SUMMARY
Huntington’s disease (HD) is an autosomal dominant progressive neurodegenerative disorder that typically begins in middle adulthood. The neurodegenerative process that underlies HD, however, likely begins many years before clinical diagnosis. Since genetic testing can identify individuals that will develop HD during this preclinical period, clinical trials aiming to slow disease progression will likely focus on this phase of the illness in an effort to delay disease onset. How to best measure the efficacy of potential disease-modifying therapies in preclinical HD remains a complex challenge. This article will review the clinical and imaging measures that have been assessed as potential markers of disease progression in preclinical and early symptomatic HD.
Huntington’s disease (HD) is an autosomal dominant neurodegenerative disorder that progresses inexorably to death, usually over a period of 10–30 years. HD by definition refers to a clinically manifest disorder that is characterized by motor, psychiatric and cognitive signs and symptoms, but the neurodegenerative process underlying these manifestations begins many years before clinical onset. In fact, the neurodegenerative process in a person with the HD gene mutation likely spans an average of approximately 40 years, comprised of 20 years before clear symptom onset (preclinical HD [pHD]) and 20 years with the progressively worsening clinical disorder.
Current therapies for HD target the signs and symptoms of the disorder but fail to alter the long-term course of the disease. Although better symptomatic therapies are needed, interventions to slow or halt the neurodegenerative process could have an even greater impact on the long-term outcomes for HD patients. In fact, the experimental therapeutics of HD have focused on potential disease-modifying therapies for several years, and numerous ongoing long-term clinical trials seek to identify therapies that can slow the progression of HD. For example, two current trials are evaluating whether the nutritional supplements coenzyme Q10 (the 2CARE trial) or creatine (the CREST-E trial) can alter the progression of mild-to-moderate HD. However, given that HD gene mutation carriers can be identified years before clinical onset through genetic testing, a natural outgrowth of this work would be to study potential disease-modifying therapies in preclinical gene mutation carriers. Nonetheless, performing clinical trials in a group of clinically normal individuals may be problematic for several reasons. One major difficulty is defining the best outcome measure for use in such trials. Currently, clinical trials in HD utilize clinical outcome measures such as the Unified Huntington’s Disease Rating Scale (UHDRS), but these measures are designed to detect changes in HD patients with manifest signs and symptoms, and may not be useful in clinically unaffected individuals. Measuring phenoconversion (i.e., progressing from pHD to diagnosed HD) as an outcome measure may be impractical as subjects in clinical trials may be many years from developing unequivocal signs of HD. This article will review the clinical and imaging methods that may be useful in monitoring disease progression in pHD and early manifest HD.
Clinical changes in pHD
Subtle clinical changes may occur in pHD years before the severity of these changes rises to the level of a confirmed clinical diagnosis. Since the HD gene mutation was identified in 1993, many studies have examined the nature of the clinical changes that occur in pHD, providing a picture of how the clinical manifestations of HD evolve from apparently nonspecific mild abnormalities to overt clinical signs of the disease.
Motor
Several studies have documented subtle changes in motor functioning predating a formal diagnosis of HD. In general, although these studies demonstrate that abnormalities can be detected and are more likely to be present as individuals approach a confirmed diagnosis [1], the variability in motor functioning, both within and between individuals, make assessments of motor function an impractical way of monitoring disease progression in pHD.
Motor abnormalities, including chorea and dystonia, oculomotor dysfunction and gait and balance changes, have been described in pHD. Subtle chorea and oculomotor abnormalities appear to be the most commonly detected changes [2,3]. The largest and most rigorous study of the motor features of pHD has been the PREDICT-HD study [1]. This ongoing multicenter study has examined the clinical features of 733 pHD subjects. For the whole cohort, the probability of clinical diagnosis within 5 years based upon CAG repeat length and age was 20%; this group had a mean UHDRS [4] motor score of five (the scale ranges from zero [normal] to a maximum of 120). Motor abnormalities were comprised of all domains of the UHDRS, including chorea, dystonia, rigidity, bradykinesia and oculomotor changes. In this cross-sectional report, subjects were divided into groups determined to be near (<9 years from predicted onset), mid (9–15 years from onset) or far (>15 years from onset) from clinical diagnosis (predicted years to onset [YTO] was determined using the CAG repeat length and current age of each subject and a published algorithm based upon actuarial data for HD clinical diagnosis [5]). All UHDRS domains increased with increasing proximity to diagnosis. The TRACK-HD study, another large observational study involving 117 premanifest HD gene carriers, has reported prospective longitudinal changes in pHD [6]. In this study, as in the PREDICT-HD study, when premanifest subjects are divided into near or far from predicted clinical onset (defined in this study as less than or greater than the mean YTO, respectively), those subjects that are relatively further along in the degenerative process (i.e., near) showed a greater increase in motor UHDRS scores. Nonetheless, for the premanifest group as a whole, there were no significant changes in motor UHDRS scores over the course of 24 months. Similar findings have been observed in smaller longitudinal progression studies in pHD. For example, in a cohort of 12 pHD subjects with a mean of ten predicted YTO, baseline mean UHDRS total motor score was 9.6 and correlated with YTO [7]. Nonetheless, the mean scores did not increase over almost 4 years of follow-up, likely because of the high rate of variability within and between subjects. In fact, this variability has been observed in all studies of motor features in pHD, including the very large Venezuela kindred [8,9]. This variability suggests that although motor abnormalities can be identified in pHD, following these changes over time may not be useful for monitoring progression in pHD. Nonetheless, it is possible that objective quantitative measures of motor function could have utility in pHD [10], although larger prospective longitudinal studies of such markers are needed. In this regard, it is worth noting that the TRACK-HD study does include quantified motor assessments such as speeded tapping, grip and tongue force variability, chorea position and orientation indices.
Cognition
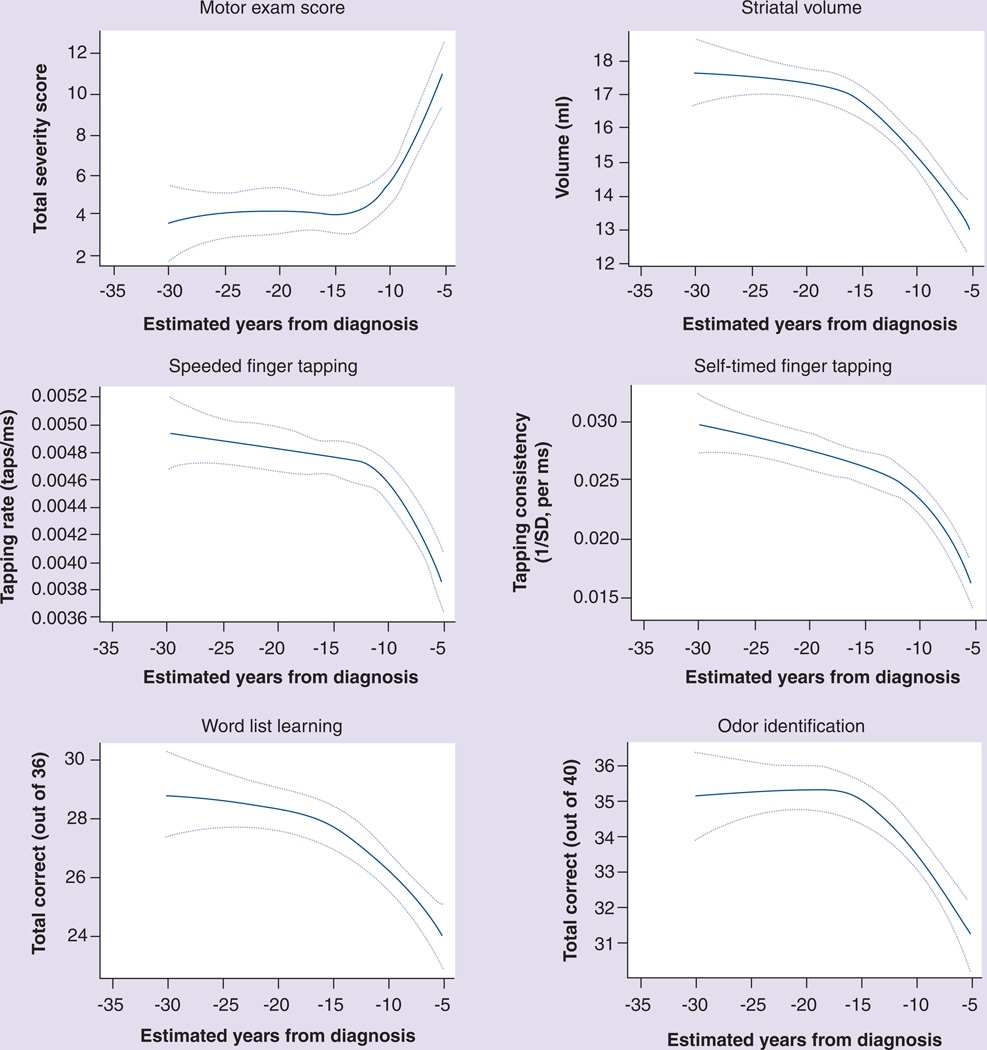
Cognitive impairment or dementia is a common feature of symptomatic HD and is perhaps the major cause of functional impairment and disability. The cognitive abnormalities of manifest HD are characterized primarily by executive dysfunction, including problems with planning and organization, flexibility and set shifting and procedural memory and attention [11]. Subclinical abnormalities of cognitive performance can also be detected many years before a clinical diagnosis is determined. The PREDICT-HD study has described impairment across multiple cognitive domains beginning 10–15 years before diagnosis (Figure 1) [12,13]. Other studies have found similar results [14–16]. Despite the presence of cognitive abnormalities in pHD, longitudinal progression studies, such as TRACK-HD, have failed to identify consistent progression in cognitive measures in this population [6]. As with motor deficits in pHD, the presence and severity of cognitive impairment in pHD is quite variable, suggesting that although psychometric testing batteries can be utilized to identify and measure change over time in pHD, using these measures in clinical trials may not be practical as primary outcome measures. For example, approximately a third of pHD individuals may meet the criteria for the diagnosis of mild cognitive impairment [13,17], suggesting that two-thirds do not. Enrollment of pHD subjects in a clinical trial could then potentially include many subjects without significant measurable cognitive abnormalities, thereby producing large degrees of variability in cognitive testing results and increasing the sample size needed to demonstrate efficacy of a potential new therapy. One way around this may be to screen potential subjects for a minimum amount of impairment before enrollment, but this may limit the generalizability of the results.
