Abstract
Background
Rabbits latent with HSV-1 strain McKrae spontaneously shed infectious virus and viral DNA into their tears and develop recurrent herpetic-specific corneal lesions. The rabbit eye model has been used for many years to assess acute ocular infections and pathogenesis, antiviral efficacy, as well as latency, reactivation, and recurrent eye diseases. This study used real-time PCR to quantify HSV-1 DNA in the saliva and tears of rabbits latent with HSV-1 McKrae.
Methods
New Zealand white rabbits used were latent with HSV-1 strain McKrae and had no ocular or oral pathology. Scarified corneas were topically inoculated with HSV-1. Eye swabs and saliva were taken from post inoculation (PI) days 28 through 49 (22 consecutive days). Saliva samples were taken four times each day from each rabbit and the DNA extracted was pooled for each rabbit for each day; one swab was taken daily from each eye and DNA extracted. Real-time PCR was done on the purified DNA samples for quantification of HSV-1 DNA copy numbers. Data are presented as copy numbers for each individual sample, plus all the copy numbers designated as positive, for comparison between left eye (OS), right eye (OD), and saliva.
Results
The saliva and tears were taken from 9 rabbits and from 18 eyes and all tested positive at least once. Saliva was positive for HSV-1 DNA at 43.4% (86/198) and tears were positive at 28.0% (111/396). The saliva positives had 48 episodes and the tears had 75 episodes. The mean copy numbers ± the SEM for HSV-1 DNA in saliva were 3773 ± 2019 and 2294 ± 869 for tears (no statistical difference).
Conclusion
Rabbits latent with strain McKrae shed HSV-1 DNA into their saliva and tears. HSV-1 DNA shedding into the saliva was similar to humans. This is the first evidence that documents HSV-1 DNA in the saliva of latent rabbits.
Keywords: HSV-1, Rabbit, Saliva, Tears, Spontaneous HSV-1 shedding, Real-time-PCR
Background
The rabbit eye model has been used for HSV-1 studies since 1960 [1-7]. One of the first studies in 1965 on HSV-1 latency in the rabbit eye model was by Laibson and Kibnick [4]. Since 1978 [8], we have utilized the rabbit eye model for HSV-1 studies on antiherpetic chemotherapy [8-13], HSV-1 latency [14,15], and spontaneous and induced viral reactivations and recurrent ocular herpetic disease [16-35]. We have also investigated up-regulation and down-regulation of host gene expression [36,37] and alterations in reactivation phenotypes in HSV-1 genomic structure by histone modifications as a result of mutations in the viral genome [38-41] that take place following induction stimuli that could induce reactivation [42-44].
Our previous studies [45-51] have focused on the cornea, tears, and trigeminal ganglia [52-54]. In this report, we document for the first time the detection of HSV-1 DNA in the saliva of rabbits latent with HSV-1 McKrae. The McKrae strain is known to be a high phenotypic reactivator in the rabbit eye model [24,53-55]. HSV-1 DNA and infectious virus from saliva of patients have been reported in many studies, the first of which appeared in 1953. We have reviewed the human studies on saliva, 10 studies on infectious virus, and 18 studies in HSV-1 DNA (Tables 1[56-64] and 2[65-77]). Miller and Danaher reviewed most of those cited [[78]. The “gold” standard of proof of HSV-1 is detection of infectious virus. The sensitivity of the PCR, as well as the increase in frequency of sampling per day, has increased the percent of positives of HSV-1 and HSV-2 to almost 100% of humans tested [73,77,79-81].
Table 1.
Asymptomatic shedding of infectious HSV-1 detected by cell culture from mouth swabs of healthy subjects
| Author(s), Year | N | Individuals: total positive/total subjects | Shedding frequency: total positiveswabs/total swabs | Mouth swabs frequency: numberof swabs/subject |
|---|---|---|---|---|
| Buddingh et al., 1953
[56] |
368* |
30/368 (8.2%) |
30/368 (8.2%) |
one |
| Buddingh et al., 1953
[56] |
185** |
5/185 (2.4%) |
5/185 (2.7%) |
one |
| Kaufman et al., 1967
[57] |
35 |
6/35 (17.1%) |
6/700 (0.9%) |
20 |
| Lindgren et al., 1968
[58] |
418 |
8/418 (1.9%) |
8/2204 (0.4%) |
5-6 |
| Douglas & Couch, 1970
[59] |
10 |
8/10 (80%) |
11/494 (2.2%) |
~49 |
| Hatherley et al., 1980
[60] |
384 |
37/384 (9.6%) |
47/1536 (3.1%) |
4 |
| Spruance, 1984
[61] |
8 |
8/8 (100%) |
47/637 (7.4%) |
105 |
| Kameyama et al., 1988
[62] |
110 |
5/110 (4.5%) |
70/7805 (0.9%) |
71 |
| Tateishi et al., 1994
[63] |
1000 |
27/1000 (2.7%) |
27/1000 (2.7%) |
one |
| Okinaga, 2000
[64] |
10 |
4/10 (40%) |
4/870 (0.5%) |
87 |
| Total | 2528 | 138/2528 (5.5%) | 255/15799 (1.6%) |
*adults.
**children.
Table 2.
Asymptomatic shedding of HSV-1 detected by PCR from mouth swabs (saliva) of healthy subjects
| Authors, Year | N | Individuals: total positive/total subjects | Shedding frequency: total positiveswabs/total swabs | Mouth swab frequency: numberof swabs/subject |
|---|---|---|---|---|
| Robinson et al., 1992
[65] |
12 |
12/12 (100%) |
0/0 (0%) |
0 |
| Robinson et al., 1992
[65] |
12* |
0/12 (0%) |
0/0 (0%) |
0 |
| Tateishi et al., 1994
[63] |
1000 |
46/1000 (4.6%) |
46/1000 (4.6%) |
one |
| Kriesel et al., 1994
[66] |
27 |
3/27 (11%) |
3/27 (11%) |
one |
| Lee et al., 1996
[67] |
87 |
12/87 (13.8%) |
12/87 (13.8%) |
one |
| Knaup et al., 2000
[68] |
30 |
19/30 (63.3%) |
39/290 (13.5%) |
9-10 |
| Druce et al., 2002
[69] |
477 |
43/477 (9%) |
43/477 (9%) |
one |
| Gleeson et al., 2002
[70] |
14 |
0/14 (0%) |
0/0 (0%) |
0 |
| Youssef et al., 2002
[71] |
5 |
1/5 (20%) |
1/5 (20%) |
one |
| Miller et al., 2004
[72] |
123 |
11/123 (8.9%) |
11/123 (8.9%) |
one |
| Miller et al., 2004
[72] |
63** |
13/63 (21%) |
17/217 (7.8%) |
3-4 |
| Kaufman et al., 2005
[73] |
50 |
46/50 (92%) |
1020/2712 (37.6%) |
54 |
| Miller et al., 2005
[74] |
58 |
1/58 (1.7%) |
1/58 (1.7%) |
one |
| da Silva et al., 2005
[75] |
25 |
25/25 (100%) |
162/309(52.4%) |
12-13 |
| Lin et al., 2005
[76] |
60 |
4/60 (6.7%) |
4/60 (6.7%) |
one |
| Kumar et al., 2009
[77] |
14***** |
12/14 (86%) |
357/840 (42%) |
60 |
| Kumar et al., 2009
[77] |
15*** |
15/15 (100%) |
465/900 (52%) |
60 |
| Kumar et al., 2009
[77] |
16**** |
15/16 (94%) |
701/961 (73%) |
60 |
| Totals | 2076 | 278/2088 (13%) | 2882/8066 (36%) |
*Had no clinical history of HSV-1.
