Abstract
Substantial experimental data and recent clinical evidence suggesting that tissue reperfusion is a better predictor of outcome after thrombolysis than recanalization necessitate that patency of microcirculation after recanalization should be reevaluated. If indeed microcirculatory blood flow cannot be sufficiently reinstituted despite complete recanalization as commonly observed in coronary circulation, it may be one of the factors contributing to low efficacy of thrombolysis in stroke. Although microvascular no-reflow is considered an irreversible process that prevents tissue recovery from injury, emerging evidence suggests that it might be reversed with pharmacological agents administered early during recanalization. Therefore, therapeutic approaches aiming at reducing microvascular obstructions may improve success rate of recanalization therapies. Importantly, promoting oxygen delivery to the tissue, where entrapped erythrocytes cannot circulate in capillaries, with ongoing serum flow may improve survival of the underreperfused tissue. Altogether, these developments bring about the exciting possibility that benefit of reperfusion therapies can be further improved by restoring microcirculatory function because survival in the penumbra critically depends on adequate blood supply. Here, we review the available evidence suggesting presence of an ‘incomplete microcirculatory reperfusion' (IMR) after focal cerebral ischemia and discuss potential means that may help investigate IMR in stroke patients after recanalization therapies despite technical limitations.
Keywords: brain ischemia, capillaries, microcirculation, no-reflow, reperfusion, thrombolysis
The best conceivable treatment for acute ischemic stroke is restoration of blood flow to the ischemic region, which is currently provided by thrombolysis. Unfortunately, a short time window limits the use of recanalization therapies, and not infrequently, thrombolysis is complicated by hemorrhage and edema because of increased vascular permeability.1, 2, 3 There is also a not well-recognized potential drawback associated with recanalization therapies, which is characterized by incomplete restoration of the microcirculatory flow in some parts of the ischemic tissue after reopening of the occluded blood vessel; the so-called ‘no-reflow phenomenon'.4, 5, 6, 7 This phenomenon enjoyed considerable scientific interest after its first description in the 1970s;8, 9, 10 later, it lost its popularity because of the claims that it might be an experimental artifact,11, 12, 13, 14 in addition to the concerns that it might be an epiphenomenon of the established tissue injury.9, 10 However, substantial experimental data accumulating since the past decades15, 16, 17, 18, 19, 20 and the recent clinical evidence suggesting that tissue reperfusion (restoration of microcirculatory blood flow) is a better predictor of outcome after thrombolysis than recanalization (reopening of the occluded artery)21, 22 necessitate that this phenomenon should be reevaluated. If indeed the microcirculatory blood flow cannot be sufficiently reinstituted after brief focal cerebral ischemia despite complete recanalization as commonly observed in the coronary circulation,5, 6 it might be one of the factors decreasing the efficacy of cerebral thrombolysis done within the time window when penumbral cells are still viable but microcirculatory obstructions have emerged. In addition to its potential use as a negative prognostic factor, understanding the conditions of reversibility of microvascular occlusions may create the possibility of improving recovery after recanalization therapies.
Here, we review the available evidence and discuss the potential means that may help investigate the presence and significance of incomplete microcirculatory reperfusion in thrombolysis patients. We also suggest to reserve the term ‘no-reflow' for the impaired perfusion of the microvessels seen after long-lasting global ischemia and after prolonged or severe focal ischemia when microvessel occlusions are irreversible and prevent recovery from injury. The term ‘incomplete microcirculatory reperfusion' (IMR) can be used to describe the potentially reversible ‘no-reflow' observed after brief focal ischemia when there is still salvageable penumbral tissue. This formulation is based on the assumption that the microvascular responses are heterogeneous within the mini-cores and mini-penumbras at the territory-at-risk after focal ischemia as proposed by del Zoppo and colleagues.23 Although the evidence is yet insufficient and the ischemic thresholds (severity, duration, etc) determining the reversibility of microvascular injury remain to be clarified, this distinction may be of clinical significance because the IMR can reduce the success of recanalization therapies as will be discussed below. The aim of this review is to bring this potentially important subject to the attention of stroke researchers with the hope of finding the imaging correlates of IMR having satisfactory resolution, and of developing therapeutic interventions.
Impaired Microvascular Patency after Ischemia
An impaired reflow because of loss of microvascular patency (no-reflow) was first noted by Ames et al8 after global cerebral ischemia. A similar microvascular impairment was subsequently reported after focal ischemia.9, 10, 24 Carbon tracer techniques and fluorescent-labeled intravascular markers showed that parts of the microcirculation were not perfused after reopening of the middle cerebral artery (MCA).7, 25, 26 Histologic evaluation of the ischemic tissue with light and electron microscopy showed that microvessel lumina were narrowed and obstructed with blood cells and fibrin.9, 15, 27 Notwithstanding technical concerns such as improper osmolarity of perfusion fluids may cause endothelial swelling, reports agree with regard to the histologic changes observed at various segments of the microvascular bed despite some differences possibly caused by varying duration of ischemia or recirculation and by experimental conditions (see excellent reviews by del Zoppo et al16, 28, 29). Briefly, precapillary arterioles generally remain open,9, 25 although circular constrictions have been observed after prolonged permanent ischemia.30 Capillaries show constrictions and luminal narrowing, which are attributed to compression by swollen astrocyte endfeet.9, 15 Their narrowed lumina are filled with entrapped erythrocytes, leukocytes, and fibrin–platelet deposits.7, 9, 19, 27, 31, 32, 33, 34 It has been proposed that increased blood–brain barrier (BBB) permeability also contributes to microvascular occlusion by promoting fibrin deposition via exposure of plasma constituents to tissue factor in the perivascular spaces.35 The lumina of postcapillary venules are surrounded with adherent leukocytes and may contain cellular aggregates made of fibrin, platelets, and leukocytes.7, 17, 25, 31, 36
Can Incomplete Microcirculatory Reperfusion Unfavorably Affect Tissue Survival after Recanalization?
The incomplete microcirculatory reperfusion after prolonged focal ischemia could be an epiphenomenon of the established tissue injury; therefore, its reversal may not be of functional significance. In contrast, incomplete restoration of microcirculatory blood flow may negatively affect tissue recovery if reopening of the occluded artery is achieved early when there is still salvageable penumbral tissue. Considering that microvascular obstructions are already present 1 hour after MCA occlusion,37, 38 and that a salvageable penumbra may exist at least 4.5 hours in some stroke patients,1 improving microcirculatory reperfusion seems to be a promising strategy after recanalization therapies despite the lack of direct evidence connecting the microvasculature obstructions to the evolution of tissue injury as well as the findings in animal models to patients.
