Abstract
The inflammatory response plays a pivotal role in propagating injury of intracerebral hemorrhage (ICH). Glucagon-like-peptide-1 (GLP-1) is a hormone with antidiabetic effect and may also have antiinflammatory properties. Despite consensus that the glucoregulatory action is mediated by the GLP-1 receptor (GLP-1R), mechanisms in the brain remain unclear. We investigated the effect of a long-acting GLP-1 analog, liraglutide, and its truncated metabolite, GLP-1(9-36)a from dipeptidyl peptidase-4 (DPP-4) cleavage in ICH-induced brain injury. Primary outcomes were cerebral edema formation, neurobehavior, and inflammatory parameters. GLP-1(9-36)a, GLP-1R inhibitor, adenosine monophosphate-activated protein kinase (AMPK) phosphorylation inhibitor and DPP-4 inhibitor were administered to examine the mechanisms of action. Liraglutide suppressed neuroinflammation, prevented brain edema and neurologic deficit following ICH, which were partially reversed by GLP-1R inhibitor and AMPK phosphorylation inhibitor. Liraglutide-mediated AMPK phosphorylation was unaffected by GLP-1R inhibitor, and was found to be induced by GLP-1(9-36)a. GLP-1(9-36)a showed salutary effects on primary outcomes that were reversed by AMPK phosphorylation inhibitor but not by GLP-1R inhibitor. Liraglutide and DPP-4 inhibitor co-administration reversed liraglutide-mediated AMPK phosphorylation and antiinflammatory effects. Liraglutide exerted duals actions and the antiinflammatory effects are partially mediated by its metabolite in a phosphorylated AMPK-dependent manner. Therapies that inhibit GLP-1 degradation may weaken the metabolite-mediated effects.
Keywords: GLP-1(9-36)a, glucagon-like peptide 1, inflammation, intracerebral hemorrhage, neuroprotection
Introduction
Intracerebral hemorrhage (ICH) induced formation of perihematomal edema remains a major challenge for which there is no effective therapy.1 A key element for the development of cerebral edema starts with the inflammatory reaction following the introduction of the hematoma.2 Blood-derived leukocytes, neutrophils in particular, infiltrate into the brain parenchyma, result in enhanced disruption of blood–brain barrier, cause increased cerebral edema formation, and subsequently deterioration in neurobehavioral function.3 Thus, to improve outcome of ICH patients, it may be important to develop new therapies against inflammation without the often-associated major adverse effects seen in existing immunosuppressants.
Glucagon-like peptide (GLP-1) was originally reported as a gut hormone derived from posttranslational proteolysis of preproglucagon that is rapidly released by the L-cells of the small intestine in response to food intake in the form of GLP-1(7-36)a.4 Its insulinotropic property and inhibitory effects on glucagon secretion, gastric emptying, appetite, and food intake formed the basis of its application as a novel lead compound for the treatment of type 2 diabetes mellitus. For review see Anagnostis et al.5 The functional importance of GLP-1 is further signified by the identical peptide sequence found in mouse, rat, and human.6 Nevertheless, circulating levels of GLP-1(7-36)a fall quickly with a half-life of 1 to 2 minutes due to enzymatic cleavage of the two N-terminal amino acids by dipeptidyl peptidase 4 (DPP-4) to form GLP-1(9-36)a.7 Glucagon-like-peptide-1 metabolites are considered inactive with no significant action on glucose metabolism and the GLP-1 receptor.8 With the aim to preserve and increase circulating intact GLP-1 level to enhance therapeutic potential, DPP-4 inhibitors and long-acting GLP-1 analogs were developed.
Emerging evidence suggests GLP-1, apart from its glucoregulatory effects, also exerts antiinflammatory actions on the vascular system in both hyperglycemic and euglycemic states.9, 10, 11, 12 However, mechanisms through which GLP-1 modulates vascular immune response are incompletely understood. At present, GLP-1 is widely believed to exert its actions through a distinct heptahelical G-protein-coupled receptor, the GLP-1 receptor (GLP-1R), functionally associated with adenylate cyclase through the stimulatory Gs protein.13 In the mammalian brain, GLP-1R expression was detected in endothelial cells, neurons, astrocytes, and microglia.10, 14, 15
The present study was designed to examine the effects of a long-acting GLP-1 analog, liraglutide and its metabolite, GLP-1(9-36)a, in ICH induced brain injury in mice with inflammatory parameters, cerebral edema formation, and neurologic function used as primary outcomes. The mechanisms of action of liraglutide and GLP-1(9-36)a were explored in the presence of exendin 9-39, a classic GLP-1R inhibitor16 and compound C, a specific inhibitor of 5′ adenosine monophosphate-activated protein kinase (AMPK).17 Finally, the relative contribution of GLP-1R and metabolite-mediated effects were examined by coadministering liraglutide and DPP-4 inhibitor.
Materials and methods
Animals
All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Loma Linda University and conducted according to the Guidelines for Animal Experimentation at Loma Linda University. Eight-week-old male CD1 mice (weight 35 to 45 g; Charles River, MA, USA) were housed in a light- and temperature-controlled environment with unlimited access to food and water.
Intracerebral Hemorrhage Mouse Model
Intracerebral hemorrhage was induced by using a double infusion model of autologous whole blood (40 μL) as previously described by our laboratory.18 Briefly, mice were randomly assigned to the experimental groups, anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg) (2:1 v/v, intraperitoneal injection) and positioned prone in a stereotactic head frame (Kopf Instruments, Tujunga, CA, USA). Arterial blood was collected in a nonheparinized capillary tube, transferred into a Hamilton syringe and with a microinjection pump 5 μL of blood was infused into the right basal ganglia at bregma anterior–posterior 0.2, mediolateral 2.0, and dorsoventral 3.0. After 5 minutes, 35 μL of blood was delivered at 3.6 mm dorsoventral giving a total injection volume of 40 μL. Sham-operated animals were subjected to needle insertion only.
Reagents
Liraglutide is FDA approved for the treatment of type 2 diabetes mellitus and was purchased from Loma Linda University pharmacy (Victoza; Novo Nordisk A/S, Bagsvaerd, Denmark). Exendin 9-39 (H-8740) and GLP-1(9-36)a (H-4012) were from Bachem (St Helens, UK). Compound C (3093) was from Tocris (Minneapolis, MN, USA). KR-62436 hydrate (K4264), a specific DPP-4 inhibitor,19 was from Sigma-Aldrich (St Louis, MO, USA). Liraglutide was diluted, whereas exendin 9-39 and GLP-1(9-36)a were reconstituted in sterile phosphate-buffered saline (PBS). Compound C and KR-62436 were reconstituted in distilled water.
Intracerebroventricular Injections
Intracerebroventricular (ICV) injection of GLP-1(9-36)a, exendin 9-39, compound C and KR-62436 was performed immediately after ICH induction in the ipsilateral lateral ventricle. Injection was at 1.1 mm lateral and 2.5 mm deep to bregma.
Experimental Design
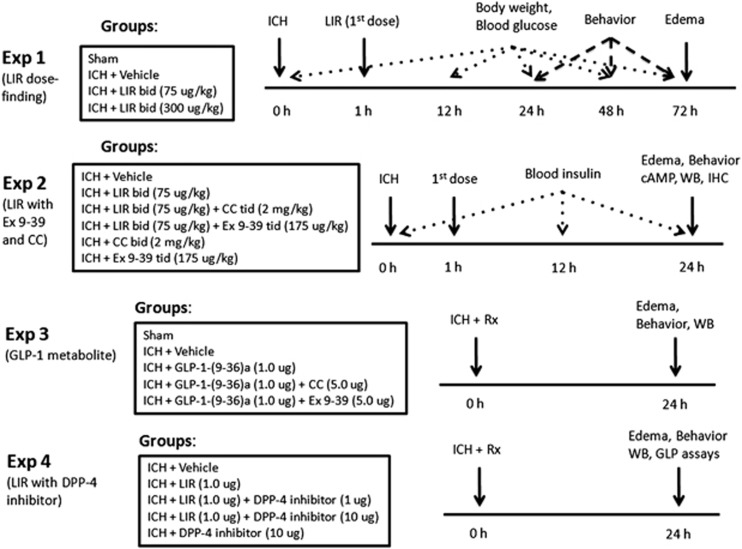
Four separate experiments were conducted (Figure 1, Experiments 1 to 4).
