SUMMARY
Background
Patients with gallbladder cancer or cholangiocarcinoma usually present with advanced disease and limited treatment options. Based on the common embryologic origin of the exocrine pancreas and gallbladder, coupled with data demonstrating effectiveness of gemcitabine in pancreatic carcinoma, this trial was pursued. The aim was to test the combination of gemcitabine 1,000 mg/m2 IV over 100 minutes on days 1 and 8, and capecitabine 650 mg/m2 BID PO days 1–14, administered every 21 days, in the treatment of patients in this population.
Patients and Methods
The primary objective of this study was to assess the response rate (confirmed complete and partial responses) of gemcitabine and capecitabine used in incurable biliary neoplasms. Secondary objectives included overall survival and toxicities.
A two-stage design was used to detect a difference in the null hypothesis of 5% response probability and the alternative 20% response probability. If at least one response occurred after the first 20 patients, another 20 were to be accrued.
Results
The study accrued 57 patients from September 2003 until April 2005. Three patients were ineligible, and two others received no treatment. Characteristics of analyzable patients: 35 (67%) cholangiocarcinoma, 17 (33%) gallbladder cancer; PS 0 (18 pts), 1 (26 pts), 2 (8 pts); 26 (50%) male; median age 58.8 years (29.5–85.6). Among 51 patients evaluated for toxicity, 6 experienced grade 4 toxicities: 1 thrombosis/embolism and muscle pain, 1 fatigue and 4 neutropenia, one of whom also had grade 4 leukopenia and grade 4 thrombocytopenia. Among 52 patients, there were 7 confirmed partial responses for a confirmed response probability of 13% (95% CI: 6% to 26%). Six patients had an unconfirmed partial response for an overall response probability of 25% (95% CI: 14% to 39%). Twelve patients (23%) demonstrated stable disease. The 6 month overall survival was 55% (95% CI: 41%–69%), and median survival was 7 months (95% CI: 5 – 8 mo.).
Conclusions
The combination of gemcitabine and capecitabine is a well tolerated regimen with activity in patients with advanced gallbladder cancer and cholangiocarcinoma.
Keywords: Capecitabine, cholangiocarcinoma, gallbladder cancer, gemcitabine, metastatic, unresectable
INTRODUCTION
Greater than 5,000 cases of gallbladder cancer, and 2,500 to 3,000 cases of cholangiocarcinoma are diagnosed each year in the United States.1 The highest prevalence of gallbladder tumors and cholangiocarcinomas in the U.S. is in Native Americans, for reasons that are unclear. Other countries with high rates of gallbladder cancer are Chile, Bolivia, and Mexico.2 The primary modality of treatment for patients with limited stage gallbladder cancers or cholangiocarcinomas is resection, but even these patients have a high recurrence rate. Most, however, present with locally invasive or advanced stage disease. Median survival for those presenting with locally advanced or metastatic gallbladder or cholangiocarcinoma is approximately 3 to 6 months, and overall five year survival for those with biliary tumors is less than 5%.1 In the past, fluoropyrimidines have been the mainstay of treatment of advanced disease, alone or in combination (with drugs such as platinums, methotrexate, and adriamycin) and reported response rates ranged from 0 – 34%.3–7 Additionally, there have been several phase II studies that have reported single-agent activity for the nucleoside analog gemcitabine.8–10 Because of a lack of randomized studies, there is no clear standard regimen. Current recommendations for treatment are based on the patient’s performance status and can range from best supportive care to single agent fluoropyrimidines or gemcitabine. 11
Lacking a randomized clinical trial, Eckel and colleagues reported a pooled analysis and review of all chemotherapy trials that had been done in biliary tract cancers. They found that the highest response rate combinations were gemcitabine with fluoropyrimidines and/or platinums.12 Specifically, the combination of gemcitabine and 5-fluorouracil might be particularly efficacious. Gemcitabine may potentiate 5-fluorouracil’s inhibition of thymidylate synthase. Gemcitabine diphsophate acts by inhibiting ribonucleotide reductase, an important enzyme for 5-FU conversion to fluorodeoxyuridine monophosphate (the active inhibitor of thymidylate synthase). This inhibition would be expected to be sequence dependent, occurring if gemcitabine were administered following fluorouracil 13 When gemcitabine administered in a 30 minute infusion was compared with a fixed-dose rate of 10 mg/m2/min, the median survival time and 1 and 2-year survival rates were superior in the fixed-dose rate arm, although with more hematologic toxicity.14
Based on these data, the combination of fixed dose rate infusion gemcitabine and capecitabine, an oral fluoropyrimidine for patients with metastatic or advanced unresectable gallbladder cancer and cholangiocarcinoma was tested in this Southwest Oncology Group study. Along with clinical outcomes, exploratory molecular and pharmacogenomic correlative studies were done on blood and tumor specimens to attempt to identify patients who may specifically benefit from therapy.