Parallel to observations in other tissues,39 the idea that incomplete microcirculatory reperfusion could compromise tissue recovery after focal cerebral ischemia was primarily put forward by del Zoppo et al.7, 40 Based on ultrastructural studies showing narrowed and clogged capillary lumens with entrapped blood cells, Garcia27 also emphasized the possibility of an impaired microcirculation after focal ischemia, and proposed that the microcirculatory collapse could account for expansion of the ischemic lesion. It is likely that coalescence of the multiple hypoperfused foci can contribute to this process.23, 27, 41, 42 Although patent arterioles may provide sufficient O2 to the immediate tissue surrounding them (∼50 μm in the rat cortex),43 the tissue where O2 supply is dependent on capillaries may have hypoxia because of impaired circulation of erythrocytes. Unlike blood cells, plasma may continue to flow through the narrowed capillaries and promote anaerobic glycolysis and lactate formation because of unsatisfactory oxygen delivery to the tissue while glucose is supplied by way of serum. Supporting this view, in ischemic MCA area in primates, extracellular glucose levels were correlated with regional cerebral blood flow, whereas heterogeneous tissue lactate levels varied with the cerebral metabolic rate of O2 (CMRO2).44 When assessed globally with electron paramagnetic resonance oxymetry in the rat cortex, the recovery of penumbral interstitial pO2 was found to be incomplete after recanalization, possibly because the compromised microcirculation did not match the increased oxygen consumption.45 Reinforcing the idea that a uniform tissue oxygenization is critical for survival, the mean distance of the center of neuronal somata to the closest microvessel is 15 μm in the rat cortex,46 and neurons distant from microvessels are the ones first injured during focal ischemia,47 whereas regions surrounding penetrating arterioles are spared from hypoperfusion-induced injury.48 Supporting the idea that microvascular obstructions can directly lead to neuronal injury, a reduction in microtubule-associated protein-2 immunoreactivity, a marker of neuronal injury, was reported to be spatially linked to local microvascular obstruction and flow after 1 hour of MCA occlusion.38 It has also been shown that secondary microvascular obstruction resulting from compression by the swollen astrocyte endfeet contributed to the evolution of disseminated selective neuronal necrosis after temporary unilateral carotid occlusion in Mongolian gerbils.49 A BBB-impermeable nitric oxide synthase inhibitor (L-N5-(1-iminoethyl)-ornithine) decreased microvascular clogging and reduced the infarct volume, suggesting that restoring microvascular patency may improve stroke outcome without direct parenchymal neuroprotection.50 Conforming with the above arguments, the ischemic area has been shown to be metabolically heterogeneous in both rodents and humans,23, 51, 52 and the infarct evolves by coalescence of multiple discrete foci where cell death advances before the neighboring less severely ischemic areas.27, 53 Finally, pharmacological agents and genetic manipulations reducing microvascular clogging by inhibiting leukocyte adherence, platelet activation, or fibrin–platelet interactions have been shown to restore microcirculation and improve stroke outcome in animal models, suggesting, although indirectly, that IMR unfavorably affects recovery of the ischemic tissue after reopening of the occluded artery.25, 33, 40, 50, 54, 55, 56, 57
Do Pericytes Contribute to Incomplete Microcirculatory Reperfusion?
Endothelial swelling as well as compression caused by swollen astrocyte endfeet were postulated as the mechanism of narrowing of capillary lumina that, together with mechanisms that involve endothelial cell adhesion, promotes aggregation of blood cells and fibrin in capillaries.15, 16, 27 Recently, it has been shown that pericytes on microvessels contract during ischemia and remain contracted despite reopening of the occluded artery in a mouse model of focal ischemia50 (Figure 1). This mechanism may significantly contribute to microvascular clogging because the active contraction of pericytes is likely to increase the microcirculatory resistance far greater than compression of the lumen by swollen endfeet because large volume changes at the periphery of a cylindrical structure causes relatively smaller changes in diameter. Moreover, segmental constrictions giving a sausage-like appearance to ischemic capillaries (see also Morris et al34) are more compatible with an active contraction rather than passive compression by the astrocyte endfeet covering capillaries all along their longitudinal course. Indeed, the constricted nodes were found to be colocalized with the contracted pericyte processes intermittently located along microvessels.50 It might be interesting to reevaluate whether the constricted capillaries illustrated in electron microscopy studies might have been caused by contracted pericytes, and the swollen endfeet then passively have filled the distorted spaces.9, 15 Because capillary luminal size hardly allows passage of one row of erythrocytes and, leukocytes, because of their size and inflexibility, are not easily able to negotiate capillaries, small decreases in capillary radius typically caused by pericyte contractions (by 17% in vivo58) as well as by endfeet compression and endothelial swelling may lead to blood cell entrapments.50, 59 For example, half of the superficial cortical microvessels (visualized through a cranial window) were still clogged 2 hours after recanalization after 2 hours of MCA occlusion in the mouse cortex.50, 60 Considering the fact that the level of local cerebral blood flow within the first hours of ischemia/reperfusion is the most critical factor determining penumbral tissue survival,61 the failure of erythrocyte circulation is expected to play a detrimental role in determining the tissue fate after recanalization therapies. Pericyte cytoplasm and mitochondria were shown to swell soon after ischemia with electron microscopy.9, 62 The pericyte dysfunction may be caused by oxygen and nitrogen radicals that are intensely generated on the microvascular wall during ischemia/reperfusion63, 64 because the unregulated pericyte contraction can be reversed by inhibiting oxidative and nitrative mechanisms.50 Reversing pericyte contraction with these agents administered during recanalization restored microcirculatory patency and promoted tissue survival, reinforcing the idea that microcirculatory obstructions may unfavorably affect recovery after recanalization as discussed above. However, the potential role of pericytes in IMR requires more compelling and direct evidence because this hypothesis is currently based on a synthesis of ex vivo studies of brain sections and intact retina as well as in vivo observations through a cranial window. The recent in situ demonstration of pericyte contraction with vasoactive agents in transgenic mouse harboring green fluorescent protein-expressing pericytes58 unequivocally shows that brain pericytes have contractile capability as seen in vitro.59, 65 However, the link between observations in the superficial pial circulation and those in the deep subcortical beds, where ‘no-reflow phenomenon' has been described, still remains indirect. In addition to their role in regulation of capillary blood flow by changing the capillary diameter with various chemical stimuli originating from neighboring astrocytes and neurons,59, 65 pericytes also play an important role in maintenance of the BBB integrity.66, 67 Therefore, it would be interesting to investigate whether pericyte dysfunction could also contribute to the ischemia/reperfusion-induced BBB leakiness. These developments might bring about the exciting possibility that effective suppression of oxidative/nitrative stress during recanalization therapies may not only restore BBB integrity (hence, bleeding and edema), but may also improve impaired microcirculation (hence, recovery).
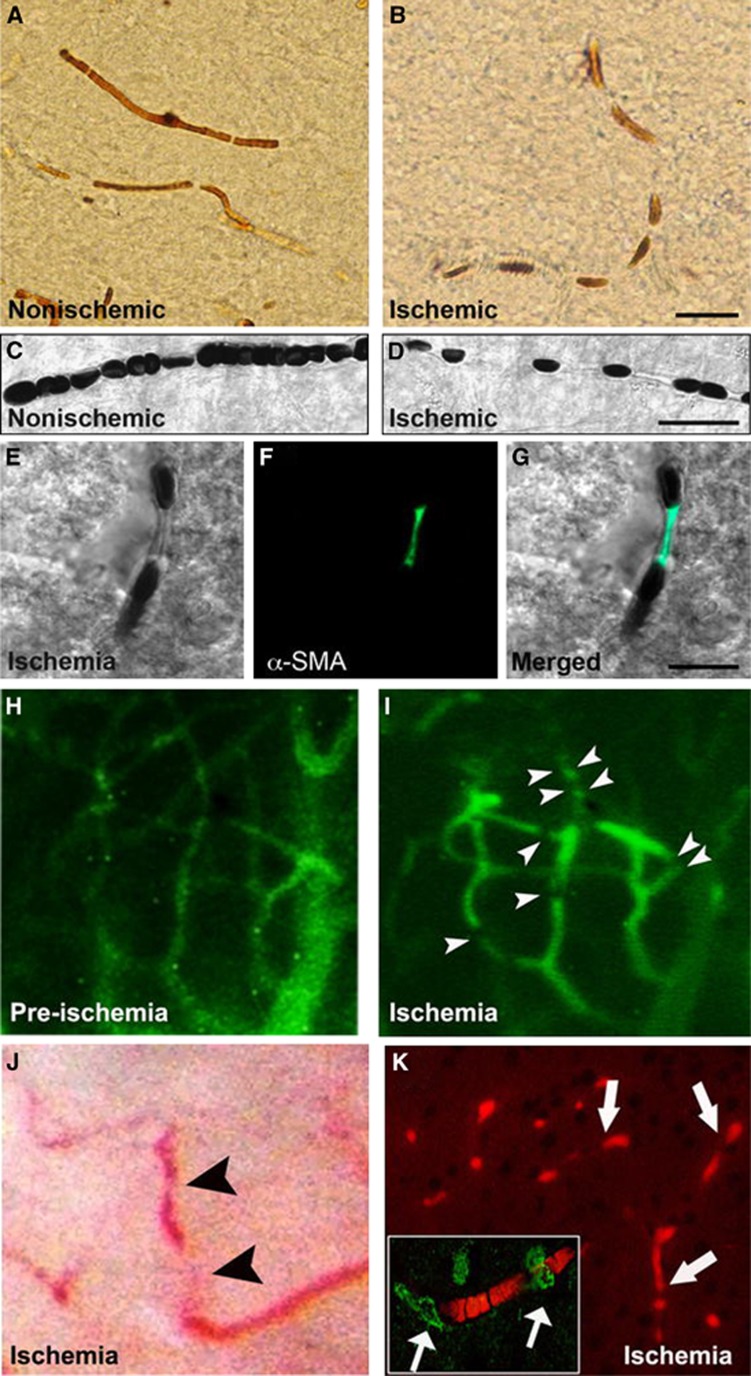
Figure 1.