**Had dental procedure prior to mouth swab.
*** Received Valtrex treatment + Placebo treatment.
****Received Valtrex treatment + Aspirin treatment.
***** Received 2 Placebo treatments.
The Kumar et al., 2009 study was double blind. Placebo and control had trials in healthy adults.
Results and discussion
Starting on PI day 28 and continuing for 22 consecutive days until PI day 49, saliva and tear swabs were taken. Real-time PCR was done on all of the saliva and tear samples. All nine rabbits shed HSV-1 DNA at least once in their tears and saliva (summarized in Table 3). Cumulative data showed 43.4% (86/198) of saliva samples were positive for HSV-1 DNA and 28.0% (111/396) of tear samples were positive.
Table 3.
Summary of data from saliva and tears
|
Characteristics |
HSV-1 Strain McKrae |
|
|---|---|---|
| Saliva/ mouth | Tears/eyes (OD & OS) | |
|
Rabbits (+/total) |
9/9 (100%) |
9/9 (100%) |
|
Swabs (+/total) |
9/9 (100%) |
18/18 (100%) |
|
Total positive swabs/total swabs |
86/198 |
111/396 |
|
Total positive swab (%) |
(43.43%) |
(28.03%) |
|
Mean ± SEM copynumbers |
3773 ± 2019 |
2287 ± 868 |
|
Lowest copy number |
28 |
20 |
|
Highest copy number |
337,894 |
205,000 |
|
Median copy number |
439 |
553 |
|
Total number of episodes |
49 |
75 |
|
Range of positives |
6-15 |
1-9 |
|
Average positives/days (Total positives/days of swabs) |
86/22 = 3.9 |
111/44 = 2.5 |
| Average duration of shedding(Total positive/rabbit saliva orrabbit eyes) | 86/9 = 9.6 days | 111/18 = 6.2 days |
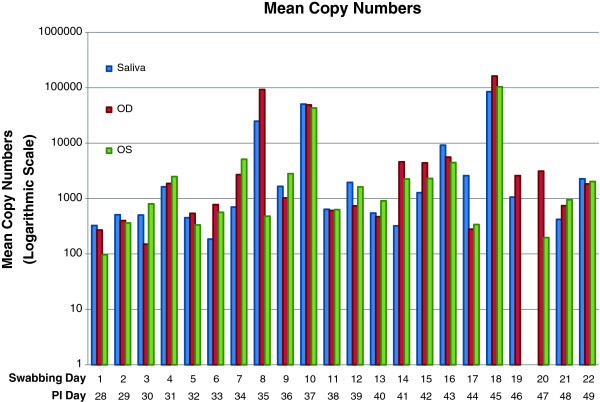
Figure 1 displays the copy numbers on a logarithmic scale obtained from left eye (OS), right eye (OD), and saliva for each day. The mean copy number ± the SEM of HSV-1 DNA in saliva was 3773 ± 2019 and for tears 2284 ± 868. The median copy number for saliva was 439 and the median copy number for tears was 553. The real-time PCR copy numbers for tears ranged from a low of 20 to a high 205,000. The real-time PCR copy numbers for saliva ranged from a low of 28 to a high 337, 900.
Figure 1.
Mean copy numbers of HSV-1 DNA per day post inoculation, taken from left eye (OS), right eye (OD), and saliva of nine rabbits.
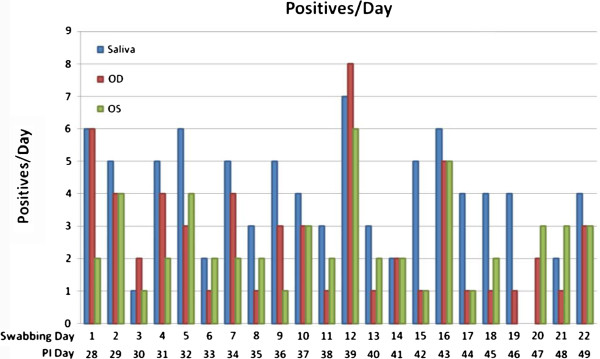
Figure 2 displays data showing positives over totals from OD, OS, and saliva, for PI days 28 to 49. Episodes of shedding are defined by finding zero virus, followed by positive virus, followed by zero virus. There were 49 episodes of shedding in saliva and 75 episodes in tear swabs. The average duration of shedding for saliva was 9.6 (86/9) days and for eyes the average duration of shedding was 6.2 (111/18) days.
Figure 2.
Number of positives per day post inoculation, taken from left eye (OS), right eye (OD), and saliva of nine rabbits.
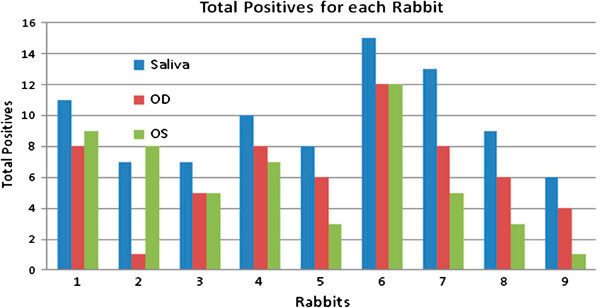
Total positives from individual rabbits are shown in Figure 3. Each positive value is the total copy number by real-time PCR for each swab. Figure 3 provides data for saliva and for left and right eyes for each rabbit.
Figure 3.
Total number of positives per rabbit, from left eye (OS), right eye (OD), and saliva.