PATIENTS AND METHODS
Patients were required to have a cytologically or pathologically verified diagnosis of advanced or metastatic adenocarcinoma of the gallbladder or cholangiocarcinoma and could not be curable with surgery or radiation. Patients with measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST) were considered eligible for the trial. Prior surgery was allowed more than 14 days prior to registration as long as patients had recovered. Patients may have received prior chemotherapy, hormonal therapy, immunotherapy, radiation therapy (to less than 25% of bone marrow) or chemoradiotherapy as neoadjuvant or adjuvant treatment. This must have been completed at least 12 months prior to documented recurrence or metastatic disease. Patients must not have received previous treatment for metastatic disease. Additional eligibility requirements included performance status Zubrod scale of 0–2, the ability to swallow and/or receive enteral medications via gastrostomy feeding tube and have the ability to absorb medication (i.e., no malabsorption syndrome). Patients must have had adequate bone marrow reserve as evidenced by AGC ≥ 1,500/μl and platelets ≥ 100,000/μl, adequate hepatic function as evidenced by serum bilirubin ≤ 3.0 × institutional upper limit of normal (IULN), serum transaminases (SGOT or SGPT) ≤ 2.5 × institutional upper limit of normal (IULN). If liver metastasis were present, SGOT or SGPT must be ≤ 5 × institutional upper limit of normal. A measured or calculated creatinine clearance of 30 mL/min (utilizing G-K equation) was required. Patients with clinically significant cardiac disease not well controlled by medication were not eligible. It was strongly recommended, but not required for entry that patients’ blood/tissue specimens be submitted as detailed below.
Study Design
This was a phase II, open-label, multi-center trial administered and monitored by the Southwest Oncology Group (SWOG). The primary objective of this study was to assess the confirmed response rate of the combination of capecitabine and gemcitabine in patients with advanced disease. Secondary objectives included (1) assessment of overall survival in these patients; (2) evaluation of quantitative and qualitative toxicities of this regimen; (3) assessment of the feasibility of accruing patients with this disease; (4) evaluation, in a preliminary fashion, of potentially relevant prognostic markers in gallbladder and cholangiocarcinoma. Patients received capecitabine 1,300 mg/m2 every day on days 1 through 14. The total daily dose of capecitabine was divided into two equal doses and given in 12 hour intervals on days 1 through 14. Capecitabine was available in 500 mg tablets, and 150 mg tablets were rounded down to the nearest 150 mg or 500 mg tablet. Patients received gemcitabine 1,000 mg/m2 IV over 100 minutes, 10 mg/m2/minute on days 1 and 8, followed by one week of rest. Each cycle was administered every 3 weeks, and patients were monitored for toxicity weekly at the treating physician’s discretion. Patients were continued on protocol treatment until disease progression or until other reason for removal from protocol treatment
Treatment Assessments
Baseline assessments included medical history and physical examination, performance status, CBC with differential and platelet count, bilirubin, SGOT and SGPT, creatinine clearance and diagnostic tumor imaging. Submission of tissue specimens for evaluation of molecular correlates of genes in the fluoropyrimidine and gemcitabine pathways was strongly recommended. Institutions were also encouraged to submit blood samples.
During the study, history, physical exam, performance status, blood counts, SGOT, SGPT and creatinine clearance were evaluated every 3 weeks. A blood count was checked weekly until after cycle 3. Toxicity assessment was performed weekly, and was based on the National Cancer Institute Common Toxicity Criteria, version 2. Tumor response was assessed after every 2 cycles of therapy (6 weeks) and coded in accordance with RECIST criteria.
Molecular Correlates
DNA- extraction
Peripheral blood or paraffin embedded tissue samples were collected from 25 patients. Genomic DNA was extracted from white blood cells or paraffinized tissue using the QiAmp kit (Qiagen, Valencia, CA). Genomic DNA was obtained in 20 patients from peripheral blood and in 5 patients from paraffin embedded tissue. We determined germline polymorphisms in these 25 patients. (Table 1)
Table 1.
