Abstract
Background
Vitiligo is characterized by the death of melanocytes in the skin. This is associated with the presence of T cell infiltrates in the lesional borders. However, at present, there is no detailed and systematic characterization on whether additional cellular or molecular changes are present inside vitiligo lesions. Further, it is unknown if the normal appearing non-lesional skin of vitiligo patients is in fact normal. The purpose of this study is to systematically characterize the molecular and cellular characteristics of the lesional and non-lesional skin of vitiligo patients.
Methods and Materials
Paired lesional and non-lesional skin biopsies from twenty-three vitiligo patients and normal skin biopsies from sixteen healthy volunteers were obtained with informed consent. The following aspects were analyzed: (1) transcriptome changes present in vitiligo skin using DNA microarrays and qRT-PCR; (2) abnormal cellular infiltrates in vitiligo skin explant cultures using flow cytometry; and (3) distribution of the abnormal cellular infiltrates in vitiligo skin using immunofluorescence microscopy.
Results
Compared with normal skin, vitiligo lesional skin contained 17 genes (mostly melanocyte-specific genes) whose expression was decreased or absent. In contrast, the relative expression of 13 genes was up-regulated. The up-regulated genes point to aberrant activity of the innate immune system, especially natural killer cells in vitiligo. Strikingly, the markers of heightened innate immune responses were also found to be up-regulated in the non-lesional skin of vitiligo patients.
Conclusions and Clinical Implications
As the first systematic transcriptome characterization of the skin in vitiligo patients, this study revealed previously unknown molecular markers that strongly suggest aberrant innate immune activation in the microenvironment of vitiligo skin. Since these changes involve both lesional and non-lesional skin, our results suggest that therapies targeting the entire skin surface may improve treatment outcomes. Finally, this study revealed novel mediators that may facilitate future development of vitiligo therapies.
Introduction
Affecting 0.5%–1% of population worldwide, vitiligo is an acquired pigmentation disorder in which melanocytes are destroyed, resulting in development of porcelain-white patches of skin [1], [2], [3]. Although non-fatal in nature, vitiligo causes severe negative psychosocial impact on the affected individuals, such as social stigmatization and decreased quality of life [4], [5].
The pathogenesis of vitiligo is largely unclear. There is evidence that vitiligo is an autoimmune disease [6], [7], [8], [9], [10], [11], [12], [13], [14], possibly involving additional factors such as oxidative stress [15] and genetic predisposition [6], [10], [11], [12], [13], [16], [17], [18]. The autoimmune hypothesis stems from the frequently observed association with other autoimmune diseases, such as hypothyroidism and diabetes [19], [20], [21], [22], [23]. This is further supported by the observation of T-lymphocyte infiltration in human vitiligo lesions and in mouse models [24] and the demonstration of melanocyte-specific antibodies in the blood of vitiligo patients. While these results suggest a role for the adaptive immune responses in melanocyte death [25], [26], [27], there are also early suggestions that innate, or natural, immunity in vitiligo is abnormal, as suggested by Jin et al [6], who demonstrated an association between vitiligo susceptibility and genetic changes in a critical innate immunity regulator gene, the NOD-like receptor 1 (NALP1), which has recently been confirmed by immunohistochemistry [28]. However, to date, direct evidence and independent confirmation of innate immunity in the skin of vitiligo patients have been sparse.
Previous vitiligo transcriptome analyses have focused on cultured melanocytes that were isolated from patients with vitiligo, demonstrating possible molecular abnormalities in vitiligo-patient's melanocytes [29], [30], [31], [32]. However, systematic detailed characterizations of vitiligo patient's skin, especially areas within well-established lesions away from the advancing border regions, as well as the normal appearing non-lesional areas, have not been performed previously, leaving unanswered the question of whether there are additional structural or molecular abnormalities present in vitiligo patient's skin other than the well-documented lack of melanocytes [33]. Answering this question may uncover additional clues to vitiligo pathogenesis and suggest novel approaches for future development of vitiligo therapies.
In this study, we performed a transcriptome analysis comparing gene expression profiles of three types of skin biopsies: (1) vitiligo lesional skin (LS); (2) normal appearing non-lesional skin (NLS) of vitiligo patients; and (3) normal skin of healthy volunteers (NS). This was followed by explant skin cultures and immunofluorescence analyses of the cellular infiltrates present in these skin biopsies. The results showed for the first time markers and cells of activated innate immune response not only in the lesional areas, but also in the normal appearing non-lesional areas of vitiligo patients' skin. This conclusion suggests that future therapeutic development needs to consider the role of innate immune activation in the affected as well as the unaffected areas of the skin in vitiligo patients. Therefore, treating the entire skin surface of vitiligo patients may have higher chances of achieving optimal therapeutic results than targeting individual lesions. Further, targeting innate immune activation may represent a promising approach for developing vitiligo therapies in the future.
Materials and Methods
Study Subjects and sample collection
Twenty-three subjects with vitiligo vulgaris and 16 healthy volunteers were recruited for this study ( Tables 1 and 2 ). The diagnosis of vitiligo was based on acquired depigmentation of skin with typical symmetrical distribution on characteristic locations such as the torso, the extremities and the face. Wood's lamp was used to help establish the diagnosis. Paired 5 mm full-thickness punch biopsies were obtained from vitiligo lesional skin (LS, at least 2 cm inside the lesional border), non-lesional skin (NLS) located at least 2 cm outside of the border of the same lesion, or normal appearing skin on the non-involved contra-lateral side of the body. To minimize tissue heterogeneity due to anatomic variations, the biopsies were obtained from the torso and proximal extremities (proximal to the elbows and the knees) while avoiding the acral and facial locations. The biopsies were bisected into two equal portions, one placed in RNAlater solution (Invitrogen, Burlington, ON, Canada) for RNA extraction, while the other was immediately immersed in Histo-freeze medium and quickly frozen at a −80°C freezer for histological confirmation and structural characterization. For explant culture (see below) another 5 mm-punch biopsy was obtained from each subject (the lesional and non-lesional skin) and placed in the culture medium immediately.
Table 1. Demographics and clinical features of vitiligo subjects.
| Subject No. | Sex | Ethnic Origin | Age (yrs) | Other Autoimmune Diseases | Family History of Autoimmune Diseases | 1Disease Extent- BSA (%) | Biopsy Site | Types of Analysis |
| VIT1 | M | Chinese | 28 | None | None | 30 | Elbow | MA, qPCR |
| VIT2 | M | Caucasian | 70 | Myasthenia gravis | None | 2 | Arms | MA, qPCR |
| VIT3 | M | S. Asian | 54 | None | None | 5 | Abd | MA, qPCR |
| VIT4 | M | Chinese | 20 | None | Vitiligo | 11 | Legs | MA, qPCR |
| VIT5 | F | S. Asian | 35 | None | None | 10 | Abd. | MA, qPCR |
| VIT6 | M | S. Asian | 75 | None | None | 3 | Neck | MA, qPCR |
| VIT7 | F | Chinese | 21 | None | None | 5 | Torso | MA, qPCR |
| VIT8 | M | S. Asian | 18 | None | None | 22 | Abd. | MA, qPCR |
| VIT9 | F | Chinese | 71 | None | None | 2 | Abd. | MA, qPCR |
| VIT10 | M | Chinese | 36 | None | None | 30 | Abd. | MA, qPCR |
| VIT11 | F | Chinese | 33 | Eczema | None | 80 | Flank | MA, qPCR |
| VIT12 | F | Korean | 28 | None | None | 20 | Flank | MA, qPCR, IF |
| VIT13 | F | S. Asian | 57 | None | None | 6 | Upper back | MA, qPCR, IF |
| VIT14 | M | Chinese | 51 | None | Diabetes | 2 | Upper back | MA, qPCR, IF |
| VIT15 | F | Caucasian | 36 | None | Vitiligo | 3 | Upper back | MA, qPCR, IF |
| VIT16 | F | S. Asian | 47 | Hypothyroidism | None | 3 | Upper back | MA, qPCR, IF |
| VIT17 | M | Chinese | 26 | None | None | 1 | Buttock | MA, qPCR, IF |
| VIT18 | M | S Asian | 71 | None | None | 10 | Back | qPCR, Exp C, IF |
| VIT19 | M | S Asian | 65 | None | None | 7 | Neck | qPCR, Exp C, IF |
| VIT20 | M | Chinese | 52 | None | Diabetes | 3 | Neck | qPCR, Exp C, IF |
| VIT21 | F | Chinese | 53 | None | None | 25 | Flank | qPCR, Exp C, IF |
| VIT22 | F | Caucasian | 32 | None | None | 5 | Flank | qPCR, Exp C, IF |
| VIT23 | M | Caucasian | 27 | None | Hypothyroidism, Vitiligo | 10 | Back | qPCR, Exp C, IF |
Abbreviations: F = Female; M = Male; BSA = body surface area; Abd. = abdomen; VIT = subjects with vitiligo; MA = microarray; qPCR = quantitative polymerase chain reaction; Exp C = Explant skin cultures; IF = immunofluorescence.