Cerebral ischemia induces intermittently spaced capillary constrictions, among which erythrocytes are entrapped. Brain sections in upper panels illustrate the cortical capillaries filled with horseradish peroxidase (HRP) injected before killing the mouse. In contrast to uninterruptedly filled nonischemic microvessels (A), the luminal HRP column is interrupted by nodal constrictions in the ischemic hemisphere (B). Differential interference contrast (DIC) images (C–G) illustrate that erythrocytes are entrapped between the constricted segments and that the nodal capillary constrictions are colocalized with α-smooth muscle actin (α-SMA) immunopositive pericytes (green). (A–G) Images were captured from the frontal cortex at 6 hours after 2 hours of middle cerebral artery occlusion (MCA) occlusion. Ischemia-induced capillary constrictions and erythrocyte entrapment can also be observed in vivo through a cranial window in mice under anesthesia (H–J). Microvessels made visible with systemically given fluorescein isothiocyanate (FITC)–dextran become better delineated after ischemia because of sluggish blood flow (allowing more fluorescence emitting), and numerous capillary constrictions appear (arrowheads) starting 1 hour after MCA occlusion (I). (H, I) Images were captured from the same cortical area before and during ischemia. Similarly, erythrocytes, which are hardly detectable under bright-field microscopy in vivo because of rapid blood flow, become visible and entrapped around the capillary constrictions during ischemia (J, arrowheads). Although it is difficult to show in vivo that all microvascular constrictions are colocalized with pericytes, processing of these brains ex vivo with NaBH4, which renders hemoglobin fluorescent (K, red), confirmed that constricted capillaries imaged during intravital microscopy were filled with trapped erythrocytes (arrows) and the capillary constrictions were colocalized with α-SMA immunopositive pericytes (green, arrows in inset). Reproduced from Dalkara et al60 with permission. The color reproduction of this figure is available at the Journal of Cerebral Blood Flow and Metabolism journal online.
Can Incomplete Microcirculatory Reperfusion Have Clinical Significance?
Although the above reviewed evidence supports a role for IMR in stroke pathophysiology, the direct evidence is still missing because of the technical limitations, both experimentally and in clinical imaging. Technical insufficiencies also preclude the investigation of microcirculatory dynamics and associated metabolic changes in required resolution, especially in patients. Recent developments in multiphoton microscopy,43, 68 optical coherence tomography, and phosphorescence quenching,69, 70 as well as magnetic resonance imaging (MRI), may provide further insight and help to assess the potential impact of this interesting phenomenon on the outcome of recanalization therapies. Contrary views reported in the past were mostly based on examination of capillary patency with Evans blue,11, 13 which may have filled the periphery of microvessel lumen with circulating serum and created the illusion of a patent lumen despite the presence of entrapped blood cells.19 Indeed, capillary patency was found to be significantly compromised 1 hour after MCA occlusion when a tracer with different characteristics (e.g. fluorescein isothiocyanate–dextran 2,000 kDa) was used.37, 38 Similarly, detection of regional cerebral blood flow with iodoantipyrine or laser Doppler flowmetry showed varying results ranging from clear local reperfusion deficits to near-normal recovery after MCA recanalization.13, 71, 72, 73, 74 Relatively free circulation of the radioactive tracer with serum may have caused the iodoantipyrine technique to underestimate the microcirculatory deficit caused by capillary cellular aggregates. Doppler shifts created by erythrocytes bypassing through patent capillaries and arteriolo-veneous anastomoses or thoroughfare channels may have led to pseudonormalized signal after recanalization.75 Therefore, labeling erythrocytes might be a more reasonable approach to study the microcirculatory occlusions in vivo as well as ex vivo (Figure 1). In conclusion, it appears that the resistance increase in some capillaries because of intraluminal cell aggregates, which aggravates capillary flow heterogeneity and reduces oxygenation,76, 77 may not be reliably assessed by some cerebral blood flow detection methods depending on experimental factors such as the tracer used, circulation time of the tracer, sampling of region of interests, and tissue processing protocols. In fact, MRI perfusion studies, which evaluated the transit time of the water molecules through a given slice of brain tissue, have clearly disclosed that tissue reperfusion is incomplete even after MCA occlusion as brief as 35 minutes, and further deteriorates with prolonged ischemia78, 79, 80 (Figure 2).
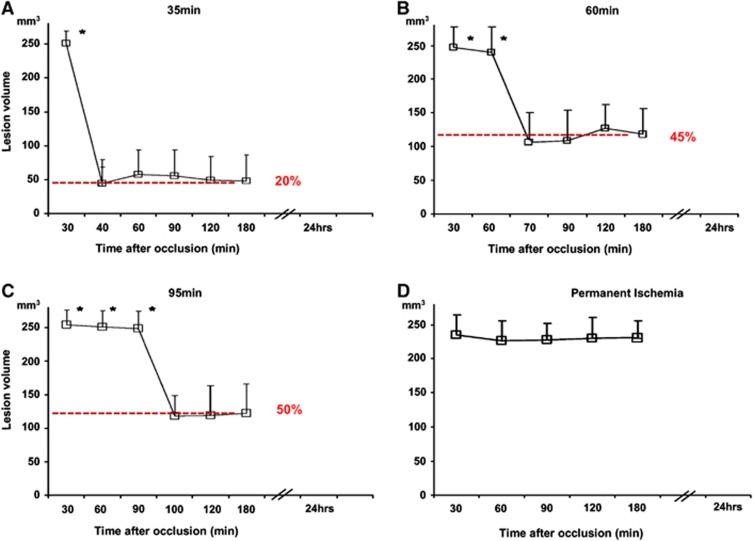
Figure 2.
Temporal evolution of cerebral blood flow changes on magnetic resonance image-derived lesion volumes after (A) 35 minutes, (B) 60 minutes, (C) 95 minutes, and (D) permanent middle cerebral artery occlusion. Tissue reperfusion is incomplete even after MCA occlusion as brief as 35 minutes, and further deteriorates with prolonged ischemia. *P<0.0001 compared with postreperfusion values. Adapted from Bardutzky et al78 with permission.
Can We Improve the Stroke Outcome after Thrombolysis?