Figure 1. Relationship between estimated years to diagnosis of Huntington’s disease and various other measures in presymptomatic gene carriers.
On each plot, the solid line represents the predicted mean response and broken lines represent the 95% CIs for the mean.
Reproduced from [12] with permission from BMJ Publishing Group Ltd.
Behavior
The psychiatric manifestations of HD are among the most disabling features of the disease and can predate the diagnosis of HD by many years. In fact, several reports suggest that the behavioral aspects of the disease may be among the earliest manifestations, perhaps beginning as early as 20 years before onset [18,19]. The common psychiatric diagnoses in HD and pHD include depression and increased suicidality, irritability and agitation, anxiety, apathy and psychosis with paranoia [20]. Subtle behavioral changes that do not rise to the level of an overt psychiatric diagnosis are also common in pHD and early HD. Although behavioral and psychiatric symptoms can be identified in a majority of HD subjects, these signs and symptoms do not appear to progress (i.e., worsen) in a reliable fashion even in symptomatic HD [21]. Similarly, neither the PREDICT-HD nor TRACK-HD studies have identified psychiatric changes that consistently worsen over time in pHD. Given the variability of the presence and progression of behavioral problems in both pHD and HD, and the additional confound of effective symptomatic therapies (e.g., antidepressants and antipsychotics), it is unlikely that assessments of progression in behavioral measures will be useful as outcome measures for clinical trials of potential disease-modifying therapies. Nonetheless, behavioral and psychiatric manifestations of HD will continue to be important targets for novel symptomatic therapies in both pHD and HD.
Monitoring progression in early clinically manifest HD
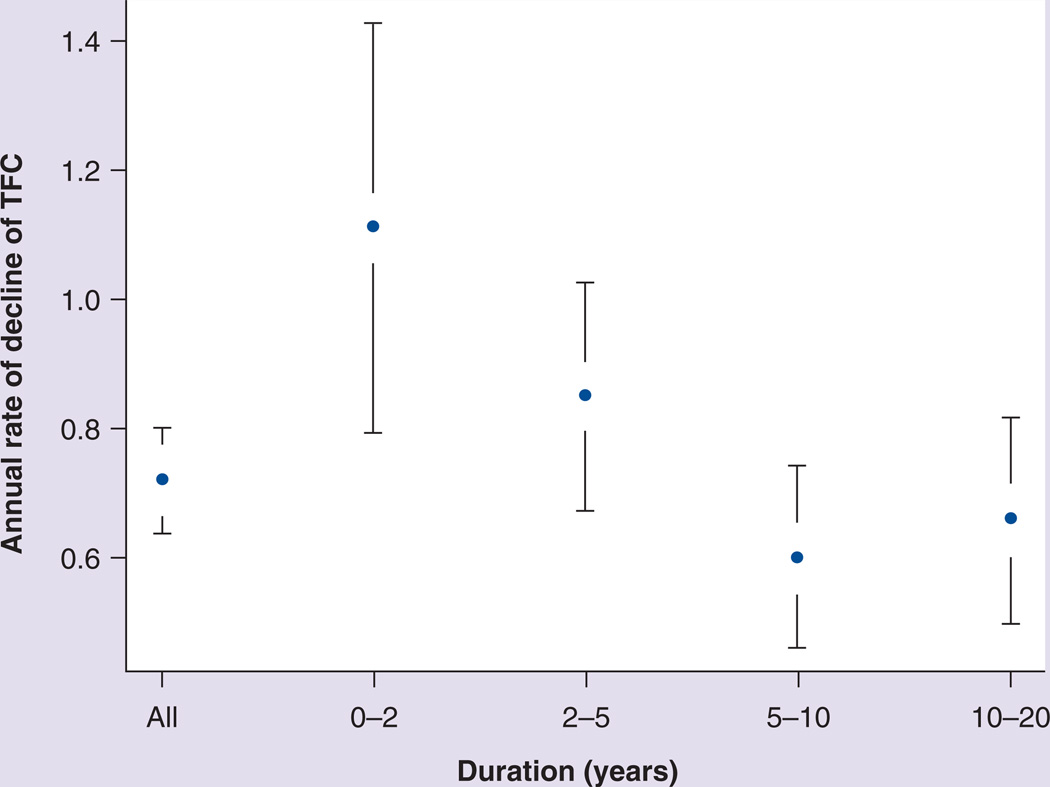
Many clinical trials have been conducted in individuals clinically affected with HD. Most of these trials have utilized the UHDRS [4], which includes four domains that measure motor, cognitive, behavioral and functional status. Individual items or portions of the UHDRS have also been utilized to focus on specific outcomes [22,23]. Many, but not all, of the UHDRS items have been shown to progress in a fairly predictable manner in early symptomatic HD. For example, the total functional capacity score declines at a rate of approximately one point per year in this group (Figure 2) [21], and all functional components of the UHDRS appear to progress in a linear fashion in early HD [24]. In addition, most of the motor items also progress in a linear fashion, while the behavioral and cognitive components may be less reliable as measures of progression [24].
Figure 2. Progressive decline of total functional capacity at varying points in the course of clinically manifest Huntington’s disease.
Bars represent the confidence intervals of the mean annual rates of decline.
TFC: Total functional capacity.
Reproduced with permission from [21].
Imaging
Although early signs of motor, cognitive and behavioral changes are present in pHD gene mutation carriers [12,18,25], these clinical measures appear to be less sensitive to disease progression than imaging biomarkers in the preclinical period [6,26,27]. Although new clinical measures could be devised that would be more sensitive to changes in pHD, especially for the period within 5–10 years of clinical disease onset, it appears that many pHD subjects spend much of the preclinical period of HD in a truly clinically normal state. To conduct clinical trials of potential disease-modifying therapies that include this group of subjects will likely require objective and quantitative measures of disease progression such as imaging [28]. In prior studies, imaging tools such as MRI and PET have been employed to assess localized changes in brain structure and function in preclinical and early symptomatic HD patients [29–39]. Recent longitudinal imaging studies have further shed light on the neural mechanisms of disease progression in HD gene carriers, especially the changes that occur in the transition between the preclinical period and the onset of symptoms [7,40].
Dopamine D2 receptor binding
Loss of striatal projection neurons is the primary neuropathological finding in HD [29] and can be demonstrated even in the earliest pathological stages [30]. Different neuroimaging methods have been employed to measure this aspect of the neurodegenerative process during the preclinical period of HD in an effort to provide a measure of progression and to predict clinical onset.
Dopamine D2 receptors are highly expressed in striatal medium spiny neurons. Dopamine signaling pathways play a key role in the pathogenesis of HD [41], and dopamine receptor alterations have been found to occur before neuronal death in transgenic mice models of HD [31,42]. Thus, [11C]raclopride (RAC) PET has been widely used to quantify striatal dopamine D2 receptor binding as a measure of projection neuron density in HD. Decreases in striatal dopamine D2 receptor binding have been documented in pHD [32,43], demonstrating early HD pathology in human HD mutation carriers before clinical onset [44]. Striatal RAC binding has also been found to decline over time as more neurons are lost in the striatum [32,43]; these changes in receptor binding have been shown to be associated with increased disease severity in clinically manifest HD [43,45]. A cross-sectional RAC PET study reported that striatal D2 receptor binding is likely to be more sensitive than assessments of regional metabolism in an assessment of early HD progression [46]. Furthermore, progressive loss of D2 receptors in the striatum has been consistently found in asymptomatic gene carriers [7,40,47] as well as in early symptomatic HD patients [48–50]. In a longitudinal PET study, striatal RAC binding was estimated to decline by an annual rate of approximately 2–3% during the preclinical period [7], which is in line with prior finding of progressive regional volume loss in pHD [51]. These results suggest that PET measures of RAC binding may have utility as a quantitative measure of disease progression in both pHD and clinical HD.
While much attention has been paid to the progressive loss of striatal D2 receptors in HD, there has been evidence showing that D2 receptor binding in extrastriatal regions, such as frontal and temporal areas, also declines as HD advances [52]. However, new data suggest that the loss of D2 receptor binding outside the striatum, although it progresses over time, is still mild in the early stage of HD [53]. Thus, unlike striatal RAC binding, extrastriatal binding may not be a useful marker in the assessment of disease progression in premanifest and early symptomatic HD. Moreover, it should be noted that most of the RAC imaging studies have had a small sample of HD subjects, often fewer than 20, and therefore require further validation in larger, prospective patient populations. An additional issue regarding RAC imaging is that it is less available than other techniques such as MRI, which potentially limits its use in large-scale clinical trials.
