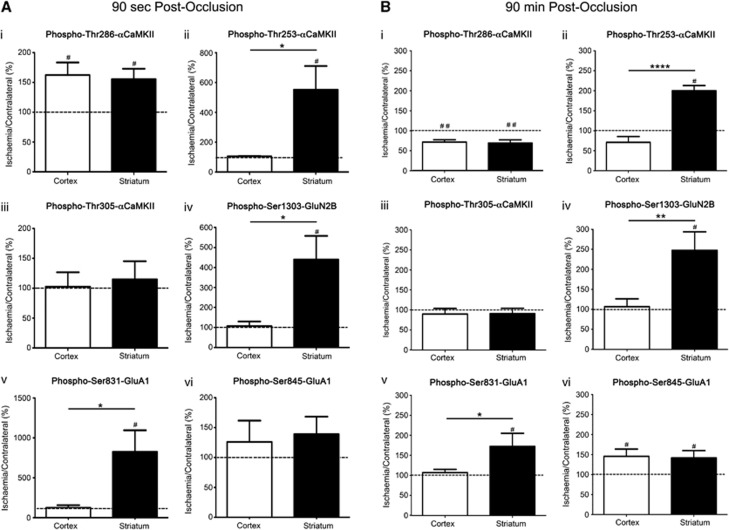
The results using microslices show that there are inherent differences in the response of CaMKII in striatum and cortex to activation by an excitotoxic stimulus. We hypothesized that this would also be true after a stroke in vivo. To investigate this prediction, we examined the changes in the phosphorylation of CaMKII, GluA1, and GluN2B in striatum and cortex after MCAo. The level of phosphorylation on the ischemic side was compared with the level on the contralateral side within the same animal.
After a short occlusion (90 seconds), the relative differences in αCaMKII phosphorylation in vivo at Thr286, Thr253, and Thr305, GluA1 at Ser831 and Ser845, and GluN2B at Ser1303 compared with contralateral (Figure 6A) were similar to those observed after a 90 second excitotoxic stimulus of brain slices in vitro (Figures 2, 3 and 4). At this time, Thr286-αCaMKII (Figure 6A(i)) and Ser845-GluA1 (Figure 6A(vi)) phosphorylation was significantly elevated in both the ischemic striatum and cortex (Figure 6A(i)), whereas Thr305-αCaMKII phosphorylation in the ischemic regions was equivalent to the contralateral side in both regions (Figure 6A(iii)). As observed after glutamate+glycine stimulation, phosphorylation at Thr253-αCaMKII (Figure 6A(ii)), Ser1303-GluN2B (Figure 6A(iv)), and Ser831-GluA1 (Figure 6A(v)) was significantly elevated in the ischemic striatum, but not in the cortex.
Figure 6.
αCaMKII, GluA1, and GluN2B phosphorylation after occlusion of the middle cerebral artery for 90 seconds or 90 minutes. (i) Thr286-αCaMKII, (ii) Thr253-αCaMKII, (iii) Thr305-αCaMKII, (iv) Ser1303-GluN2B, (v) Ser831-GluA1, and (vi) Ser845-GluA1 phosphorylation in the striatum and cortex of Sprague-Dawley (SD) rats after (A) 90 seconds or (B) 90 minutes occlusion of their middle cerebral artery (n=6/time point). Rats were decapitated, and the striatum and cortex were removed and homogenized. Phosphorylation levels are normalized to total protein expression (i.e., phosphorylation/ total expression), and are expressed as a percentage (ischemia side/contralateral side). #Denotes statistical significance from contralateral side (P<0.05); *denotes statistical significance between the two brain regions (P<0.05). CaMKII, calcium/calmodulin-stimulated protein kinase II.
At the end of the occlusion period (90 minutes with no reperfusion), Thr286-αCaMKII (Figure 6B(i)) and Ser845-GluA1 (Figure 6B(vi)) phosphorylation was significantly decreased in both the ischemic cortex and striatum, whereas the levels of phosphorylation at Thr253-αCaMKII (Figure 6B(ii)), Ser1303-GluN2B (Figure 6B(iv)), and Ser831-GluA1 (Figure 6B(v)) were significantly elevated in the ischemic striatum, but not in the cortex. Thr305-αCaMKII phosphorylation was not increased in the ischemic tissue compared with the contralateral side for either brain region (Figure 6B(iii)).