Unlike the situation in most stroke patients, the arterial occlusion was not produced with a clot, and recanalization was not attained with thrombolytic agents in most of the animal studies reviewed above. This may be of clinical significance because protracted recanalization as typically seen during thrombolysis may differently affect the microcirculation from prompt recanalization induced by the release of mechanical occlusion. However, a few studies in which MCA was embolized with autologous clots and tissue plasminogen activator (tPA) was used for recanalization reported that tissue reperfusion was only partly restored despite complete recanalization.79, 80, 81 This finding conforms to the idea of an impaired microcirculation despite recanalization and also raises the possibility that microemboli originating from the disintegrated thrombus could contribute to the clogging of microvessels. In stroke patients, clots occluding the arteries fragment, either spontaneously or by thrombolytics, may move downstream to obstruct distal arterial branches and sometimes arterioles (>50 μm).82, 83 Microthrombi have also been found in brain microvessels of stroke patients who died within a month after the ictus.84 Microthrombi may originate from proximal clots or be formed in situ within the microvessels.33 Moreover, Hossmann and his colleagues80 proposed that low perfusion pressure during slow recanalization process might hinder clearance of microthrombi from microcirculation. An elegant study has recently shown that part of the microemboli (8 to 20 μm) are cleared from microcirculation within 2 hours after their infusion into the carotid artery in mice.85 Yet, a substantial number of emboli are retained in the microvasculature and interrupt the blood flow. Obstructed microvessel lumina reopen after extravasation of the clot to the perivascular area within the following days. Although this study has revealed an interesting and novel mechanism, the clinical relevance of such massive microembolism observed in superficial pial vessels under conditions of normal flow to the acute arterial occlusion with a large clot and subsequent fragmentation is hard to predict. In fact, all cortical microvessels were found to be perfused 1 to 3 hours after clot injection to the origin of the MCA in rats, suggesting that cerebral circulation has the capacity to rapidly and effectively lyse fibrin.86 However, the latter study assessed microvascular patency with Evans blue, which may have failed to detect microvascular cell aggregates as discussed above. In conclusion, the available evidence, although indirect, suggests that microcirculation may remain clogged by cellular aggregates as well as fibrin and microthrombi after opening of the occluded artery with thrombolysis. Therefore, therapeutic approaches aiming at reducing microvascular obstruction might improve the success rate of recanalization therapies.4, 25, 33, 40, 50, 54, 55, 56, 57 Importantly, promoting oxygen delivery to the tissue where erythrocytes cannot circulate by means of ongoing serum flow (e.g., with hyperoxia or oxygen carriers) may decrease lactic acidosis and improve survival of the underreperfused tissue.19, 87 However, current approaches also have down sides; for example, hyperoxia may cause vascular and tissue injury, whereas oxygen carriers like perfluorocarbons may induce alterations in coagulation and endothelial cell activation.87, 88
Clinical Evidence Suggesting a Role for Incomplete Microcirculatory Reperfusion in Recanalization Therapies
Although recanalization is vital for the treatment of stroke, a subset of patients do not show any clinical improvement despite recanalization.89 Among many other factors, incomplete reperfusion at the microcirculatory level might be one of the reasons for this discrepancy. Despite the well-established nature of IMR in experimental models of cerebral ischemia, the incidence and relevance of this phenomenon in the clinical setting has not been analyzed systematically. One major reason for the paucity of human data in this regard was the absence of clinical tools that provided temporal information regarding the status of recanalization and reperfusion in patients with acute ischemic stroke. Moreover, the current imaging techniques are far from to match the spatial resolution obtained by neuropathologic examination of microvascular bed in experimental animals. However, with the advent and widespread use of modern neuroimaging tools like computed tomography (CT) angiography, CT perfusion, MRI angiography, and MRI perfusion, together with more common application of intravenous and intraarterial thrombolysis in the acute stroke setting, we have started to get a better picture of the relationship between recanalization and reperfusion in humans (Table 1). The development and standardization of new imaging modalities that enable repetitive monitoring of tissue perfusion status, like arterial spin labeling MRI perfusion or transcranial Doppler ultrasound perfusion, will hopefully help us to better understand the nature of these complex pathophysiological events in humans in the near future.
Table 1. Studies serially analyzing recanalization and reperfusion in ischemic stroke patients.
| Type of treatment | Time to initiation of treatment | Tool for assessing reperfusion | Tool for assessing recanalization | Time of evaluation | Number of patients with successful recanalization | Percentage (95% CI) of patients without reperfusion | |
|---|---|---|---|---|---|---|---|
| Baird et al90 | IA SK | 4 to 24 hours | SPECT | CA | 24 to 48 hours | 4 | 25% (13–78%) |
| Yasaka et al91 | IV SK or placebo | <4 hours | SPECT | TCD | 24 hours | 8 | 50% (17–83%) |
| Khatri et al92 | IV+IA tPA | <3 hours | CA | CA | Completion of angiographya | 43 | 23% (12–39) |
| Tomsick et al93 | IV tPA or IV+IA tPA | <3 hours | CA | CA | Completion of angiographya | 26 | 19% (7–40%) |
| Albers et al94 | IV tPA | 3 to 6 hours | MRP | MRA | 3 to 6 hours after tPAb | 19 | 21% (7–46%) |
| De Silva et al22 | IV tPA or placebo | 3 to 6 hours | MRP | MRA | 3 to 5 days | 13 | 31% (10–61%) |
| Soares et al21 | IV tPA or IA MT or IV tPA+IA MT or standard treatment | <5 hours | CTP | CTA | 24 hours | 13 | 38% (15–68%) |
| Overall | 26% (19–35%) |
CA, conventional angiography; CI, confidence interval; CTA, computed tomography angiography; CTP, computed tomography perfusion; IA, intraarterial; IV, intravenous; MRA, magnetic resonance imaging angiography; MRP, magnetic resonance imaging perfusion; MT, mechanical thrombolysis; SK, streptokinase; SPECT, single-photon emission computed tomography; TCD, transcranial Doppler ultrasonography; tPA, tissue plasminogen activator.
Intravenous tPA had to be started within 3 hours of symptom onset; this was followed with initiation of intraarterial tPA (with or without intraarterial sonography) infusion that lasted for a maximum of 2 hours.
The median door to needle time was 328 minutes in the study.
The first hints regarding the presence of impaired reperfusion despite successful recanalization in humans came from studies that carried out serial single-photon emission CT studies combined with transcranial Doppler ultrasound or digital subtraction angiography in patients receiving streptokinase as part of clinical trials.90, 91 In one study, out of five patients with an initial perfusion defect on serial single photon emission CT together with an intracranial artery occlusion, one patient had no cerebral reperfusion despite partial recanalization of MCA with intraarterial streptokinase.90 Recanalization and reperfusion status were fairly correlated in the other three patients, and one additional patient had undergone partial reperfusion despite unsuccessful recanalization.90 In another study, which compared the efficacy of intravenous streptokinase against placebo, out of 10 patients with initial vessel occlusion, 4 had successful recanalizaton, yet impaired reperfusion.91 Reperfusion and recanalization were correlated in the remaining eight patients; six attained successful recanalization and reperfusion, whereas the remaining two reached neither end point.91 Both studies showed that perfusion status, rather than successful or unsuccessful recanalization, had a significant impact on clinical outcome at follow-up.90, 91
These studies were followed by analyses carried out in cohorts of patients receiving intravenous or intraarterial tPA in the acute ischemic stroke setting. In the International Management of Stroke-I (IMS-I) trial, which tested the efficacy and safety of intravenous and intraarterial tPA combination therapy, digital subtraction angiography was used for assessing both recanalization and reperfusion.92 The analyses have indicated that despite a partial or complete recanalization of the proximal occlusion (graded as arterial occlusive lesion (AOL) score II or III), there was no evidence of distal branch filling (graded as Thrombolysis in Myocardial Infarction (TIMI) score of 1) in 10 out of 43 patients.92 In the following IMS-II trial, the same treatment approach was combined with low-energy sonography applied by a microcatheter. Similar to IMS-I, an AOL score of II or III was observed in 26 patients, but optimal reperfusion was achieved (graded as Thrombolysis in Cerebral Infarction (TICI) score of 2 or 3) in only 21 patients.93 Both studies showed, once again, that clinical outcome was better correlated with reperfusion status rather than recanalization.92, 93 Although angiography by itself cannot be considered as an appropriate tool for assessing tissue perfusion, these results highlight that there is a discordance between proximal and distal vascular beds in terms of vascular patency in a subset of stroke patients after recanalization.