MRI
Structural MRI has been widely used as a direct measure of local neuronal loss in HD gene carriers to quantify volumetric changes in the striatum [45]. Ample evidence suggests that striatal volume loss begins more than a decade before predicted years to clinical onset and then progresses linearly in premanifest and early HD individuals [54–57]. In recent longitudinal studies, the rate of disease progression has been more accurately estimated by measuring atrophy rates in the caudate and putamen in pHD subjects relative to healthy controls [26,58,59]. Based on these data, volumetric measurements of striatal atrophy have been implicated as a useful marker for disease progression in pHD [45,60]. Based on longitudinal data, it has also been postulated that striatal volumetric loss may be used to predict HD phenoconversion for as many as 20 years before the onset of the disease [45]. These estimates, although very informative, require careful interpretation given that they were based on a small sample and have yet to be validated in prospective longitudinal studies. Moreover, the sensitivity of these regional MRI measures may suffer from several potential limitations: the low signal-to-noise ratio of local striatal measures causes relatively large inter- and intra-subject variation; and the estimated rates of striatal loss are relatively slow (i.e., insensitive to disease progression) due to the long HD preclinical period and a probable floor effect with advancing disease. Indeed, a new study found that the use of striatal atrophic measures would require a large number of subjects (~650 in each of the treatment and placebo arms) to detect a 30% change of the progression rate in a 2-year clinical trial for pHD [58], likely owing to a relatively greater variability of the regional data. In addition, it has also been reported that abnormal changes in striatal dopamine D2 receptor binding and metabolic activity may precede volumetric loss in this region in pHD gene carriers [7,46].
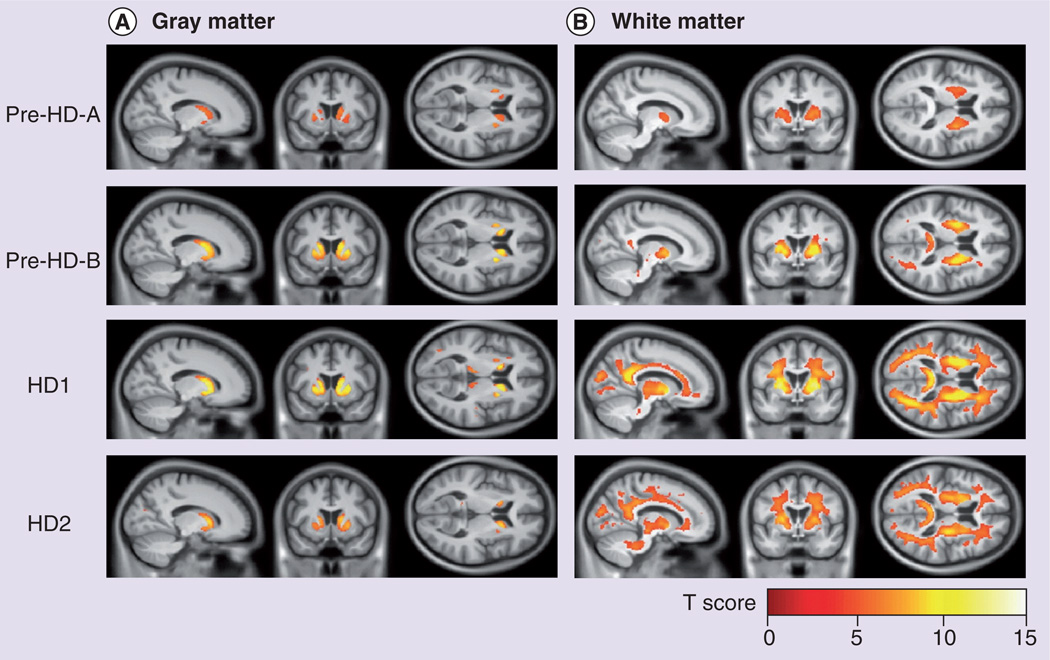
Despite the fact that the earliest and most profound histopathological changes in HD occur in the striatum, striatal measures fail to capture the extensive pathological and functional changes outside the striatum in HD. Widespread cortical and subcortical neurodegeneration has been documented outside the striatum in early disease stages [61–63]. Structural MRI has been used to measure these extrastriatal changes in pHD [61,64], which have been found to be associated with early cognitive dysfunction and motor impairment in premanifest gene carriers [65,66]. It was also reported that white matter volume loss could be detected even before the appearance of gray matter atrophy and correlated with predicted YTO in pHD subjects [67]. Furthermore, recent longitudinal data from the TRACK-HD study has revealed that atrophic loss of white matter, particularly in the frontal and posterior lobes, correlated with disease progression in prodromal and early HD subjects (Figure 3) [26,58]. Interestingly, although the striatum is the most profoundly affected structure in HD, some of the extrastriatal measures appear to be more sensitive to progression in both premanifest and manifest HD subjects [27], thus potentially requiring a smaller sample size in a case–control clinical trial [58].
Figure 3. MRI volumetric changes in preclinical and early symptomatic Huntington’s disease.
Statistical parametric maps showing regions in which (A) gray matter and (B) white matter atrophy rates in the presymptomatic (pre-HD-A and pre-HD-B groups) and early symptomatic (HD1 and HD2 groups) HD patients were significantly different from the healthy control group (family-wise error at p < 0.05 level).
HD: Huntington’s disease.
Reproduced from [26] © 2011 with permission from Elsevier.
In addition to structural MRI, new advances in other MRI techniques in the last decade have allowed for the development of alternative measures in the assessment of progression in HD. Studies with diffusion-weighted imaging showed abnormally increased diffusivity in the caudate and putamen in premanifest [68] and early symptomatic HD [39]. Nevertheless, a 2-year longitudinal study with both diffusion-weighted imaging and volumetric MRI found that the diffusivity may be less sensitive than volume loss in the assessment of striatal degeneration in early HD [69]. Furthermore, diffusion-tensor imaging studies have revealed significant changes in fractional anisotropy in subcortical and cortical white matter in pHD and HD [38,70], associated with cognitive performance in individual subjects [71,72]. Longitudinal changes in white matter fractional anisotropy have also been reported in a diffusion-tensor imaging study in which HD mutation carriers were followed over a 1-year period [73]. Interestingly, data have suggested that microstructural changes in white matter may be present and detectable before volume loss in the progression of early HD [13].
Abnormal iron accumulation in the brain has been implicated in the pathogenesis of HD. An MRI study found increased iron concentrations in the caudate and putamen and decreased levels in the frontal lobe white matter in HD patients, associated with concurrent tissue loss in these regions [74]. The latest study with susceptibility- weighted imaging MRI further revealed, importantly, that the increases in striatal iron levels begin years before symptom onset and continue in symptomatic subjects, while the increases in cortical areas are present only in the symptomatic stages of HD [75]. These findings, along with the animal data that clioquinol treatment improved the pathologic and behavioral measures of transgenic Huntington’s mice [76], suggest a novel therapeutic strategy of using metal-binding compounds to normalize brain iron levels and the use of susceptibility-weighted imaging MRI as a potential biomarker for the evaluation of treatment effects in pHD.
Imaging of regional brain activity has the potential advantage of capturing the functional changes associated with HD cellular dysfunction prior to cell death and volume loss. Recent studies using an arterial-spin labeling MRI technique to evaluate alterations in regional cerebral blood flow (rCBF) found that premanifest HD subjects showed decreased rCBF in the prefrontal cortex and the putamen and increased rCBF in the precuneus and the hippocampus [77]. In early symptomatic HD patients, rCBF reductions were further detected in the caudate and in the sensorimotor, paracentral, temporal and occipital cortical regions, which were found to be associated with cognitive dysfunction in these subjects [78]. These results suggest that quantifying abnormalities of brain function may provide a unique and sensitive method for assessing potential disease-modifying therapies in pHD and early HD.
Metabolism & brain networks
Functional imaging of cerebral blood flow with H2 15O PET has also played an important role in investigating the pathophysiology of HD as well as the mechanisms of cognitive abnormalities in pHD gene mutation carriers [79,80]. By quantifying brain activation during impaired motor sequence learning in pHD subjects, it was found that activation responses were abnormally elevated in the thalamus and orbitofrontal cortex. These data suggest that enhanced activation of thalamocortical pathways during motor learning may compensate for caudate degeneration in HD gene carriers prior to the onset of clinical symptoms. Nonetheless, this compensatory mechanism may not be sufficient to maintain normal performance of motor learning during the preclinical period of the disease [80].