| Gene | Forward primer | Reverse primer | Enzyme | Annealing |
|---|---|---|---|---|
| MTHFR 677 | CTTTGGGGAGCTGAAGGACTACTAC | CACTTTGTGACCCCG GTTTG | Hinf I | 62° |
| MTHFR 1298 | CTTTGGGGAGCTGAAGGACTACTAC | CACTTTGTGACCCCGGTTTG | Mbo II | 60° |
| TS 3′ UTR | CAAATCTGAGGGAGCTGAGT | CAGATAAGTGGCAGTACAGA | Dra I | 58° |
| TS 5′ repeat | GTGGCTCCTGCGTTTCCCCC | GCTCCGAGCCGGCCACAGGCATGGCGCGG | Hae III | 65° |
| CDA A79C | GGTACCAACATGGCCCAGAA | CCTTTGAAGATTCTCCCCTCC | n.a. | 62° |
| RRMI | TTCCTTGTAGGGTTTGAAGA | AGGATCCACACATCA GACAT | n.a. | 57° |
Genotyping
Samples were tested using polymerase chain reaction restriction fragment length polymorphism (PCR-RFLP) technique. Briefly, forward and reverse primers were used for PCR amplification, PCR products were digested by restriction enzymes (New England Biolab, Massachussetts, USA) and alleles were separated on 4 % NuSieve ethidium bromide stained agarose gel. Forward and reverse primer, restriction enzymes and annealing temperatures are listed in Table 1.
Statistical Design
The primary objective of this study was to evaluate the response rate (confirmed complete and partial responses) in patients with cholangiocarcinoma and gallbladder carcinoma treated with gemcitabine and capecitabine. Survival was a secondary endpoint. An additional goal was to assess the feasibility of studying these tumors in a cooperative group setting. For the primary endpoint, the regimen of Gemcitabine and capecitabine would not be judged to be of further interest if the true confirmed response rate was 5% or less, but of considerable interest if it was 20% or more. A two-stage design was used for patient accrual. Initially, 20 eligible patients were accrued. If none of these first 20 patients responded, then the regimen was considered to be of no further interest. If one or more patients responded, an additional 20 eligible patients would be accrued. Five or more responders of a total of 40 patients (observed confirmed response rate of 13%) was considered as evidence that this regimen warranted further testing, provided other factors, such as toxicity and survival, appeared favorable. The planned design had a significance level of .047 and a power of .92. Forty patients would have been sufficient to estimate the 6-month survival rate and the probability of a particular toxicity to within ± 16%. There was a surge in accrual just before study closure, in order to maintain the same significance level the threshold for rejection of the null hypothesis was revised down to an observed confirmed response rate of 12%. The power of this test based on actual accrual was 96%. It was proposed that molecular correlates for genes in the fluoropyrimidine and folate pathway would be explored in a very preliminary fashion.
RESULTS
Patient Characteristics
This study was activated in September 2003, satisfied the first stage response criterion, and continued to a second stage of accrual. At closure in April 2005, fifty-seven patients were accrued. Characteristics of analyzable patients: 35 (67%) cholangiocarcinoma, 17 (33%) gallbladder cancer; PS 0 (18 pts), 1 (26 pts), 2 (8 pts); 26 (50%) male; median age 58.8 years (29.5–85.6). [Table 2] Three patients were found to be ineligible (one due to a baseline CT scan being done too early; one did not have pathological confirmation of adenocarcinoma; and another did not have measurable disease). Two patients never received treatment and are not analyzable for any endpoint. Thus, 52 patients were eligible and evaluable for response and survival outcomes. One additional patient went off study prior to any assessment of side effects, and is not evaluable for toxicity. Fourteen patients discontinued treatment for adverse events or side effects, 6 patients refused therapy unrelated to adverse events, 26 patients progressed and there was 1 death. Five patients were off therapy for other reasons not specified.
Table 2.