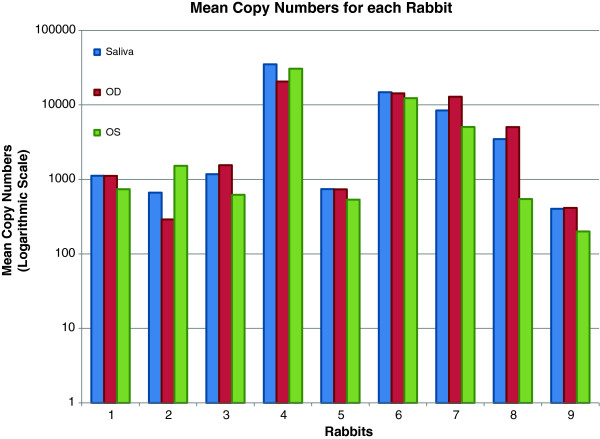
Figure 4 shows the mean copy number for each of the 9 rabbits for saliva, right eye, and left eye.
Figure 4.
Mean copy numbers of HSV-1 DNA for each rabbit for left eye (OS), right eye (OD), and saliva. All rabbits tested positive for virus in both tears and saliva at least once.
We speculate that the potential source of HSV-1 that appears in the tears and saliva could be from many ganglia in the head and neck region. Richter et al [82]. assessed 8 different types of human ganglia in the head and neck regions of 36 formalin-fixed cadavers, assaying for HSV-1, HSV-2 and VZV from a total of 415 ganglia samples. For HSV-1, 36% (150/415) were positive; 89% (32/36) were positive in at least one sample. Certainly the TG could be the source of most of the virus that appears in the saliva and tears since the TG has been studied most frequently and is the largest ganglia in the head and neck region. However, we suggest, based on evidence from Shimeld et al [83] that the virus is not latent in a specific region for the ganglia but could be in all three regions, referred to as V1, V2, and V3.
The first isolation of infectious HSV-1 from the oral cavity (saliva) was in 1953 by Buddingh et al [56] took place at the LSU School of Medicine in New Orleans. Saliva was obtained from 571 healthy volunteers (386 children >15 years of age and 185 adults <15 years of age). The oral swabs were assayed using the chorioallantois of 12-day-old chick embryos. The results showed that infectious HSV-1 was detected at a rate three times greater in the saliva from children than adults. Although these experiments were conducted almost 60 years ago, comparison of these data with more recent studies from humans (see review [84]) demonstrates a strong general correlation [57] and attests to the efficiency of the HSV-1 detection system used by Buddingh et al [58].
The first report of detection of infectious HSV-1 in human tears by Kaufman et al. appeared in 1967 [57]. Our review article in 2009 [84] summarized the numerous reports of asymptomatic shedding of infectious HSV-1 from human tears and saliva. Also, our other reviews [53,54,85] have summarized results from our and other laboratories of the detection of infectious HSV-1 and viral DNA in tears from HSV-1 latent animals such as rabbits and mice.
The development of very sensitive real-time PCR for detection of HSV-1 DNA has resulted in many studies of tears from humans and animal eye models; however, we know of no reports of either infectious virus or HSV-1 DNA detected in the oral cavity (saliva) of animals latent with HSV-1.
This study used the McKrae strain of HSV-1 known to be a high phenotypic reactivator in rabbits that can cause spontaneous shedding into the tear film of infectious virus, HSV-1 DNA, and spontaneous development of HSV–specific corneal epithelial lesions. This is the first study that documents shedding of HSV-1 DNA in the saliva and compares that to the shedding of HSV-1 DNA that occurs in the tears of these latently infected rabbits.
HSV-1 strains that are high phenotypic reactivators, such as 17 Syn + and McKrae, are excellent for studying ocular latency, reactivation, and recurrent ocular diseases in the rabbit eye model. There are disadvantages of using these strains in rabbits: the mortality is approximately 45%, and 5-10% of the surviving rabbits have eyes with stromal opacity or that are otherwise not completely healed. We never use any eyes that are not completely healed. Thus, the ability to assess saliva has benefits and practicality when corneas are not completely healed.
The frequency (43.4%) of positives assessed by real-time PCR of the saliva was almost twice that of the positive tears (28.0%). Table 3 gives the average positives per days of swabs for the saliva as 3.9 (86/22). For both eyes, the average positive was 2.5 (111/44). The average duration of shedding for saliva is 9.6 days (86/9). For the 18 eyes, the average duration of shedding was 6.2 days (111/18). The HSV-1 copy numbers in the tears have a higher median copy number (533) compared to the saliva, which had a median copy number of 439. There was no statistical difference in the mean copy numbers from the saliva and tears. The frequency of coincidence of simultaneous shedding of the right eye, left eye, and saliva being positive was 12.6% (25/198). It is impractical to repeatedly swab the eyes, since this can induce epithelial trauma and neovascularization. As a result, saliva swabs were taken four times each day from each rabbit while only one swab was taken each day for tears. Higher frequency of positives from the saliva could be due to greater frequency of swabs or another explanation is that the saliva is a combination of the flow of tears into the oral cavity from both eyes as well as shedding of the virus from the nerve endings in the oral cavity. More studies with different strains of HSV-1 need to be conducted to determine patterns of HSV-1 copy numbers and frequencies (percent positives).
Conclusions
The rabbit eye model has been used to investigate vaccines to prevent acute ocular herpes (prophylactically) or block recurrent ocular herpes (therapeutically) [86-90]. Many immunological and virological assays can be conducted to assess the efficiency of an HSV vaccine as to whether it is prophylactic and/or therapeutic. This new discovery that latent rabbits shed HSV-1 DNA in their saliva reliably and with very high frequency can be used to assess the efficacy of HSV-1 vaccines, as well as to determine the efficacy of antiviral chemotherapy. This discovery that rabbits latent with HSV-1 strain McKrae spontaneously shed viral DNA in their saliva significantly increases the utility and practicality of the rabbit model and highlights its similarity to humans.
Methods
Rabbits, cells, and virus
All experimental procedures were performed in accordance with the ARVO Resolution for the Use of Animals in Ophthalmic and Vision Research and were approved by the LSU Health Sciences Center (LSUHSC) Institutional Animal Care and Use Committee (IACUC #2850). New Zealand white (NZW) rabbits were obtained from McNeil Rabbitry (Moss Point, MS). The HSV-1 strain employed was McKrae (obtained as a low passage isolate from H.E. Kaufman). Strain McKrae was used to inoculate rabbit corneas. Before inoculation, numbers of plaque-forming units (PFU) were determined in a standard plaque assay procedure using CV-1 cells. All of the viral stocks were grown at a very low multiplicity of infection in primary rabbit kidney cells. Rabbits were equilibrated for seven days in the LSUHSC animal care facility prior to viral inoculation.