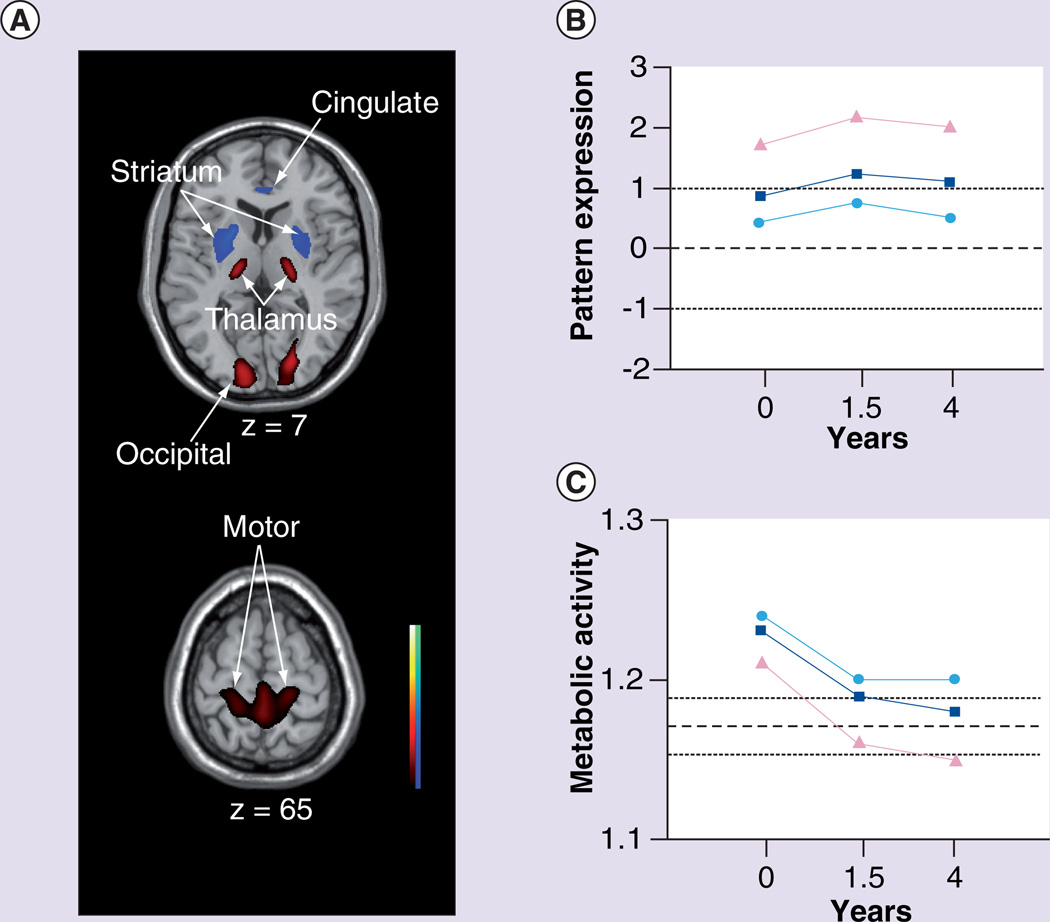
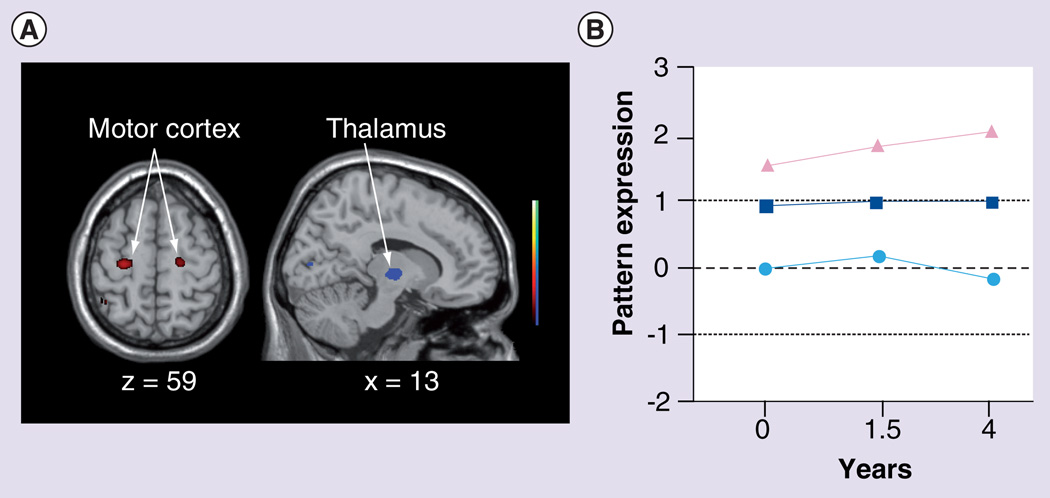
The role of thalamic compensation in pHD was further substantiated in a subsequent longitudinal imaging study of HD gene carriers [7]. In this study, PET imaging with [18F]fluorodeoxyglucose (FDG) and RAC was utilized to study 12 pHD subjects at baseline and again at 1.5 and 4 years. Using a cross-sectional network modeling approach based on principal component analysis (see [81] for a review), the FDG PET data was analyzed in the combined group of pHD carriers at baseline and 12 age-matched healthy control subjects. Network modeling identified a specific HD-related spatial covariance pattern (HDRP). This pattern is characterized by reductions in striatal and anterior cingulate metabolic activity, covarying with metabolic increases in the ventrolateral thalamus, cerebellum/dentate nucleus and in the primary motor cortex (Figure 4A). The spatial topography of the HDRP network provided validation for a previously identified metabolic network derived utilizing a different method of analysis and a separate population of pHD subjects [33]. HDRP network activity was abnormally elevated in the pHD cohort, with higher scores in the pHD subjects who subsequently developed unequivocal clinical signs of HD within the 4 years of follow-up (Figure 4B); in addition, individual subject HDRP expression correlated with estimated YTO [5]. Thus, elevated HDRP expression in pHD subjects implies closer proximity to symptom onset.
Figure 4. The Huntington’s disease-related metabolic pattern.
(A) This pattern was identified by network analysis of baseline [18F]fluorodeoxyglucose PET scans from 12 presymptomatic Huntington’s disease (HD) gene carriers and 12 age-matched healthy volunteer subjects. The pattern was characterized by metabolic decreases in the striatum and cingulate cortex associated with relative increases in the ventral thalamus, motor cortex and occipital lobe. Subject scores for this pattern discriminated the preclinical HD (pHD) subjects from the controls (p < 0.01). The display represents voxels that contribute significantly to the network at p < 0.005. Voxels with positive region weights (metabolic increases) are color coded from red to yellow; those with negative region weights (metabolic decreases) are color coded from blue to purple. (B) Mean HD-related metabolic pattern activity in the pHD group (squares) at baseline, and at the 1.5 and 4 year follow-up visits. Mean network activity is represented separately for the four pHD subjects (triangles) who subsequently developed symptoms, and for the eight remaining pHD subjects (circles) who had not phenoconverted by the third time point. Mean HD-related metabolic pattern activity is abnormally elevated at all time points, although a significant decline (p < 0.05) in pattern expression was evident between 1.5 and 4 years. The broken and dotted lines represent the mean ± standard deviation for the 12 healthy control subjects. (C) The pHD subjects demonstrated an elevation of thalamic metabolism at baseline (p < 0.05), which was more pronounced in the subgroup who did not phenoconvert during the follow-up period (circles). Elevated thalamic metabolism persisted in these nonconverters. Although elevations in thalamic metabolism were also present in the baseline scans of those subjects who subsequently phenoconverted (triangles), this local metabolic response declined to subnormal levels over time in this subgroup. The broken and dotted lines represent the mean ± standard error for the 12 healthy control subjects.
(A & C) Reproduced from [7] with permission from Oxford University Press.
Longitudinal analysis revealed a significant increase in HDRP expression from baseline to 1.5 years and a subsequent decrease from 1.5 to 4 years, showing a tendency to decline after reaching a peak value (Figure 4B). In this study, multitracer PET data was used to examine the relationship between the longitudinal changes in HDRP expression and concurrent declines in caudate and putamen RAC binding. Despite a significant correlation between RAC binding and local glucose utilization in the striatum, the longitudinal changes in network activity did not correlate with the declines in striatal RAC binding in the same pHD subjects. Therefore, the longitudinal changes in HDRP expression, demonstrated with FDG PET, are likely to represent a unique feature of disease progression that differs from the focal loss of striatal projection neurons measured by RAC PET. This disassociation between longitudinal changes in the two imaging measures suggests that the HDRP topography encompasses local striatal pathology as well as functional changes occurring downstream in striato-pallido-thalamic-cortical pathways [82].
The nonlinear trajectory of HDRP progression in the preclinical period raises the possibility that adaptive or compensatory processes are involved prior to symptom onset. These compensatory processes could be viewed as targets for new therapies (i.e., methods to enhance compensatory mechanisms in relatively normally functioning brain regions could ameliorate symptoms of HD) or they could be viewed as complicating imaging measures of disease progression. In any event, to explore the possibility that compensatory brain changes were altering the measurements of HD brain network progression, the time course of changes in regional metabolism within key nodes of the HDRP network was analyzed. Striatal activity was found to be significantly reduced throughout the follow-up period with relatively greater declines in the pHD subjects who were subsequently observed to become clinically manifest (phenoconversion) during the 4 years of follow-up. Interestingly, elevations in thalamic metabolism were evident in the baseline scans of the pHD subjects (Figure 4C). This is consistent with the earlier finding that activation in the thalamus was abnormally elevated during impaired motor sequence learning in pHD subjects [80]. A progressive loss of this compensatory thalamic response (i.e., declining thalamic metabolism to subnormal levels) was found in the pHD subjects who proceeded to phenoconvert (Figure 4C). This association of symptom onset with thalamic hypometabolism is likely attributed to the loss of inhibitory pallidothalamic output and consequent increases in thalamocortical excitatory activity [83]. Notably, the observed longitudinal decrease in thalamic metabolism remained significant even after correction for atrophy based upon concurrent MRI data acquired from the same subjects. Therefore, thalamic hypometabolism related to symptom onset is independent from, and likely to precede, volume loss in this region that was observed in early symptomatic HD [26]. Thalamic atrophy was found to be linked to the impairment of executive function [34]. That said, it remains a controversial issue whether thalamic volume loss can be reliably detected in early HD given that minimal thalamic atrophy has been reported in other MRI studies [84,85].