Figure 1.
Experimental design and animal groups classification. ICH, autologous arterial blood-induced intracerebral hemorrhage; LIR, liraglutide; bid, twice daily; tid, four times daily; CC, compound C; Ex 9-39, exendin 9-39; DPP-4, dipeptidyl peptidase-4; WB, Western blot; IHC, immunohistochemistry; Rx, treatment; GLP, glucagon-like peptide.
Experiment 1
Liraglutide was administered subcutaneously (SC) in a high-dose regimen (300 μg/kg SC: LIR 300) twice daily (bid) and a previously reported weight neutral dose (75 μg/kg SC bid: LIR 75)20 in 200 μL, with the first dose given at 1 hour post-ICH. The high-dose regimen is a dose between the maximum tolerated dose of 400 μg/kg SC bid and the antidiabetic dose of 200 μg/kg SC bid in mice (data on file, Novo Nordisk). Mice were divided into four groups: sham (n=4), vehicle (ICH+PBS injection, n=7), LIR 75 (n=7), and LIR 300 (n=7). Assessments included body weight (0, 24, 48, and 72 hours), neurobehavior (24, 48, and 72 hours), brain edema (72 hours), and spot blood glucose level (0, 12, 24, 48, and 72 hours).
Experiment 2
LIR 75 was tested in the presence and absence of exendin 9-39 and compound C. The dose for exendin 9-39 is based on the knowledge that the free fraction of liraglutide is 1% to 2%, and the half-lives of liraglutide and exendin 9-39 are 12 and 2 hours, respectively.21 Since exendin 9-39 in 300-fold excess to GLP-1 was found to antagonize the glucoregulatory effects of liraglutide,22 we administered exendin 9-39 175 μg/kg SC four times daily (tid) to achieve plasma concentration in 400 to 600-fold excess to LIR 75. Compound C was administrated SC 2 mg/kg tid. Total injection volume was 100 μL each time. (Part A) Mice were divided into six groups: vehicle (n=6), LIR 75 (n=6), LIR 75+exendin 9-39 (n=6), LIR 75+compound C (n=6), compound C alone (n=4), exendin 9-39 alone (n=4). All mice were subjected to ICH. Assessments included neurobehavior (24 hours) and brain edema (24 hours). (Part B) Mice were divided into three groups: sham (n=2), vehicle (n=5), and LIR 75 (n=5). Assessment included immunohistochemistry (24 hours). (Part C) Mice were divided into four groups: vehicle (n=5), LIR 75 (n=5), LIR 75+compound C (n=5), and LIR 75+exendin 9-39 (n=5). Assessments included neurobehavior (24 hours), brain edema (24 hours), immunohistochemistry (24 hours), serum insulin level (0, 12, 24 hours), brain cyclic AMP (cAMP) content (24 hours), and Western blot (24 hours).
Experiment 3
All compounds were delivered by ICV injection. The GLP-1(9-36)a (1.0 μg), exendin 9-39 (5.0 μg) and compound C (5.0 μg) were injected in 5 μL. (Part A) Mice were divided into four groups: vehicle (n=6), GLP-1(9-36)a (n=6), GLP-1-(9-36)a+compound C (n=6), and GLP-1(9-36)a+exendin 9-39 (n=6). Assessments included neurobehavior (24 hours) and brain edema (24 hours). (Part B) Mice were divided into four groups: sham (n=4), vehicle (n=6), GLP-1(9-36)a (n=6), and GLP-1-(9-36)a+compound C (n=6). Assessments included neurobehavior (24 hours), brain tissue nuclear extraction, and Western blot (24 hours).
Experiment 4
(Part A) Mice were divided into five groups: vehicle (n=6), LIR (n=6), LIR+DPP-4 inhibitor 1 μg (n=6), LIR+DPP-4 inhibitor 10 μg (n=6), and DPP-4 inhibitor 10 μg (n=4). All compounds were delivered ICV in 5 μL. The amount of liraglutide was 1 μg. Assessments included neurobehavior (24 hours) and brain edema (24 hours). (Part B) Group assignment included the first four groups of part A (n=6 each group). Assessments included GLP assays (24 hours) and Western blot (24 hours).
Measurement of Glucose and Insulin Levels (Experiments 1 and 2)
Nonfasting blood glucose measurements were obtained through a tail nick using a handheld glucometer and One-Touch glucometer strips (Accu-Chek Aviva Test Strips, Roche, Indianapolis, IN, USA, rc04538412001) as described previously.20 Blood (50 μL) was collected and serum insulin level was determined using an insulin enzyme-linked immunosorbent assay (Ultra Sensitive Mouse Insulin ELISA kit, Crystal Chem, Downers Grove, IL, USA, 90080).
Brain Water Content Measurement (Experiments 1 to 4)
Brain water content was measured as previously described.18 Briefly, mice were decapitated under deep anesthesia. The brains were immediately removed and cut into 4 mm slabs around the needle track. Each slab was divided into four parts: hemorrhagic and contralateral basal ganglia and cortex. The cerebellum was collected as an internal control. Each part was weighed on an electronic analytical balance (APX-60, Denver Instrument, New York, NY, USA) giving the WW (wet weight) and then dried at 100°C for 24 hours to determine the DW (dry weight). The brain water content (%) was calculated as [(WW−DW)/WW] × 100.
Neurobehavioral Function Test (Experiments 1 to 4)
An evaluator blinded to animal groups tested the mice. Two tests were implemented for evaluation of neurologic deficits. For modified Garcia test, mice were given a score of 0 to 21.23 The scoring system consists of seven tests (spontaneous activity, axial sensation, vibrissae proprioception, limb outstretching, lateral turning, forelimb walking, and climbing) with a possible score range of 0 to 3 (0=worst; 3=best). The minimum total score is 0 and the maximum is 21. Wire hanging test23 utilized a bridge of 550 cm wire between two platforms on which the mice were placed in the center. Mice were evaluated according to the six criteria based on the subject's ability to reach the platform and/or use its limbs in a symmetrical manner, for which they were assigned a score of 0 to 5 (normal). The average of three trials per test for each animal was calculated.
Histological Assessment (Experiment 2)
Twenty-four hours after ICH, mice were perfused under deep anesthesia with 30 mL of ice-cold PBS, followed by infusion of 10 mL of 10% paraformaldehyde. The brains were removed and immersed in the same fixative at 4 °C for 24 hours, then in 30% sucrose and PBS until saturation. The brains were cut into 10 μm thick coronal sections in cryostat (CM3050S; Leica Microsystems, Bannockburn, IL, USA). Immunohistochemistry was performed with antimyeloperoxidase (MPO) antibody (ab45977, Abcam, Cambridge, MA, USA). The positive cell numbers were counted as previously described.18 The number of immunoreactive cells from 12 locations per mouse (three sections per mouse, four fields per section, n=5, microscopic field × 20) were averaged and expressed as positive cells per field.
Sample Collection and Protein Extraction (Experiments 2 to 4)
Mice were euthanized 24 hours after ICH. Perfusion with 30 mL of ice-cold PBS was performed, followed by removal and collection of the hemorrhagic hemisphere. For experiment 2 part C, brain tissue was instantly frozen in liquid nitrogen and grinded into powder where a fraction was allocated for cAMP assay (see below) and the other for Western blot. For experiment 2 part C and 4, protein extraction was performed with RIPA Lysis Buffer System (sc-24948, Santa Cruz Biotechnology, Santa Cruz, CA, USA) according to the manufacturer's instruction with added protease inhibitor (P8340, Sigma), phosphatase inhibitor cocktails 2 and 3 (P5726, P0044, Sigma). For experiment 3, nuclear extraction of fresh unfrozen brain samples was performed with nuclear and cytoplasmic extraction reagents (NE-PER Nuclear and Cytoplasmic Extraction Reagents, Therma Scientific, Rockford, IL, USA, 78835) according to the manufacturer's instruction with added protease inhibitor and phosphatase inhibitors as above. Extracted samples were stored at −80 °C for Western blot and ELISA analysis.