Experimental design
For corneal inoculation, the rabbits were anesthetized by intramuscular administration of xylazine (6.6 mg/kg of body weight) and ketamine (100 mg/kg of body weight). The eyes were topically anesthetized with 1.0% tropicamide. The corneas were mildly scarified in a 2 × 2 cross-hatch pattern and each cornea was inoculated with 5 × 105 PFU of strain McKrae. All corneas developed dendritic lesions as verified by slit-lamp examination. The rabbits were visually examined daily for health and ocular conditions for 27 days. The eyes were examined with a slit lamp on PI day 27 to verify the absence of ocular abnormalities; none were found in any rabbit used. Also, visual examinations by the LSUHSC veterinarian found no observable lip or oral lesions; thus, no rabbit had symptoms of HSV-1 infections in the head and neck region or any other areas on its body.
Collection of saliva from HSV-1 latent rabbits
Saliva was collected on polyester swabs (Puritan Medical Products Co., LLC, Gilford, ME) from rabbits beginning on PI day 28 and continuing for 20 consecutive days. The saliva was collected each day in the early morning four consecutive times approximately 20–40 minutes between the repeated samples; thus each rabbit had four saliva swabs taken each day, which were combined so that there was one saliva sample per rabbit per day. All swabs were processed either on the day taken or within 72 hours. If not immediately processed, they were frozen at −80°C, until DNA was extracted similar to other studies that we have published [77].
DNA elution of swabs of rabbit saliva
Each individual swab was processed as previously described [77] with the exception that prior to the supernate extraction and before precipitation, all samples were pooled for each day from each rabbit into one saliva sample. The DNA from the swabs was extracted with a DNA elution kit (Gentra Puregene; Qiagen Sciences, Germantown, MD), and the DNA samples were stored in DNA hydration buffer (provided with the kit) at 4°C until processed by real-time PCR. Sterile unused swabs were processed as a negative control. Other swabs were spiked with either infectious virus of each strain or purified HSV-1 DNA; these were processed as positive controls for DNA extraction by the same method noted above. We determined that the efficiency of extraction of HSV-1 particles and HSV-1 DNA and the efficacy ranged between 58-66%. Oral swabs from naïve rabbits were used as an additional negative saliva control.
Collection of tears and DNA elution from eye swabs
The procedures and protocols used for these studies were the same as previously reported [15,73,77]. One swab was taken daily from each eye for 22 consecutive days.
HSV-1 DNA quantification
HSV-1 copy numbers from the DNA samples were determined by calculating the number of DNA polymerase genes in the sample according to the previously described methods [15,77,91]. The sequences of forward and reverse primers were 5’-AGA GGG ACA113 TCC AGG ACT TTG T-3’ and 5’-CAG GCG CTT GTT GGGT GTA C-3’, respectively (Integrated DNA Technologies [IDT], Coralville, IA). The primer pair was synthesized by IDT. The probe was 5’6-FAM/ACC GCC GAA CTG AGC A/3’ BHQ-1 (IDT). All reactions were done in a total volume of 20 μl. The 20 μl of reaction mixture contained 1 TaqMan® Universal Mastermix (Applied Biosystems, Inc., Foster City, CA), 100 nM of primers and probe, and 5 μl of DNA sample. All reactions were performed in 96-well plates (Bio-Rad, Hercules, CA), which were centrifuged for 1 minute at 1000 g and room temperature in a swing-bucket rotor (CRU 5000 centrifuge; Damon/IEC, Needham, MA) to remove any air bubbles. The reaction conditions were as follows: 95°C for denaturation for 10 sec, 55°C for annealing for 30 sec, and 72°C extension for 10 sec in the real-time PCR (iCycler iQ; Bio-Rad) system for 45 cycle repeats. All samples were analyzed in triplicate. Only samples in which all three were determined to be positive were used. Each reaction plate contained both positive and negative controls as described above as well as HSV-1 DNA standards. The cosmid containing the HSV-1 DNA polymerase gene was obtained from Dr. David C. Bloom (University of Florida, Gainesville, FL) and used as a standard for this study. The cosmid contained a copy of the 4.8 kb restriction fragment (HindIIIA) encompassing the HSV-1 DNA polymerase gene from the HSV-1 strain 17Syn+. A standard curve was generated from both 10-fold and 2-fold serial dilutions of the pHindIIIA cosmid. The lowest dilutions assessed in the 10-fold and 2-fold series were below 1 copy.
Statistical analysis
Standard statistical procedures were used to determine the mean, the median, and the SEM. Detailed statistics were used to determine P-values among the two groups (saliva vs. tears) for the HSV-1 DNA copy number and the total positives. Longitudinal series of observations were analyzed to include intersubject correlation. The data were analyzed by repeated-measures analysis of variance (ANOVA). Our main interest was the comparison of significance between the two groups to determine a between-subject effect. P-values given were those for the F test of the repeated measures ANOVAs. This analysis is similar to that done by Kumar et al [15,77].
Abbreviations
HSV-1: Herpes simplex virus type 1; PI: Post-inoculation; RT-PCR: Real-time-polymerase chain reaction; HPR: High phenotypic reactivator.
Competing interests
The authors have no competing interest in anything mentioned in this article.
Authors’ contributions
All authors collaborated on the experimental design, the calculation and preparation of tables and figures, and discussion of the experimental results. JMH conceived the basic strategic plan. All authors participated in the manuscript preparation. NMN, CC, HEM, and PSB participated in the inoculations, slit lamp experiments, and collections of eye swabs and saliva and performing the real-time PCR. HWT did statistical analyses.
Contributor Information
James M Hill, Email: jhill@lsuhsc.edu.
Nicole M Nolan, Email: nnolan1@tulane.edu.
Harris E McFerrin, Email: hmcferri@xula.edu.
Christian Clement, Email: cclem1@lsuhsc.edu.
Timothy P Foster, Email: tfoste@lsuhsc.edu.
William P Halford, Email: halford@siumed.edu.
Konstantin G Kousoulas, Email: vtgusk@lsu.edu.
Walter J Lukiw, Email: WLukiw@lsuhsc.edu.
Hilary W Thompson, Email: hthomp2@lsuhsc.edu.
Ethan M Stern, Email: estee1@lsuhsc.edu.
Partha S Bhattacharjee, Email: pbhattac@xula.edu.