At the network level, symptom onset was also found to be associated with the decline in HDRP expression, which is linked to the loss of compensation of the thalamic node in this network as the disease advances to early symptomatic stage. Because of its curvilinear trajectory with an initial increase followed by subsequent decline, the HDRP is althought to be a compensatory network during the preclinical phase of HD. These findings suggest a role of functional metabolic networks as useful biomarkers of disease progression in the preclinical period. Quantitative assessment of the integrity of compensatory functional pathways with the HDRP could potentially improve the accuracy of actuarial predictions of clinical onset, although further investigation is needed to validate this network before its application in clinical trials of HD.
The identification of compensatory changes in brain function raises the possibility of identifying brain networks that underlie the clinical manifestations of HD distinct from brain changes that purely reflect disease progression. Such measures could also be useful for assessing novel symptomatic therapies. To this purpose, we have attempted to identify spatial covariance patterns that are expressed specifically in newly symptomatic HD patients but not in pHD subjects. We performed a network analysis on the longitudinal pHD data at 4 years of follow-up, which was comprised of FDG PET scans from four members of the original pHD cohort who had phenoconverted by this time point, and six others who remained presymptomatic. We identified a candidate phenoconversion pattern (Figure 5A) characterized by metabolic increases in primary motor and premotor cortex associated with relative reductions in the ventral thalamus [86]. At the 4-year time point, the expression of this pattern was elevated in the early symptomatic patients as compared with the healthy controls and the nonphenoconverted pHD subjects (Figure 5B). In this cohort, subject scores for this pattern correlated significantly with UHDRS motor ratings. Retrospective quantification of this pattern in the scans of these subjects at baseline and 1.5 years revealed that network activity was already elevated in the four phenoconverters at the times when they were considered to be presymptomatic on clinical grounds.
Figure 5. The Huntington’s disease symptom-related metabolic pattern.
(A) This pattern was identified by network analysis of [18F]fluorodeoxyglucose PET scans from the ten original preclinical Huntington’s disease subjects who returned for follow-up at 4 years, including four subjects who had phenoconverted by this time point and six others who remained presymptomatic. This candidate pattern was characterized by metabolic increases in primary motor and premotor regions associated with relative decreases in the ventral thalamus. The display represents voxels that contribute significantly to the network at p < 0.01. Voxels with positive region weights (metabolic increases) are color coded from red to yellow; those with negative region weights (metabolic decreases) are color coded from blue to purple. (B) Subject scores for this pattern were elevated in the four subjects who had phenoconverted by 4 years (triangles). Elevations in network activity were also present in the baseline and 1.5-year scans of these subjects. By contrast, network values for the remaining nonphenoconverters (circles) were in the normal range at all three time points. Squares represent the mean of all preclinical Huntington’s disease subjects at the three time points. The broken and dotted lines represent the mean ± standard deviation for the 12 healthy control subjects.
Electrophysiological data have revealed the presence of abnormal motor cortex plasticity in pHD [87]. However, in contrast to our observation on the role of the thalamus (a key node in the symptom-related network) in the manifestation of HD, this abnormality may not be associated directly with the emergence of motor signs and symptoms in HD [88]. In the current study, given the small number of early symptomatic patients available at the third time point and their very mild motor manifestations, the candidate pattern in its current form is unlikely to be used as a robust imaging marker of HD symptoms to assess response to symptomatic therapy. Additional gene-positive early symptomatic subjects, if added in the network analysis, may help to capture a larger proportion of data variability associated with emerging motor manifestations. This may allow for identification of a more significant symptom-related network marker specifically related to phenotype, resulting in a more accurate discrimination between symptomatic HD patients and clinically unaffected pHD carriers. It is conceivable that, in contrast to the compensatory HDRP expression that declines after reaching a peak value before symptom onset, the activity of the symptom-related pattern continues to rise as pHD gene carriers approach phenoconversion and as symptoms worsen in the early clinical phase of the disease. Such a network marker may also have practical value as a means of objectively assessing the effects of new therapies for HD motor symptoms.
Novel network assessments
New theoretical advances in multivariate analysis have allowed for substantial improvements in the predictive accuracy of network quantification approaches. Specifically, the ordinal trends (OrT) model can be used in longitudinal imaging data to identify a special class of progression networks [89]. Rather than detecting covariance patterns with increasing (or decreasing) activity at the group level, this novel approach specifies networks in which these changes are present at the individual subject level. In other words, using nonparametric permutation approaches [89, 90], the OrT analysis seeks trends in the progression of network activity that appear in most, if not all, individual members of the cohort. The resulting OrT patterns based on the longitudinal changes within the same subjects are likely to be more specifically related to disease progression than other patterns derived from traditional network analysis of cross-sectional data, which primarily separate the subjects from healthy controls. Moreover, by substantially reducing the large variability of imaging data from individual regions, the OrT network reflects co-varying changes across regions and is likely to be a superior measure of disease progression over regional imaging measures. For those reasons, the rigorous OrT computational approach is especially useful in longitudinal studies of neurodegenerative processes such as HD in which the assumption of monotonicity is reasonable on biological grounds.
In an ongoing investigation, we have applied the OrT analysis to FDG PET scan data across multiple time points from pHD subjects to assess longitudinal changes in metabolic activity at the network level [86]. Activity of the resulting metabolic network (referred to as HD progression pattern) increases predictably over time in both pHD and symptomatic HD, and correlates with predicted YTO based upon CAG repeat length and age. Our preliminary data suggest that the application of this pattern to longitudinal FDG PET scans has the potential to detect significant brain dysfunction more than a decade before HD can be clinically diagnosed. Thus, this functional measure of metabolic abnormalities in HD has great potential as an objective means of assessing new neuroprotective therapies in pHD. This novel network approach may also have the advantage of substantially reducing the number of subjects needed for clinical trials of new therapies aimed at slowing progression of HD.
Conclusion
As disease-modifying therapies are sought for neurodegenerative disorders, novel biomarkers are needed to assess these therapies. In HD, individuals that will ultimately develop HD can be identified through genetic testing many years before clinical onset, raising the prospect of delaying or forestalling disease onset. Although reliable and valid clinical measures, such as the UHDRS, are available for studying therapies in clinically manifest HD subjects, existing clinical measures are not likely to be very useful for demonstrating disease modification in pHD. Imaging, however, has shown great promise for detecting abnormalities in pHD and for measuring progression over time even in the absence of clinical findings. It is noted that the estimated sample size based on imaging data for future clinical trials varies between studies, likely due to the differences in imaging methodology, study design, subject characteristics and other factors. That said, the use of validated imaging measures as biomarkers, in addition to the primary clinical end points, may reduce the number of subjects needed for clinical trials and improve the cost efficiency and chance for success of these studies. Nonetheless, the utilization of imaging as an outcome measure in clinical trials will need to be undertaken with caution. In Parkinson’s disease, the use of imaging measures (e.g., dopamine transporter ligands or fluorodopa) in clinical trials has demonstrated that many of the same confounds that apply to clinical measures (e.g., unknown effects of drugs on the outcome measure and variability between sites) may also apply to imaging measures [91]. To truly validate, new outcome measures will require their use initially as adjunctive measures in clinical trials, and this will likely occur with imaging measures in future HD clinical trials. Finally, utilizing a combination of clinical, imaging and biological measures (e.g., CAG repeat length and other cerebrospinal fluid or blood measures [92]) may lead to a composite biomarker that can be used to accurately predict disease onset or rate of progression.
Future perspective
Many people that carry the genetic mutation for HD, but are not yet affected by the disease, function at a normal level, especially when they are many years from the expected time of onset based upon actuarial data. In fact, these individuals may achieve high levels of success in all aspects of professional and personal life. Nonetheless, pathological changes predate the onset of overt clinical signs of HD by many years and are often accompanied by subtle clinical changes. Whether these changes begin at some point during the individual’s life or actually during early developmental periods remains unknown. Certainly, studying the progression of an underlying neurodegenerative process in individuals at risk for HD raises many challenging ethical and scientific questions. Nonetheless, it is likely that over the next 5–10 years there will be new attempts to slow HD progression and delay or forestall disease onset in this population. These clinical trials will likely involve a combination of clinical, imaging and perhaps other biological outcome measures. Although much data has been collected in longitudinal observational studies in an effort to prepare for these clinical trials, it is likely that the data collected during these trials will have an even greater impact on the selection and design of future outcome measures.
Practice Points.
-
▪
Huntington’s disease (HD) is an autosomal dominant neurodegenerative disorder that usually starts in mid-adult life.
-
▪
The clinical disease progresses to death over an average of 20 years.
-
▪
Genetic testing can identify gene mutation carriers years before disease onset, suggesting the prospect that potential disease-modifying therapies could be used in preclinical HD to delay or forestall disease onset.
-
▪
Many clinical trials have been conducted in clinically affected HD patients.
-
▪
The goals of these trials have been to develop better symptomatic therapies or to identify therapies to slow disease progression.