Brain cAMP, GLP-1-(7-36)a and GLP-1 Metabolite Assays (Experiments 2 and 4)
In experiment 2 part C, the hemorrhagic hemisphere was instantly frozen in liquid nitrogen, grinded into powder and homogenized using lysis buffer provided in the cAMP enzyme immunoassay (EIA) kit from Enzo (ADI-900-066, Enzo, Farmingdale, NY, USA), with which cAMP level in brain extracts were determined. In experiment 4, the hemorrhagic hemisphere was assayed for GLP-1. GLP-1(7-36)a (‘active GLP-1') was measured using ELISA (EGLP-35K, Millipore, Billerica, MA, USA) with undetectable cross-reactivity with other forms of GLP, including GLP-1(1-36)a, GLP-1(1-37), GLP-1(9-36)a, GlP-1(9-37), GLP-2 and glucagon. The ‘total GLP-1' is determined by ELISA (EZGLP1T-36K, Millipore) with antibody pair directed towards GLP-1(7-36)a and GLP-1(9-36)a and has no significant cross-reactivity with GLP-2, GIP, glucagon, and oxyntomodulin. GLP-1(9-36)a was derived by subtracting ‘total GLP-1' from ‘active GLP-1'.
Semiquantitative Western Blotting (Experiments 2 to 4)
Extracted samples were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis. The separated proteins were transferred to polyvinylidene difluoride membrane with iBlot (IB4010, Invitrogen, Grand Island, NY, USA) and incubated with primary antibodies overnight at 4°C. The primary antibodies were anti-intracellular adhesion molecule 1 (ICAM-1) (ab25375, Abcam), anti-E-selectin (ab18981, Abcam), antiMPO (ab45977, Abcam), anti-AMPK α 1 (ab32047, Abcam), anti-phospho-AMPKa (Thr 172) (2531, Cell Signaling, Danvers, MA, USA), anti- nuclear factor-κB (NF-κB) p65 (ab7970, Abcam), anti-Histone H1 (ab71580, Abcam), and anti-Tubulin (ab21057, Abcam). Polyvinylidene difluoride membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (Santa Cruz) for 1 hour at room temperature, probed with ECL Plus chemiluminescence reagent (Amersham Biosciences, Arlington Heights, IL, USA) and binding was visualized on autoradiography films (E3012, Denville Scientific, Metuchen, NJ, USA). Band intensities were quantified using Image J (Bethesda, MD, USA).
Statistics
Data were expressed as mean values±s.e.m. Analysis was performed using SigmaPlot 11.0 (Systat Software, San Jose, CA, USA). Statistical differences between the two groups were analyzed using Student's unpaired, two-tailed t-test. Multiple comparisons (without rating scale data) were statistically analyzed with one-way analysis of variance followed by Student–Newman–Keuls test. Statistical significance was defined as P<0.05. For the rating scale data (neurobehavior), data were expressed as median±25th to 75th percentile. We used the Kruskal–Wallis One-Way Analysis of Variance on Ranks, followed by the Steel–Dwass multiple comparisons tests.
Results
Effect of Liraglutide on Neurobehavioral Function and Brain Edema (Experiments 1 and 2)
Since a regimen of liraglutide >200 μg/kg bid was previously reported to induce weight loss in mice,20 we studied the effect of liraglutide in a parallel group of mice after administering of a lower dose of liraglutide (LIR 75) that did not produce significant weight loss.20
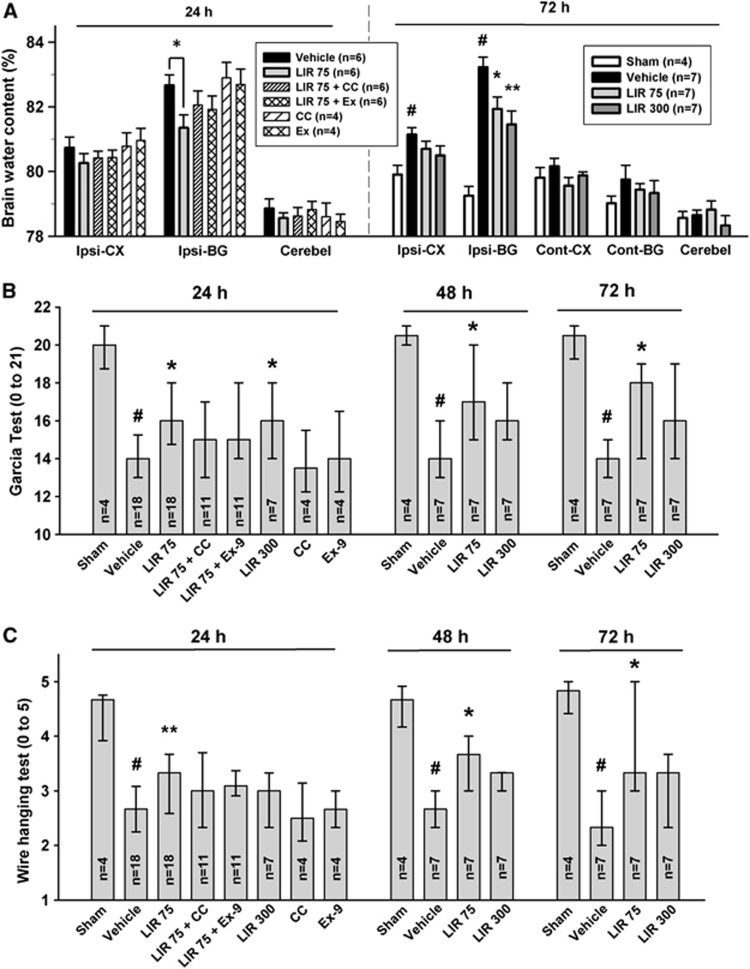
At 24 hours, LIR 75 significantly decreased cerebral edema in the ispilateral basal ganglia compared with vehicle (P<0.05; Figure 2A). This salutary effect was partially abolished by compound C and exendin 9-39. Exendin 9-39 per se in ICH did not show any change in cerebral edema compared with vehicle while compound C in ICH displayed tendency for increased cerebral edema.
Figure 2.
Liraglutide reduced brain edema and improved neurologic functions at 24, 48, and 72 hours following intracerebral hemorrhage (ICH). Liraglutide was administered 1 hour after immunohistochemistry (IHC) in 75 μg/kg body weight twice daily (LIR 75) or 300 μg/kg body weight twice daily (LIR 300). Brain edema at 24 and 72 hours (A) following operation in sham, vehicle, LIR 75, LIR 300, compound C (CC; 2 mg/kg four times daily) and exendin 9-39 (Ex; 175 μg/kg four times daily). The brain sections (4 mm) were divided into four parts: ispilateral basal ganglia (Ipsi-BG), ispilateral cortex (Ipsi-CX), contralateral basal ganglia (Cont-BG), contralateral cortex (Cont-CX). Cerebellum (Cerebel) is the internal control. Modified Garcia test (B) and wire hanging test (C) at 24, 48, and 72 hours following ICH. Error bars represent mean±standard error of the mean (A) or median±25th to 75th percentiles (B, C). #P<0.01 versus sham; *P<0.05 versus vehicle, **P<0.01 versus vehicle. GLP, glucagon-like peptide.
At the delayed stage of 72 hours, vehicle-treated animals showed significantly increased cerebral edema compared with sham animals in both the ipsilateral basal ganglia and cerebral cortex (P<0.01). Liraglutide significantly decreased brain edema in the ipsilateral basal ganglia in a dose-dependent manner (versus vehicle, LIR 75 P<0.05, LIR 300, P<0.01).
For neurobehavior, the vehicle group demonstrated severe deficits compared with sham animals in both modified Garcia and wire hanging test at 24, 48, and 72 hours (P<0.01; Figures 2B and 2C). LIR 75 significantly improved neurologic score in modified Garcia test (P<0.05 at all times) and wire hanging test (P<0.01 at 24 hours; P<0.05 at 48 and 72 hours) compared with vehicle. Consistent with cerebral edema finding, improvement in neurobehavior was partially abolished by compound C and exendin 9-39. Compound C and exendin 9-39 per se in ICH did not change neurobehavior compared with vehicle. In contrast, although LIR 300 led to greater reduction in cerebral edema than LIR 75, significant improvement in neurobehavioral function was only observed in modified Garcia at 24 hours but not at 48 and 72 hours post-ICH. For wire hanging test, LIR 300 showed trends toward improved performance without reaching statistical significance. However, post hoc analysis of modified Garcia test, after omitting scores for motor strength consisted of lateral turning, forelimb walking, and climbing, LIR 300 group showed significantly better performance than vehicle (P<0.05) at all times (data not shown).