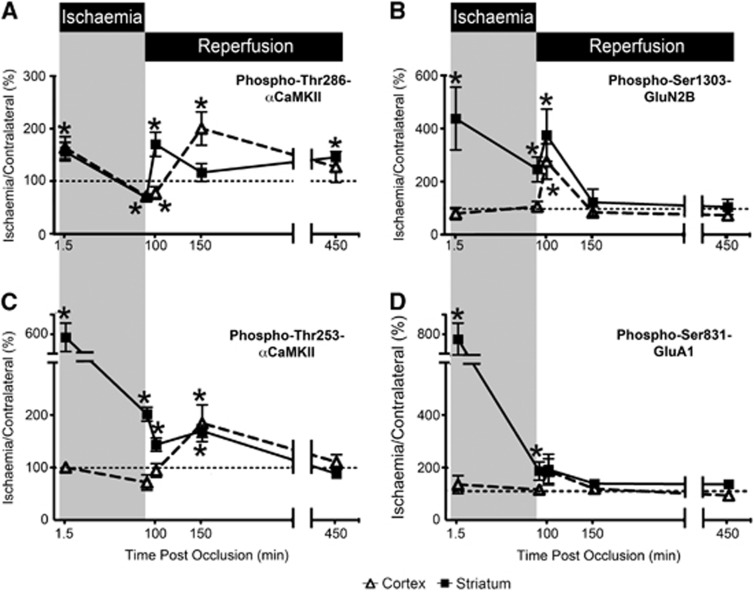
Next, we examined the changes in phosphorylation that occurred at various reperfusion times after 90 minutes MCAo. After 10 minutes of reperfusion, the level of phosphorylation of Thr286- and Thr253-αCaMKII was significantly increased in ischemic striatum, whereas the level of phosphorylation in ischemic cortex was not significantly different from contralateral (Figures 7A and 7C). However, after 60 minutes of reperfusion, Thr286-αCaMKII phosphorylation was elevated in the ischemic cortex but not in the striatum (Figure 7C), whereas Thr253-αCaMKII phosphorylation was elevated in both the ischemic cortex and striatum (Figure 7A). By 6 hours after reperfusion, Thr253-αCaMKII phosphorylation had returned to control levels in both regions (Figure 7A), whereas Thr286-αCaMKII phosphorylation levels had decreased in the cortex, but had risen in the striatum (Figure 7C). Reperfusion produced a significant transient increase in the phosphorylation of Ser1303-GluN2B in both striatum and cortex at 10 minutes after reperfusion, but was not different at 60 minutes or 6 hours after reperfusion (Figure 7D). Additionally, the phosphorylation of Ser831-GluA1 in cortex and striatum did not show a statistically significant change after reperfusion (Figure 7B).
Figure 7.
αCaMKII, GluA1, and GluN2B phosphorylation after varying periods of ischemia and reperfusion. (A) Thr286-αCaMKII, (B) Ser1303-GluN2B, (C) Thr253-αCaMKII, and (D) Ser831-GluA1 phosphorylation in the cortex and striatum of Sprague-Dawley (SD) rats after occlusion of their middle cerebral artery (n=6/time point, except for Ser831-GluA1 and Ser1303-GluN2B 150 and 450 minutes after occlusion where n=3/time point). Rats were decapitated, and the striatum and cortex were removed and homogenized. Phosphorylation levels are normalized to total protein expression (i.e., phosphorylation/total expression), and are expressed as a percentage (ischemia side/contralateral side). Ratios >100% indicate that the level of phosphorylation in the ischemic side is greater than that observed in the contralateral side. Times postocclusion (min) are shown. The data at 1.5 and 90 minutes ischemia are the same as those presented in Figures 7 and 8. *Denotes statistical significance from the contralateral side (P<0.05). CaMKII, calcium/calmodulin-stimulated protein kinase II.
These results show that striatum and cortex show different αCaMKII phosphorylation responses in vivo to ischemia-reperfusion and support the conclusions from the microslice experiments that there is an endogenous difference between these two tissues in their response to ischemia.