-
▪
Since these trials have been conducted in clinically manifest HD patients, clinical outcome measures such as the Unified Huntington’s Disease Rating Scale have been utilized.
-
▪
Future clinical trials will likely focus on preclinical HD gene mutation carriers prompting the need for novel outcome measures.
-
▪
Brain imaging with MRI and PET consistently demonstrates abnormalities in preclinical and early HD.
-
▪
Changes in regional brain volumes, dopamine D2 receptor binding and abnormal brain network expression can be measured over time in preclinical HD, and these changes correlate with predicted years to disease onset.
-
▪
These imaging measures may have utility for measuring the efficacy of potential disease-modifying agents in preclinical HD.
-
▪
Future clinical trials to assess novel therapies aimed at slowing disease progression and delaying disease onset in preclinical carriers of the HD gene mutation will likely utilize a combination of clinical and imaging outcome measures.
-
▪
The exact measures that are used will likely evolve as more data is collected during the conduct of actual clinical trials.
Acknowledgments
A Feigin was supported by grants from the Huntington’s Disease Society of America, Cure Huntington’s Disease Initiative Foundation, Inc., and the National Institute of Neurological Disorders and Stroke (R01 NS 37564). A Feigin has served on the speaker’s bureau for Allergan Inc. and Teva Pharmaceuticals.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Biglan KM, Ross CA, Langbehn DR, et al. Motor abnormalities in premanifest persons with Huntington’s disease: the PREDICT-HD study. Mov. Disord. 2009;24(12):1763–1772. doi: 10.1002/mds.22601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirkwood SC, Siemers E, Hodes ME, et al. Subtle changes among presymptomatic carriers of the Huntington’s disease gene. J. Neurol. Neurosurg. Psychiatry. 2000;69(6):773–779. doi: 10.1136/jnnp.69.6.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCusker E, Richards F, Sillence D, Wilson M, Trent RJ. Huntington’s disease: neurological assessment of potential gene carriers presenting for predictive DNA testing. J. Clin. Neurosci. 2000;7(1):38–41. doi: 10.1054/jocn.1998.0151. [DOI] [PubMed] [Google Scholar]
- 4.Huntington Study Group. Unified Huntington’s Disease Rating Scale: reliability and consistency. Mov. Disord. 1996;11(2):136–142. doi: 10.1002/mds.870110204. [DOI] [PubMed] [Google Scholar]
- 5.Langbehn DR, Brinkman RR, Falush D, Paulsen JS, Hayden MR. A new model for prediction of the age of onset and penetrance for Huntington’s disease based on CAG length. Clin. Genet. 2004;65(4):267–277. doi: 10.1111/j.1399-0004.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- 6. Tabrizi SJ, Reilmann R, Roos RA, et al. Potential endpoints for clinical trials in premanifest and early Huntington’s disease in the TRACK-HD study: analysis of 24 month observational data. Lancet Neurol. 2012;11(1):42–53. doi: 10.1016/S1474-4422(11)70263-0. ▪▪ The most recent findings from TRACK-HD, reporting prospective longitudinal data on clinical and imaging findings in preclinical Huntington’s disease (HD).
- 7. Feigin A, Tang C, Ma Y, et al. Thalamic metabolism and symptom onset in preclinical Huntington’s disease. Brain. 2007;130(Pt 11):2858–2867. doi: 10.1093/brain/awm217. ▪▪ Longitudinal [18F]fluorodeoxyglucose PET study of presymptomatic HD gene carriers over 4 years. Subjects also underwent concurrent MRI and [11C]raclopride PET. A thalamic metabolic compensatory mechanism was identified during the preclinical period, and a loss of this mechanism was associated with symptom onset in HD.
- 8.Penney JB, Jr, Young AB, Shoulson I, et al. Huntington’s disease in Venezuela, 7 years of follow-up on symptomatic and asymptomatic individuals. Mov. Disord. 1990;5(2):93–99. doi: 10.1002/mds.870050202. [DOI] [PubMed] [Google Scholar]
- 9.Hogarth P, Kayson E, Kieburtz K, et al. Interrater agreement in the assessment of motor manifestations of Huntington’s disease. Mov. Disord. 2005;20(3):293–297. doi: 10.1002/mds.20332. [DOI] [PubMed] [Google Scholar]
- 10.Rao AK, Muratori L, Louis ED, Moskowitz CB, Marder KS. Spectrum of gait impairments in presymptomatic and symptomatic Huntington’s disease. Mov. Disord. 2008;23(8):1100–1107. doi: 10.1002/mds.21987. [DOI] [PubMed] [Google Scholar]
- 11.Kosinski CM, Landwehrmeyer B. Huntington’s disease. In: Beal MF, Lang AE, Ludolph AC, editors. Neurodegenerative Diseases. Neurobiology, Pathogenesis and Therapeutics. Cambridge, UK: Cambridge University Press; 2005. pp. 847–860. [Google Scholar]
- 12. Paulsen JS, Langbehn DR, Stout JC, et al. Detection of Huntington’s disease decades before diagnosis: the PREDICT-HD study. J. Neurol. Neurosurg. Psychiatry. 2008;79(8):874–880. doi: 10.1136/jnnp.2007.128728. ▪ Large cross-sectional MRI study demonstrating that subtle cognitive, motor, psychiatric and imaging signs can be detected one to two decades before clinical diagnosis of HD.
- 13.Paulsen JS. Early detection of Huntington’s disease. Future Neurol. 2010;5(1):85–104. doi: 10.2217/fnl.09.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farrow M, Churchyard A, Chua P, et al. Attention, inhibition, and proximity to clinical onset in preclinical mutation carriers for Huntington’s disease. J. Clin. Exp. Neuropsychol. 2007;29(3):235–246. doi: 10.1080/13803390600657693. [DOI] [PubMed] [Google Scholar]
- 15.Lemiere J, Decruyenaere M, Evers-Kiebooms G, Vandenbussche E, Dom R. Cognitive changes in patients with Huntington’s disease (HD) and asymptomatic carriers of the HD mutation – a longitudinal follow-up study. J. Neurol. 2004;251(8):935–942. doi: 10.1007/s00415-004-0461-9. [DOI] [PubMed] [Google Scholar]
- 16.Solomon AC, Stout JC, Weaver M, et al. Ten-year rate of longitudinal change in neurocognitive and motor function in prediagnosis Huntington disease. Mov. Disord. 2008;23(13):1830–1836. doi: 10.1002/mds.22097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duff K, Paulsen JS, Mills JA, et al. Mild cognitive impairment in Huntington’s disease. Clin. Genet. 2009;76:125. [Google Scholar]
- 18.Duff K, Paulsen JS, Beglinger LJ, Langbehn DR, Stout JC. Psychiatric symptoms in Huntington’s disease before diagnosis: the PREDICT-HD study. Biol. Psychiatry. 2007;62(12):1341–1346. doi: 10.1016/j.biopsych.2006.11.034. [DOI] [PubMed] [Google Scholar]
- 19.Julien CL, Thompson JC, Wild S, et al. Psychiatric disorders in preclinical Huntington’s disease. J. Neurol. Neurosurg. Psychiatry. 2007;78(9):939–943. doi: 10.1136/jnnp.2006.103309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paulsen JS, Ready RE, Hamilton JM, Mega MS, Cummings JL. Neuropsychiatric aspects of Huntington’s disease. J. Neurol. Neurosurg. Psychiatry. 2001;71(3):310–314. doi: 10.1136/jnnp.71.3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marder K, Zhao H, Myers RH, et al. Rate of functional decline in Huntington’s disease. Huntington Study Group. Neurology. 2000;54(2):452–458. doi: 10.1212/wnl.54.2.452. [DOI] [PubMed] [Google Scholar]
- 22.Puri BK, Leavitt BR, Hayden MR, et al. Ethyl-EPA in Huntington disease: a double-blind, randomized, placebo-controlled trial. Neurology. 2005;65(2):286–292. doi: 10.1212/01.wnl.0000169025.09670.6d. [DOI] [PubMed] [Google Scholar]
- 23.van Vugt JP, Siesling S, Vergeer M, van der Velde EA, Roos RA. Clozapine versus placebo in Huntington’s disease: a double blind randomised comparative study. J. Neurol. Neurosurg. Psychiatry. 1997;63(1):35–39. doi: 10.1136/jnnp.63.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meyer C, Landwehrmeyer B, Schwenke C, et al. Rate of change in early Huntington’s disease: a clinicometric analysis. Mov. Disord. 2012;27(1):118–124. doi: 10.1002/mds.23847. ▪▪ The most recent longitudinal 3-year study of clinical measures in early HD. Linear progression was found in the Unified Huntington’s Disease Rating Scale motor score and in many of its item scores as well as other outcome measures such as the total functional capacity score.
- 25.Tabrizi SJ, Langbehn DR, Leavitt BR, et al. Biological and clinical manifestations of Huntington’s disease in the longitudinal TRACK-HD study: cross-sectional analysis of baseline data. Lancet Neurol. 2009;8(9):791–801. doi: 10.1016/S1474-4422(09)70170-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tabrizi SJ, Scahill RI, Durr A, et al. Biological and clinical changes in premanifest and early stage Huntington’s disease in the TRACK-HD study: the 12-month longitudinal analysis. Lancet Neurol. 2011;10(1):31–42. doi: 10.1016/S1474-4422(10)70276-3. ▪▪ Large multicenter longitudinal MRI study of premanifest and early HD subjects over months of follow-up. Volume loss of gray matter and white matter was found and atrophy rates in these areas were also estimated. The imaging changes were further associated with changes in a broad spectrum of clinical and functional measures in the preclinical period.