Patient and tumor characteristics
| Characteristics (n=52) | |
|---|---|
| Age | |
| Median | 58.8 |
| Range | 29.5–85.6 |
| Sex | |
| Male | 26 (50%) |
| Female | 26 (50%) |
| Race | |
| White | 37 (71%) |
| Black | 6 (12%) |
| Asian | 6 (12%) |
| Native American | 1 (2%) |
| Unknown | 2 (4%) |
| Ethnicity | |
| Non-hispanic | 47 (90%) |
| Hispanic | 4 (8%) |
| unknown | 1 (2%) |
| Zubrod Performance Status | |
| 0 | 18 (35%) |
| 1 | 26 (50%) |
| 2 | 8 (15%) |
| Primary Site | |
| Cholangiocarcinoma | 35 (67%) |
| Gallbladder Cancer | 17 (33%) |
| Site of metastasis | |
| Liver | 45 (87%) |
| Lung | 16 (31%) |
| Abdominal disease ?peritoneal | 12 (23%) |
| Regional Lymph nodes | 21 (40%) |
| Prior Surgery | 23 (44%) |
| Prior Adjuvant Therapy | |
| Radiation | 3 (6%) |
| Chemotherapy | 2 (4%) |
Treatment Efficacy
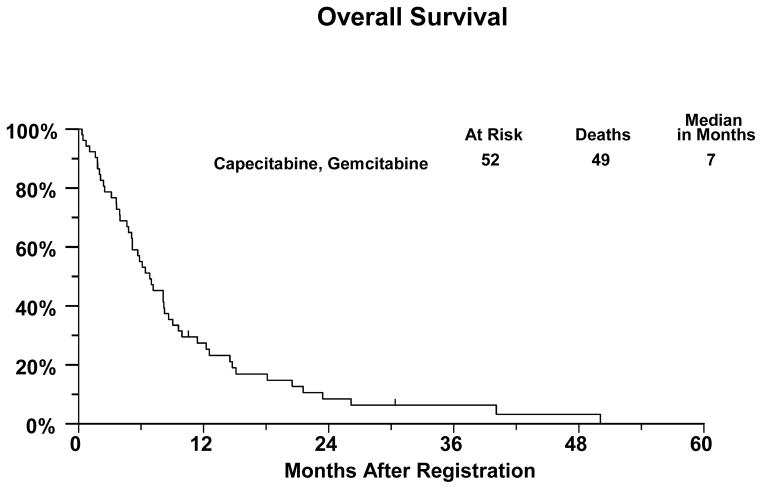
There were 25 eligible patients accrued in the first stage and 1 confirmed and two unconfirmed partial responses were observed, which met the initial objective of the study. The objective responses to the combination of gemcitabine and capecitabine for the entire trial are summarized in Table 3. There were 7 confirmed partial responses, for a confirmed response probability of 13% (95% CI: 6 to 26%). Six patients had unconfirmed partial responses for an overall response probability of 25% (95% CI: 14 to 39%). Forty-nine of the 52 eligible patients have died, with a median overall survival of 7 months (95% CI: 5 to 8 months). [Table 3 and Figure 1] Of the three patients last known to be alive, the median follow-up was 11 months.
Table 3.
Responses
| Complete Response | 0 |
| Partial Response | 7 (13%) |
| Unconfirmed Complete Response | 0 |
| Unconfirmed Partial Response | 6 (12%) |
| Stable Disease | 12 (23%) |
| Progressive Disease | 15 (29%) |
| Symptomatic Deterioration | 3 (6%) |
| Early Death | 1 (2%) |
| Inadequate Assessment | 8 (15%) |
Figure 1.
Kaplan-Meier Curve for overall survival in patients with unresectable or metastatic gallbladder cancer or cholangiocarcinoma treated with gemcitabine and capecitabine
Toxicity
Among the 51 patients evaluated for toxicity. The most frequent toxicities reported were grade 1 or 2 and included liver function abnormalities, diarrhea, fatigue nausea, vomiting and hematologic toxicity. The grade 3 toxicities included liver function abnormalities, fatigue, diarrhea, hand foot syndrome and hematologic toxicity. There were six patients who experienced grade 4 toxicities: one patient experienced grade 4 muscle pain and grade 4 thrombosis/embolism, one patient experienced grade 4 fatigue and 4 patients experienced grade 4 neutropenia, one of whom also experienced grade 4 leukopenia and grade 4 thrombocytopenia.
Fourteen patients were removed from protocol therapy due to adverse events: hematologic (5 patients), hepatic toxicities (4), fatigue (2), elevated alkaline phosphatase (2) and hand/foot syndrome (1). [Table 4] One patient should have been removed from protocol therapy for recurrence of neutropenia and thrombocytopenia after having been dose reduced twice.
Table 4.