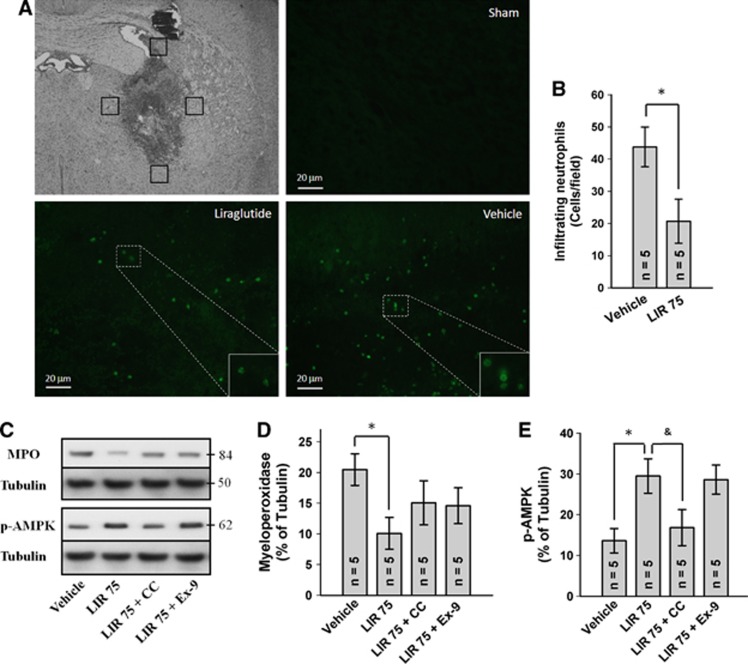
Effect of Liraglutide on Neutrophil Infiltration and Adenosine Monophosphate-Activated Protein Kinase Phosphorylation (Experiment 2)
To determine the effect of liraglutide on neutrophil infiltration, we histologically quantified the number of neutrophils in the perihematoma region by staining for MPO. LIR 75 treatment at 24 hours significantly reduced the number of MPO-positive cells compared with vehicle-treated animals (P<0.05; Figures 3A and 3B). Sham animals were largely devoid of neutrophils in the brain. Similarly, Western blot analysis of homogenized brain tissue revealed reduced MPO intensity in LIR 75-treated animals (P<0.05 versus vehicle), which was again partially reversed by compound C and exendin 9-39 (Figures 3C and 3D). Furthermore, LIR 75 significantly increased brain phosphorylated AMPK (p-AMPK) level (P<0.05 versus vehicle), which appears to be independent of GLP-1R as suggested by the lack of suppression by exendin 9-39 (Figures 3C and 3E). In contrast, compound C suppressed LIR 75-mediated AMPK phosphorylation.
Figure 3.
Effect of liraglutide on neutrophil infiltration and adenosine monophosphate-activated protein kinase (AMPK) phosphorylation. (A) Representative photograph of immunofluorescence staining for myeloperoxidase (MPO) showing that the MPO-positive cells were increased in vehicle group and decreased in liraglutide treatment (75 μg/kg twice daily) at 24 hours after intracerebral hemorrhage (ICH). Sections from mice brain were probed with anti-MPO antibody and rabbit FITC green secondary antibody (green). Scale bars, 20 μm. (B) Bar graph illustrating the quantification of MPO-positive cells in the perihematomal region at 24 hours in vehicle and liraglutide treatment (12 fields/brain). Representative Western blots (C) and effects of liraglutide (75 μg/kg twice daily), compound C (CC; 2 mg/kg four times daily) and exendin 9-39 (Ex-9; 175 μg/kg four times daily) on MPO (D) and p-AMPK (E) levels in the ispilateral cerebral hemisphere at 24 hours after ICH. Expression levels of each protein have been normalized against β-tubulin. *P<0.05; &P=0.059. The color reproduction of this figure is available on the Journal of Cerebral Blood Flow and Metabolism journal online.
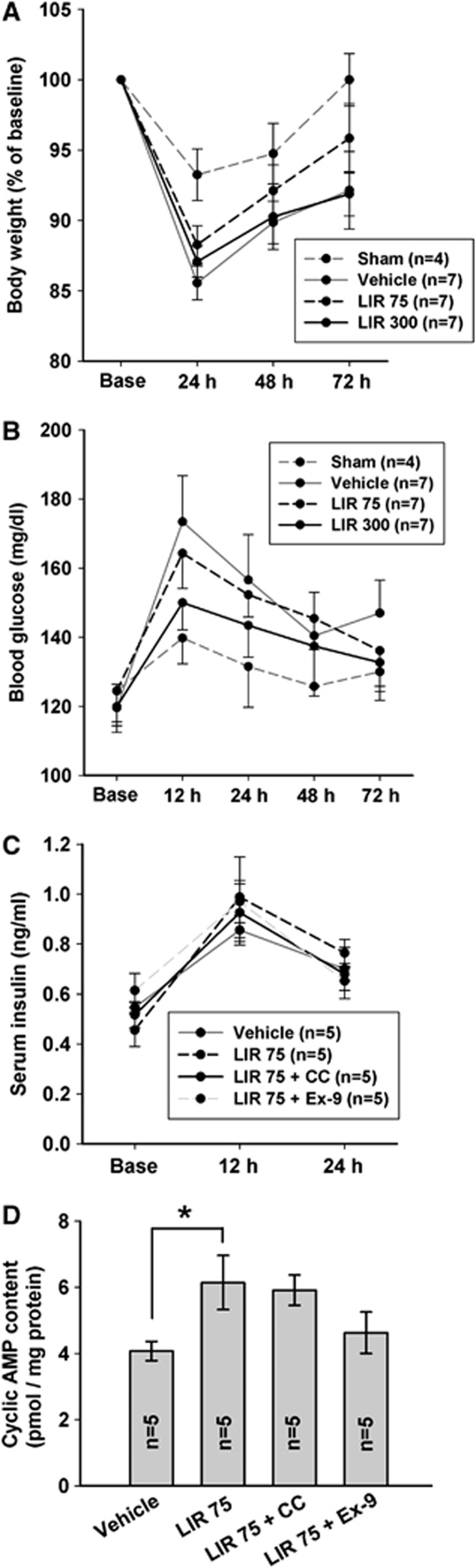
Effect of Liraglutide on Physiologic Parameters (Experiments 1 and 2)
At 24 hours, body weight reduction was significantly greater in all ICH groups compared with sham (P<0.05) (Figure 4A). However, progressively up to 72 hours, LIR 75 group showed trend for recovery of body weight whereas LIR 300 and vehicle groups lack behind. Serial changes in blood glucose measured during experiment 1 until 72 hours after ICH were similar between vehicle and LIR 75-treated groups (Figure 4B). LIR 300 group showed trend for blunting acute hyperglycemia at 12 hours post-ICH compared with vehicle (P=0.13). Serum insulin level was unaffected by LIR 75, compound C and exendin 9-39 treatment compared with vehicle (Figure 4C).
Figure 4.
Physiologic parameters and brain cAMP level. Changes in body weight (A), blood glucose (B), and serum insulin (C) levels in the sham, vehicle, liraglutide 75 μg/kg twice daily (LIR 75) and 300 μg/kg twice daily (LIR 300) after intracerebral hemorrhage (ICH). Base, nonoperation state. cAMP level (D) in the ispilateral cerebral hemisphere 24 hours after ICH in vehicle, LIR 75, LIR 75+compound C (CC; 2 mg/kg four times daily) and LIR 75+exendin 9-39 (Ex-9; 175 μg/kg four times daily) treatment. *P<0.05.
Effect of Liraglutide on Brain Cyclic AMP Level (Experiment 2)
We examined the correlation between liraglutide and cAMP response because activation of GLP-1R is known to increase intracellular levels of cAMP and modulate cell-survival mechanisms in various types of cells.24 LIR 75 treatment for 24 hours led to significantly higher brain cAMP level than vehicle (P<0.05), which was suppressed by exendin 9-39 but not by compound C (Figure 4D).