- 27.Esmaeilzadeh M, Ciarmiello A, Squitieri F. Seeking brain biomarkers for preventive therapy in Huntington disease. CNS Neurosci. Ther. 2011;17(5):368–386. doi: 10.1111/j.1755-5949.2010.00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weir DW, Sturrock A, Leavitt BR. Development of biomarkers for Huntington’s disease. Lancet Neurol. 2011;10(6):573–590. doi: 10.1016/S1474-4422(11)70070-9. ▪▪ Comprehensive review of the development of clinical, cognitive, neuroimaging and other biomarkers for HD. The implications of such scientific advances to future clinical trials are also discussed in depth.
- 29.Braak H, Braak E. Allocortical involvement in Huntington’s disease. Neuropathol. Appl. Neurobiol. 1992;18(6):539–547. doi: 10.1111/j.1365-2990.1992.tb00824.x. [DOI] [PubMed] [Google Scholar]
- 30.Vonsattel JP, Myers RH, Stevens TJ, et al. Neuropathological classification of Huntington’s disease. J. Neuropathol. Exp. Neurol. 1985;44:559–577. doi: 10.1097/00005072-198511000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Cha JH, Frey AS, Alsdorf SA, et al. Altered neurotransmitter receptor expression in transgenic mouse models of Huntington’s disease. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1999;354(1386):981–989. doi: 10.1098/rstb.1999.0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antonini A, Leenders KL, Spiegel R, et al. Striatal glucose metabolism and dopamine D2 receptor binding in asymptomatic gene carriers and patients with Huntington’s disease. Brain. 1996;119(Pt 6):2085–2095. doi: 10.1093/brain/119.6.2085. [DOI] [PubMed] [Google Scholar]
- 33.Feigin A, Leenders KL, Moeller JR, et al. Metabolic network abnormalities in early Huntington’s disease: an [18F]FDG PET study. J. Nucl. Med. 2001;42(11):1591–1595. [PubMed] [Google Scholar]
- 34.Kassubek J, Juengling FD, Ecker D, Landwehrmeyer GB. Thalamic atrophy in Huntington’s disease co-varies with cognitive performance: a morphometric MRI analysis. Cereb. Cortex. 2005;15(6):846–853. doi: 10.1093/cercor/bhh185. [DOI] [PubMed] [Google Scholar]
- 35.Peinemann A, Schuller S, Pohl C, et al. Executive dysfunction in early stages of Huntington’s disease is associated with striatal and insular atrophy: a neuropsychological and voxel-based morphometric study. J. Neurol. Sci. 2005;239(1):11–19. doi: 10.1016/j.jns.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Politis M, Pavese N, Tai YF, et al. Hypothalamic involvement in Huntington’s disease: an in vivo PET study. Brain. 2008;131(Pt 11):2860–2869. doi: 10.1093/brain/awn244. [DOI] [PubMed] [Google Scholar]
- 37. Rosas HD, Salat DH, Lee SY, et al. Cerebral cortex and the clinical expression of Huntington’s disease: complexity and heterogeneity. Brain. 2008;131(Pt 4):1057–1068. doi: 10.1093/brain/awn025. ▪ High-resolution MRI study of HD identifying widespread volume loss in multiple cortical areas. These atrophic changes outside the striatum were found to be associated with various motor, cognitive and other functional measures in this disease.
- 38.Rosas HD, Tuch DS, Hevelone ND, et al. Diffusion tensor imaging in presymptomatic and early Huntington’s disease: selective white matter pathology and its relationship to clinical measures. Mov. Disord. 2006;21(9):1317–1325. doi: 10.1002/mds.20979. [DOI] [PubMed] [Google Scholar]
- 39.Seppi K, Schocke MF, Mair KJ, et al. Diffusion-weighted imaging in Huntington’s disease. Mov. Disord. 2006;21(7):1043–1047. doi: 10.1002/mds.20868. [DOI] [PubMed] [Google Scholar]
- 40.van Oostrom JC, Dekker M, Willemsen AT, et al. Changes in striatal dopamine D2 receptor binding in pre-clinical Huntington’s disease. Eur. J. Neurol. 2009;16(2):226–231. doi: 10.1111/j.1468-1331.2008.02390.x. [DOI] [PubMed] [Google Scholar]
- 41.Tang TS, Chen X, Liu J, Bezprozvanny I. Dopaminergic signaling and striatal neurodegeneration in Huntington’s disease. J. Neurosci. 2007;27(30):7899–7910. doi: 10.1523/JNEUROSCI.1396-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Benchoua A, Trioulier Y, Diguet E, et al. Dopamine determines the vulnerability of striatal neurons to the N-terminal fragment of mutant huntingtin through the regulation of mitochondrial complex II. Hum. Mol. Genet. 2008;17(10):1446–1456. doi: 10.1093/hmg/ddn033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Antonini A, Leenders KL, Eidelberg D. [11C]raclopride-PET studies of the Huntington’s disease rate of progression: relevance of the trinucleotide repeat length. Ann. Neurol. 1998;43(2):253–255. doi: 10.1002/ana.410430216. [DOI] [PubMed] [Google Scholar]
- 44.Dunah AW, Jeong H, Griffin A, et al. Sp1 and TAFII130 transcriptional activity disrupted in early Huntington’s disease. Science. 2002;296(5576):2238–2243. doi: 10.1126/science.1072613. [DOI] [PubMed] [Google Scholar]
- 45.Aylward EH. Change in MRI striatal volumes as a biomarker in preclinical Huntington’s disease. Brain Res. Bull. 2007;72(2–3):152–158. doi: 10.1016/j.brainresbull.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 46. van Oostrom JC, Maguire RP, Verschuuren-Bemelmans CC, et al. Striatal dopamine D2 receptors, metabolism, and volume in preclinical Huntington disease. Neurology. 2005;65(6):941–943. doi: 10.1212/01.wnl.0000176071.08694.cc. ▪ Multitracer imaging study of preclinical HD gene carriers. Striatal volume loss, dopamine D2 receptor binding and metabolism were measured and compared. Among these, striatal D2 receptor binding was suggested to be the most sensitive imaging marker of preclinical HD.
- 47.Weeks RA, Piccini P, Harding AE, Brooks DJ. Striatal D1 and D2 dopamine receptor loss in asymptomatic mutation carriers of Huntington’s disease. Ann. Neurol. 1996;40(1):49–54. doi: 10.1002/ana.410400110. [DOI] [PubMed] [Google Scholar]
- 48.Andrews TC, Weeks RA, Turjanski N, et al. Huntington’s disease progression. PET and clinical observations. Brain. 1999;122(Pt 12):2353–2363. doi: 10.1093/brain/122.12.2353. [DOI] [PubMed] [Google Scholar]
- 49.Ginovart N, Lundin A, Farde L, et al. PET study of the pre- and post-synaptic dopaminergic markers for the neurodegenerative process in Huntington’s disease. Brain. 1997;120(Pt 3):503–514. doi: 10.1093/brain/120.3.503. [DOI] [PubMed] [Google Scholar]
- 50.Brandt J, Folstein SE, Wong DF, et al. D2 receptors in Huntington’s disease: positron emission tomography findings and clinical correlates. J. Neuropsychiatry Clin. Neurosci. 1990;2(1):20–27. doi: 10.1176/jnp.2.1.20. [DOI] [PubMed] [Google Scholar]
- 51.Kipps CM, Duggins AJ, Mahant N, et al. Progression of structural neuropathology in preclinical Huntington’s disease: a tensor based morphometry study. J. Neurol. Neurosurg. Psychiatry. 2005;76(5):650–655. doi: 10.1136/jnnp.2004.047993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pavese N, Andrews TC, Brooks DJ, et al. Progressive striatal and cortical dopamine receptor dysfunction in Huntington’s disease: a PET study. Brain. 2003;126(Pt 5):1127–1135. doi: 10.1093/brain/awg119. [DOI] [PubMed] [Google Scholar]
- 53.Esmaeilzadeh M, Farde L, Karlsson P, et al. Extrastriatal dopamine D2 receptor binding in Huntington’s disease. Hum. Brain Mapp. 2011;32(10):1626–1636. doi: 10.1002/hbm.21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aylward EH, Codori AM, Rosenblatt A, et al. Rate of caudate atrophy in presymptomatic and symptomatic stages of Huntington’s disease. Mov. Disord. 2000;15(3):552–560. doi: 10.1002/1531-8257(200005)15:3<552::AID-MDS1020>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 55.Douaud G, Gaura V, Ribeiro MJ, et al. Distribution of grey matter atrophy in Huntington’s disease patients: a combined ROI-based and voxel-based morphometric study. Neuroimage. 2006;32(4):1562–1575. doi: 10.1016/j.neuroimage.2006.05.057. [DOI] [PubMed] [Google Scholar]
- 56.Paulsen JS, Magnotta VA, Mikos AE, et al. Brain structure in preclinical Huntington’s disease. Biol. Psychiatry. 2006;59(1):57–63. doi: 10.1016/j.biopsych.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 57.Ruocco HH, Bonilha L, Li LM, Lopes-Cendes I, Cendes F. Longitudinal analysis of regional grey matter loss in Huntington disease: effects of the length of the expanded CAG repeat. J. Neurol. Neurosurg. Psychiatry. 2008;79(2):130–135. doi: 10.1136/jnnp.2007.116244. [DOI] [PubMed] [Google Scholar]
- 58. Aylward EH, Nopoulos PC, Ross CA, et al. Longitudinal change in regional brain volumes in prodromal Huntington disease. J. Neurol. Neurosurg. Psychiatry. 2011;82(4):405–410. doi: 10.1136/jnnp.2010.208264. ▪ Longitudinal MRI study of a large group of preclinical HD gene carriers and healthy controls. Atrophy rates were estimated in multiple brain regions and power and sample size calculations were performed accordingly.