More recent studies, using noninvasive tools like CT or MRI to assess vessel status and tissue reperfusion, have also identified the presence of patients with discordant recanalization and reperfusion. In the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE) trial, there were 4 patients out of 19 who did not show evidence for reperfusion on MRI after 3 to 6 hours of intravenous tPA despite recanalization.94 The correlation between recanalization and reperfusion was significant, but moderate with a κ value of 0.58.94 In the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET), among 18 patients receiving intravenous tPA and with technically adequate serial angiography and MRI perfusion studies, recanalization was attained in 13 cases at days 3 to 5, 4 of whom with no success in reperfusion.22 In multivariate analyses, reperfusion but not recanalization was significantly associated with good neurologic and functional outcome.22 Consistently, another study has shown that reperfusion was a significant predictor of both clinical and tissue outcomes, whereas none of these outcome parameters were related to recanalization.21 Out of 13 patients in this study, 5 had impaired reperfusion despite recanalization21 (Figure 3). Although the supporting data continue to accumulate, we still do not know the exact mechanism of these observations in humans. Multiple mechanisms, ranging from fragmentation and migration of thrombotic particles into distal arterial beds to disturbed flow within the capillaries secondary to IMR, might be related to impaired perfusion on CT or MRI studies, despite restored patency in proximal arteries (hence, recanalization).95 One promising aspect regarding the potential role of IMR in humans is that both the no-reflow phenomenon and most of the phenomena that are now considered critical in acute ischemic stroke, like the penumbra concept, have been shown to be present in non-human primates.7, 96
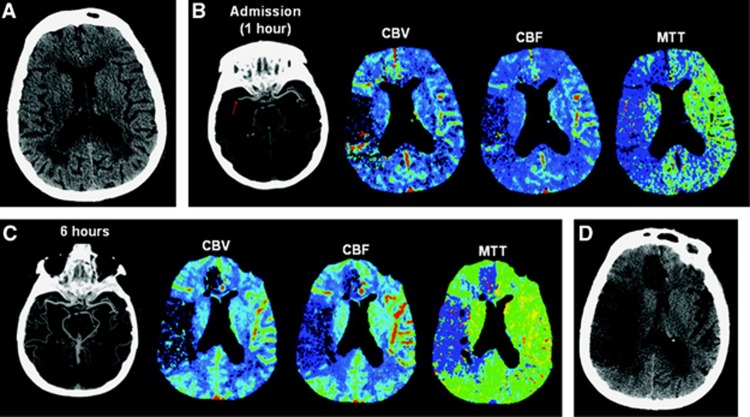
Figure 3.
Absence of reperfusion, even in the setting of complete recanalization, may result in a large follow-up infarct volume. (A) Axial noncontrast computed tomography (CT) carried out on admission (2.5 hours after onset of symptoms) shows subtle hypoattenuation of the right putamen but no sulcal effacement in the right middle cerebral artery (MCA) territory. (B) Axial maximum-intensity projection image from computed tomography angiography (CTA) carried out admission shows occlusion of the M1 segment of the right MCA (arrow). Perfusion CT (PCT) maps show decreased cerebral blood volume (CBV) and cerebral blood flow (CBF) in the right frontal and temporal lobes and a larger region of prolonged mean transit time (MTT) that also involves the right anterior cerebral artery (ACA) territory. The region of decreased CBV corresponds to the infarct core, whereas the surrounding mismatch region of prolonged MTT represents the ischemic penumbra. The patient received endovascular thrombectomy with a MERCI device. (C) Axial maximum-intensity projection image from CTA and PCT carried out 6 hours after admission show that, despite complete recanalization of the right MCA, PCT maps show that the region of decreased CBV and CBF has expanded to include the right anterior ACA territory that was previously considered tissue at risk. MTT is still abnormally increased in the right superficial MCA territory and in a portion of the right ACA territory. (D) Axial noncontrast CT carried out 48 hours after admission shows marked hypoattenuation and edema in the territories matching the perfusion deficit on the reperfusion PCT. Reproduced from Soares et al21 with permission.
In conclusion, clinical studies suggest that a state of incomplete reperfusion is observed in approximately one quarter of patients with successful recanalization. The understanding of potential mechanisms that contribute to this microcirculatory insufficiency and development of therapeutic strategies to restore microcirculation should become one of the priorities of stroke research in the near future as both tissue and clinical outcomes heavily depend on successful reperfusion.
The authors declare no conflict of interest.
References
- Donnan GA, Davis SM, Parsons MW, Ma H, Dewey HM, Howells DW. How to make better use of thrombolytic therapy in acute ischemic stroke. Nat Rev Neurol. 2011;7:400–409. doi: 10.1038/nrneurol.2011.89. [DOI] [PubMed] [Google Scholar]
- Pfefferkorn T, Rosenberg GA. Closure of the blood-brain barrier by matrix metalloproteinase inhibition reduces rtPA-mediated mortality in cerebral ischemia with delayed reperfusion. Stroke. 2003;34:2025–2030. doi: 10.1161/01.STR.0000083051.93319.28. [DOI] [PubMed] [Google Scholar]
- Yang Y, Estrada EY, Thompson JF, Liu W, Rosenberg GA. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- Al-Ali F, Jefferson A, Barrow T, Cree T, Louis S, Luke K, et al. The capillary index score: rethinking the acute ischemic stroke treatment algorithm. Results from the Borgess Medical Center Acute Ischemic Stroke Registry J Neurointerv Surgdoi: 10.1136/neurintsurg-2011-010146in press). [DOI] [PubMed]
- Ito H. No-reflow phenomenon in patients with acute myocardial infarction: its pathophysiology and clinical implications. Acta Med Okayama. 2009;63:161–168. doi: 10.18926/AMO/31817. [DOI] [PubMed] [Google Scholar]
- Bekkers SC, Yazdani SK, Virmani R, Waltenberger J. Microvascular obstruction: underlying pathophysiology and clinical diagnosis. J Am Coll Cardiol. 2010;55:1649–1660. doi: 10.1016/j.jacc.2009.12.037. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ, Schmid-Schonbein GW, Mori E, Copeland BR, Chang CM. Polymorphonuclear leukocytes occlude capillaries following middle cerebral artery occlusion and reperfusion in baboons. Stroke. 1991;22:1276–1283. doi: 10.1161/01.str.22.10.1276. [DOI] [PubMed] [Google Scholar]
- Ames A, Wright RL, Kowada M, Thurston JM, Majno G. Cerebral ischemia. II. The no-reflow phenomenon. Am J Pathol. 1968;52:437–453. [PMC free article] [PubMed] [Google Scholar]
- Little JR, Kerr FWL, TM Sundt. Microcirculatory obstruction in focal cerebral ischemia: an electron microscopic investigation in monkeys. Stroke. 1976;7:25–30. doi: 10.1161/01.STR.7.1.25. [DOI] [PubMed] [Google Scholar]
- Little JR, Kerr FW, Sundt TM. Microcirculatory obstruction in focal cerebral ischemia. Relationship to neuronal alterations. Mayo Clin Proc. 1975;50:264–270. [PubMed] [Google Scholar]
- Li PA, Vogel J, Smith M, He QP, Kuschinsky W, Siesjo BK. Capillary patency after transient middle cerebral artery occlusion of 2 h duration. Neurosci Lett. 1998;253:191–194. doi: 10.1016/s0304-3940(98)00643-0. [DOI] [PubMed] [Google Scholar]
- Li PA, Gisselsson L, Keuker J, Vogel J, Smith ML, Kuschinsky W, et al. Hyperglycemia-exaggerated ischemic brain damage following 30 min of middle cerebral artery occlusion is not due to capillary obstruction. Brain Res. 1998;804:36–44. doi: 10.1016/s0006-8993(98)00651-9. [DOI] [PubMed] [Google Scholar]