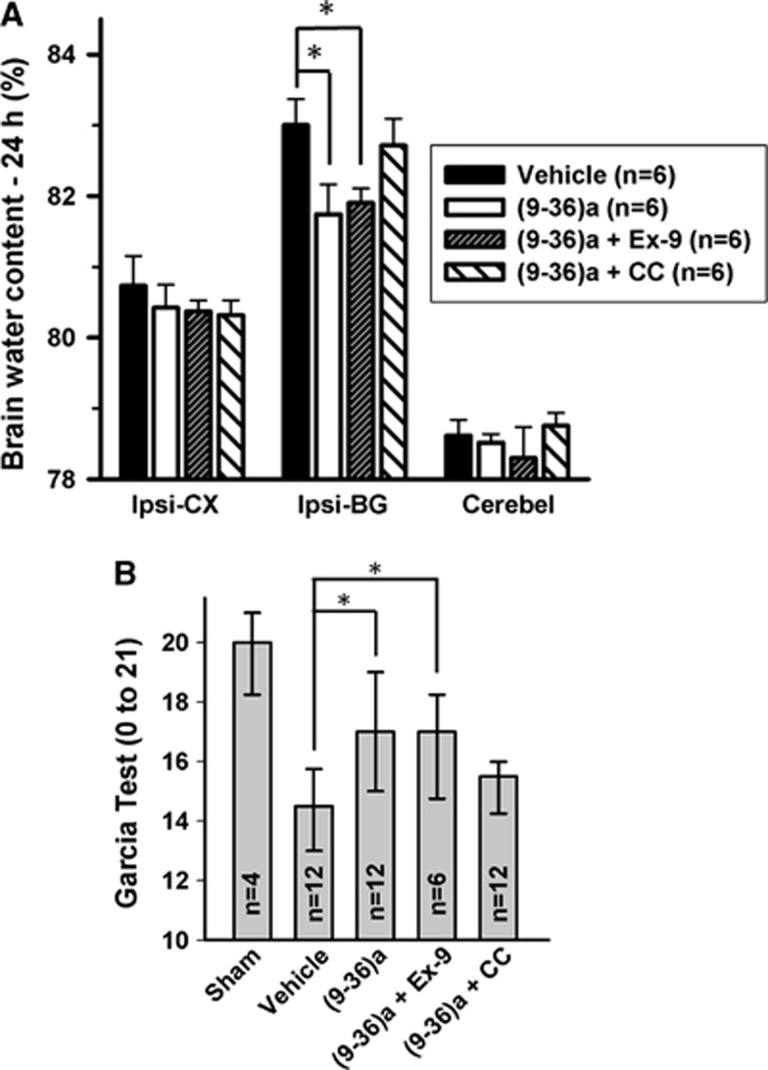
GLP-1-(9-36)a Alleviates Cerebral Edema Through a p-Adenosine Monophosphate-Activated Protein Kinase-Mediated Pathway Independent of Glucagon-like-Peptide-1 Receptor (Experiment 3)
We next examined whether GLP-1(9-36)a, a metabolite generated from liraglutide by DPP-4-mediated cleavage, might be responsible for some of the actions previously attributed to intact GLP-1. Notably, treatment with GLP-1(9-36)a led to significant reduction in cerebral edema formation in the ispilateral basal ganglia at 24 hours after ICH compared with vehicle (P<0.05; Figure 5A). Coadministered of compound C markedly reversed this effect whereas exendin 9-39 caused minimal changes. Consistent with the cerebral edema finding, modified Garcia test showed similar results—GLP-1(9-36)a significantly improved neurobehavior compared with vehicle (P<0.05), which was reversed by compound C but unaffected by exendin 9-39 (Figure 5B).
Figure 5.
Effects of GLP-1(9-36)a on brain edema (A) and modified Garcia test (B) at 24 hours after intracerebral hemorrhage (ICH). *P<0.05. GLP, glucagon-like peptide.
GLP-1(9-36)a-Mediated p-Adenosine Monophosphate-Activated Protein Kinase Amplification Suppresses Neuroinflammation and NFκB Nuclear Translocation (Experiment 3)
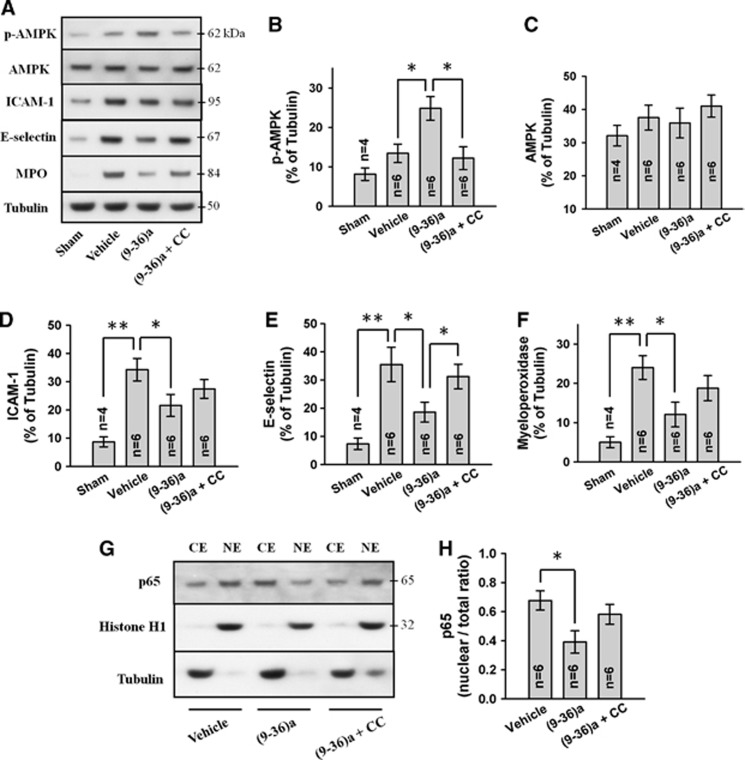
Since compound C markedly suppressed GLP-1(9-36)a-mediated antiinflammatory, we determined the level of p-AMPK and AMPK.
Western blot analysis of cytoplasmic fraction revealed that ICH induces tendency towards increased AMPK phosphorylation (vehicle versus sham, P=0.14) while the total AMPK level was largely unchanged (Figures 6A to 6C). GLP-1(9-36)a further amplified p-AMPK level (P<0.05 versus vehicle) without altering total AMPK whereas compound C cotreatment suppressed p-AMPK to near vehicle group level.
Figure 6.
Representative Western blots (A) of cytoplasmic fraction and effects of GLP-1(9-36)a (1 μg) and compound C (CC; 5 μg) on p-adenosine monophosphate-activated protein kinase (p-AMPK) (B), AMPK (C), ICAM-1 (D), E-selectin (E), and myeloperoxidase (F) levels. Representative Western blots of cytoplasmic (CE) and nuclear (NE) fractions (G) and effects of GLP-1(9-36)a and compound C on NF-κB p65 levels (H). All Western blots were performed on ispilateral cerebral hemisphere at 24 hours after intracerebral hemorrhage (ICH). Expression levels of each protein have been normalized against β-tubulin (CE) or histone H1 (NE). Ex-9=exendin 9-39 (5 μg). GLP-1(9-36)a is abbreviated as (9-36)a. *P<0.05; **P<0.01. GLP, glucagon-like peptide.
The effect of GLP-1(9-36)a on neuroinflammation was determined by characterizing the expression of proinflammatory mediators ICAM-1, E-selectin and MPO intensity after GLP-1(9-36)a and compound C treatment.
ICAM-1 and E-selectin were induced by ICH (vehicle versus sham, P<0.01), suppressed by GLP-1(9-36)a treatment (P<0.05 versus vehicle) and partially reversed by compound C (Figures 6A). Consistently, with reduced cell-adhesion molecules, GLP-1(9-36)a treatment also decreased MPO level in the brain (P<0.05 versus vehicle) indicates less neutrophil infiltration, while this effect was also partially reversed by compound C (Figures 6A and 6F). Correlation of brain levels of proinflammatory molecules and brain edema with neurobehavioral parameters are shown in Supplementary Table 1.