Acknowledgements
This publication was made possible in part by grant numbers R01-EY006311 (JMH), R01-EY14289 (JMH) from the National Eye Institute; grant numbers AG18031 (WJL) and AG038834 (WJL) and AG23085 (JMH) from the National Institute on Aging National Institutes of Health; NIH-R21-EY019144 (PSB); grant numbers P20RR016456 and P20GM103424 (HEM, JHM) from the National Center of Research Resources and P20GM103501 (TPF) the National Institute of General Medical Sciences; and grant number AS143000, National Institute of Allergy and Infectious Disease (GS). This research was funded in part by an unrestricted research grant from the LSU Health Sciences Center; Transitional Research Initiative Grants (JMH, WJL); LSU School of Veterinary Medicine funds (KS); Research to Prevent Blindness (RPB) Senior Scientific Investigator Award (JMH); an unrestricted grant to the LSU Eye Center from RPB, New York, NY; the Louisiana Biotechnology Research Network (WJL, JMH); an Alzheimer Association Investigator-Initiated Research Grant IIRG-09-131729 (WJL); the Louisiana Lions Eye Foundation, New Orleans (JMH); the Louisiana Vaccine Center (JMH, KGK, WPH) sponsored by the Louisiana Board of Regents; and Lions International USA (JMH); and Louisiana Cancer Research Consortium (LCRC) (PSB). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NEI, NIA, or NIH.
References
- Kaufman HE, Maloney ED. IDU and hydrocortisone in experimental herpes simplex keratitis. Arch Ophthalmol. 1962;68:396–398. doi: 10.1001/archopht.1962.00960030400014. [DOI] [PubMed] [Google Scholar]
- Laibson PR, Kibrick S. Recurrence of herpes simplex virus in rabbit eyes: Results of a three-year study. Invest Ophthalmol Vis Sci. 1969;8:346–350. [PubMed] [Google Scholar]
- Nesburn AB, Elliott JH, Leibowitz HM. Spontaneous reactivation of experimental herpes simplex keratitis in rabbits. Arch Ophthalmol. 1967;78:523–529. doi: 10.1001/archopht.1967.00980030525021. [DOI] [PubMed] [Google Scholar]
- Laibson PR, Kibrick S. Reactivation of herpetic keratitis by epinephrine in rabbit. Arch Ophthalmol. 1966;75:254–260. doi: 10.1001/archopht.1966.00970050256020. [DOI] [PubMed] [Google Scholar]
- Kaufman HE. Treatment of herpes simplex and vaccinia keratitis by 5-iodo- and 5-bromo- 2' -deoxyuridine. Perspective Virology. 1963;111:90–107. [Google Scholar]
- Kaufman HE, Martola EL, Dohlman CH. Herpes simplex treatment with IDU and corticosteroids. Trans Am Acad Ophthalmol Otolaryngol. 1963;67:695–701. [PubMed] [Google Scholar]
- Kaufman HE. Clinical cure of herpes simplex keratitis by 5-iodo-2-deoxyuridine. Proceedings of the Society for Experimental Biology and Medicine. 1962;109:251–252. doi: 10.3181/00379727-109-27169. [DOI] [PubMed] [Google Scholar]
- Hill JM, Park NH, Gangarosa LP, Hull DS, Tuggle CL, Bowman K, Green K. Iontophoresis of vidarabine monophosphate into rabbit eyes. Invest Ophthalmol Vis Sci. 1978;17:473–476. [PubMed] [Google Scholar]
- Kwon BS, Gangarosa LP, Park NH, Hull DS, Fineberg E, Wiggins C, Hill JM. Effect of iontophoretic and topical application of antiviral agents in treatment of experimental HSV-1 keratitis in rabbits. Invest Ophthalmol Vis Sci. 1979;18:984–988. [PubMed] [Google Scholar]
- Hill JM, Kwon B, Burch KD, Deback J, Whang I, Jones GT, Luke B, Harp R, Shimomura Y, Hull DS, Gangarosa LP. Acyclovir and vidarabine monophosphate: Comparison of iontophoretic and intravenous administration for the treatment of HSV-1 stromal keratitis. Am J Med. 1982;73:300–304. doi: 10.1016/0002-9343(82)90110-3. [DOI] [PubMed] [Google Scholar]
- Demangone M, Hill JM, Kwon BS. Effects of acyclovir therapy during simultaneous reactivation of latent HSV-1 in rabbits. Antiviral Res. 1987;7:237–243. doi: 10.1016/0166-3542(87)90032-5. [DOI] [PubMed] [Google Scholar]
- Rootman DS, Haruta Y, Hill JM. Reactivation of HSV-1 in primates by transcorneal iontophoresis of adrenergic agents. Invest Ophthalmol Vis Sci. 1990;31:597–600. [PubMed] [Google Scholar]
- Majumdar S, Nashed YE, Patel K, Jain R, Itahashi M, Neumann DM, Hill JM, Mitra AK. Dipeptide monoester ganciclovir prodrugs for treating HSV-1-induced corneal epithelial and stromal keratitis: in vitro and in vivo evaluations. J Ocul Pharmacol Ther. 2005;21:463–474. doi: 10.1089/jop.2005.21.463. [DOI] [PubMed] [Google Scholar]
- Gebhardt BM, Hill JM. T lymphocytes in the trigeminal ganglia of rabbits during corneal HSV infection. Invest Ophthalmol Vis Sci. 1988;29:1683–1691. [PubMed] [Google Scholar]
- Kumar M, Kaufman HE, Clement C, Bhattacharjee PS, Huq TS, Varnell ED, Thompson HW, Hill JM. Effect of high versus low oral doses of valacyclovir on herpes simplex virus-1 DNA shedding into tears of latently infected rabbits. Invest Ophthalmol Vis Sci. 2010;51:4703–4706. doi: 10.1167/iovs.09-4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JM, Haruta Y, Rootman DS. Adrenergically induced recurrent HSV-1 corneal epithelial lesions. Curr Eye Res. 1987;6:1065–1071. doi: 10.3109/02713688709034878. [DOI] [PubMed] [Google Scholar]
- Haruta Y, Rootman DS, Hill JM. Recurrent HSV-1 corneal epithelial lesions induced by timolol iontophoresis in latently infected rabbits. Invest Ophthalmol Vis Sci. 1988;29:387–392. [PubMed] [Google Scholar]
- Hill JM, Wiggins CA, Kwon BS, Gentry GA, Gangarosa LP S. Thymine arabinoside (Ara-T) topical and iontophoretic application for herpes simplex virus type 1 and type 2 skin infections in hairless mice. Meth Find Exptl Clin Pharmacol. 1984;6:17–20. [PubMed] [Google Scholar]
- Shimomura Y, Gangarosa LP Sr, Kataoka M, Hill JM. HSV-1 shedding by iontophoresis of 6-hydroxydopamine followed by topical epinephrine. Invest Ophthalmol Vis Sci. 1983;24:1588–1594. [PubMed] [Google Scholar]