Discussion
Different brain regions exhibit differing sensitivities to ischemia; for example, the striatum is more vulnerable to ischemic damage than the overlying cortex after MCAo.27 While factors such as collateral blood supply could account for this variation, there is an unresolved controversy about whether endogenous differences in such brain regions could be responsible for this differential susceptibility. Herein, we report that rapid changes in protein phosphorylation involving CaMKII, an enzyme involved in ischemia-induced cell death, are strikingly different in striatum and cortex after MCAo in vivo. Furthermore, these rapid changes are faithfully mimicked by excitotoxic pharmacological stimulation of microslices of striatum and cortex in vitro, where potential differences in blood supply can have no role. These data support the hypothesis that there are endogenous differences between these brain regions that account for their differential vulnerabilities to ischemia/excitotoxicity.
Autophosphorylation of αCaMKII at Thr253 induced by either in vivo ischemia or in vitro excitotoxicity occurred more rapidly, to a higher stoichiometry, and was longer lasting in the striatum (highly sensitive to ischemia/excitotoxicity) than in cortex (relatively resistant to ischemia/excitotoxicity) (Figures 2, 3, 4, 6 and 7). Additionally, there was a corresponding difference in the phosphorylation of AMPA-R and NMDA-R (Figures 2, 3, 4, 6 and 7) mediated by CaMKII (Figure 5). Our data are strengthened by previous findings which showed that Ser831-GluA1 phosphorylation is increased in the CA1 (sensitive to ischemia), but not in the CA3/dentate gyrus (relatively resistant to ischemia) regions of the hippocampus after transient global brain ischemia.28
We have shown that the differences in CaMKII activation and control between the striatum and cortex after ischemia/excitotoxicity are not accounted for by differences in the levels of expression of αCaMKII, AMPA-R, or NDMA-R, or the density of synapses in these regions. One explanation for the different CaMKII responses in striatum and cortex is the presence of different CaMKII interacting proteins that alter the molecular microenvironment for CaMKII by complex formation leading to allosteric modification and/or localization to different cellular microdomains. As Thr253 phosphorylation has no direct effect on CaMKII activity, but alters CaMKII targeting through interactions with particular binding proteins, and the pattern of expression of CaMKII binding proteins varies between brain regions and cell types,3 tissue differences in phospho-Thr253-mediated CaMKII targeting may be responsible for the differences in sensitivity to ischemia/excitotoxicity between the striatum and cortex.
CaMKII has been implicated in neuronal death and survival pathways after excitotoxic insults, with reports of CaMKII inhibition either being neuroprotective29, 30, 31 or exacerbating cell death.32, 33, 34 While there is some controversy surrounding this issue, the balance of available data favors the hypothesis that short-term CaMKII inhibition postischemia, or after an excitotoxic stimulus, is neuroprotective in mature brain. Recent studies have shown that inhibitors of autonomously active CaMKII (calcium/calmodulin-independent activity) are neuroprotective when applied after an ischemic/excitotoxic insult, both in vivo after MCAo in mice and in vitro in cultured neurons after glutamate receptor stimulation,7, 29 but inhibitors of the calcium/calmodulin-dependent activity were only protective when applied before the stimulus.7 This suggests that ischemia/excitotoxicity induces a long-lasting autonomous activity in CaMKII that is important for cell death.
Since autophosphorylation at Thr286 makes CaMKII autonomously active,2 it is assumed that the long-lasting excitotoxicity/ischemia-induced autonomous CaMKII activity is due to long-lasting autophosphorylation at Thr286. The finding that overexpression of a wild-type, but not a Thr286 phospho-null (T286A) form of αCaMKII increased glutamate induced cell death in primary hippocampal cultures7 supports this assumption. In this light, it is interesting to consider our results with Thr286 phosphorylation after MCA occlusion and reperfusion. We observed an oscillatory pattern of phosphorylation–dephosphorylation of Thr286-αCaMKII in both the striatum and cortex (Figure 7A) that is consistent with events known to elevate intracellular calcium during ischemia and reperfusion: the onset of ischemia after occlusion, the early consequences of reperfusion and the waves of spreading depression associated with infarct evolution.35 This suggests that repeated CaMKII activation by calcium/calmodulin maintains or boosts Thr286 autophosphorylation and autonomous CaMKII activity in mature brain in vivo. This is an interesting phenomena, and indicates that the reactivation of CaMKII after reperfusion