Commonly Observed Toxicities (adverse events which were unlikely or not related to treatment were excluded)
| ADVERSE EVENT | Capecitabine, Gemcitabine (n=51) | |||||
|---|---|---|---|---|---|---|
| Grade
| ||||||
| 0 | 1 | 2 | 3 | 4 | 5 | |
|
|
|
|||||
| Blood/Bone Marrow | 6 | 7 | 13 | 21 | 4 | 0 |
| Cardiac Arrhythmia | 49 | 0 | 1 | 1 | 0 | 0 |
| Cardiac General | 48 | 1 | 2 | 0 | 0 | 0 |
| Constitutional symptoms | 11 | 21 | 11 | 7 | 1 | 0 |
| Dermatology/Skin | 24 | 11 | 12 | 4 | 0 | 0 |
| Gastrointestinal | 7 | 21 | 13 | 10 | 0 | 0 |
| Hemorrhage/Bleeding | 49 | 0 | 1 | 1 | 0 | 0 |
| Hepatobiliary/Pancreas | 47 | 0 | 4 | 0 | 0 | 0 |
| Infection | 44 | 0 | 5 | 2 | 0 | 0 |
| Lymphatics | 43 | 6 | 2 | 0 | 0 | 0 |
| Metabolic/Laboratory | 15 | 11 | 14 | 11 | 0 | 0 |
| Musculoskeletal/Soft Tissue | 46 | 0 | 4 | 1 | 0 | 0 |
| Neurology | 43 | 7 | 1 | 0 | 0 | 0 |
| Ocular/Visual | 48 | 3 | 0 | 0 | 0 | 0 |
| Pain | 30 | 10 | 8 | 2 | 1 | 0 |
| Pulmonary/Upper Respiratory | 45 | 4 | 2 | 0 | 0 | 0 |
| Renal/Genitourinary | 48 | 0 | 3 | 0 | 0 | 0 |
| Vascular | 48 | 0 | 2 | 0 | 1 | 0 |
| MAXIMUM GRADE ANY ADVERSE EVENT Number | 0 | 2 | 12 | 31 | 6 | 0 |
Biologic Markers
The SWOG Statistical Center received genotyping on 23 patients registered to this trial. One patient was ineligible and is not included in this analysis.
Of the 22 patients evaluated, one is last known to be alive, with follow-up time of 30 months. Median overall survival for this group was 7 months (95% confidence interval of 4 to 9 months. The characteristics of this subset of patients were consistent with those of the entire patient population of this study.
Three polymorphisms in the TS gene have been identified. TSER polymorphism, the promoter-enhancer region is a tandem repeat upstream of the TS translational start site and contains either double (2R) or triple (3R) repeats of 28-bp sequences. These have been found to be associated with the autoregulation of TS transcription and translation. Another functional variant within the 5′UTR region of the TS gene have been identified and the TS 2R/3R repeat is now studied together with a G to C single nucleotide polymorphism within the second repeat of the 3R allele (TSER 3R G/C). The TSER 3RC/3RC genotype caused lower transcriptional activity of TS comparable with the TS 2R/2R genotype. Another functional TS polymorphism is the 6-bp deletion/insertion within the 3′-UTR region of the TS gene. This has been shown to decrease RNA stability and influence TS mRNA and TS protein expression. 15,16
The TS 2R/3R repeat and the TSER 3R G/C were analyzed jointly and classified into TS 5′ UTR functional status as 5′UTR Low (2R/2R, 2R/3R(C), or 3R(C)/3R(C)), 5′UTR Intermediate (2R/3R(G) or 3R(C)/3R(G)) and 5′UTR High (3/R(G)/3R(G)) as classified by Lurje et al. 16
Other polymorphisms that have been associated with the pathways of these agents, MTHFR, RRMI and CDA, were also evaluated.
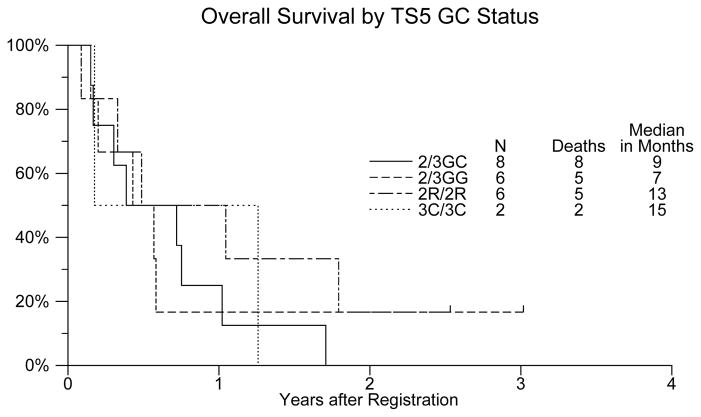
Given the small sample number and the limitations of such, overall survival in the TS 5′ GC combined group was reportedly the longest at 15 months for the 3R(C)/3R(C). [Table 5, Figure 2] There was no association of any of the polymorphisms with response rate. Further, of all the polymorphisms evaluated, this was the only one that correlated clinically.
Table 5.