- Shimomura Y, Dudley JB, Gangarosa LP Sr, Hill JM. HSV-1 quantitation from rabbit neural tissues after epinephrine-induced reactivation. Invest Ophthalmol Vis Sci. 1985;26:121–125. [PubMed] [Google Scholar]
- Berman EJ, Hill JM. Spontaneous ocular shedding of HSV-1 in latently infected rabbits. Invest Ophthalmol Vis Sci. 1985;26:587–590. [PubMed] [Google Scholar]
- Abbott KC, McLendon EF, Gangarosa LP Sr, Hill JM. Adrenergic induction of HSV-1 ocular shedding in rabbits. J Ocular Pharmacol. 1986;2:41–54. doi: 10.1089/jop.1986.2.41. [DOI] [PubMed] [Google Scholar]
- Hill JM, Dudley JB, Shimomura Y, Kaufman HE. Quantitation and kinetics of induced HSV-1 ocular shedding. Curr Eye Res. 1986;5:241–246. doi: 10.3109/02713688609020049. [DOI] [PubMed] [Google Scholar]
- Hill JM, Rayfield MA, Haruta Y. Strain specificity of spontaneous and adrenergically induced HSV-1 ocular reactivation in latently infected rabbits. Curr Eye Res. 1987;6:91–97. doi: 10.3109/02713688709020074. [DOI] [PubMed] [Google Scholar]
- Hill JM, Shimomura Y, Dudley JB, Berman E, Haruta Y, Kwon BS, Maguire LJ. Timolol induces HSV-1 ocular shedding in the latently infected rabbit. Invest Ophthalmol Vis Sci. 1987;28:585–590. [PubMed] [Google Scholar]
- Haruta Y, Maguire LJ, Rootman DS, Hill JM. Recurrent herpes simplex virus type 1 corneal epithelial lesions after radial keratotomy in the rabbit. Arch Ophthalmol. 1987;105:692–694. doi: 10.1001/archopht.1987.01060050110048. [DOI] [PubMed] [Google Scholar]
- Hill JM, Haruta Y, Yamamoto Y, Jones MD, Wingate HL, Jemison MT. Lack of efficacy of adenosine-5'-monophosphate against HSV-1 ocular shedding in rabbits. J Ocular Pharmacol. 1987;3:31–38. doi: 10.1089/jop.1987.3.31. [DOI] [PubMed] [Google Scholar]
- Rootman DS, Hill JM, Haruta Y, Reidy JJ, Kaufman HE. Trifluridine decreases ocular HSV-1 recovery, but not herpetic lesions after timolol iontophoresis. Invest Ophthalmol Vis Sci. 1989;30:678–683. [PubMed] [Google Scholar]
- Rivera L, Beuerman RW, Hill JM. Corneal nerves contain intra-axonal HSV-1 after virus reactivation by epinephrine iontophoresis. Curr Eye Res. 1988;7:1001–1008. doi: 10.3109/02713688809015146. [DOI] [PubMed] [Google Scholar]
- Haruta Y, Rootman DS, Xie LX, Kiritoshi A, Hill JM. Recurrent HSV-1 corneal lesions in rabbits induced by cyclophosphamide and dexamethasone. Invest Ophthalmol Vis Sci. 1989;30:371–376. [PubMed] [Google Scholar]
- Beyer CF, Tepper D, Hill JM. Cryogenic induced ocular HSV-1 reactivation is enhanced by an inhibitor of the lipoxygenase pathway. Curr Eye Res. 1989;8:1287–1292. doi: 10.3109/02713688909013908. [DOI] [PubMed] [Google Scholar]
- Beyer CF, Hill JM, Reidy JJ, Beuerman RW. Corneal nerve disruption reactivates virus in rabbits latently infected with HSV-1. Invest Ophthalmol Vis Sci. 1990;31:925–932. [PubMed] [Google Scholar]
- Portnoy SL, Beyer CF, Hill JM, Kaufman HE. The coincidence of HSV-1 ocular cultures with HSV-1 corneal epithelial defects in rabbits after experimental penetrating keratoplasty. Cornea. 1991;10:17–20. [PubMed] [Google Scholar]
- Kaufman HE, Varnell ED, Gebhardt BM, Thompson HW, Hill JM. Propanolol suppression of ocular HSV-1 recurrence and associated corneal lesions following spontaneous reactivation in the rabbit. Curr Eye Res. 1996;15:680–684. doi: 10.3109/02713689609008909. [DOI] [PubMed] [Google Scholar]
- Garza HH Jr, Hill JM. Effect of a beta-adrenergic antagonist, propranolol, on induced HSV-1 ocular recurrence in latently infected rabbits. Curr Eye Res. 1997;16:453–458. doi: 10.1076/ceyr.16.5.453.7051. [DOI] [PubMed] [Google Scholar]
- Clement C, Popp MP, Bloom DC, Schultz G, Liu L, Neumann DM, Bhattacharjee PS, Hill JM. Microarray analysis of host gene expression for comparison between naive and HSV-1 latent rabbit trigeminal ganglia. Mol Vis. 2008;14:1209–1221. [PMC free article] [PubMed] [Google Scholar]
- Clement C, Bhattacharjee PS, Kaufman HE, Hill JM. Heat-induced reactivation of HSV-1 in latent mice: Upregulation in the TG of CD83 and other immune response genes and their LAT-ICP0 locus. Invest Ophthalmol Vis Sci. 2009;50:2855–2861. doi: 10.1167/iovs.08-2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner EK, Flanagan WM, Devi-Rao GB, Zhang YF, Hill JM, Anderson KP, Stevens JG. The herpes simplex virus latency-associated transcript is spliced during the latent phase of infection. J Virol. 1988;62:4577–4585. doi: 10.1128/jvi.62.12.4577-4585.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JM, Sedarati F, Javier RT, Wagner EK, Stevens JG. Herpes simplex virus latent phase transcription facilitates in vivo reactivation. Virology. 1990;174:117–125. doi: 10.1016/0042-6822(90)90060-5. [DOI] [PubMed] [Google Scholar]
- Cook SD, Paveloff MJ, Doucet JJ, Cottingham AJ, Sedarati F, Hill JM. Ocular herpes simplex virus reactivation in mice latently infected with latency-associated transcript mutants. Invest Ophthalmol Vis Sci. 1991;32:1558–1561. [PubMed] [Google Scholar]