Percent body surface area involvement are estimations using estimated number of palm areas covered by the white patches of skin, with each palm area representing as 1% body surface area.
Table 2. Demographics and clinical features of normal control subjects.
| Subject No. | Sex | Ethnic Origin | Age (yrs) | Other Autoimmune Diseases | Family History of Autoimmune Diseases | 1Disease Extent- BSA (%) | Biopsy Site | Types of Analysis |
| NS1 | M | Chinese | 48 | None | NA | NA | Back | MA, qPCR |
| NS2 | F | S Asian | 55 | None | NA | NA | Back | MA, qPCR |
| NS3 | M | Chinese | 43 | None | NA | NA | buttock | MA, qPCR |
| NS4 | M | Chinese | 70 | None | NA | NA | Back | MA, qPCR |
| NS5 | F | Caucasian | 28 | None | NA | NA | Abdo | MA, qPCR |
| NS6 | M | Caucasian | 48 | None | NA | NA | Chest | MA, qPCR |
| NS7 | M | Caucasian | 65 | None | NA | NA | Chest | MA, qPCR |
| NS8 | F | Chinese | 49 | None | NA | NA | Abdo | MA, qPCR |
| NS9 | M | Chinese | 78 | None | NA | NA | Thigh | MA, qPCR |
| NS10 | F | S Asian | 50 | None | NA | NA | Abdo | MA, qPCR |
| NS11 | F | Caucasian | 25 | None | NA | NA | Back | MA, qPCR, IF |
| NS12 | M | S Asian | 34 | None | NA | NA | Neck | MA, qPCR, Exp C, IF |
| NS13 | F | Caucasian | 36 | None | NA | NA | Back | MA, qPCR, Exp C, IF |
| NS14 | M | S Asian | 31 | None | NA | NA | Abdo | MA, qPCR, Exp C, IF |
| NS15 | F | Chinese | 26 | None | NA | NA | Flank | MA, qPCR, Exp C, IF |
| NS16 | M | Caucasian | 22 | None | NA | NA | buttock | MA, qPCR, Exp C, IF |
Abbreviations: F = Female; M = Male; BSA = body surface area; Abd. = abdomen; NS = normal skin from healthy volunteers; NA = not available; MA = microarray; qPCR = quantitative polymerase chain reaction; Exp C = Explant skin cultures; IF = immunofluorescence.
Percent body surface area involvement are estimations using estimated number of palm areas covered by the white patches of skin, with each palm area representing as 1% body surface area.
The study was approved by the Ethical Review Board of the University of British Columbia (Certificate Number C98-0493) in accordance with the contents of the Declaration of Helsinki, and by the Institutional Review Board of the University of British Columbia. Collection of skin biopsies was undertaken after the subjects have signed the informed consent.
RNA extraction
Skin samples were trimmed to remove visible adipose tissue and homogenized in Trizol (Invitrogen, Burlington, ON, Canada) using the tissue homogenizer (Model 398; Biospec Products Inc, Bartlesville, OK, USA). RNeasy Fibrous Tissue Mini Kit (Qiagen, Valencia, CA, USA) was used to extract total cellular RNA according to the manufacturer's protocol. The quality of the RNA was assessed by the Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, USA); and the concentration of the RNA samples was determined by the Nanodrop 1000 spectrophotometer (Thermo Scientific, Ottawa, ON, CA).
Transcriptome analysis using DNA microarrays and molecular pathway analysis
For the initial screening of gene expression differences in vitiligo skin, RNA from 17 pairs of full-thickness vitiligo skin and 16 normal skin biopsies were used in two-colored DNA microarray analysis following a protocol we previously described [34], [35], [36].. Briefly, total cellular RNA (500 ng) was reverse-transcribed into cDNA and linearly amplified by in vitro transcription in the presence of fluorescent-labeled CTP using the Low RNA Input Linear Amplification Kit, PLUS, Two-Color from Agilent following the manufacturer's instructions. Each microarray was hybridized with 825 ng of amplified cDNA labelled with Cy5 (each individual RNA sample) or Cy3 (pooled control skin RNA from 16 individual donors) at a specific activity between 8 and 15 pmol/µg. Hybridizations were performed on Whole Human Genome Oligo microarrays (G4112F, Agilent Technologies, Santa Clara, CA, USA), comprising 41,059 60-nt oligonucleotide probes, mostly represented as single spots. Image scanning was performed with the Agilent DNA Microarray Scanner and quantified using Agilent's Feature Extraction software. The results were imported and analyzed with the GeneSpring GX 7.3 software (Agilent Technologies, Santa Clara, CA, USA) for statistical computing and visualization. Data normalization was performed within and across the arrays using per gene, per chip normalization according to the Agilent recommendation. To detect the differentially expressed genes between vitiligo LS and NLS and NS, non-parametric Mann-Whitney U tests were used based on group analysis. The genes were ranked according to their false discovery rate-adjusted p-values (with cut off set at <0.05 after Bonferroni corrections). The threshold of expression differences was set at a 2.0-fold increase or decrease in gene expression levels as compared with NS.
Pathway analysis on the differentially expressed genes in vitiligo skin was performed using Database for Annotation, Visualization and Integrated Discovery (DAVID) Bioinformatics Resources 6.7, which is a standard bioinformatics tool for functional analysis of large gene list derived from high-throughput genomic scanning [37], [38]. It systematically maps a large number of genes in a list to the associated biological annotation terms (e.g. GO Terms or Pathways), and then examine the statistical significance of the gene enrichment by comparing the outcome to the reference controls [38]. Vitiligo differentially-expressed gene lists were also mapped to the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway database. All annotated pathways were ranked by enrichment score and Benjamini adjusted p values.
Confirmation of gene expression changes by quantitative polymerase chain reaction
The candidate differentially-expressed genes that were identified by transcriptome analysis were verified by quantitative real time polymerase chain reaction (qRT-PCR) according to methods we previous reported [34], [39] on all samples used in the transcriptome analysis plus 6 additional pairs of vitiligo samples. Briefly, total cellular RNA was extracted using the RNeasy Mini Kit (Qiagen, Mississauga, ON, Canada) following manufacturer's protocol. The isolated RNA was reverse transcribed using random primers and SuperScript III reverse transcriptase (Invitrogen, Burlington, ON, Canada). Real-time PCR was performed on a DNA Engine Opticon™ System (Bio-Rad Laboratories, Mississauga, ON, Canada) using the SYBR® Green method and analyzed with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the internal control. The statistical significance of the gene expression differences was calculated using paired two-tailed Student t tests (for comparisons between LS and NLS) or two-tailed non-parametric Whitney U tests (between vitiligo skin biopsies and healthy control skin biopsies).
Explant culture of natural killer cells
Immune cells from the skin biopsies of 6 vitiligo patients and 5 healthy individuals were isolated and cultured as previously described [40], [41]. Briefly, Cell-foam matrices (Cellsciences Pte Ltd, Singapore) were treated with rat tail collagen I (BD Biosciences, Bedford, MA, USA) and served as three-dimensional scaffolds that separate dermal fibroblasts and skin-resident lymphocytes. The skin explants were minced and placed on the surface of the matrices and cultured in 12-well 0.4 mm pore size polyester trans-well culture plates (Corning, Corning, NY, USA) in the presence of 25 ng/ml IL-2 and 20 ng/ml IL-15 (R&D systems, Minneapolis, MN, USA) in IMDM (Stemcell Technologies, Vancouver, BC, Canada). The cultures were supplemented with 10% heat-inactivated fetal bovine serum (Hyclone; Thermo Scientific, Ottawa, ON, CA), penicillin and streptomycin (Sigma-Aldrich, Oakville, ON, Canada). The cells that have migrated out of the explants were analyzed after 3 weeks by flow cytometry.