may cause the rephosphorylation of CaMKII substrates (such as glutamate receptors), thereby reactivating downstream signaling pathways potentially involved in excitotoxic responses. Additionally, this mechanism may result in the rephosphorylation of Thr253, thereby accounting for its longer stability. By contrast, in cultured neurons, the autonomous CaMKII activity produced by the excitotoxic stimulus is maintained for some hours apparently without the need for further rephosphorylation. This is showed by recent studies showing that inhibitors of calcium/calmodulin-dependent CaMKII activity are neuroprotective only if applied before the excitotoxic stimulus while inhibitors of autonomous CaMKII activity are neuroprotective when applied after an excitotoxic stimulus.29, 34
The changes in Thr253-αCaMKII phosphorylation in striatum and cortex after MCAo indicate that it is associated with brain region sensitivity to ischemia/excitotoxicity. Furthermore, the ability of tissue slices in vitro to reproduce the changes seen in vivo indicates that these regional differences are not related to differences in tissue perfusion (Figures 2, 3 and 4). In striatum, Thr253-αCaMKII phosphorylation increased after MCAo, and remained high until at least 90 minutes after reperfusion (Figure 7B). This phenomenon has previously been observed in the ischemia-sensitive hippocampus after global ischemia and reperfusion, as well as drug-induced status epilepticus.5 By contrast, in the less ischemia-sensitive cortex, an increase in phosphorylation at Thr253-αCaMKII was only observed on reperfusion (Figure 7B). Additionally, the patterns of change in the CaMKII-mediated phosphorylation of the AMPA- and NMDA-R subunits in striatum resembled those of Thr253-αCaMKII (Figures 7C and 7D), consistent with a role for CaMKII targeting in their phosphorylation. In view of the close parallel between the early phosphorylation responses observed in the stimulated slices in vitro (Figure 4) and the ischemic brain regions in vivo (Figure 6A), we cannot rule out the possibility that MCAo caused changes in the phosphorylation of Thr253-αCaMKII, Ser1303-GluN2B, or Ser831-GluA1 in cortex since measurements were only made 90 seconds and 90 minutes after occlusion. However, if such changes did take place in the ischemic cortex, then they must have been slower in onset, shorter in duration, and most likely smaller in extent than those in striatum. Additionally, it is not known whether these changes are occurring at the synapse, cell membrane, or within intracellular vesicular compartments, and future studies examining fractionated samples would address this.
As we were interested in rapid phosphorylation responses after excitotoxicity/ischemia we chose to measure changes in the phosphorylation of GluA1 and GluN2B because they are well-characterized substrates for CaMKII that are concentrated at the PSD, where CaMKII is also concentrated. Although overstimulation of most glutamate receptors causes cell death,36 the calcium-permeable NMDA-Rs appear to be the most sensitive ‘death receptors'.30 The phosphorylation of the GluA1 subunit of the AMPA-R at Ser831 has been proposed to be a death enhancing effect of CaMKII activation,30 due to increased single-channel conductance of GluA1 after phosphorylation of this site.25 By contrast, phosphorylation of the GluN2B subunit of the NMDA-R at Ser1303 desensitizes the NMDA-R,22 and therefore may inhibit cell death pathways. However, it is not clear whether the CaMKII-mediated phosphorylation of these receptors is involved in the cell death response or whether the key protein targets regulated by CaMKII are downstream of calcium entry.
All animals used in these studies were anesthetized with isoflurane for 1 hour before kill, so that direct comparisons could be made between the in vitro microslice experiments and in vivo micropunch experiments. As isoflurane is neuroprotective,37 it may have influenced the phosphorylation/expression of proteins examined herein. However, whatever effect isoflurane had should be the same throughout the brain, and if responses to isoflurane were different between brain regions, this provides further evidence that an endogenous molecular difference exists between these regions.
After periods of experimental cerebral ischemia, CaMKII translocates to one of the two sites: (1) the PSD38 and (2) extrasynaptic clusters.39 Many CaMKII binding partners, including GluA1 and GluN2B, are present in the PSD, whereas extrasynaptic clustering most likely occurs after aggregation of multiple CaMKII holoenzymes. These translocations are also associated with a reduction in calcium/calmodulin-stimulated CaMKII activity.38 The precise mechanisms and functional outcomes involved in these translocations/alterations in activity remain to be determined, but highlight the importance of targeting in regulating CaMKII activity and hence function.