- Tsuchidate R, He QP, Smith ML, Siesjo BK. Regional cerebral blood flow during and after 2 hours of middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab. 1997;17:1066–1073. doi: 10.1097/00004647-199710000-00008. [DOI] [PubMed] [Google Scholar]
- de la Torre JC, Fortin T, Saunders JK, Butler K, Richard MT. The no-reflow phenomenon is a post-mortem artifact. Acta Neurochir (Wien) 1992;115:37–42. doi: 10.1007/BF01400588. [DOI] [PubMed] [Google Scholar]
- Garcia JH, Liu KF, Yoshida Y, Chen S, Lian J. Brain microvessels: factors altering their patency after the occlusion of a middle cerebral artery (Wistar rat) Am J Pathol. 1994;145:728–740. [PMC free article] [PubMed] [Google Scholar]
- del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab. 2003;23:879–894. doi: 10.1097/01.WCB.0000078322.96027.78. [DOI] [PubMed] [Google Scholar]
- Hallenbeck JM, Dutka AJ, Tanishima T, Kochanek PM, Kumaroo KK, Thompson CB, et al. Polymorphonuclear leukocyte accumulation in brain regions with low blood flow during the early postischemic period. Stroke. 1986;17:246–253. doi: 10.1161/01.str.17.2.246. [DOI] [PubMed] [Google Scholar]
- Hossmann KA. Pathophysiology and therapy of experimental stroke. Cell Mol Neurobiol. 2006;26:1055–1081. doi: 10.1007/s10571-006-9008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Connor J, Peterson S, Shuttleworth CW, Liu KJ. Direct visualization of trapped erythrocytes in rat brain after focal ischemia and reperfusion. J Cereb Blood Flow Metab. 2002;22:1222–1230. doi: 10.1097/01.wcb.0000037998.34930.83. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Lindauer U.Microcirculatory disturbances in cerebral ischemiaIn: Ginsberg MD, Bogousslavsky J (eds)Cerebrovascular Disease: Pathophysiology, Diagnosis, and Management vol. 1Blackwell Science: Malden, MA; 1998343–357. [Google Scholar]
- Soares BP, Tong E, Hom J, Cheng SC, Bredno J, Boussel L, et al. Reperfusion is a more accurate predictor of follow-up infarct volume than recanalization: a proof of concept using CT in acute ischemic stroke patients. Stroke. 2010;41:e34–e40. doi: 10.1161/STROKEAHA.109.568766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva DA, Fink JN, Christensen S, Ebinger M, Bladin C, Levi CR, et al. Assessing reperfusion and recanalization as markers of clinical outcomes after intravenous thrombolysis in the echoplanar imaging thrombolytic evaluation trial (EPITHET) Stroke. 2009;40:2872–2874. doi: 10.1161/STROKEAHA.108.543595. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ, Sharp FR, Heiss WD, Albers GW. Heterogeneity in the penumbra. J Cereb Blood Flow Metab. 2011;31:1836–1851. doi: 10.1038/jcbfm.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell RM, Olsson Y. Impaired microvascular filling after focal cerebral ischemia in the monkey. Modification by treatment. Neurology. 1972;22:500–504. doi: 10.1212/wnl.22.5.500. [DOI] [PubMed] [Google Scholar]
- Belayev L, Pinard E, Nallet H, Seylaz J, Liu Y, Riyamongkol P, et al. Albumin therapy of transient focal cerebral ischemia: in vivo analysis of dynamic microvascular responses. Stroke. 2002;33:1077–1084. doi: 10.1161/hs0402.105555. [DOI] [PubMed] [Google Scholar]
- Anwar M, Buchweitz-Milton E, Weiss HR. Effect of prazosin on microvascular perfusion during middle cerebral artery ligation in the rat. Circ Res. 1988;63:27–34. doi: 10.1161/01.res.63.1.27. [DOI] [PubMed] [Google Scholar]
- Garcia JH.Evolution of the brain leison induced by experimental focal ischemiaIn: Welch KMA, Caplan LR, Reis DJ, Siesjö BK, Weir B (eds)Primer on Cerebrovascular Diseases Academic Press: San Diego, CA; 1997107–111. [Google Scholar]
- del Zoppo GJ, Hamann GF.The cerebral microvasculature and responses to ischemiaIn: Mohr JP, Wolf PA, Grotta JC, Moskowitz MA, Mayberg MR, R vK (eds)Stroke: Pathophysiology, Diagnosis and Management5th edn. (Elsevier: Philadelphia; 201116–28. [Google Scholar]
- del Zoppo GJ. Microvascular changes during cerebral ischemia and reperfusion. Cerebrovasc Brain Metab Rev. 1994;6:47–96. [PubMed] [Google Scholar]
- Ohtake M, Morino S, Kaidoh T, Inoue T. Three-dimensional structural changes in cerebral microvessels after transient focal cerebral ischemia in rats: scanning electron microscopic study of corrosion casts. Neuropathology. 2004;24:219–227. doi: 10.1111/j.1440-1789.2004.00560.x. [DOI] [PubMed] [Google Scholar]
- Garcia JH, Liu KF, Yoshida Y, Lian J, Chen S, del Zoppo GJ. Influx of leukocytes and platelets in an evolving brain infarct (Wistar rat) Am J Pathol. 1994;144:188–199. [PMC free article] [PubMed] [Google Scholar]
- Zhang ZG, Chopp M, Goussev A, Lu D, Morris D, Tsang W, et al. Cerebral microvascular obstruction by fibrin is associated with upregulation of PAI-1 acutely after onset of focal embolic ischemia in rats. J Neurosci. 1999;19:10898–10907. doi: 10.1523/JNEUROSCI.19-24-10898.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhri TF, Hoh BL, Zerwes HG, Prestigiacomo CJ, Kim SC, Connolly ES, et al. Reduced microvascular thrombosis and improved outcome in acute murine stroke by inhibiting GP IIb/IIIa receptor-mediated platelet aggregation. J Clin Invest. 1998;102:1301–1310. doi: 10.1172/JCI3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris DC, Davies K, Zhang Z, Chopp M. Measurement of cerebral microvessel diameters after embolic stroke in rat using quantitative laser scanning confocal microscopy. Brain Res. 2000;876:31–36. doi: 10.1016/s0006-8993(00)02543-9. [DOI] [PubMed] [Google Scholar]
- Okada Y, Copeland BR, Fitridge R, Koziol JA, del Zoppo GJ.Fibrin contributes to microvascular obstructions and parenchymal changes during early focal cerebral ischemia and reperfusion Stroke 1994251847–1853.discussion 1853-1844. [DOI] [PubMed] [Google Scholar]
- Ritter LS, Orozco JA, Coull BM, McDonagh PF, Rosenblum WI. Leukocyte accumulation and hemodynamic changes in the cerebral microcirculation during early reperfusion after stroke. Stroke. 2000;31:1153–1161. doi: 10.1161/01.str.31.5.1153. [DOI] [PubMed] [Google Scholar]
- Buchweitz-Milton E, Weiss HR. Perfused microvascular morphometry during middle cerebral artery occlusion. Am J Physiol. 1988;255:H623–H628. doi: 10.1152/ajpheart.1988.255.3.H623. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Davies K, Prostak J, Fenstermacher J, Chopp M. Quantitation of microvascular plasma perfusion and neuronal microtubule-associated protein in ischemic mouse brain by laser-scanning confocal microscopy. J Cereb Blood Flow Metab. 1999;19:68–78. doi: 10.1097/00004647-199901000-00008. [DOI] [PubMed] [Google Scholar]
- Schmid-Schonbein GW, Engler RL. Granulocytes as active participants in acute myocardial ischemia and infarction. Am J Cardiovasc Pathol. 1987;1:15–30. [PubMed] [Google Scholar]
- Mori E, del Zoppo GJ, Chambers JD, Copeland BR, Arfors KE. Inhibition of polymorphonuclear leukocyte adherence suppresses no-reflow after focal cerebral ischemia in baboons. Stroke. 1992;23:712–718. doi: 10.1161/01.str.23.5.712. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Hallenbeck JM. Acute focal ischemia-induced alterations in MAP2 immunostaining: description of temporal changes and utilization as a marker for volumetric assessment of acute brain injury. J Cereb Blood Flow Metab. 1996;16:170–174. doi: 10.1097/00004647-199601000-00020. [DOI] [PubMed] [Google Scholar]