Analysis of natural killer cells by flow cytometry
Flow cytometry of the immune cells migrating out of the skin biopsies in culture dishes was performed using fluorophore-conjugated antibodies against T cell receptors and natural killer (NK) cell markers. Cells were labeled with Fixable Viability Stain 450 and antibodies against CD3 APC (Ms IgG1: SK7), CD56 FITC (Ms IgG2b: NCAM16.2), Ki67 PE (Ms IgG1: B56) and granzyme B APC (Ms IgG1: GB11) (BD Biosciences, Bedford, MA, USA), where NK cells were distinguished from the rest of the lymphocytes via positive expression of CD56 and negative expression of CD3. The samples were analyzed on a BD FACSCanto flow cytometer (BD Biosciences, Bedford, MA, USA) and data was analyzed with FCS Express Pro Software Version 3 (De Novo Software, Los Angeles, CA, USA). A total of 10,000 events were collected for each sample, and live cells (positive for the viability stain) were analyzed for the proportion of CD3-CD56+ NK cells. These cells were further gated to obtain the proportion of NK cells expressing granzyme B.
Assessment of natural killer cells in skin biopsies by immunofluorescence microscopy
Biopsies were embedded in Histo-freeze medium upon collection and transferred to −80°C for storage. Sections of 14 to 20 µm in thickness were cut using a cryostat and subjected to standard immunofluorescence staining protocol. Briefly, the sections were fixed in 4% paraformaldehyde (Sigma-Aldrich, Oakville, ON, Canada) at 4°C for 20 min. For permeabilization, 0.3% Triton X (Sigma-Aldrich, Oakville, ON, Canada) was applied to the sections for 10 min at 4°C. After blocking in 10% normal goat serum (Sigma-Aldrich, Oakville, ON, Canada) for 60 min at room temperature, the sections were incubated overnight in mouse monoclonal anti-human NKG2D (Abcam, Cambridge, MA, USA), which labels NK cells; rabbit polyclonal anti-human CD3 (Dako, Burlington, ON, CA), which labels T lymphocytes; or rabbit polyclonal anti-human Melan-A/MART1 (Sigma-Aldrich, Oakville, ON, Canada), which identifies the melanocytes. Then, the slides were treated with Alexa Fluor® 594 goat anti-mouse IgG and Alexa Fluor® 488 goat anti-rabbit IgG (Invitrogen, Burlington, ON, Canada). Finally, the sections were counter-stained with DAPI (Sigma-Aldrich, Oakville, ON, CA) and mounted in Gold anti-fade medium (Invitrogen, Burlington, ON, CA). The slides were visualized with a Zeiss Axiovert 200M inverted fluorescence microscope (Zeiss, Toronto, ON, Canada). Image processing and quantification were performed with AxioVision Rel. 4.6 software (Zeiss, Toronto, ON, Canada) using the interactive measurement module. Quantification of cells was performed across entire tissue sections, with the resulting data expressed as the mean number of cells per 400× field of view over a range of 20–32 fields of view depending on the size of the specimens.
Statistical analysis
The transcriptome analyses were performed using GeneSpring GX 7.3 software (Agilent Technologies, Santa Clara, CA, USA). Additional statistical analyses were performed with GraphPad Prism software (GraphPad Software, Inc, La Jolla, CA, USA). Non-parametric Mann-Whitney U tests were used to compare the difference in gene expression and the quantity of natural killer cells between vitiligo skin and the normal healthy skin.
Results
1. Demographics and clinical characterization of study subjects
All study subjects had the diagnosis of vitiligo vulgaris, with a mean of 17.8% (range: 1% to 80%) body skin area depigmented ( Table 1 ). The subjects were from the Vancouver General Hospital Skin Care Centre. There were 11 East Asians (Chinese and Koreans), 8 South Asians (Indians and Pakistanis) and 4 Caucasians. Twelve were males. The average age was 43.7 years (Range 18–75). Eight of the 23 subjects (34.8%) had a personal or family history of autoimmune diseases such as thyroid diseases or vitiligo. The healthy volunteers were of similar gender and ethnic composition as the vitiligo subjects but without vitiligo or other skin diseases.
2. Gene expression changes in vitiligo lesional skin
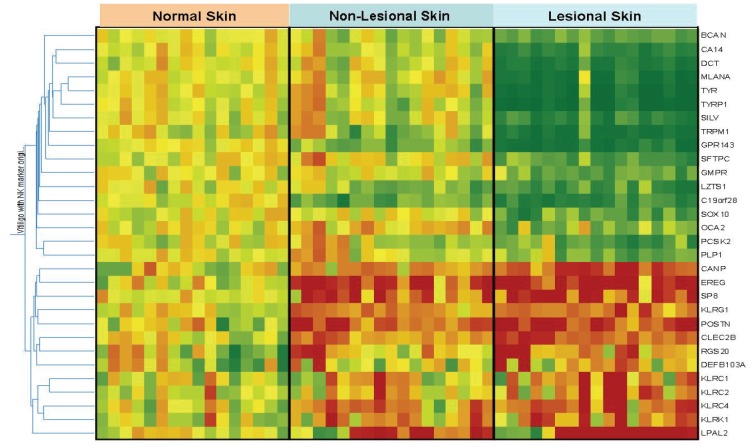
We first examined gene expression changes in vitiligo lesional skin (LS) using the skin from healthy subjects as the controls ( Figure 1 and Tables 3 and 4 ). Based on microarray analysis, there were 30 genes with significant expression changes (>2 fold up- or down- regulation, p<0.05, non-parametric Mann-Whitney U tests, with Bonferroni correction for multiple-testing). Of these, 17 were down-regulated ( Table 3 ) and 13 up-regulated ( Table 4 ) in vitiligo LS compared with healthy normal skin (NS), which are further confirmed with qRT-PCR analysis of additional samples. As expected, most of the down-regulated genes encode lineage markers or functional components of melanocytes, including TYRP1, TYR, Melan-A, and SILV. Several neuron-related genes, including PLP1, which encodes the major myelin associated protein (a specific marker for the Schwann cells) were also decreased. Among the up-regulated genes, the vast majority encode innate immunity regulators, such as β-defensin and CLEC2B (an activating ligand for natural killer cells that is primarily expressed by macrophages), as well as multiple activation markers of the NK cells (another important player in innate immunity). Among the other notable up-regulated genes, CANP codes for calpains, a family of proteases strongly associated with oxidative stress [42], which may participate in melanocyte destruction in vitiligo [15]; POSTN codes for periostin, a protein involved in tissue injury, repair and remodeling [43], although its role in vitiligo is unclear at present.
Figure 1. Transcriptome Analysis of Vitiligo and Normal Skin Biopsies.
A heat map is constructed by Gene Spring software (see methods) comparing the relative expression levels of the 30 significantly altered genes in vitiligo skin. Depicted are the expression levels of these genes in individual samples relative to their corresponding expression reference levels, which are the averages of expression in the 16 normal skin biopsies. Red squares: Genes with up-regulation in that sample compared with normal skin of healthy volunteers. Green squares: Genes with down-regulation in that specific sample compared with normal skin of healthy volunteers. Yellow squares: no significant change between the sample and the normal skin of healthy volunteers.