In an in vitro study of human endothelial cells, liraglutide was found to markedly suppress TNF-α (tumor necrosis factor-α) and hyperglycemia-induced NF-κB activation and NF-κB-dependent transcription of proinflammatory genes, where compound C and AMPK small interfering RNA attenuated this effect.11 As a result, we investigated NF-κB nuclear translocation dynamics. Nuclear extraction of brain tissue and Western blot analysis revealed effective suppression of NF-κB nuclear translocation after GLP-1(9-36)a treatment (P<0.05 versus vehicle) in a p-AMPK-dependent manner (Figures 6G and 6H).
Coadministering of Liraglutide and Dipeptidyl Peptidase-4 Inhibitor Dampens Antiinflammatory Effects (Experiment 4)
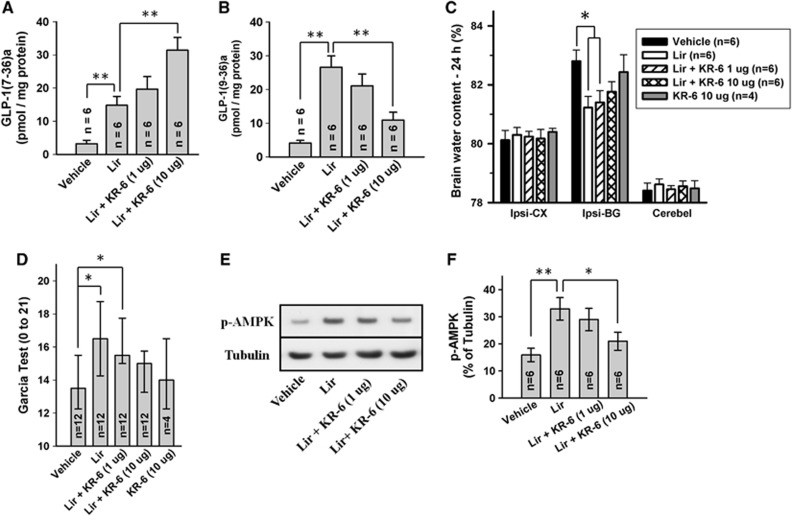
To determine the relative contribution of GLP-1R and GLP-1(9-36)a in liraglutide-mediated antiinflammation, we coinjected liraglutide and incremental doses of KR-62436, a specific DPP-4 inhibitor, to prevent the formation of GLP-1(9-36)a from liraglutide. Assays of extracted brain tissue revealed that liraglutide ICV injection significantly increased the level of GLP-1(7-36)a and GLP-1(9-36)a (P<0.01 versus vehicle) (Figures 7A and 7B). Furthermore, KR-62436 dose dependently increased GLP-1(7-36)a and decreased GLP-1(9-36)a. Despite increased intact GLP-1 levels, KR-62436 cotreated animals reversed liraglutide-mediated alleviation of cerebral edema formation and neurobehavioral improvement in a dose-dependent manner (Figures 7C and 7D). Western blot analysis of whole-cell fraction reiterated the finding of previous experiments that liraglutide increases p-AMPK level (P<0.01 versus vehicle) (Figures 7E and 7F). This effect was attenuated dose dependently by KR-62436, asserted above findings that AMPK phosphorylation is indeed caused by GLP-1 degradation products and that they may be the primary mediators of liraglutide facilitated antiinflammatory effects.
Figure 7.
Effect of liraglutide (1 μg) and coadministered KR-62436 on brain GLP-1(7-36)a (A) and GLP-1(9-36)a (B) levels. Effect on brain edema (C) and modified Garcia test (D) are shown. Representative Western blots (E) and effect of treatment on p-adenosine monophosphate-activated protein kinase (p-AMPK) (F). All analyses were performed on ispilateral cerebral hemisphere 24 hours after intracerebral hemorrhage (ICH). Phosphorylated AMPK levels have been normalized against β-tubulin. *P<0.05; **P<0.01. GLP, glucagon-like peptide.
Discussion
Spontaneous ICH remains a devastating condition with a 1-month mortality rate of 42%.25 Many survivors are left permanently disabled. In the 2010 American Heart Association/American Stroke Association management guideline, there is not a single primary treatment shown to be effective by level A evidence.1
Accumulating evidence suggests that inflammatory mechanisms are involved in ICH-induced brain injury.2, 3 Infiltrating neutrophils were observed as early as 4 hours after blood injection ICH model in rat26 where they damage brain tissue directly by generating reactive oxygen species and secreting proinflammatory proteases.27 In addition, the contents of dying neutrophils can promote inflammatory tissue injury indirectly by stimulating macrophages to release proinflammatory mediators.28 Taken together, regulation of inflammatory response may be a potential strategy for ICH therapy.
The present study was aimed to investigate the effect of GLP-1 and its metabolite, GLP-1(9-36)a, in ICH-induced brain injury in mice. The long-acting GLP-1 analog, liraglutide, was studied. It has 97% amino-acid sequence identity to the native GLP-1(7-36)a, but differs from the native hormone by replacement of Lys34 by Arg and derivatization of GLP-1 protein backbone in the Lys26 position with a glutamate spacer bound to a 16C fatty acid.29 The fatty acid chain enables reversible binding to plasma albumin, leading to decreased clearance and protracted pharmacological activity with a half-life of 12 hours.21
In terms of primary outcomes, SC administered liraglutide ameliorated ICH-induced cerebral edema formation and improved neurologic function at 24 and 72 hours in a dose-dependent manner. Here, the antiinflammatory effect of liraglutide was likely to involve suppression of inflammatory reactions after ICH. We found liraglutide treatment decreased the number of neutrophils in the perihematoma region on histological sections. The validity of quantification of infiltrating neutrophils was confirmed by examination of immunoreactivity against MPO. LIR 75 resulted in progressive recovery of body weight and improvement in neurobehavior and may be better tolerated than the high-dose regimen of LIR 300 in the absence of critical care nutritional support.
Insulin and glucose levels may influence brain injury. Particularly, hyperglycemia is associated with greater hematoma expansion,30 whereas the clinical benefit of insulin therapy in ICH remains elusive.1 In the ICH mouse model, acute hyperglycemia was found 12 hours after ICH, possibly in response to stress caused by surgery and ICH. High-dose liraglutide treatment (LIR 300) exhibited trend in blunting acute hyperglycemia. Previous clamp experiments report that liraglutide acts as a euglycemic agent where stimulation of insulin secretion and suppression of glucagon does not occur at hypoglycemic plasma glucose concentrations.31 Hence in ICH, liraglutide may be an alternative or adjunct to insulin therapy in a conservative approach to the management of hyperglycemia with possible lowered risk of hypoglycemic events. In contrast, blood glucose and serum insulin levels remained largely unchanged with the lower-dose regimen of LIR 75.
The relationship between GLP-1 and cAMP has been examined previously.15 In the present study, liraglutide increased intracellular cAMP level, which was reversed by exendin 9-39 but not by compound C, suggests that this is a direct consequence of GLP-1R activation unrelated to p-AMPK. In accordance with the previous studies, GLP-1R is functional associated with adenylate cyclase to increase cAMP levels in various cells including β-cells of the pancreas4 and brain tissue.15 Elevation of cAMP levels may be beneficial in suppressing the generation of superoxide and hydrogen peroxide.32 In addition, upregulated cAMP can trigger phosphorylation of CREB at serine 133. The role of CREB phosphorylation in neurons has been studies extensively, and is known to be important in neuronal development, synaptic plasticity, and memory formation.33 Furthermore, CREB phosphorylation leads to the expression of neuroprotective genes such as B-cell lymphoma 2 and brain-derived neurotrophic factor,34 and therefore may play a role in neuroprotection against ICH-induced brain injury. Indeed, in this study, exendin 9-39 partially reversed LIR 75-mediated neuroprotection suggests some GLP-1R-mediated effects.