- Cook SD, Hill JM, Lynas C, Maitland NJ. Latency-associated transcripts in corneas and ganglia of HSV-1 infected rabbits. Br J Ophthalmol. 1991;75:644–648. doi: 10.1136/bjo.75.11.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann DM, Bhattacharjee PS, Giordani NV, Bloom DC, Hill JM. In vivo changes in the patterns of chromatin structure associated with the latent herpes simplex virus type 1 genome in mouse trigeminal ganglia can be detected at early times after butyrate treatment. J Virol. 2007;81:13248–13253. doi: 10.1128/JVI.01569-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement C, Bhattacharjee PS, Kumar M, Foster TP, Thompson HW, Hill JM. Upregulation of mouse genes in HSV-1 latent TG after butyrate treatment implicates the multiple roles of the LAT-ICP0 locus. Invest Ophthalmol Vis Sci. 2011;52:12770–1779. doi: 10.1167/iovs.09-5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordani NV, Neumann DM, Kwiatkowski DL, Bhattacharjee PS, McAnany PK, Hill JM, Bloom DC. During herpes simplex virus type 1 infection of rabbits, the ability to express the latency-associated transcript increases latent-phase transcription of lytic genes. J Virol. 2008;82:6056–6060. doi: 10.1128/JVI.02661-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JM, Maggioncalda JB, Garza HH Jr, Su YH, Fraser NW, Block TM. In vivo epinephrine reactivation of ocular herpes simplex virus type 1 in the rabbit is correlated to a 370-base-pair region located between the promoter and the 5' end of the 2.0-kilobase latency-associated transcript. J Virol. 1996;70:7270–7274. doi: 10.1128/jvi.70.10.7270-7274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell MJ, Hill JM, Margolis TP, Stevens JG, Wagner EK, Feldman LT. The herpes simplex virus type 1 reactivation function lies outside the latency-associated transcript open reading frame ORF-2. J Virol. 1993;67:3653–3655. doi: 10.1128/jvi.67.6.3653-3655.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom DC, Devi-Rao GB, Hill JM, Stevens JG, Wagner EK. Molecular analysis of herpes simplex virus type 1 during epinephrine-induced reactivation of latently infected rabbits in vivo. J Virol. 1994;68:1283–1292. doi: 10.1128/jvi.68.3.1283-1292.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa T, Hill JM, Stanberry LR, Bourne N, Kurawadwala JF, Krause PR. The characteristic site-specific reactivation phenotypes of HSV- 1 and HSV-2 depend upon the latency-associated transcript region. J Exp Med. 1996;184:659–664. doi: 10.1084/jem.184.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devi-Rao GB, Aguilar JS, Rice MK, Garza HH Jr, Bloom DC, Hill JM, Wagner EK. Herpes simplex virus genome replication and transcription during induced reactivation in the rabbit eye. J Virol. 1997;71:7039–7047. doi: 10.1128/jvi.71.9.7039-7047.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JM, Garza HH Jr, Su YH, Meegalla R, Hanna LA, Loutsch JM, Thompson HW, Varnell ED, Bloom DC, Block TM. A 437-base-pair deletion at the beginning of the latency-associated transcript promoter significantly reduced adrenergically induced herpes simplex virus type 1 ocular reactivation in latently infected rabbits. J Virol. 1997;71:6555–6559. doi: 10.1128/jvi.71.9.6555-6559.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom DC, Stevens JG, Hill JM, Tran RK. Mutagenesis of a cAMP response element within the latency-associated transcript promoter of HSV-1 reduces adrenergic reactivation. Virology. 1997;236:202–207. doi: 10.1006/viro.1997.8723. [DOI] [PubMed] [Google Scholar]
- Hill JM, Wen R, Halford WP. In: Herpes Simplex Virus Protocols Totowa. Brown MS, MacLean AR, editor. NJ: Humana Press/Wiley; 1998. Pathogenesis and molecular biology of ocular HSV in the rabbit; pp. 291–315. [Google Scholar]
- Toma HS, Murina AT, Areaux RG Jr, Neumann DM, Bhattacharjee PS, Foster TP, Kaufman HE, Hill JM. Ocular HSV-1 latency, reactivation and recurrent disease. Sem Ophthalmol. 2008;23:249–273. doi: 10.1080/08820530802111085. [DOI] [PubMed] [Google Scholar]
- Al-Dujaili LJ, Clerkin PP, Clement C, McFerrin HE, Bhattacharjee PS, Varnell ED, Kaufman HE, Hill JM. Ocular herpes simplex virus: how are latency, reactivation, recurrent disease and therapy interrelated? Future Microbiology. 2011;6:877–907. doi: 10.2217/fmb.11.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt BM, Varnell ED, Hill JM, Kaufman HE. In: Handbook of Animal Models of Infection: Experimental Models in Antimicrobial Chemotherapy. Zak O, Sande M, editor. CA: Academic Press, San Diego; 1999. Animal models of ocular herpes simplex virus infection (rabbits, primates, mice) pp. 919–926. [Google Scholar]
- Buddingh GJ, Schrum DI, Lanier JC, Guidry DJ. Studies of the natural history of herpes simplex infections. Pediatrics. 1953;11:595–610. [PubMed] [Google Scholar]
- Kaufman HE, Brown DC, Ellison EM. Recurrent herpes in the rabbit and man. Science. 1967;156:1628–1629. doi: 10.1126/science.156.3782.1628. [DOI] [PubMed] [Google Scholar]
- Lindgren KM, Douglas RG Jr, Couch RB. Significance of Herpesvirus hominis in respiratory secretions of man. N Engl J Med. 1968;278:517–523. doi: 10.1056/NEJM196803072781001. [DOI] [PubMed] [Google Scholar]
- Douglas RG Jr, Couch RB. A prospective study of chronic herpes simplex virus infection and recurrent herpes labialis in humans. J Immunol. 1970;104:289–295. [PubMed] [Google Scholar]
- Hatherley LI, Hayes K, Jack I. Herpes virus in an obstetric hospital. II: Asymptomatic virus excretion in staff members. Med J Aust. 1980;2:273–275. [PubMed] [Google Scholar]
- Spruance SL. Pathogenesis of herpes simplex labialis: Excretion of virus in the oral cavity. J Clin Microbiol. 1984;19:675–679. doi: 10.1128/jcm.19.5.675-679.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyama T, Sajaku C, Yamamoto S, Hwang CB, Shillitoe EJ. Shedding of herpes simplex virus Type 1 into saliva. J Oral Pathol. 1988;17:478–481. doi: 10.1111/j.1600-0714.1988.tb01320.x. [DOI] [PubMed] [Google Scholar]
- Tateishi K, Toh Y, Minagawa H, Tashiro H. Detection of herpes simplex virus (HSV) in the saliva from 1,000 oral surgery outpatients by the polymerase chain reaction (PCR) and virus isolation. J Oral Pathol Med. 1994;23:80–84. doi: 10.1111/j.1600-0714.1994.tb00261.x. [DOI] [PubMed] [Google Scholar]