Table 3. Down-regulated genes in vitiligo skin.
| Gene | Chrom | 1Microarray | 2qRT-PCR | Function | ||||
| osome | LS/NS | NLS/NS | LS/NLS | LS/NS | NLS/NS | LS/NLS | ||
| TYRP1 | 9 | 0.06 * | 0.97 | 0.06 * | 0.02 * | 1.34 | 0.01 * | Melanogenesis |
| TYR | 11 | 0.08 * | 1.09 | 0.07 * | 0.02 * | 1.25 | 0.02 * | Melanogenesis |
| MLANA | 9 | 0.13 * | 1.08 | 0.12 * | 0.04 * | 1.44 * | 0.02 * | Melanogenesis |
| TRPM1 | 15 | 0.11 * | 0.89 | 0.12 * | 0.07 * | 1.82 * | 0.04 * | Melanogenesis and signal transduction |
| DCT | 13 | 0.13 * | 0.90 | 0.14 * | 0.01 * | 0.51 * | 0.02 * | Melanogenesis |
| PMEL | 12 | 0.21 * | 1.02 | 0.21 * | 0.22 * | 2.16 * | 0.10 * | Melanogenesis |
| CA14 | 1 | 0.21 * | 0.89 | 0.24 * | 0.33 * | 1.98 * | 0.17 * | Physiological processes |
| SFTPC | 8 | 0.35 * | 1.21 | 0.29 * | 0.14 * | 2.53 * | 0.06 * | Surfactant |
| SOX10 | 22 | 0.31 * | 0.80 | 0.39 * | 0.02 * | 0.20 * | 0.08 * | Stem cell development |
| OCA2 | 15 | 0.58 * | 1.44 | 0.40 * | 0.46 * | 1.06 | 0.43 * | Melanogenesis |
| PCSK2 | 20 | 0.36 * | 0.89 | 0.40 * | 0.18 * | 1.05 | 0.17 * | Hormone synthesis |
| PLP1 | X | 0.50 * | 1.14 | 0.44 * | 0.23 * | 0.57 * | 0.41 * | Myelin sheath protein |
| GMPR | 6 | 0.40 * | 0.84 | 0.48 * | 0.25 * | 0.69 * | 0.37 * | Nucleoside reductase |
| BCAN | 1 | 0.37 * | 0.78 | 0.47 * | 0.15 * | 0.87 | 0.17 * | Cell motility |
| LZTS1 | 8 | 0.31 * | 0.63 * | 0.49 * | 0.10 * | 0.44 * | 0.23 * | Tumor suppressor |
| GPR143 | X | 0.10 * | 0.51 * | 0.20 * | 0.09 * | 0.76 * | 0.12 * | Melanogenesis and signal transduction |
| C19orf28 | 19 | 0.22 * | 0.44 * | 0.50 * | 0.21 * | 0.87 | 0.25 * | Unknown |
Ratios (LS/NS, NLS/NS, LS/NLS) are calculated based on the mean expression levels in 17 vitiligo skin and the mean expression levels in 16 normal skin. * p<0.05 after Bonferroni correction (Whitney U tests).
Ratios (LS/NS, NLS/NS, LS/NLS) are calculated based on the mean expression levels in 23 vitiligo skin and the mean expression levels in 16 normal skin. * p<0.05 (Whitney U tests).
Table 4. Up-regulated genes in vitiligo skin.
| Gene | Chrom | 1Microarray | 2qRT-PCR | Function | ||||
| osome | LS/NS | NLS/NS | LS/NLS | LS/NS | NLS/NS | LS/NLS | ||
| KLRC1 | 12 | 6.27 * | 1.71 | 3.67 * | 7.39 * | 4.71 * | 1.57 * | Natural killer cell receptor |
| KLRC2 | 12 | 5.88 * | 1.44 | 4.08 * | 8.55 * | 9.26 * | 0.92 | Natural killer cell activating receptor |
| NKG2D (KLRK1) | 12 | 2.48 * | 2.02 * | 1.23 | 6.07 * | 5.98 * | 1.01 | Natural killer cell activating receptor |
| KLRG1 | 12 | 2.46 * | 2.05 * | 1.20 | 3.92 * | 2.86 * | 1.37 | Natural killer cell receptor |
| KLRC4 | 12 | 3.91 * | 2.27 * | 1.72 | 7.91 * | 5.37 * | 1.47 * | Natural killer cell receptor |
| LPAL2 | 6 | 6.37 * | 3.28 * | 1.94 * | 7.65 * | 5.14 * | 1.49 * | Pseudogene |
| CANP | 11 | 3.56 * | 1.92 * | 1.85 * | 1.62 * | 1.69 * | 0.96 | Oxidative stress |
| DEFB103A | 8 | 2.27 * | 1.38 | 1.64 | 11.62 * | 9.27 * | 1.25 | Innate immunity |
| CLEC2B | 12 | 3.18 * | 2.01 * | 1.58 | 9.81 * | 11.13 * | 0.88 | Ligand for natural killer cell receptor |
| SP8 | 7 | 6.48 * | 4.15 * | 1.56 | 5.53 * | 8.15 * | 0.68 * | Transcription factor |
| POSTN | 13 | 5.70 * | 3.68 * | 1.55 | 4.23 * | 2.76 * | 1.53 * | Tissue injury and repair |
| RGS20 | 8 | 3.10 * | 2.02 * | 1.53 | 10.31 * | 6.11 * | 1.69 * | Signal transduction |
| EREG | 4 | 7.46 * | 5.37 * | 1.39 | 7.09 * | 6.74 * | 1.05 | Epidermal growth factor |
Ratios (LS/NS, NLS/NS, LS/NLS) are calculated based on the mean expression levels in 17 vitiligo skin and the mean expression levels in 16 normal skin. * p<0.05 after Bonferroni correction (Whitney U tests).
Ratios (LS/NS, NLS/NS, LS/NLS) are calculated based on the mean expression levels in 23 vitiligo skin and the mean expression levels in 16 normal skin. * p<0.05 (Whitney U tests).
Pathway analysis with Database for Annotation, Visualization and Integrated Discovery (DAVID) Bioinformatics Resources 6.7 [37], [38] showed that the differentially expressed genes in vitiligo lesional skin point to four enriched pathways: (1) tyrosine metabolism; (2) melanin biosynthesis, (3) natural killer cell cytotoxicity, and (4) antigen processing ( Table 5 ). This further highlights the potential importance of innate immunity and natural killer (NK) cells in vitiligo skin.
Table 5. Enriched molecular pathways in vitiligo differentially expressed genes*.
| Pathway | p-Value | p-value (Benjamini adjusted) |
| Tyrosine metabolism | 0.0025 | 0.023 |
| Antigen processing and presentation | 0.0088 | 0.039 |
| Melanogenesis | 0.012 | 0.037 |
| Natural killer cell mediated cytotoxicity | 0.022 | 0.048 |
3. Gene expression changes in non-lesional skin of vitiligo patients
Further comparisons were made between the NLS of vitiligo patients and NS from healthy subjects. As shown in Figure 1 and Tables 3 and 4 , most of the genes whose expression was down-regulated in vitiligo LS (including the melanocyte markers) showed no such change in NLS. In contrast, the expression levels of most up-regulated genes in vitiligo LS, including all of the innate immunity activation markers, were also increased in the normal appearing NLS skin of vitiligo patients, suggesting that the activation of the innate immunity is not just limited to the LS. Rather, innate immunity activation may be present throughout the entire skin surface of vitiligo patients.
4. Analysis and quantification of natural killer cells in the lesional and non-lesional skin of vitiligo patients
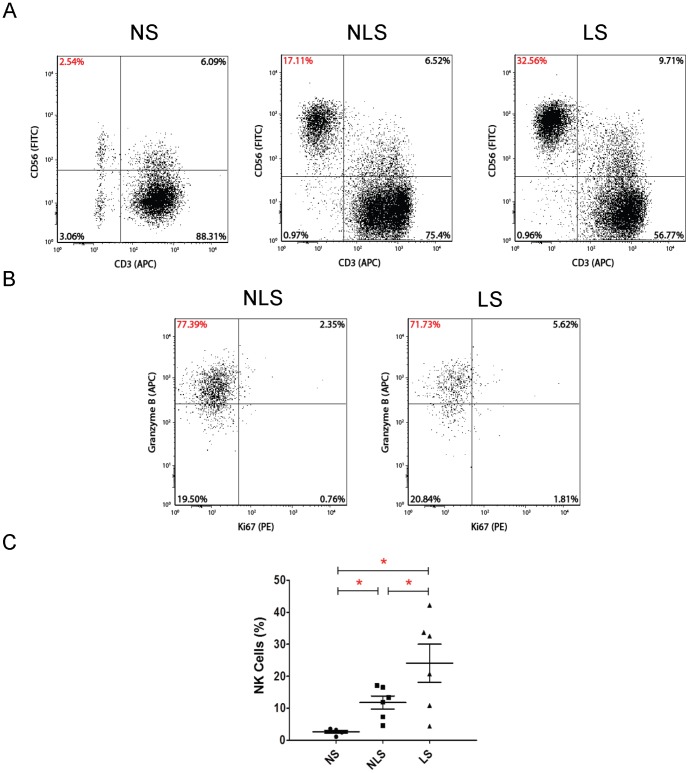
Since the gene expression analyses revealed markedly increased NK cell markers in NLS and LS of vitiligo patients, we speculated that the skin of vitiligo subjects contain abnormal infiltration of NK cells. To test this speculation, skin resident immune cells were isolated from cultured vitiligo skin explants [40], [41] and analysed for cellular compositions and activation status using antibodies against CD3 (a pan T cell marker), CD56 (a specific natural killer cell marker) and granzyme B (a cytotoxicity marker for NK cells). Skin from healthy volunteers served as the controls. The explant culture method has previously been shown to accurately reflect the in situ immune cell compositions in the tissues [41]. As shown in Figure 2A and C , NS contained only a small percentage of NK cells (less than 5% of total immune cells present). In contrast, in the skin obtained from vitiligo patients, there was a significant increase in the proportion of NK cells not only in the LS (24%), but also in the normal-appearing NLS (12%). In addition, the NK cells cultured from vitiligo skin explants exhibited high levels of the serine protease, granzyme B ( Figure 2B ), indicating that these cells are highly active and capable of exerting cytotoxicity on the target cells by contact [44].