Based on our findings, we hypothesize that CaMKII involvement in ischemia-induced neuronal death requires autophosphorylation of CaMKII at two sites: (1) Thr286, which induces CaMKII to become autonomously active, therefore allows CaMKII to continue to phosphorylate key substrates and to maintain sustained activation of cell death pathways; and (2) Thr253 that targets the autonomously active CaMKII to the key substrates necessary to activate these pathways. Furthermore, we hypothesize that brain regions that are inherently more sensitive to excitotoxicity/ischemia express enhanced levels of one or more proteins that bind to CaMKII in a way that allosterically favors autophosphorylation of CaMKII at Thr253 and localizes CaMKII near calcium channels. This would allow a more rapid activation of CaMKII after a rise in intracellular calcium (as occurs after initial occlusion and reperfusion), and may account for the more rapid CaMKII and substrate phosphorylation that was observed in brain regions that are more sensitive to excitotoxicity/ischemia.
Based on strong preclinical data, CaMKII is an attractive target for the development of neuroprotective therapies for brain pathologies involving excitotoxic pathways. However, as CaMKII is expressed in virtually all tissues, existing inhibitors that target CaMKII kinase activity are unlikely to be useful therapeutically, as they are likely to cause many side effects. By contrast, drugs that interfere with CaMKII targeting to specific signaling complexes, such as AMPA- and/or NMDA-Rs, offer the potential for cell and stimulus-specific inhibition that may be beneficial therapeutically, either when administered on their own or in conjunction with existing therapies such as tissue plasminogen activator. Our results suggest that CaMKII targeting mediated by Thr253 phosphorylation may be an attractive focus for such drug development.
Acknowledgments
The authors would like to thank Ms A'qilah Banu Binte Abdul Majeed, Mrs Helen Carpenter, Ms S Phoebe Chung, Ms Sarah McCann, Mr Matthew Morten, Ms Lucy Murtha, Ms Debbie Pepperall, and Ms Amelia Tomkins for their technical assistance on various aspects of this project.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This work was supported by research funds from the National Health and Medical Research Council of Australia, the Hunter Medical Research Institute, and the University of Newcastle. Dr Spratt was supported by an NHMRC Health Professional Research Training Fellowship (455632).
Supplementary Material
References
- Skelding KA, Rostas JA, Verrills NM. Controlling the cell cycle: The role of calcium/calmodulin-stimulated protein kinases I and II. Cell Cycle. 2011;10:631–639. doi: 10.4161/cc.10.4.14798. [DOI] [PubMed] [Google Scholar]
- Skelding KA, Rostas JAP. Regulation of CaMKII in vivo: the importance of targeting and the intracellular microenvironment. Neurochem Res. 2009;34:1792–1804. doi: 10.1007/s11064-009-9985-9. [DOI] [PubMed] [Google Scholar]
- Skelding KA, Suzuki T, Gordon SL, Xue J, Verrills NM, Dickson PW, et al. Regulation of CaMKII by phospho-Thr253 or phospho-Thr286 sensitive targeting alters cellular function. Cell Signal. 2010;22:759–769. doi: 10.1016/j.cellsig.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Migues PV, Lehmann IT, Fluechter L, Cammarota M, Gurd JW, Sim ATR, et al. Phosphorylation of CaMKII at Thr253 occurs in vivo and enhances binding to isolated postsynaptic densities. J Neurochem. 2006;98:289–299. doi: 10.1111/j.1471-4159.2006.03876.x. [DOI] [PubMed] [Google Scholar]
- Gurd JW, Rawof S, Zhen Hou J, Dykstra C, Bissoon N, Teves L, et al. Ischemia and status epilepticus result in enhances phosphorylation of calcium and calmodulin-stimulated protein kinase II on threonine 253. Brain Res. 2008;1218:158–165. doi: 10.1016/j.brainres.2008.04.040. [DOI] [PubMed] [Google Scholar]