Polymorphisms of genes in folic pathway and overall survival
| Genotype | Frequency (%) | Median Overall Survival in months (95% CI) | |
|---|---|---|---|
| TS 3′ | +/+ | 14/22 (64%) | 7 (4 – 13) |
| +/− | 6/22 (27%) | 7 (2–7) | |
| −/− | 2/22 (9%) | 9 (2 - n.r.) | |
| TS 5′ | 2R/2R | 6/22 (27%) | 13 (4 – 13) |
| 2R/3R | 14/22 (64%) | 7 (2–9) | |
| 3R/3R | 2/22 (9%) | 15 (2-n.r.) | |
| TS 5′ GC | 2R/2R | 6/22 (27%) | 13 (4–13) |
| 2R/3R(C) | 8/22 (36%) | 9 (2–9) | |
| 3R(C)/3R(C) | 2/22 (9%) | 15 (2-n.r.) | |
| 2R/3R(G) | 6/22 (27%) | 7 (2–7) | |
| 3R(G)/3R(C) | 0/22 (0%) | · | |
| 3R(G)/3R(G) | 0/22 (0%) | · | |
| TS 5′ Functional Significance | LOW | 16/22 (73%) | 9 (4–12) |
| INTERMEDIATE | 6/22 (27%) | 7 (2–7) | |
| HIGH | 0/22 (0%) | · | |
| MTHFR C677T | C/C | 11/22 (50%) | 6 (4–9) |
| C/T | 11/22 (50%) | 7 (2–13) | |
| T/T | 0/22 (0%) | · | |
| MTHFR A1298C | A/A | 11/22 (50%) | 7 (4–12) |
| A/C | 8/22 (36%) | 4 (2–6) | |
| C/C | 3/22 (14%) | 9 (5–9) | |
| RRMI G/A | G/G | 9/22 (41%) | 7 (4–7) |
| G/A | 10/22 (45%) | 9 (2–12) | |
| A/A | 3/22 (14%) | 5 (4–5) | |
| CDA A79C | A/A | 8/21 (38%) | 4 (2–5) |
| A/C | 12/21 (57%) | 7 (5–9) | |
| C/C | 1/21 (5%) | · | |
Figure 2.
Overall survival by TS5 status in patients with unresectable or metastatic gallbladder cancer or cholangiocarcinoma treated with gemcitabine and capecitabine
DISCUSSION
There is currently no standard regimen for the treatment of advanced biliary cancer. Most commonly, single-agent gemcitabine or 5FU is used, with occasional combinations (including gemcitabine plus platinums) being offered. In our trial, the combination of gemcitabine and capecitabine was well tolerated, with 7 confirmed responses and an additional 6 unconfirmed responses, giving an overall response probability of 25% and an overall survival of 7 months. Generally, the reported toxicities were hematologic and manageable.
There have been three other phase II studies reported using the combination of gemcitabine and capecitabine in patients with biliary tumors. All were single institution trials. Riechelmann and colleagues at Princess Margaret in Canada report on a total of 75 patients treated with gemcitabine and capecitabine for advanced biliary cancer, detailing a response rate of 29% and an overall survival of 12.7 months.17 A second study performed in South Korea with a total of 44 patients had a response rate of 32% and median overall survival of 14 months.18 In this trial, 23% of patients accrued had locally advanced disease and 16% of patients had cancer of the ampulla, both of which tend to have a better prognosis. A third trial from Roswell Park accrued a total of 12 patients over 2 years with a response rate of 16% (the lowest response rate reported of the three studies).19 In these studies, there did not appear to be any significant issues with toxicity.
In our study, the confirmed responses and overall survival appear to be lower than that which has been generally reported. The overall response rate is consistent with the larger of the 3 previously reports using this combination. Although our overall response rate was similar, this did not translate into a median overall survival of a year, as described in the other trials.
Our study was conducted through the cooperative group setting, which usually offers a patient selection more representative of the community than single institution trials. Cooperative group studies usually report efficacy results inferior to those seen in highly selected patients from single institution trials. Further, the patient characteristics in these trials differed, and the Korean trial potentially reported on a better prognostic group of patients, thereby resulting in a higher response rate and median overall survival. There may also be potential inherent differences in the Korean population, related to the unidentified genetic differences within these populations in terms of the natural course of the disease, response and tolerability to chemotherapy.