Figure 2. Explant culture analysis of natural killer cell infiltrates in biopsies of vitiligo lesional and non-lesional skin.
Natural killer (NK) cells from 6 pairs of vitiligo skin explants (lesional and non-lesional) and 5 normal skin explants were cultured on Cellfoam matrices (see methods section) and analyzed using flow cytometry, with the gate set on total live cells A: Skin-resident CD56bright CD3-ve NK cells in normal control skin, vitiligo non-lesional skin and lesional skin by scatter plot. B: Further gating on the CD56bright CD3-ve cells revealed that majority of the NK cells in vitiligo skin were granzyme B-positive. C: Dot plot of all samples analyzed for CD56bright CD3- natural killer cells. The difference in the proportion of resident natural killer cells between normal skin and the respective vitiligo non-lesional and lesional skin is statistically significant (p = 0.0043; mean ± SEM). Comparisons between the respective groups are indicated in the figure by lines with an asterisk (*) denoting statistical significance (p<0.05). Abbreviations: NS: normal skin; NLS: non-lesional skin; LS: lesional-skin.
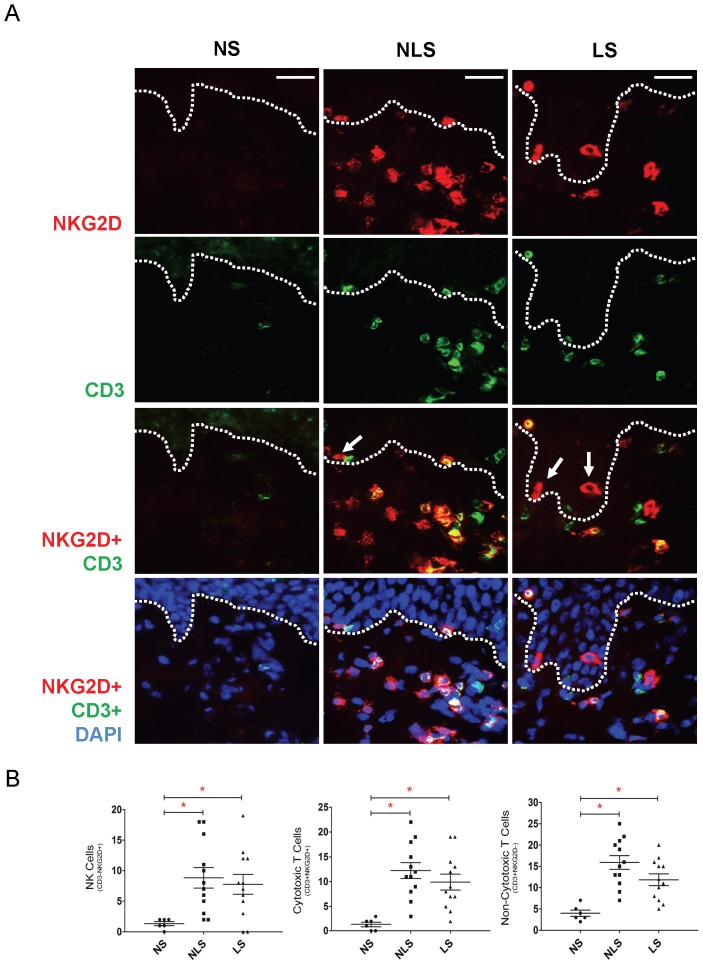
To further confirm the increased NK cell infiltration within the skin of vitiligo patients, we next examined the biopsies by immunofluorescence microscopy. As shown in Figure 3A and 3B , both lesional and non-lesional vitiligo skin harboured markedly increased number of NK cells as demonstrated by the presence of CD3-NKG2D+ cells as compared to the control skin of healthy individuals. Some of these CD3-NKG2D+ NK cells were in the epidermis and/or and were in close proximity to the basal layer (arrows), where cutaneous melanocytes normally reside. In addition, increased numbers of CD3+ NKG2D+ (cytotoxic T cells) cells and CD3+ NKG2D− cells (non-cytotoxic T cells) were also observed in vitiligo skin, which is consistent with previous findings [28] , [45] .
Figure 3. Distribution of natural killer cells and cytotoxic T cells in vitiligo lesional and non-lesional skin.
Skin biopsies taken from 12 vitiligo patients and 6 normal individuals were subjected to immunofluorescence analysis of natural killer (NK) cells. A: Micrographs showing natural killer cells (CD3−/NKG2D+) (red) present in vitiligo lesional and non-lesional skin but absent from the normal skin of healthy volunteers. Some NK cells are in close proximity to the basal epidermal layer where melanocytes reside (arrows). In addition, increased numbers of cytotoxic T cells (CD3+/NKG2D+) (yellow: co-localization of red and green) as well as non-cytotoxic T cells (CD3+/NKG2D−) (green) were also found in both vitiligo peri-lesional and lesional skin. B: Quantification of cells demonstrates a statistically significant increase in NK cells, cytotoxic T cells and non-cytotoxic T cells in vitiligo non-lesional skin (p = 0.0021, 0.0015, 0.001; mean ± SEM) and lesional skin (p = 0.021, 0.0017, 0.0023; mean ± SEM) as compared with normal skin. Color keys: Green: CD3 (a pan-T cell marker); Red: NKG2D (NK cell activation receptor); and blue: DAPI (nuclear stain). Comparisons between the respective groups are indicated in the figure by lines with an asterisk (*) denoting statistical significance (p<0.05). Abbreviations: NS: normal skin; NLS: non-lesional skin; LS: lesional-skin. Magnification: 400×; scale bar: 20 µm.
Discussion
This study examined the gene expression profiles of skin tissues from patients with vitiligo using skin biopsies of healthy individuals as the controls. A potential technical challenge of the current study arises from the fact that melanocytes only account for a small proportion of total cells in the full thickness skin biopsies, which theoretically makes it difficult to detect melanocyte-related gene changes. The fact that the down-regulated genes identified by this non-cell-targeted analysis included the melanocyte markers among the most significantly altered genes demonstrated the robustness of the current approach in picking up gene expression changes even if these changes only account for a small fraction of the total cells present in the skin biopsy tissues.
Most of the down-regulation of melanocyte-related genes (such as Tyr, TYRP1) in the well-established vitiligo lesional skin (LS) most likely is the result of melanocyte death in the lesional skin. The expression down-regulation of several other genes, including LZTS1, GPR143 and C19orf28 does not have clear explanation at the present. It is possible that these genes are also melanocyte-expressed genes although this remains to be clearly established. The down regulation of SOX10 and PLP1 in vitiligo lesional skin may also be partially explained by the fact that they can be found in melanocytes [46], [47]. However, they are also found in Schwann cells [48], raising the possibility that these cells may be damaged although direct results are lacking at present. This possibility is consistent with previous reports of degenerative changes in Schwann cells in vitiligo skin as revealed by electron microscopy [49], [50].
The significance of the down-regulation of some melanocyte related genes (such as DCT, SOX10 and PLP1) in vitiligo non-lesional skin as compared with normal skin of healthy individual is not entirely clear, but may represent subclinical melanocyte damage even in the lack of overt death of melanocytes. Alternatively, they may reflect inherent abnormalities present in the vitiligo individuals' melanocytes, as have been suggested to be present by the observation of gene expression abnormalities in purified melanocytes from vitiligo individuals [29], [30], [31], [32].