- Szydlowska K, Tymianski M. Calcium, ischemia and excitotoxicity. Cell Calcium. 2010;47:122–129. doi: 10.1016/j.ceca.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Vest RS, O'Leary H, Coultrap SJ, Kindy MS, Bayer KU. Effective post-insult neuroprotection by a novel Ca(2+)/calmodulin-dependent protein kinase II (CaMKII) inhibitor. J Biol Chem. 2010;285:20675–20682. doi: 10.1074/jbc.M109.088617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- Markus R, Reutens DC, Kazui S, Read S, Wright P, Chambers BR, et al. Topography and temporal evolution of hypoxic viable tissue identified by 18F-fluoromisonidazole positron emission tomography in humans after ischemic stroke. Stroke. 2003;34:2646–2652. doi: 10.1161/01.STR.0000094422.74023.FF. [DOI] [PubMed] [Google Scholar]
- Bozzao L, Fantozzi LM, Bastianello S, Bozzao A, Fieschi C. Early collateral blood supply and late parenchymal brain damage in patients with middle cerebral artery occlusion. Stroke. 1989;20:735–740. doi: 10.1161/01.str.20.6.735. [DOI] [PubMed] [Google Scholar]
- Pantoni L, Garcia JH, Gutierrez JA. Cerebral white matter is highly vulnerable to ischemia. Stroke. 1996;27:1641–1647. doi: 10.1161/01.str.27.9.1641. [DOI] [PubMed] [Google Scholar]
- Pignataro G, Maysami S, Studer FE, Wilz A, Simon RP, Boison D. Downregulation of hippocampal adenosine kinase after focal ischemia as potential endogenous neuroprotective mechanism. J Cereb Blood Flow Metab. 2008;28:17–23. doi: 10.1038/sj.jcbfm.9600499. [DOI] [PubMed] [Google Scholar]
- Lecrux C, Nicole O, Chazalviel L, Catone C, Chuquet J, MacKenzie ET, et al. Spontaneously hypertensive rats are highly vulnerable to AMPA-induced brain lesions. Stroke. 2007;38:3007–3015. doi: 10.1161/STROKEAHA.107.491126. [DOI] [PubMed] [Google Scholar]
- Coyle P. Different susceptibilities to cerebral infarction in spontaneously hypertensive (SHR) and normotensive Sprague-Dawley rats. Stroke. 1986;17:520–525. doi: 10.1161/01.str.17.3.520. [DOI] [PubMed] [Google Scholar]
- McIlwain H, Buddle HL. Techniques in tissue metabolism: 1. A mechanical chopper. Biochemistry. 1953;53:412–420. doi: 10.1042/bj0530412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh JM, Bunn SJ, Boyd TL, Rostas JA. Developmental changes in glutamate receptor stimulated inositol phospholipid metabolism and 45Ca(2+)-accumulation in posthatch chicken forebrain. Neurosci Lett. 1995;194:161–164. doi: 10.1016/0304-3940(95)11749-m. [DOI] [PubMed] [Google Scholar]
- Spratt NJ, Fernandez J, Chen M, Rewell S, Cox S, van Raay L, et al. Modification of the method of thread manufacture improves stroke induction rate and reduces mortality after thread-occlusion of the middle cerebral artery in young or aged rats. J Neurosci Methods. 2006;155:285–290. doi: 10.1016/j.jneumeth.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Heijtz RD, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J, Soler-Llavina GJ, Fuccillo MV, Malenka RC, Sudhof TC. Neuroglins/LRRTMs prevent activity- and Ca2+/calmodulin-dependent synapse elimination in cultured neurons. J Cell Biol. 2011;194:323–334. doi: 10.1083/jcb.201101072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone FC, Price WJ, White RF, Willette RN, Feuerstein GZ. Genetic hypertension and increased susceptibility to cerebral ischemia. Neurosci Biobehav Rev. 1992;16:219–233. doi: 10.1016/s0149-7634(05)80182-4. [DOI] [PubMed] [Google Scholar]
- Liao GY, Wagner DA, Hsu MH, Leonard JP. Evidence for direct protein kinase-C mediated modulation of N-methyl-D-aspartate receptor current. Mol Pharmacol. 2001;59:960–964. doi: 10.1124/mol.59.5.960. [DOI] [PubMed] [Google Scholar]
- Sessoms-Sikes S, Honse Y, Lovinger DM, Colbran RJ. CaMKIIalpha enhances the desensitization of NR2B-containing NMDA receptors by an autophosphorylation-dependent mechanism. Mol Cell Neurosci. 2005;29:139–147. doi: 10.1016/j.mcn.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Barria A, Muller D, Derkach V, Griffith LC, Soderling TR. Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science. 1997;276:2042–2045. doi: 10.1126/science.276.5321.2042. [DOI] [PubMed] [Google Scholar]