Other trials with gemcitabine-containing regimens have also been conducted, including combinations with docetaxel, oxaliplatin, cisplatin and carboplatin.20–22 Gemcitabine with oxaliplatin was reported by GERCOR, with the combination reporting a response rate of 33% and a median overall survival of 8.3 months.22 Other platinum containing regimens report 20 to 24% response rates and similar median overall survivals.20,21 The tolerability of these regimens vary. Most recently, Valle and colleagues reported a randomized phase II with 314 patients with advanced biliary cancer randomized to gemcitabine/cisplatin vs. gemcitabine alone. The median overall survival was greater with the combination of gemcitabine/cisplatin than the single agent, 11.7 vs. 8.2 months (p=0.002), as was progression free survival 8.5 vs. 6.5 months, (p=0.003). 23
Nevertheless the combination of gemcitabine and capecitabine is reasonable to consider in patients with metastatic or advanced biliary cancer. The study reached the primary objective with evaluation of confirmed response rates. The regimen was well tolerated with the most significant toxicities being hematologic, consistent with what has been reported with gemcitabine as a single agent. Furthermore, the gemcitabine was administered as a fixed dose rate infusion, which tends to cause more hematologic toxicity as well. Other more common grades 3 and 4 toxicities were related to liver function abnormalities, which may be a function of the patients underlying biliary disease. Fatigue was also more commonly reported. Given these clinical data, the combination of gemcitabine and capecitabine is a reasonable option in the treatment of patients with advanced biliary cancer.
Evaluation of the molecular correlates was limited due to the small number of samples and frequency of the polymorphisms. Although no clear associations or conclusions regarding outcome can be made, the longest overall survival was reported in the functional polymorphism TS 5′GC, which causes a lower transcriptional activity of TS, consistent with previous data with treatment with fluoropyrimidines. The data are limited but are interesting in that evaluation in larger clinical studies is warranted. The combination of gemcitabine and capecitabine is a reasonable approach to the treatment of patients with advanced biliary cancer, a disease that has limited treatment options. Further studies to evaluating chemotherapy should consider the role of novel targeted therapies in this disease process. Both EGFR1 and EGFR2 have been shown to be over-expressed, and combining chemotherapy with such agents may offer improved outcome. Other novel targets to consider in this disease include anti-angiogenic therapies. Clearly, there is a need for improved therapeutics in biliary cancer and this reported combination offers another option of a combination as a potential starting point upon which to build.
Acknowledgments
FUNDING
This work was supported in part by the following Public Health Service Cooperative Agreement grant numbers awarded by the National Cancer Institute, Department of Health and Human Services: CA32102, CA38926, CA20319, CA58882, CA45808, CA58723, CA35176, CA76132, CA46441, CA63844, CA35178, CA58658, CA67575, CA45560, CA11083, CA46113, CA35128, CA58861, CA46368, CA35261, CA58416, CA42777, CA35431; and supported in part by Roche
Footnotes
Clinical and correlative results presented in part at the 42rd Annual Meeting of the American Society of Clinical Oncology, June 2–6, 2006, Atlanta, GA
Disclaimer: None
ClinicalTrials.govIdentifier: NCT00033540
References
- 1.Cancer Facts & Figures. Atlanta, GA: American Cancer Society, Inc; 2008. [Google Scholar]
- 2.Serra I, Calvo A, Baez S, et al. Risk factors for gallbladder cancer.An international collaborative case-control study. Cancer. 1996;78:1515–7. [PubMed] [Google Scholar]
- 3.Falkson G, MacIntyre JM, Moertel CG. Eastern Cooperative Oncology Group experience with chemotherapy for inoperable gallbladder and bile duct cancer. Cancer. 1984;54:965–9. doi: 10.1002/1097-0142(19840915)54:6<965::aid-cncr2820540603>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 4.Takada T, Kato H, Matsushiro T, et al. Prospective randomized trial comparing 1/2 FAM (5-fluorouracil (5-FU) + adriamycin + mitomycin C) versus palliative therapy for the treatment of unresectable pancreatic and biliary tract carcinomas (the 2nd trial in non-resectable patients) Japanese Study Group of Surgical Adjuvant Therapy for Carcinomas of the Pancreas and Biliary Tract Gan To Kagaku Ryoho. 1996;23:707–14. [PubMed] [Google Scholar]