The unique findings from the current study include notably the discovery of the up-regulation of multiple genes of the killer cell lectin-like receptor (KLR) family including multiple NK cell activation markers such as KLRK1 (also known as NKG2D), KLRC2 and KLRC4 [51], [52], [53]. NK cells are an important component of the innate immune system. Although the most notable role of NK cells is in the defense against bacterial, viral and parasitic infections, there is strong evidence that NK cells play an important role in the initiation and/or perpetuation of autoimmune diseases [54], [55]. In particular, elevated numbers of CD56bright NK cells are found in the blood and target lesions of patients with systemic lupus erythematosus and rheumatoid arthritis [56], [57], [58]. NK cell receptors and their ligands are also implicated in autoimmune cholangitis, multiple sclerosis and psoriasis [59], [60], [61], [62].
Previous studies have shown an increase in the number of circulating NK cells in the blood of vitiligo patients [63], [64], [65], [66], [67]. Our present work has demonstrated the infiltration of NK cells in the skin microenvironment of melanocytes in both the lesional and non-lesional area of the skin of vitiligo patients. Further, these cells express high levels of granzyme B, which is characteristic of activated NK cells [44], [68], [69], [70]. In addition to the commonly attributed cytotoxic role via activation of the caspase pathway, granzyme B has been implicated in the generation and presentation of auto-antigens [71].
The fact that increased NK cell infiltration was observed in the normal-appearing NLS of vitiligo patients may indicate an unfavorable generalized melanocyte-microenvironment in the skin compartment for the melanocyte to survive. However, what gives rise to the NK cell/innate immunity activation in vitiligo skin is not clear. It has been documented that NK cells respond to cellular signals released by cells under stress [72]. In particular, it has been shown previously that ligands for the NKG2D receptor, which are MHC class I-like proteins in the ULBP and MIC families, can be induced in stressed cells and via the inflammatory cascade, and can result in marked alteration in the immune microenvironment [51], [73]. One of the well-known ligands in the ULBP family—ULBP2, has been up-regulated in vitiligo skin in our microarray analysis as well (around 1.5 folds increase in vitiligo skin), although the data did not meet our strict cut-off criteria (at 2 fold). Further, our transcriptome analysis has revealed increased expression of stress indicators (CANP and POSTN) in vitiligo skin, especially the oxidative stress marker CANP, which is consistent with (although not directly confirm) the oxidative stress theory of vitiligo pathogenesis [74]. The current study, combined with previously reported abnormal gene expression profile of melanocytes isolated from vitiligo skin [29], [30], [31], [32], raised the speculation that aberrant expression of stress markers on melanocytes (either as a result of intrinsic functional defect or external stimuli) may recruit and activate NK cells in the skin microenvironment, which may play an important role in the pathogenic process of vitiligo. CLEC2B gene, which encodes an activating ligand of the NK cells, is also found in our study to be up-regulated in vitiligo skin. This gene is mainly expressed by monocytes and macrophages, and can activate NK cells by binding to the NK cell-activating receptor NKp80 [75], [76], [77]. Since CLEC2B can be up-regulated by toll-like receptor stimulation [76], and topical imiquimod (an activator of the toll-like receptors) has been shown to induce vitiligo [78], [79], [80], we speculate that the increased expression of CLEC2B is a reflection of the activated innate immune system in vitiligo skin.
In addition to increased NK cells, this study revealed the presence of cytotoxic T cells in both vitiligo LS and NLS ( Figure 3 ), whereas previous reports documented that these cells were present in the lesions and at the advancing borders of the lesions [28], [45]. Although our microarray analysis has also found increased expression of genes related to the Th1 pathway (including IFN-γ and IL-12), the Th17 pathway (IL-6), as well as genes associated with inflammation in general in vitiligo skin, they did not meet our strict cut-off criteria (2 or more fold changed with p<0.05 with Bonferonni Correction) and thus were not shown. It is currently unknown whether innate immune activation in vitiligo is a consequence of adaptive immune reaction to the melanocytes, or a contributing factor leading to the activation of adaptive immune reaction. More research is needed in the future to clarify this issue.
To our knowledge, this is the first genomic expression analysis on the skin of patients with vitiligo. Our results strengthened the notion that immunity and inflammation play important roles in vitiligo pathogenesis. Moreover, our study specifically highlights the potential pathogenic role of the aberrantly heightened innate immunity, especially NK cells, in the local microenvironment of melanocytes in vitiligo skin. Further studies in the future are needed to verify if increased NK cells and heightened innate immunity are the direct cause of melanocyte death in vitiligo. Finally, the observation of activated innate immunity in the normal appearing non-lesional skin far away from the lesional border suggests that therapeutically targeting the activated innate immune responses in both lesional and non-lesional vitligo skin may represent a viable approach for developing vitiligo therapies in the future.
Acknowledgments
RY, SZ, and SW are Canadian Institutes of Health Research-University of British Columbia Skin Research Training Centre Scholars; YZ is an Establish Clinician Scientist of Vancouver Hospital Foundation. JPD is a Michael Smith Foundation for Health Research Scholar and Child and Family Research Institute Scholar.
Funding Statement
This work was supported by grants from National Natural Sciences Foundation of China (No. 30628021), Canadian Institutes of Health Research, Canadian Dermatology Foundation, and Astellas Pharma Research Competition, Canada. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Whitton ME, Ashcroft DM, Gonzalez U (2008) Therapeutic interventions for vitiligo. J Am Acad Dermatol 59: 713–717. [DOI] [PubMed] [Google Scholar]
- 2. Grimes PE (2005) New insights and new therapies in vitiligo. JAMA 293: 730–735. [DOI] [PubMed] [Google Scholar]
- 3. Abu Tahir M, Pramod K, Ansari SH, Ali J (2010) Current remedies for vitiligo. Autoimmun Rev 9: 516–520. [DOI] [PubMed] [Google Scholar]
- 4. Porter JR, Beuf AH (1991) Racial variation in reaction to physical stigma: a study of degree of disturbance by vitiligo among black and white patients. J Health Soc Behav 32: 192–204. [PubMed] [Google Scholar]
- 5. Thompson AR, Kent G, Smith JA (2002) Living with vitiligo: dealing with difference. Br J Health Psychol 7: 213–225. [DOI] [PubMed] [Google Scholar]
- 6. Jin Y, Mailloux CM, Gowan K, Riccardi SL, LaBerge G, et al. (2007) NALP1 in vitiligo-associated multiple autoimmune disease. N Engl J Med 356: 1216–1225. [DOI] [PubMed] [Google Scholar]
- 7. Spritz RA (2011) The genetics of vitiligo. J Invest Dermatol 131: E18–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jin Y, Birlea SA, Fain PR, Gowan K, Riccardi SL, et al. (2010) Variant of TYR and autoimmunity susceptibility loci in generalized vitiligo. N Engl J Med 362: 1686–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jin Y, Birlea SA, Fain PR, Spritz RA (2007) Genetic variations in NALP1 are associated with generalized vitiligo in a Romanian population. J Invest Dermatol 127: 2558–2562. [DOI] [PubMed] [Google Scholar]