- Mammen AL, Kameyama K, Roche KW, Huganir RL. Phosphorylation of the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. J Biol Chem. 1997;272:32528–32533. doi: 10.1074/jbc.272.51.32528. [DOI] [PubMed] [Google Scholar]
- Roche KW, O'Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- Tu W, Xu X, Peng L, Zhong X, Zhang W, Soundarapandian MM, et al. DAPK1 interaction with NMDA receptor NR2B subunits mediates brain damage in stroke. Cell. 2010;140:222–234. doi: 10.1016/j.cell.2009.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JH, Liu KF, Ye ZR, Gutierrez JA. Incomplete infarct and delayed neuronal death after transient middle cerebral artery occlusion in rats. Stroke. 1997;28:2303–2309. doi: 10.1161/01.str.28.11.2303. [DOI] [PubMed] [Google Scholar]
- Takagi Y, Takagi N, Besshoh S, Miyake-Takagi K, Takeo S. Transient global ischemia enhances phosphorylation of the GluR1 subunit of the alpha-amino-3-hydroxy-5-methylisozazole-4-propionate receptor in the hippocampal CA1 region in rats. Neurosci Lett. 2003;341:33–36. doi: 10.1016/s0304-3940(03)00153-8. [DOI] [PubMed] [Google Scholar]
- Ashpole NM, Hudmon A. Excitotoxic neuroprotection and vulnerability with CaMKII inhibition. Mol Cell Neurosci. 2011;46:720–730. doi: 10.1016/j.mcn.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Coultrap SJ, Vest RS, Ashpole NM, Hudmon A, Bayer KU. CaMKII in cerebral ischemia. Acta Pharmacol Sin. 2011;32:861–872. doi: 10.1038/aps.2011.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Xu J, Shen X, Lv C, Xu T, Pei D. CaMKII antisense oligodeoxynucleotides protect against ischemia-induced neuronal death in the rat hippocampus. J Neurol Sci. 2012;314:104–110. doi: 10.1016/j.jns.2011.10.012. [DOI] [PubMed] [Google Scholar]
- Waxham MN, Grotta JC, Silva AJ, Strong R, Aronowski J. Ischemia-induced neuronal damage: a role for calcium/calmodulin-dependent protein kinase II. J Cereb Blood Flow Metab. 1996;16:1–6. doi: 10.1097/00004647-199601000-00001. [DOI] [PubMed] [Google Scholar]
- Hansen MR, Bok J, Devaiah AK, Zha X-M, Green SH. Ca2+/calmodulin-dependent protein kinases II and IV both promote survival but differ in their effects on axon growth in sprial ganglion neurons. J Neurosci Res. 2003;72:169–184. doi: 10.1002/jnr.10551. [DOI] [PubMed] [Google Scholar]
- Ashpole NM, Song W, Brustovetsky T, Engleman EA, Brustovetsky N, Cummins TR, et al. Calcium/calmodulin-dependent protein kinase II (CaMKII) inhibition induces neurotoxicity via dysregulation of glutamate/calcium signaling and hyperexcitability. J Biol Chem. 2012;287:8495–8506. doi: 10.1074/jbc.M111.323915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumagai T, Walberer M, Nakamura H, Endepols H, Sue M, Vollmar S, et al. Distinct spatiotemporal patterns of spreading depolarizations during early infarct evolution: evidence from real-time imaging. J Cereb Blood Flow Metab. 2011;31:580–592. doi: 10.1038/jcbfm.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frandsen A, Drejer J, Schousboe A. Direct evidence that excitotoxicity in cultured neurons is mediated via N-methyl-D-aspartate (NMDA) as well as non-NMDA receptors. J Neurochem. 1989;53:297–299. doi: 10.1111/j.1471-4159.1989.tb07327.x. [DOI] [PubMed] [Google Scholar]
- Schifilliti D, Grasso G, Conti A, Fodale V. Anaesthetic-related neuroprotection: intravenous or inhalational agents. CNS Drugs. 2010;24:893–907. doi: 10.2165/11584760-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Mengesdorf T, Althausen S, Mies G, Olah L, Paschen W. Phosphorylation state, solubility, and activity of calcium/calmodulin-dependent protein kinase II alpha in transient focal ischemia in mouse brain. Neurochem Res. 2002;27:471–484. doi: 10.1023/a:1019844518704. [DOI] [PubMed] [Google Scholar]
- Hudmon A, LeBel E, Roy H, Sik A, Schulman H, Waxham MN, et al. A mechanism for Ca2+/calmodulin-dependent protein kinase II clustering at synaptic and nonsynaptic sites based on self-association. J Neurosci. 2005;25:6971–6983. doi: 10.1523/JNEUROSCI.4698-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.