- 5.Kajanti M, Pyrhonen S. Epirubicin-sequential methotrexate-5-fluorouracil-leucovorin treatment in advanced cancer of the extrahepatic biliary system. A phase II study. Am J Clin Oncol. 1994;17:223–6. doi: 10.1097/00000421-199406000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Patt YZ, Jones DV, Jr, Hoque A, et al. Phase II trial of intravenous flourouracil and subcutaneous interferon alfa-2b for biliary tract cancer. J Clin Oncol. 1996;14:2311–5. doi: 10.1200/JCO.1996.14.8.2311. [DOI] [PubMed] [Google Scholar]
- 7.Sanz-Altamira PM, Ferrante K, Jenkins RL, et al. A phase II trial of 5-fluorouracil, leucovorin, and carboplatin in patients with unresectable biliary tree carcinoma. Cancer. 1998;82:2321–5. [PubMed] [Google Scholar]
- 8.Verderame F, Mandina P, Abruzzo F, et al. Biliary tract cancer: our experience with gemcitabine treatment. Anticancer Drugs. 2000;11:707–8. doi: 10.1097/00001813-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Castro MP. Efficacy of gemcitabine in the treatment of patients with gallbladder carcinoma: a case report. Cancer. 1998;82:639–41. doi: 10.1002/(sici)1097-0142(19980215)82:4<639::aid-cncr4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 10.Eng C, Ramanathan RK, Wong MK, et al. A Phase II trial of fixed dose rate gemcitabine in patients with advanced biliary tree carcinoma. Am J Clin Oncol. 2004;27:565–9. doi: 10.1097/01.coc.0000135924.94955.16. [DOI] [PubMed] [Google Scholar]
- 11.Network NCC. NCCN Practice Guidelines in Oncology: Hepatobiliary Cancers. 2008. (ed V.2.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer. 2007;96:896–902. doi: 10.1038/sj.bjc.6603648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braakhuis BJ, Ruiz van Haperen VW, Boven E, et al. Schedule-dependent antitumor effect of gemcitabine in in vivo model system. Semin Oncol. 1995;22:42–6. [PubMed] [Google Scholar]
- 14.Tempero M, Plunkett W, Ruiz Van Haperen V, et al. Randomized phase II comparison of dose-intense gemcitabine: thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. J Clin Oncol. 2003;21:3402–8. doi: 10.1200/JCO.2003.09.140. [DOI] [PubMed] [Google Scholar]
- 15.Mandola MV, Stoehlmacher J, Zhang W, et al. A 6 bp polymorphism in the thymidylate synthase gene causes message instability and is associated with decreased intratumoral TS mRNA levels. Pharmacogenetics. 2004;14:319–27. doi: 10.1097/00008571-200405000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Lurje G, Zhang W, Yang D, et al. Thymidylate synthase haplotype is associated with tumor recurrence in stage II and stage III colon cancer. Pharmacogenet Genomics. 2008;18:161–8. doi: 10.1097/FPC.0b013e3282f4aea6. [DOI] [PubMed] [Google Scholar]
- 17.Riechelmann RP, Townsley CA, Chin SN, et al. Expanded phase II trial of gemcitabine and capecitabine for advanced biliary cancer. Cancer. 2007;110:1307–12. doi: 10.1002/cncr.22902. [DOI] [PubMed] [Google Scholar]
- 18.Cho JY, Paik YH, Chang YS, et al. Capecitabine combined with gemcitabine (CapGem) as first-line treatment in patients with advanced/metastatic biliary tract carcinoma. Cancer. 2005;104:2753–8. doi: 10.1002/cncr.21591. [DOI] [PubMed] [Google Scholar]
- 19.Iyer RV, Gibbs J, Kuvshinoff B, et al. A phase II study of gemcitabine and capecitabine in advanced cholangiocarcinoma and carcinoma of the gallbladder: a single-institution prospective study. Ann Surg Oncol. 2007;14:3202–9. doi: 10.1245/s10434-007-9539-9. [DOI] [PubMed] [Google Scholar]
- 20.Kim ST, Park JO, Lee J, et al. A Phase II study of gemcitabine and cisplatin in advanced biliary tract cancer. Cancer. 2006;106:1339–46. doi: 10.1002/cncr.21741. [DOI] [PubMed] [Google Scholar]
- 21.Julka PK, Puri T, Rath GK. A phase II study of gemcitabine and carboplatin combination chemotherapy in gallbladder carcinoma. Hepatobiliary Pancreat Dis Int. 2006;5:110–4. [PubMed] [Google Scholar]
- 22.Andre T, Reyes-Vidal JM, Fartoux L, et al. An international multicenter phase II trial of gemcitabine and oxaliplatin (GEMOX) in patients with advanced biliary tract cancer. Journal of Clinical Oncology, ASCO Annual Meeting Proceedings Part I. 2006 Jun 20;24(18S):4135. Supplement. [Google Scholar]
- 23.Valle JW, Wasan HS, Palmer DD, et al. Gemcitabine with or without cisplatin in patients (pts) with advanced or metastatic biliary tract cancer (ABC): Results of a multicenter randomized phase III trial (the UK ABC-02 trial). J Clin Oncol; 2009; Annual Meeting Proceedings; Chicago. 2009. [Google Scholar]