- 10. Zhu KJ, Lv YM, Yin XY, Wang ZX, Sun LD, et al. (2011) Psoriasis regression analysis of MHC loci identifies shared genetic variants with vitiligo. PLoS One 6: e23089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Quan C, Ren YQ, Xiang LH, Sun LD, Xu AE, et al. (2010) Genome-wide association study for vitiligo identifies susceptibility loci at 6q27 and the MHC. Nat Genet 42: 614–618. [DOI] [PubMed] [Google Scholar]
- 12. Ren Y, Yang S, Xu S, Gao M, Huang W, et al. (2009) Genetic variation of promoter sequence modulates XBP1 expression and genetic risk for vitiligo. PLoS Genet 5: e1000523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liang Y, Yang S, Zhou Y, Gui J, Ren Y, et al. (2007) Evidence for two susceptibility loci on chromosomes 22q12 and 6p21-p22 in Chinese generalized vitiligo families. J Invest Dermatol 127: 2552–2557. [DOI] [PubMed] [Google Scholar]
- 14. Wang X, Erf GF (2003) Melanocyte-specific cell mediated immune response in vitiliginous Smyth line chickens. J Autoimmun 21: 149–160. [DOI] [PubMed] [Google Scholar]
- 15. Slominski A, Paus R, Bomirski A (1989) Hypothesis: possible role for the melatonin receptor in vitiligo: discussion paper. J R Soc Med 82: 539–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xu S, Zhou Y, Yang S, Ren Y, Zhang C, et al. (2010) Platelet-derived growth factor receptor alpha gene mutations in vitiligo vulgaris. Acta Derm Venereol 90: 131–135. [DOI] [PubMed] [Google Scholar]
- 17. Spritz RA, Gowan K, Bennett DC, Fain PR (2004) Novel vitiligo susceptibility loci on chromosomes 7 (AIS2) and 8 (AIS3), confirmation of SLEV1 on chromosome 17, and their roles in an autoimmune diathesis. Am J Hum Genet 74: 188–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang XJ, Chen JJ, Liu JB (2005) The genetic concept of vitiligo. J Dermatol Sci 39: 137–146. [DOI] [PubMed] [Google Scholar]
- 19. Boelaert K, Newby PR, Simmonds MJ, Holder RL, Carr-Smith JD, et al. (2010) Prevalence and relative risk of other autoimmune diseases in subjects with autoimmune thyroid disease. Am J Med 123: e181–189, 183, e181-189. [DOI] [PubMed] [Google Scholar]
- 20. Deretzi G, Kountouras J, Koutlas E, Zavos C, Polyzos S, et al. (2010) Familial prevalence of autoimmune disorders in multiple sclerosis in Northern Greece. Mult Scler 16: 1091–1101. [DOI] [PubMed] [Google Scholar]
- 21. Kocer B, Nazliel B, Oztas M, Batur HZ (2009) Vitiligo and multiple sclerosis in a patient treated with interferon beta-1a: a case report. Eur J Neurol 16: e78–79. [DOI] [PubMed] [Google Scholar]
- 22. Ramagopalan SV, Dyment DA, Valdar W, Herrera BM, Criscuoli M, et al. (2007) Autoimmune disease in families with multiple sclerosis: a population-based study. Lancet Neurol 6: 604–610. [DOI] [PubMed] [Google Scholar]
- 23. Rashtak S, Pittelkow MR (2008) Skin involvement in systemic autoimmune diseases. Curr Dir Autoimmun 10: 344–358. [DOI] [PubMed] [Google Scholar]
- 24. Harris JE, Harris TH, Weninger W, Wherry EJ, Hunter CA, et al. (2012) A Mouse Model of Vitiligo with Focused Epidermal Depigmentation Requires IFN-gamma for Autoreactive CD8(+) T-Cell Accumulation in the Skin. J Invest Dermatol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Glassman SJ (2011) Vitiligo, reactive oxygen species and T-cells. Clin Sci (Lond) 120: 99–120. [DOI] [PubMed] [Google Scholar]
- 26. Kemp EH, Emhemad S, Akhtar S, Watson PF, Gawkrodger DJ, et al. (2011) Autoantibodies against tyrosine hydroxylase in patients with non-segmental (generalised) vitiligo. Exp Dermatol 20: 35–40. [DOI] [PubMed] [Google Scholar]
- 27. Le Poole IC, Luiten RM (2008) Autoimmune etiology of generalized vitiligo. Curr Dir Autoimmun 10: 227–243. [DOI] [PubMed] [Google Scholar]
- 28. Wang CQ, Cruz-Inigo AE, Fuentes-Duculan J, Moussai D, Gulati N, et al. (2011) Th17 cells and activated dendritic cells are increased in vitiligo lesions. PLoS One 6: e18907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kitamura R, Tsukamoto K, Harada K, Shimizu A, Shimada S, et al. (2004) Mechanisms underlying the dysfunction of melanocytes in vitiligo epidermis: role of SCF/KIT protein interactions and the downstream effector, MITF-M. J Pathol 202: 463–475. [DOI] [PubMed] [Google Scholar]
- 30. Kingo K, Aunin E, Karelson M, Philips MA, Ratsep R, et al. (2007) Gene expression analysis of melanocortin system in vitiligo. J Dermatol Sci 48: 113–122. [DOI] [PubMed] [Google Scholar]
- 31. Kingo K, Aunin E, Karelson M, Ratsep R, Silm H, et al. (2008) Expressional changes in the intracellular melanogenesis pathways and their possible role in the pathogenesis of vitiligo. J Dermatol Sci 52: 39–46. [DOI] [PubMed] [Google Scholar]
- 32. Stromberg S, Bjorklund MG, Asplund A, Rimini R, Lundeberg J, et al. (2008) Transcriptional profiling of melanocytes from patients with vitiligo vulgaris. Pigment Cell Melanoma Res 21: 162–171. [DOI] [PubMed] [Google Scholar]
- 33. Spritz RA (2007) The genetics of generalized vitiligo and associated autoimmune diseases. Pigment Cell Res 20: 271–278. [DOI] [PubMed] [Google Scholar]
- 34. Wang Y, Su M, Zhou LL, Tu P, Zhang X, et al. (2011) Deficiency of SATB1 expression in Sezary cells causes apoptosis resistance by regulating FasL/CD95L transcription. Blood 117: 3826–3835. [DOI] [PubMed] [Google Scholar]
- 35. Kennah E, Ringrose A, Zhou LL, Esmailzadeh S, Qian H, et al. (2009) Identification of tyrosine kinase, HCK, and tumor suppressor, BIN1, as potential mediators of AHI-1 oncogene in primary and transformed CTCL cells. Blood 113: 4646–4655. [DOI] [PubMed] [Google Scholar]
- 36. Ringrose A, Zhou Y, Pang E, Zhou L, Lin AE, et al. (2006) Evidence for an oncogenic role of AHI-1 in Sezary syndrome, a leukemic variant of human cutaneous T-cell lymphomas. Leukemia 20: 1593–1601. [DOI] [PubMed] [Google Scholar]
- 37. Huang da W, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57. [DOI] [PubMed] [Google Scholar]
- 38. Huang da W, Sherman BT, Lempicki RA (2009) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhou Y, Lee HS, Kooshesh F, Fujisawa H, Sauder DN, et al. (1996) Effects of UVB irradiation on keratinocyte growth factor (KGF) and receptor (KGFR) expression in cultured human keratinocytes. Exp Dermatol 5: 138–144. [DOI] [PubMed] [Google Scholar]
- 40. Broady R, Yu J, Chow V, Tantiworawit A, Kang C, et al. (2010) Cutaneous GVHD is associated with the expansion of tissue-localized Th1 and not Th17 cells. Blood 116: 5748–5751. [DOI] [PubMed] [Google Scholar]
- 41. Clark RA, Chong BF, Mirchandani N, Yamanaka K, Murphy GF, et al. (2006) A novel method for the isolation of skin resident T cells from normal and diseased human skin. J Invest Dermatol 126: 1059–1070. [DOI] [PubMed] [Google Scholar]
- 42. Yuan L, Zhou LJ, Li WM (2010) Oxidative stress-induced apoptosis is mediated by calpains in alcoholic cardiomyopathy. Int J Cardiol [DOI] [PubMed] [Google Scholar]
- 43. Dorn GW 2nd (2007) Periostin and myocardial repair, regeneration, and recovery. N Engl J Med 357: 1552–1554. [DOI] [PubMed] [Google Scholar]
- 44. Afonina IS, Cullen SP, Martin SJ (2010) Cytotoxic and non-cytotoxic roles of the CTL/NK protease granzyme B. Immunol Rev 235: 105–116. [DOI] [PubMed] [Google Scholar]
- 45. van den Boorn JG, Konijnenberg D, Dellemijn TA, van der Veen JP, Bos JD, et al. (2009) Autoimmune destruction of skin melanocytes by perilesional T cells from vitiligo patients. J Invest Dermatol 129: 2220–2232. [DOI] [PubMed] [Google Scholar]