- Dereski MO, Chopp M, Knight RA, Rodolosi LC, Garcia JH. The heterogeneous temporal evolution of focal ischemic neuronal damage in the rat. Acta Neuropathol. 1993;85:327–333. doi: 10.1007/BF00227730. [DOI] [PubMed] [Google Scholar]
- Kasischke KA, Lambert EM, Panepento B, Sun A, Gelbard HA, Burgess RW, et al. Two-photon NADH imaging exposes boundaries of oxygen diffusion in cortical vascular supply regions. J Cereb Blood Flow Metab. 2011;31:68–81. doi: 10.1038/jcbfm.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frykholm P, Hillered L, Langstrom B, Persson L, Valtysson J, Enblad P. Relationship between cerebral blood flow and oxygen metabolism, and extracellular glucose and lactate concentrations during middle cerebral artery occlusion and reperfusion: a microdialysis and positron emission tomography study in nonhuman primates. J Neurosurg. 2005;102:1076–1084. doi: 10.3171/jns.2005.102.6.1076. [DOI] [PubMed] [Google Scholar]
- Liu S, Shi H, Liu W, Furuichi T, Timmins GS, Liu KJ. Interstitial pO2 in ischemic penumbra and core are differentially affected following transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2004;24:343–349. doi: 10.1097/01.WCB.0000110047.43905.01. [DOI] [PubMed] [Google Scholar]
- Tsai PS, Kaufhold JP, Blinder P, Friedman B, Drew PJ, Karten HJ, et al. Correlations of neuronal and microvascular densities in murine cortex revealed by direct counting and colocalization of nuclei and vessels. J Neurosci. 2009;29:14553–14570. doi: 10.1523/JNEUROSCI.3287-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabuchi T, Lucero J, Feng A, Koziol JA, del Zoppo GJ. Focal cerebral ischemia preferentially affects neurons distant from their neighboring microvessels. J Cereb Blood Flow Metab. 2005;25:257–266. doi: 10.1038/sj.jcbfm.9600027. [DOI] [PubMed] [Google Scholar]
- Welsh FA, O'Connor MJ, Langfitt TW. Regions of cerebral ischemia located by pyridine nucleotide fluorescence. Science. 1977;198:951–953. doi: 10.1126/science.201026. [DOI] [PubMed] [Google Scholar]
- Ito U, Hakamata Y, Kawakami E, Oyanagi K. Temporary [corrected] cerebral ischemia results in swollen astrocytic end-feet that compress microvessels and lead to delayed [corrected] focal cortical infarction. J Cereb Blood Flow Metab. 2011;31:328–338. doi: 10.1038/jcbfm.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yemisci M, Gursoy-Ozdemir Y, Vural A, Can A, Topalkara K, Dalkara T. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nat Med. 2009;15:1031–1037. doi: 10.1038/nm.2022. [DOI] [PubMed] [Google Scholar]
- Belayev L, Zhao W, Busto R, Ginsberg MD. Transient middle cerebral artery occlusion by intraluminal suture: I. Three-dimensional autoradiographic image-analysis of local cerebral glucose metabolism-blood flow interrelationships during ischemia and early recirculation. J Cereb Blood Flow Metab. 1997;17:1266–1280. doi: 10.1097/00004647-199712000-00002. [DOI] [PubMed] [Google Scholar]
- Nicoli F, Lefur Y, Denis B, Ranjeva JP, Confort-Gouny S, Cozzone PJ. Metabolic counterpart of decreased apparent diffusion coefficient during hyperacute ischemic stroke: a brain proton magnetic resonance spectroscopic imaging study. Stroke. 2003;34:e82–e87. doi: 10.1161/01.STR.0000078659.43423.0A. [DOI] [PubMed] [Google Scholar]
- Garcia JH, Yoshida Y, Chen H, Li Y, Zhang ZG, Lian J, et al. Progression from ischemic injury to infarct following middle cerebral artery occlusion in the rat. Am J Pathol. 1993;142:623–635. [PMC free article] [PubMed] [Google Scholar]
- Wong CH, Bozinovski S, Hertzog PJ, Hickey MJ, Crack PJ. Absence of glutathione peroxidase-1 exacerbates cerebral ischemia-reperfusion injury by reducing post-ischemic microvascular perfusion. J Neurochem. 2008;107:241–252. doi: 10.1111/j.1471-4159.2008.05605.x. [DOI] [PubMed] [Google Scholar]
- Abumiya T, Fitridge R, Mazur C, Copeland BR, Koziol JA, Tschopp JF, et al. Integrin alpha(IIb)beta(3) inhibitor preserves microvascular patency in experimental acute focal cerebral ischemia Stroke 2000311402–1409.discussion 1409-1410. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Vowinkel T, Stokes KY, Arumugam TV, Yilmaz G, Nanda A, et al. CD40/CD40 ligand signaling in mouse cerebral microvasculature after focal ischemia/reperfusion. Circulation. 2005;111:1690–1696. doi: 10.1161/01.CIR.0000160349.42665.0C. [DOI] [PubMed] [Google Scholar]
- Schulz JB, Panahian N, Chen YI, Beal MF, Moskowitz MA, Rosen BR, et al. Facilitation of postischemic reperfusion with alpha-PBN: assessment using NMR and Doppler flow techniques. Am J Physiol. 1997;272:H1986–H1995. doi: 10.1152/ajpheart.1997.272.4.H1986. [DOI] [PubMed] [Google Scholar]
- Fernandez-Klett F, Offenhauser N, Dirnagl U, Priller J, Lindauer U. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc Natl Acad Sci USA. 2010;107:22290–22295. doi: 10.1073/pnas.1011321108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton NB, Attwell D, Hall CN. Pericyte-mediated regulation of capillary diameter: a component of neurovascular coupling in health and disease. Front Neuroenergetics. 2010;2:pii: 5. doi: 10.3389/fnene.2010.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalkara T, Gursoy-Ozdemir Y, Yemisci M. Brain microvascular pericytes in health and disease. Acta Neuropathol. 2011;122:1–9. doi: 10.1007/s00401-011-0847-6. [DOI] [PubMed] [Google Scholar]
- Zhao W, Belayev L, Ginsberg MD. Transient middle cerebral artery occlusion by intraluminal suture: II. Neurological deficits, and pixel-based correlation of histopathology with local blood flow and glucose utilization. J Cereb Blood Flow Metab. 1997;17:1281–1290. doi: 10.1097/00004647-199712000-00003. [DOI] [PubMed] [Google Scholar]
- Liu KF, Li F, Tatlisumak T, Garcia JH, Sotak CH, Fisher M, et al. Regional variations in the apparent diffusion coefficient and the intracellular distribution of water in rat brain during acute focal ischemia. Stroke. 2001;32:1897–1905. doi: 10.1161/01.str.32.8.1897. [DOI] [PubMed] [Google Scholar]
- Gursoy-Ozdemir Y, Bolay H, Saribas O, Dalkara T.Role of endothelial nitric oxide generation and peroxynitrite formation in reperfusion injury after focal cerebral ischemia Stroke 2000311974–1980.discussion 1981. [DOI] [PubMed] [Google Scholar]
- Gursoy-Ozdemir Y, Can A, Dalkara T. Reperfusion-induced oxidative/nitrative injury to neurovascular unit after focal cerebral ischemia. Stroke. 2004;35:1449–1453. doi: 10.1161/01.STR.0000126044.83777.f4. [DOI] [PubMed] [Google Scholar]
- Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14:1398–1405. doi: 10.1038/nn.2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- Lecoq J, Parpaleix A, Roussakis E, Ducros M, Houssen YG, Vinogradov SA, et al. Simultaneous two-photon imaging of oxygen and blood flow in deep cerebral vessels. Nat Med. 2011;17:893–898. doi: 10.1038/nm.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaseen MA, Srinivasan VJ, Sakadzic S, Radhakrishnan H, Gorczynska I, Wu W, et al. Microvascular oxygen tension and flow measurements in rodent cerebral cortex during baseline conditions and functional activation. J Cereb Blood Flow Metab. 2011;31:1051–1063. doi: 10.1038/jcbfm.2010.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakadzic S, Roussakis E, Yaseen MA, Mandeville ET, Srinivasan VJ, Arai K, et al. Two-photon high-resolution measurement of partial pressure of oxygen in cerebral vasculature and tissue. Nat Methods. 2010;7:755–759. doi: 10.1038/nmeth.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan AM, Xue D, Slivka A. A new model of temporary focal neocortical ischemia in the rat. Stroke. 1992;23:273–279. doi: 10.1161/01.str.23.2.273. [DOI] [PubMed] [Google Scholar]
- Nakai A, Kuroda S, Kristian T, Siesjo BK. The immunosuppressant drug FK506 ameliorates secondary mitochondrial dysfunction following transient focal cerebral ischemia in the rat. Neurobiol Dis. 1997;4:288–300. doi: 10.1006/nbdi.1997.0146. [DOI] [PubMed] [Google Scholar]
- Shigeno T, Teasdale GM, McCulloch J, Graham DI. Recirculation model following MCA occlusion in rats. Cerebral blood flow, cerebrovascular permeability, and brain edema. J Neurosurg. 1985;63:272–277. doi: 10.3171/jns.1985.63.2.0272. [DOI] [PubMed] [Google Scholar]
- Nagasawa H, Kogure K. Correlation between cerebral blood flow and histologic changes in a new rat model of middle cerebral artery occlusion. Stroke. 1989;20:1037–1043. doi: 10.1161/01.str.20.8.1037. [DOI] [PubMed] [Google Scholar]
- Hudetz AG. Blood flow in the cerebral capillary network: a review emphasizing observations with intravital microscopy. Microcirculation. 1997;4:233–252. doi: 10.3109/10739689709146787. [DOI] [PubMed] [Google Scholar]
- Jespersen SN, Ostergaard L. The roles of cerebral blood flow, capillary transit time heterogeneity, and oxygen tension in brain oxygenation and metabolism. J Cereb Blood Flow Metab. 2012;32:264–277. doi: 10.1038/jcbfm.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita Y, Tomita M, Schiszler I, Amano T, Tanahashi N, Kobari M, et al. Moment analysis of microflow histogram in focal ischemic lesion to evaluate microvascular derangement after small pial arterial occlusion in rats. J Cereb Blood Flow Metab. 2002;22:663–669. doi: 10.1097/00004647-200206000-00004. [DOI] [PubMed] [Google Scholar]
- Bardutzky J, Shen Q, Henninger N, Schwab S, Duong TQ, Fisher M. Characterizing tissue fate after transient cerebral ischemia of varying duration using quantitative diffusion and perfusion imaging. Stroke. 2007;38:1336–1344. doi: 10.1161/01.STR.0000259636.26950.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke C, Brinker G, Pillekamp F, Hoehn M. Probability of metabolic tissue recovery after thrombolytic treatment of experimental stroke: a magnetic resonance spectroscopic imaging study in rat brain. J Cereb Blood Flow Metab. 2000;20:583–591. doi: 10.1097/00004647-200003000-00016. [DOI] [PubMed] [Google Scholar]
- Busch E, Kruger K, Allegrini PR, Kerskens CM, Gyngell ML, Hoehn-Berlage M, et al. Reperfusion after thrombolytic therapy of embolic stroke in the rat: magnetic resonance and biochemical imaging. J Cereb Blood Flow Metab. 1998;18:407–418. doi: 10.1097/00004647-199804000-00009. [DOI] [PubMed] [Google Scholar]
- Niessen F, Hilger T, Hoehn M, Hossmann KA. Thrombolytic treatment of clot embolism in rat: comparison of intra-arterial and intravenous application of recombinant tissue plasminogen activator. Stroke. 2002;33:2999–3005. doi: 10.1161/01.str.0000038096.60932.f4. [DOI] [PubMed] [Google Scholar]
- Babikian VL, Caplan LR. Brain embolism is a dynamic process with variable characteristics. Neurology. 2000;54:797–801. doi: 10.1212/wnl.54.4.797. [DOI] [PubMed] [Google Scholar]
- Heye N, Cervos-Navarro J. Microthromboemboli in acute infarcts: analysis of 40 autopsy cases. Stroke. 1996;27:431–434. doi: 10.1161/01.str.27.3.431. [DOI] [PubMed] [Google Scholar]
- Heye N, Paetzold C, Steinberg R, Cervos-Navarro J. The topography of microthrombi in ischemic brain infarct. Acta Neurol Scand. 1992;86:450–454. doi: 10.1111/j.1600-0404.1992.tb05122.x. [DOI] [PubMed] [Google Scholar]
- Lam CK, Yoo T, Hiner B, Liu Z, Grutzendler J. Embolus extravasation is an alternative mechanism for cerebral microvascular recanalization. Nature. 2010;465:478–482. doi: 10.1038/nature09001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CX, Todd KG, Yang Y, Gordon T, Shuaib A. Patency of cerebral microvessels after focal embolic stroke in the rat. J Cereb Blood Flow Metab. 2001;21:413–421. doi: 10.1097/00004647-200104000-00010. [DOI] [PubMed] [Google Scholar]
- Shi H, Liu KJ. Cerebral tissue oxygenation and oxidative brain injury during ischemia and reperfusion. Front Biosci. 2007;12:1318–1328. doi: 10.2741/2150. [DOI] [PubMed] [Google Scholar]
- Spiess BD. Perfluorocarbon emulsions as a promising technology: a review of tissue and vascular gas dynamics. J Appl Physiol. 2009;106:1444–1452. doi: 10.1152/japplphysiol.90995.2008. [DOI] [PubMed] [Google Scholar]
- Alexandrov AV, Hall CE, Labiche LA, Wojner AW, Grotta JC. Ischemic stunning of the brain: early recanalization without immediate clinical improvement in acute ischemic stroke. Stroke. 2004;35:449–452. doi: 10.1161/01.STR.0000113737.58014.B4. [DOI] [PubMed] [Google Scholar]
- Baird AE, Donnan GA, Austin MC, Fitt GJ, Davis SM, McKay WJ. Reperfusion after thrombolytic therapy in ischemic stroke measured by single-photon emission computed tomography. Stroke. 1994;25:79–85. doi: 10.1161/01.str.25.1.79. [DOI] [PubMed] [Google Scholar]
- Yasaka M, O'Keefe GJ, Chambers BR, Davis SM, Infeld B, O'Malley H, et al. Streptokinase in acute stroke: effect on reperfusion and recanalization. Australian Streptokinase Trial Study Group. Neurology. 1998;50:626–632. doi: 10.1212/wnl.50.3.626. [DOI] [PubMed] [Google Scholar]
- Khatri P, Neff J, Broderick JP, Khoury JC, Carrozzella J, Tomsick T. Revascularization end points in stroke interventional trials: recanalization versus reperfusion in IMS-I. Stroke. 2005;36:2400–2403. doi: 10.1161/01.STR.0000185698.45720.58. [DOI] [PubMed] [Google Scholar]
- Tomsick T, Broderick J, Carrozella J, Khatri P, Hill M, Palesch Y, et al. Revascularization results in the Interventional Management of Stroke II trial. AJNR Am J Neuroradiol. 2008;29:582–587. doi: 10.3174/ajnr.A0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers GW, Thijs VN, Wechsler L, Kemp S, Schlaug G, Skalabrin E, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006;60:508–517. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- Soares BP, Chien JD, Wintermark M. MR and CT monitoring of recanalization, reperfusion, and penumbra salvage: everything that recanalizes does not necessarily reperfuse! Stroke. 2009;40:S24–S27. doi: 10.1161/STROKEAHA.108.526814. [DOI] [PubMed] [Google Scholar]
- Astrup J, Symon L, Branston NM, Lassen NA. Cortical evoked potential and extracellular K+ and H+ at critical levels of brain ischemia. Stroke. 1977;8:51–57. doi: 10.1161/01.str.8.1.51. [DOI] [PubMed] [Google Scholar]