However, liraglutide also confers antiinflammatory effect through a p-AMPK-mediated mechanism independent of GLP-1R. Notably, we found liraglutide-induced AMPK phosphorylation was actually mediated by its degradation products, likely by GLP-1(9-36)a. These postulations are supported by findings that both liraglutide and GLP-1(9-36)a amplified p-AMPK and that inhibition of liraglutide degradation with coadministered DPP-4 decreased p-AMPK in a dose-dependent manner. Liraglutide-mediated AMPK phosphorylation was attenuated by compound C but not by the classic GLP-1R inhibitor, exendin 9-39, suggests GLP-1R independence. Similarly, GLP-1(9-36)a impeded ICH-induced cerebral edema formation and effectively improved neurologic outcome, again inhibited by compound C but not by exendin 9-39. In addition, compound C restored GLP-1(9-36)a-mediated inhibition of ICH-induced NF-κB nuclear translocation, an ubiquitous transcription factor that plays a detrimental role in the acute phase of ICH where it induces proinflammatory and proapoptotic responses.35, 36 Consequently, GLP-1(9-36)a suppressed the expression of cell-adhesion molecules ICAM-1 and E-selectin and reduced MPO intensity in a p-AMPK-dependent manner. These data suggest that the DPP-4 cleavage product, GLP-1(9-36)a, rather than the intact liraglutide, activates AMPK independently of GLP-1R and is responsible for the alleviation of ICH-induced inflammatory response.
While it is generally agreed that GLP-1(7-36)a, acting at the GLP-1R, is largely responsible for the effects on glucoregulation, the extent to which GLP-1(9-36)a may be responsible for some, or even most of the antiinflammatory actions is unknown. It has been shown in human endothelial cells that liraglutide markedly enhanced AMPK phosphorylation independently of cAMP activation, possible by cleaved products generated from the DPP-4 enzyme found on endothelial cell membrane37 (ref own interpretation), where it reduced TNF-α and hyperglycemia-mediated phosphorylation and subsequent degradation of IκB-α, and thereby suppressed NF-κB activity as determined by luciferase report gene linked analysis. Consequently, cell-adhesion molecule transcripts were also reduced.11 These findings support the present study that liraglutide demonstrate a range of antiinflammatory actions, acting in part through an AMPK-dependent pathway to inhibit NF-κB-induced inflammatory response. However, in another report, exenatide, an extensively modified GLP-1 analog resistant to DPP-4 degradation, inhibited leukocyte adhesion to endothelial cells and attenuated atherosclerotic lesions in apoliporpotein E-deficient mice, where these effects were reversed by cAMP inhibitor and protein kinase A inhibitor, suggests GLP-1R-dependent effects.10 The different mechanisms of action might have been caused by the different GLP-1 analog studied. In the present study, suppression of the antiinflammatory effects of liraglutide by the DPP-4 inhibitor, KR-62436, suggests that GLP-1(9-36)a may function as a key intermediary in a subset of the neuroprotective effects of GLP-1, possibly more dominant than GLP-1R-mediated effects. Thus, it is likely that the beneficial effect of liraglutide might be mediated through a distinct receptor for GLP-1(9-36)a. Since neuroinflammation is a multifactorial phenomenon, we hypothesize that liraglutide exert action on more than one cell types that compose the neurovascular unit, with major action on the endothelium, as was shown in previous in vitro studies of cultured endothelial cells.10, 11 The adhesion molecules measured in the present study, such as ICAM-1, are mainly expressed in endothelial cells and to a lesser extent in neurons.38 Glucagon-like-peptide-1R and AMPK signaling takes place in both endothelial cells and neurons.39 The finding that locally and systemically administered liraglutide showed similar effects indicates that the effects on neutrophils were possibly less significant. We provide a schematic diagram showing how liraglutide could suppress ICH-induced neuroinflammation possibly through actions on cells that compose the neurovascular unit (Supplementary Figure 1). We do not exclude the possibility that direct antiapoptotic effects on neurons are also involved in the protective effect of liraglutide and that even GLP-1(9-36)a degradation products, such as GLP-1(28-36)a may play a role. Furthermore, even though the inhibitors used in the present study were specific, they may also exert action on related molecules. For example, KR-62436, a DPP-4 inhibitor, is highly selective for DPP-4 (IC50 0.49 μmol/L) but also has residual effects on DPP-2 (IC50 56.1 μmol/L) and Prolyl oligopeptidase (IC50 59.1 μmol/L).19
Three therapeutic strategies based on potentiation of GLP-1 action are now used to treat type 2 diabetes mellitus: (1) GLP-1 analogs with attached fatty acid chain to enable binding to serum albumin and thereby escapes renal clearance while still allows DPP-4-mediated degradation (e.g., liraglutide); (2) extensively modified GLP-1 resistant to cleavage by the DPP-4 enzyme and may not give rise to GLP-1(9-36)a in vivo but retains GLP-1R agonistic property (e.g., exenatide); and (3) DPP-4 inhibitors to prevent the enzymatic cleavage of native GLP-1 to GLP-1(9-36)a (e.g., sitagliptin). Indeed, previous pharmacokinetic studies have shown liraglutide is fully metabolized within the body into, among others, GLP-1(9-36)a, by the widely distributed endogenous enzyme DPP-4, before eliminated by the liver, kidney, and gastrointestinal tract.40 In contrast, circulating exenatide is a modified GLP-1 analog resistant to enzyme degradation and is thus primarily cleared by glomerular filtration in intact form.41 Consequently, these three classes of drugs previously assumed to be functionally identical might not hold the same therapeutic potential in the setting of cerebrovascular accidents. For future clinical trials, the present study provides evidence that GLP-1 exerts dual actions, where therapies that inhibit GLP-1 degradation weaken the metabolite-mediated antiinflammatory effects.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This study was supported by NIH RO1 NS045694 and NIH RO1 NS053407 to John H Zhang.
Supplementary Material
References
- Morgenstern LB, Hemphill JC, Anderson C, Becker K, Broderick JP, Connolly ES, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals form the American Heart Association/American Stroke Association. Stroke. 2010;41:276–309. doi: 10.1161/STR.0b013e3181ec611b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronowski J, Zhao X. Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke. 2011;42:1781–1786. doi: 10.1161/STROKEAHA.110.596718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Doré S. Inflammation after intracerebral hemorrhage. J Cereb Blood Flow Metab. 2007;27:894–908. doi: 10.1038/sj.jcbfm.9600403. [DOI] [PubMed] [Google Scholar]
- Larsen PJ, Holst JJ. Glucagon-related peptide 1 (GLP-1): hormone and neurotransmitter. Regul Pept. 2005;128:97–107. doi: 10.1016/j.regpep.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Anagnostis P, Athyros VG, Adamidou F, Panagiotou A, Kita M, Karagiannis A, et al. Glucagon-like peptide-1-based therapies and cardiovascular disease: looking beyond glycaemic control. Diabetes Obes Metab. 2011;13:302–312. doi: 10.1111/j.1463-1326.2010.01345.x. [DOI] [PubMed] [Google Scholar]
- Irwin DM. Molecular evolution of proglucagon. Regul Pept. 2001;98:1–12. doi: 10.1016/s0167-0115(00)00232-9. [DOI] [PubMed] [Google Scholar]
- Deacon CF, Johnsen AH, Holst JJ. Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo. J Clin Endocrinol Metab. 1995;80:952–957. doi: 10.1210/jcem.80.3.7883856. [DOI] [PubMed] [Google Scholar]