- 59.Hobbs NZ, Henley SM, Wild EJ, et al. Automated quantification of caudate atrophy by local registration of serial MRI: evaluation and application in Huntington’s disease. Neuroimage. 2009;47(4):1659–1665. doi: 10.1016/j.neuroimage.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 60.Squitieri F, Cannella M, Simonelli M, et al. Distinct brain volume changes correlating with clinical stage, disease progression rate, mutation size, and age at onset prediction as early biomarkers of brain atrophy in Huntington’s disease. CNS Neurosci. Ther. 2009;15(1):1–11. doi: 10.1111/j.1755-5949.2008.00068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rosas HD, Liu AK, Hersch S, et al. Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology. 2002;58(5):695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- 62.Rosas HD, Feigin AS, Hersch SM. Using advances in neuroimaging to detect, understand, and monitor disease progression in Huntington’s disease. NeuroRx. 2004;1(2):263–272. doi: 10.1602/neurorx.1.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thieben MJ, Duggins AJ, Good CD, et al. The distribution of structural neuropathology in pre-clinical Huntington’s disease. Brain. 2002;125(Pt 8):1815–1828. doi: 10.1093/brain/awf179. [DOI] [PubMed] [Google Scholar]
- 64.Paulsen JS, Zimbelman JL, Hinton SC, et al. fMRI biomarker of early neuronal dysfunction in presymptomatic Huntington’s disease. Am. J. Neuroradiol. 2004;25(10):1715–1721. [PMC free article] [PubMed] [Google Scholar]
- 65.Rosas HD, Hevelone ND, Zaleta AK, et al. Regional cortical thinning in preclinical Huntington disease and its relationship to cognition. Neurology. 2005;65(5):745–747. doi: 10.1212/01.wnl.0000174432.87383.87. [DOI] [PubMed] [Google Scholar]
- 66.Hobbs NZ, Henley SM, Ridgway GR, et al. The progression of regional atrophy in premanifest and early Huntington’s disease: a longitudinal voxel-based morphometry study. J. Neurol. Neurosurg. Psychiatry. 2010;81(7):756–763. doi: 10.1136/jnnp.2009.190702. [DOI] [PubMed] [Google Scholar]
- 67.Ciarmiello A, Cannella M, Lastoria S, et al. Brain white-matter volume loss and glucose hypometabolism precede the clinical symptoms of Huntington’s disease. J. Nucl. Med. 2006;47(2):215–222. [PubMed] [Google Scholar]
- 68.Stoffers D, Sheldon S, Kuperman JM, et al. Contrasting gray and white matter changes in preclinical Huntington disease: an MRI study. Neurology. 2010;74(15):1208–1216. doi: 10.1212/WNL.0b013e3181d8c20a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vandenberghe W, Demaerel P, Dom R, Maes F. Diffusion-weighted versus volumetric imaging of the striatum in early symptomatic Huntington disease. J. Neurol. 2009;256(1):109–114. doi: 10.1007/s00415-009-0086-0. [DOI] [PubMed] [Google Scholar]
- 70.Magnotta VA, Kim J, Koscik T, et al. Diffusion tensor imaging in preclinical Huntington’s disease. Brain Imaging Behav. 2009;3(1):77–84. doi: 10.1007/s11682-008-9051-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rosas HD, Lee SY, Bender AC, et al. Altered white matter microstructure in the corpus callosum in Huntington’s disease: implications for cortical ‘disconnection’. Neuroimage. 2010;49(4):2995–3004. doi: 10.1016/j.neuroimage.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Della Nave R, Ginestroni A, Tessa C, et al. Regional distribution and clinical correlates of white matter structural damage in Huntington disease: a tract-based spatial statistics study. Am. J. Neuroradiol. 2010;31(9):1675–1681. doi: 10.3174/ajnr.A2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weaver KE, Richards TL, Liang O, et al. Longitudinal diffusion tensor imaging in Huntington’s disease. Exp. Neurol. 2009;216(2):525–529. doi: 10.1016/j.expneurol.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bartzokis G, Lu PH, Tishler TA, et al. Myelin breakdown and iron changes in Huntington’s disease: pathogenesis and treatment implications. Neurochem. Res. 2007;32(10):1655–1664. doi: 10.1007/s11064-007-9352-7. [DOI] [PubMed] [Google Scholar]
- 75.Rosas HD, Chen YI, Doros G, et al. Alterations in bain transition metals in Huntington disease: an evolving and intricate story. Arch. Neurol. 2012 doi: 10.1001/archneurol.2011.2945. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nguyen T, Hamby A, Massa SM. Clioquinol down-regulates mutant huntingtin expression in vitro and mitigates pathology in a Huntington’s disease mouse model. Proc. Natl Acad. Sci. USA. 2005;102(33):11840–11845. doi: 10.1073/pnas.0502177102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wolf RC, Gron G, Sambataro F, et al. Magnetic resonance perfusion imaging of resting-state cerebral blood flow in preclinical Huntington’s disease. J. Cereb. Blood Flow Metab. 2011;31(9):1908–1918. doi: 10.1038/jcbfm.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen JJ, Salat DH, Rosas HD. Complex relationships between cerebral blood flow and brain atrophy in early Huntington’s disease. Neuroimage. 2012;59(2):1043–1051. doi: 10.1016/j.neuroimage.2011.08.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ma Y, Eidelberg D. Functional imaging of cerebral blood flow and glucose metabolism in Parkinson’s disease and Huntington’s disease. Mol. Imaging Biol. 2007;9(4):223–233. doi: 10.1007/s11307-007-0085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Feigin A, Ghilardi MF, Huang C, et al. Preclinical Huntington’s disease: compensatory brain responses during learning. Ann. Neurol. 2006;59(1):53–59. doi: 10.1002/ana.20684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eidelberg D. Metabolic brain networks in neurodegenerative disorders: a functional imaging approach. Trends Neurosci. 2009;32(10):548–557. doi: 10.1016/j.tins.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Smith Y, Raju DV, Pare JF, Sidibe M. The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci. 2004;27(9):520–527. doi: 10.1016/j.tins.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 83.Vitek JL, Giroux M. Physiology of hypokinetic and hyperkinetic movement disorders: model for dyskinesia. Ann. Neurol. 2000;47(4 Suppl. 1):S131–S140. [PubMed] [Google Scholar]
- 84.Kassubek J, Juengling FD, Kioschies T, et al. Topography of cerebral atrophy in early Huntington’s disease: a voxel based morphometric MRI study. J. Neurol. Neurosurg. Psychiatry. 2004;75(2):213–220. [PMC free article] [PubMed] [Google Scholar]
- 85.Rosas HD, Koroshetz WJ, Chen YI, et al. Evidence for more widespread cerebral pathology in early HD: an MRI-based morphometric analysis. Neurology. 2003;60(10):1615–1620. doi: 10.1212/01.wnl.0000065888.88988.6e. [DOI] [PubMed] [Google Scholar]
- 86.Tang C, Feigin A, Bussa M, et al. Identification and validation of an abnormal metabolic network associated with phenoconversion of preclinical Huntington’s disease. Neurology. 2010;74(Suppl. 2):A197. [Google Scholar]
- 87.Schippling S, Schneider SA, Bhatia KP, et al. Abnormal motor cortex excitability in preclinical and very early Huntington’s disease. Biol. Psychiatry. 2009;65(11):959–965. doi: 10.1016/j.biopsych.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Orth M, Schippling S, Schneider SA, et al. Abnormal motor cortex plasticity in premanifest and very early manifest Huntington disease. J. Neurol. Neurosurg. Psychiatry. 2010;81(3):267–270. doi: 10.1136/jnnp.2009.171926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Habeck C, Krakauer JW, Ghez C, et al. A new approach to spatial covariance modeling of functional brain imaging data: ordinal trend analysis. Neural. Comp. 2005;17:1602–1645. doi: 10.1162/0899766053723023. [DOI] [PubMed] [Google Scholar]
- 90.Moeller JR, Habeck CG. Reciprocal benefits of mass-univariate and bilinear modeling in brain mapping: applications to event-related functional MRI H2 15O- and FDG-PET. Int. J. Biomed. Imaging. 2006;2006:1–13. doi: 10.1155/IJBI/2006/79862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hauser RA, Zesiewicz TA. Clinical trials aimed at detecting neuroprotection in Parkinson’s disease. Neurology. 2006;66(10 Suppl. 4):S58–S68. doi: 10.1212/wnl.66.10_suppl_4.s58. [DOI] [PubMed] [Google Scholar]
- 92.Fang Q, Strand A, Law W, et al. Brain-specific proteins decline in the cerebrospinal fluid of humans with Huntington disease. Mol. Cell. Proteomics. 2009;8(3):451–466. doi: 10.1074/mcp.M800231-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]