- 46. Hoek KS, Schlegel NC, Eichhoff OM, Widmer DS, Praetorius C, et al. (2008) Novel MITF targets identified using a two-step DNA microarray strategy. Pigment Cell Melanoma Res 21: 665–676. [DOI] [PubMed] [Google Scholar]
- 47. Nonaka D, Chiriboga L, Rubin BP (2008) Sox10: a pan-schwannian and melanocytic marker. Am J Surg Pathol 32: 1291–1298. [DOI] [PubMed] [Google Scholar]
- 48. Kamholz J, Sessa M, Scherer S, Vogelbacker H, Mokuno K, et al. (1992) Structure and expression of proteolipid protein in the peripheral nervous system. J Neurosci Res 31: 231–244. [DOI] [PubMed] [Google Scholar]
- 49. Al'Abadie MS, Warren MA, Bleehen SS, Gawkrodger DJ (1995) Morphologic observations on the dermal nerves in vitiligo: an ultrastructural study. Int J Dermatol 34: 837–840. [DOI] [PubMed] [Google Scholar]
- 50. Breathnach AS, Bor S, Wyllie LM (1966) Electron microscopy of peripheral nerve terminals and marginal melanocytes in vitiligo. J Invest Dermatol 47: 125–140. [DOI] [PubMed] [Google Scholar]
- 51. Champsaur M, Lanier LL (2010) Effect of NKG2D ligand expression on host immune responses. Immunol Rev 235: 267–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Huang H, Wang X, Zhang Y, Zheng X, Wei H, et al. (2010) Up-regulation of NKG2F receptor, a functionally unknown killer receptor, of human natural killer cells by interleukin-2 and interleukin-15. Oncol Rep 24: 1043–1048. [DOI] [PubMed] [Google Scholar]
- 53. Kim DK, Kabat J, Borrego F, Sanni TB, You CH, et al. (2004) Human NKG2F is expressed and can associate with DAP12. Mol Immunol 41: 53–62. [DOI] [PubMed] [Google Scholar]
- 54. French AR, Yokoyama WM (2004) Natural killer cells and autoimmunity. Arthritis Res Ther 6: 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Perricone R, Perricone C, De Carolis C, Shoenfeld Y (2008) NK cells in autoimmunity: a two-edg'd weapon of the immune system. Autoimmun Rev 7: 384–390. [DOI] [PubMed] [Google Scholar]
- 56. Dalbeth N, Callan MF (2002) A subset of natural killer cells is greatly expanded within inflamed joints. Arthritis Rheum 46: 1763–1772. [DOI] [PubMed] [Google Scholar]
- 57. Pridgeon C, Lennon GP, Pazmany L, Thompson RN, Christmas SE, et al. (2003) Natural killer cells in the synovial fluid of rheumatoid arthritis patients exhibit a CD56bright,CD94bright,CD158negative phenotype. Rheumatology (Oxford) 42: 870–878. [DOI] [PubMed] [Google Scholar]
- 58. Schepis D, Gunnarsson I, Eloranta ML, Lampa J, Jacobson SH, et al. (2009) Increased proportion of CD56bright natural killer cells in active and inactive systemic lupus erythematosus. Immunology 126: 140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yamagiwa S, Kamimura H, Ichida T (2009) Natural killer cell receptors and their ligands in liver diseases. Med Mol Morphol 42: 1–8. [DOI] [PubMed] [Google Scholar]
- 60. Karlsen TH, Boberg KM, Olsson M, Sun JY, Senitzer D, et al. (2007) Particular genetic variants of ligands for natural killer cell receptors may contribute to the HLA associated risk of primary sclerosing cholangitis. J Hepatol 46: 899–906. [DOI] [PubMed] [Google Scholar]
- 61. Trachtenberg EA (2009) Understanding the role of natural killer cell receptors and their human leukocyte antigen ligands in multiple sclerosis. Ann Neurol 65: 626–628. [DOI] [PubMed] [Google Scholar]
- 62. Jones CD, Guckian M, el-Ghorr AA, Gibbs NK, Norval M (1996) Effects of phototherapy on the production of cytokines by peripheral blood mononuclear cells and on systemic antibody responses in patients with psoriasis. Photodermatol Photoimmunol Photomed 12: 204–210. [DOI] [PubMed] [Google Scholar]
- 63. Durham-Pierre DG, Walters CS, Halder RM, Pham HN, Vanderpool EA (1995) Natural killer cell and lymphokine-activated killer cell activity against melanocytes in vitiligo. J Am Acad Dermatol 33: 26–30. [DOI] [PubMed] [Google Scholar]
- 64. Ghoneum M, Grimes PE, Gill G, Kelly AP (1987) Natural cell-mediated cytotoxicity in vitiligo. J Am Acad Dermatol 17: 600–605. [DOI] [PubMed] [Google Scholar]
- 65. Mozzanica N, Villa ML, Foppa S, Vignati G, Cattaneo A, et al. (1992) Plasma alpha-melanocyte-stimulating hormone, beta-endorphin, met-enkephalin, and natural killer cell activity in vitiligo. J Am Acad Dermatol 26: 693–700. [DOI] [PubMed] [Google Scholar]
- 66. Mozzanica N, Frigerio U, Negri M, Tadini G, Villa ML, et al. (1989) Circadian rhythm of natural killer cell activity in vitiligo. J Am Acad Dermatol 20: 591–596. [DOI] [PubMed] [Google Scholar]
- 67. Basak PY, Adiloglu AK, Koc IG, Tas T, Akkaya VB (2008) Evaluation of activatory and inhibitory natural killer cell receptors in non-segmental vitiligo: a flow cytometric study. J Eur Acad Dermatol Venereol 22: 970–976. [DOI] [PubMed] [Google Scholar]
- 68. Tak PP, Kummer JA, Hack CE, Daha MR, Smeets TJ, et al. (1994) Granzyme-positive cytotoxic cells are specifically increased in early rheumatoid synovial tissue. Arthritis Rheum 37: 1735–1743. [DOI] [PubMed] [Google Scholar]
- 69. Ronday HK, van der Laan WH, Tak PP, de Roos JA, Bank RA, et al. (2001) Human granzyme B mediates cartilage proteoglycan degradation and is expressed at the invasive front of the synovium in rheumatoid arthritis. Rheumatology (Oxford) 40: 55–61. [DOI] [PubMed] [Google Scholar]
- 70. Goldbach-Mansky R, Suson S, Wesley R, Hack CE, El-Gabalawy HS, et al. (2005) Raised granzyme B levels are associated with erosions in patients with early rheumatoid factor positive rheumatoid arthritis. Ann Rheum Dis 64: 715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Darrah E, Rosen A (2010) Granzyme B cleavage of autoantigens in autoimmunity. Cell Death Differ 17: 624–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Long EO, Rajagopalan S (2002) Stress signals activate natural killer cells. J Exp Med 196: 1399–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Strid J, Roberts SJ, Filler RB, Lewis JM, Kwong BY, et al. (2008) Acute upregulation of an NKG2D ligand promotes rapid reorganization of a local immune compartment with pleiotropic effects on carcinogenesis. Nat Immunol 9: 146–154. [DOI] [PubMed] [Google Scholar]
- 74. Boissy RE, Spritz RA (2009) Frontiers and controversies in the pathobiology of vitiligo: separating the wheat from the chaff. Exp Dermatol 18: 583–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Renedo M, Arce I, Montgomery K, Roda-Navarro P, Lee E, et al. (2000) A sequence-ready physical map of the region containing the human natural killer gene complex on chromosome 12p12.3-p13.2. Genomics 65: 129–136. [DOI] [PubMed] [Google Scholar]
- 76. Welte S, Kuttruff S, Waldhauer I, Steinle A (2006) Mutual activation of natural killer cells and monocytes mediated by NKp80-AICL interaction. Nat Immunol 7: 1334–1342. [DOI] [PubMed] [Google Scholar]
- 77. Kuttruff S, Koch S, Kelp A, Pawelec G, Rammensee HG, et al. (2009) NKp80 defines and stimulates a reactive subset of CD8 T cells. Blood 113: 358–369. [DOI] [PubMed] [Google Scholar]
- 78. Jacob SE, Blyumin M (2008) Vitiligo-like hypopigmentation with poliosis following treatment of superficial basal cell carcinoma with imiquimod. Dermatol Surg 34: 844–845. [DOI] [PubMed] [Google Scholar]
- 79. Serrao VV, Paris FR, Feio AB (2008) Genital vitiligo-like depigmentation following use of imiquimod 5% cream. Eur J Dermatol 18: 342–343. [DOI] [PubMed] [Google Scholar]
- 80. Senel E, Seckin D (2007) Imiquimod-induced vitiligo-like depigmentation. Indian J Dermatol Venereol Leprol 73: 423. [DOI] [PubMed] [Google Scholar]