- Rolin B, Deacon CF, Carr RD, Ahrén B. The major glucagon-like peptide-1 metabolite, GLP-1-(9-36)-amide, does not affect glucose or insulin levels in mice. Eur J Pharmacol. 2004;494:283–288. doi: 10.1016/j.ejphar.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Dozier KC, Cureton EL, Kwan RO, Curran B, Sadjadi J, Victorino GP. Glucagon-like peptide-1 protects mesenteric endothelium from injury during inflammation. Peptides. 2009;30:1735–1741. doi: 10.1016/j.peptides.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa M, Mita T, Azuma K, Ebato C, Goto H. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes. 2010;59:1030–1037. doi: 10.2337/db09-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori Y, Jojima T, Tomizawa A, Satoh H, Hattori S, Kasai K, et al. A glucagon-like peptide-1 (GLP-1) analogue, liraglutide, upregulates nitric oxide production and exerts anti-inflammatory action in endothelial cells. Diabetologia. 2010;53:2256–2263. doi: 10.1007/s00125-010-1831-8. [DOI] [PubMed] [Google Scholar]
- Kodera R, Shikata K, Kataoka HU, Takatsuka T, Miyamoto S, Sasaki M, et al. Glucagon-like peptide-1 receptor agonist ameliorates renal injury through its anti-inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia. 2011;54:965–978. doi: 10.1007/s00125-010-2028-x. [DOI] [PubMed] [Google Scholar]
- Thorens B. Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc Natl Acad Sci USA. 1992;89:8641–8645. doi: 10.1073/pnas.89.18.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai T, Ito S, Tanimitsu K, Udagawa S, Oka J. Glucagon-like peptide-1 inhibits LPS-induced IL-1beta production in cultured rat astrocytes. Neurosci Res. 2006;55:352–360. doi: 10.1016/j.neures.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Teramoto S, Miyamoto N, Yatomi K, Oishi H, Arai H, Hattori N, et al. Exendin-4, a glucagon-like peptide-1 receptor agonist, provides neuroprotection in mice transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2011;31:1696–1705. doi: 10.1038/jcbfm.2011.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolligs F, Fehmann HC, Göke R, Göke B. Reduction of the incretin effect in rats by the glucagon-like peptide 1 receptor antagonist exendin (9-39) amide. Diabetes. 1995;44:16–19. doi: 10.2337/diab.44.1.16. [DOI] [PubMed] [Google Scholar]
- Ramamurthy S, Ronnett GV. Developing a head for energy sensing: AMP-activated protein kinase as a multifunctional metabolic sensor in the brain. J Physiol. 2006;574:85–93. doi: 10.1113/jphysiol.2006.110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Huang B, Khatibi N, Rolland W, Suzuki H, Zhang JH, et al. PDGFR-α inhibition preserves blood-brain barrier after intracerebral hemorrhage. Ann Neurol. 2011;70:920–931. doi: 10.1002/ana.22549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KR, Rhee SD, Kim HY, Jung WH, Yang SD, Kim SS, et al. KR-62436, 6-{2-[2-(5-cyano-4,5-dihydropyrazol-1-yl)-2-oxoethylamino]ethylamino}nicotinonitrile, is a novel dipeptidyl peptidase-IV (DPP-IV) inhibitor with anti-hyperglycemic activity. Eur J Pharmacol. 2005;518:63–70. doi: 10.1016/j.ejphar.2005.05.030. [DOI] [PubMed] [Google Scholar]
- Noyan-Ashraf MH, Momen MA, Ban K, Sadi AM, Zhou YQ, Riazi AM, et al. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes. 2009;58:975–983. doi: 10.2337/db08-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen LB, Nielsen PF, Huusfeldt PO, Johansen NL, Madsen K, Pedersen FZ, et al. Potent derivatives of glucagon-like peptide-1 with pharmacokinetic properties suitable for once daily administration. J Med Chem. 2000;43:1664–1669. doi: 10.1021/jm9909645. [DOI] [PubMed] [Google Scholar]
- Schirra J, Sturm K, Leicht P, Arnold R, Göke B, Katschinski M. Exendin(9-39)amide is an antagonist of glucagon-like peptide-1(7-36)amide in humans. J Clin Invest. 1998;101:1421–1430. doi: 10.1172/JCI1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manaenko A, Lekic T, Sozen T, Tsuchiyama R, Zhang JH, Tang J. Effect of gap junction inhibition on intracerebral hemorrhage-induced brain injury in mice. Neurol Res. 2009;31:173–178. doi: 10.1179/174313209X393591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry T, Greig NH. The glucagon-like peptides: a double-edged therapeutic sword. Trends Pharmacol Sci. 2003;24:377–383. doi: 10.1016/S0165-6147(03)00160-3. [DOI] [PubMed] [Google Scholar]
- Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2:43–53. doi: 10.1016/s1474-4422(03)00266-7. [DOI] [PubMed] [Google Scholar]
- Xue M, Del Bigio MR. Intracortical hemorrhage injury in rats: relationship between blood fractions and brain cell death. Stroke. 2000;31:1721–1727. doi: 10.1161/01.str.31.7.1721. [DOI] [PubMed] [Google Scholar]
- Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320:325–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- Stern M, Savill J, Haslett C. Human monocyte-derived macrophage phagocytosis of senescent eosinophils undergoing apoptosis. Mediation by alpha v beta 3/CD36/thrombospondin recognition mechanism and lack of phlogistic response. Am J Pathol. 1996;149:911–921. [PMC free article] [PubMed] [Google Scholar]
- Knudsen LB. Glucagon-like peptide-1: the basis of a new class of treatment for type 2 diabetes. J Med Chem. 2004;47:4128–4134. doi: 10.1021/jm030630m. [DOI] [PubMed] [Google Scholar]
- Kimura K, Iguchi Y, Inoue T, Shibazaki K, Matsumoto N, Kobayashi K, et al. Hyperglycemia independently increases the risk of early death in acute spontaneous intracerebral hemorrhage. J Neurol Sci. 2007;255:90–94. doi: 10.1016/j.jns.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Nauck MA, Heimesaat MM, Behle K, Holst JJ, Nauck MS, Ritzel R, et al. Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metab. 2002;87:1239–1246. doi: 10.1210/jcem.87.3.8355. [DOI] [PubMed] [Google Scholar]
- Takei K, Tokuyama K, Kato M, Morikawa A. Role of cyclic adenosine monophosphate in reducing superoxide anion generation in guinea pig alveolar macrophages. Pharmacology. 1998;57:1–7. doi: 10.1159/000028219. [DOI] [PubMed] [Google Scholar]
- Stevens CF. CREB and memory consolidation. Neuron. 1994;13:769–770. doi: 10.1016/0896-6273(94)90244-5. [DOI] [PubMed] [Google Scholar]
- Kitagawa K. CREB and cAMP response element-mediated gene expression in the ischemic brain. FEBS J. 2007;274:3210–3217. doi: 10.1111/j.1742-4658.2007.05890.x. [DOI] [PubMed] [Google Scholar]
- Hickenbottom SL, Grotta JC, Strong R, Denner LA, Aronowski J. Nuclear factor-kappaB and cell death after experimental intracerebral hemorrhage in rats. Stroke. 1999;30:2472–2477. doi: 10.1161/01.str.30.11.2472. [DOI] [PubMed] [Google Scholar]
- Zhao X, Zhang Y, Strong R, Zhang J, Grotta JC, Aronowski J. Distinct patterns of intracerebral hemorrhage-induced alterations in NF-kappaB subunit, iNOS, and COX-2 expression. J Neurochem. 2007;101:652–663. doi: 10.1111/j.1471-4159.2006.04414.x. [DOI] [PubMed] [Google Scholar]
- Pala L, Pezzatini A, Dicembrini I, Ciani S, Gelmini S, Vannelli BG, et al. Different modulation of dipeptidyl peptidase-4 activity between microvascular and macrovascular human endothelial cells Acta Diabetol 2010[e-pub ahead of print]. [DOI] [PubMed]
- Gong C, Hoff JT, Keep RF. Acute inflammatory reaction following experimental intracerebral hemorrhage in rat. Brain. 2000;871:57–65. doi: 10.1016/s0006-8993(00)02427-6. [DOI] [PubMed] [Google Scholar]
- Osuka K, Watanabe Y, Usuda N, Atsuzawa K, Yoshida J, Takayasu M. Modification of endothelial nitric oxide synthase through AMPK after experimental subarachnoid hemorrhage. J Neurotrauma. 2009;26:1157–1165. doi: 10.1089/neu.2008.0836. [DOI] [PubMed] [Google Scholar]
- Malm-Erjefält M, Bjørnsdottir I, Vanggaard J, Helleberg H, Larsen U, Oosterhuls B, et al. Metabolism and excretion of the once-daily human glucagon-like peptide-1 analog liraglutide in healthy male subjects and its in vitro degradation by dipeptidyl peptidase IV and neutral endopeptidase. Drug Metab Dispos. 2010;38:1944–1953. doi: 10.1124/dmd.110.034066. [DOI] [PubMed] [Google Scholar]
- Simonsen L, Holst JJ, Deacon CF. Exendin-4 but not glucagon-like peptide-1, is cleared exclusively by glomerular filtration in anaesthetised pigs. Diabetologia. 2006;49:706–712. doi: 10.1007/s00125-005-0128-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.