- Okinaga S. Shedding of herpes simplex virus type 1 into tears and saliva in healthy Japanese adults. Kurume Med J. 2000;47:273–277. doi: 10.2739/kurumemedj.47.273. [DOI] [PubMed] [Google Scholar]
- Robinson PA, High AS, Hume WJ. Rapid detection of human herpes simplex virus type 1 in saliva. Arch Oral Biol. 1992;37:797–806. doi: 10.1016/0003-9969(92)90113-M. [DOI] [PubMed] [Google Scholar]
- Kriesel JD, Pisani PL, McKeough MB, Baringer JR, Spruance SL. Correlation between detection of herpes simplex virus in oral secretions by PCR and susceptibility to experimental UV radiation-induced herpes labialis. J Clin Microbiol. 1994;32:3088–3090. doi: 10.1128/jcm.32.12.3088-3090.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Bang D, Cho YH, Lee ES, Sohn S. Polymerase chain reaction reveals herpes simplex virus DNA in saliva of patients with Behcet's disease. Arch Dermatol Res. 1996;288:179–183. doi: 10.1007/BF02505221. [DOI] [PubMed] [Google Scholar]
- Knaup B, Schunemann S, Wolff MH. Subclinical reactivation of herpes simplex virus type 1 in the oral cavity. Oral Microbiol Immunol. 2000;15:281–283. doi: 10.1034/j.1399-302x.2000.150502.x. [DOI] [PubMed] [Google Scholar]
- Druce J, Catton M, Chibo D, Minerds K, Tyssen D, Kostecki R, Maskill B, Leong-Shaw W, Gerrard M, Birch C. Utility of a multiplex PCR assay for detecting herpesvirus DNA in clinical samples. J Clin Microbiol. 2002;40:1728–1732. doi: 10.1128/JCM.40.5.1728-1732.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson M, Pyne DB, Austin JP, Lynn Francis J, Clancy RL, McDonald WA, Fricker PA. Epstein-Barr virus reactivation and upper-respiratory illness in elite swimmers. Med Sci Sports Exerc. 2002;34:411–417. doi: 10.1097/00005768-200203000-00005. [DOI] [PubMed] [Google Scholar]
- Youssef R, Shaker O, Sobeih S, Mashaly H, Mostafa WZ. Detection of herpes simplex virus DNA in serum and oral secretions during acute recurrent herpes labialis. J Dermatol. 2002;29:404–410. doi: 10.1111/j.1346-8138.2002.tb00294.x. [DOI] [PubMed] [Google Scholar]
- Miller CS, Cunningham LL, Lindroth JE, Avdiushko SA. The efficacy of valacyclovir in preventing recurrent herpes simplex virus infections associated with dental procedures. J Am Dent Assoc. 2004;135:1311–1318. doi: 10.14219/jada.archive.2004.0407. [DOI] [PubMed] [Google Scholar]
- Kaufman HE, Azcuy AM, Varnell ED, Sloop GD, Thompson HW, Hill JM. HSV-1 DNA in tears and saliva of normal adults. Invest Ophthalmol Vis Sci. 2005;46:241–247. doi: 10.1167/iovs.04-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CS, Avdiushko SA, Kryscio RJ, Danaher RJ, Jacob RJ. Effect of prophylactic valacyclovir on the presence of human herpesvirus DNA in saliva of healthy individuals after dental treatment. J Clin Microbiol. 2005;43:2173–2180. doi: 10.1128/JCM.43.5.2173-2180.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva LM, Guimaraes AL, Victoria JM, Gomes CC, Gomez RS. Herpes simplex virus type 1 shedding in the oral cavity of seropositive patients. Oral Dis. 2005;11:13–16. doi: 10.1111/j.1601-0825.2004.01058.x. [DOI] [PubMed] [Google Scholar]
- Lin SS, Chou MY, Ho CC, Kao CT, Tsai CH, Wang L, Yang CC. Study of the viral infections and cytokines associated with recurrent aphthous ulceration. Microbes Infect. 2005;7:635–644. doi: 10.1016/j.micinf.2004.12.023. [DOI] [PubMed] [Google Scholar]
- Kumar M, Hill JM, Clement C, Varnell ED, Thompson H, Kaufman HE. A double-blind placebo-controlled study to evaluate valacyclovir alone and with aspirin for asymptomatic HSV-1 DNA shedding in human tears and saliva. Invest Ophthalmol Vis Sci. 2009;50:5601–5608. doi: 10.1167/iovs.09-3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CS, Danaher RJ. Asymptomatic shedding of herpes simplex virus (HSV) in the oral cavity. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:43–50. doi: 10.1016/j.tripleo.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Schiffer JT, Magaret A, Selke S, Corey L, Wald A. Detailed analysis of mucosal herpes simplex virus-2 replication kinetics with and without antiviral therapy. J Antimicrob Chemother. 2011;66:2593–2600. doi: 10.1093/jac/dkr346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald A, Corey L, Cone R, Hobson A, Davis G, Zeh J. Frequent genital herpes simplex virus 2 shedding in immunocompetent women: Effect of acyclovir treatment. J Clin Invest. 1997;99:1092–1097. doi: 10.1172/JCI119237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald A, Zeh J, Barnum G, Davis LG, Corey L. Suppression of subclinical shedding of herpes simplex virus type 2 with acyclovir. Ann Intern Med. 1996;124:8–15. doi: 10.7326/0003-4819-124-1_part_1-199601010-00002. [DOI] [PubMed] [Google Scholar]
- Richter ER, Dias JK, Gilbert JE II, Atherton SS. Distribution of herpes simplex virus type 1 and varicella zoster virus in ganglia of the human head and neck. J Infect Dis. 2009;200:1901–1906. doi: 10.1086/648474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimeld C, Tullo AB, Hill TJ, Blyth WA, Easty DL. Spread of herpes simplex virus and distribution of latent infection after intraocular infection of the mouse. Arch Virol. 1985;85:175–187. doi: 10.1007/BF01314229. [DOI] [PubMed] [Google Scholar]
- Cohrs RJ, Koelle DM, Schuette MC, Mehta S, Pierson D, Gilden DH, Hill JM. In: Herpesviridae:Viral Structure, Life Cycle and Infections. Gluckman TR, editor. Hauppauge, NY: Nova Science Publishers Inc; 2009. Asymptomatic alphaherpesvirus reactivation; pp. 133–168. [Google Scholar]
- Webre JM, Hill JM, Clement C, Nolan NM, McFerrin HE, Bhattacharjee PS, Hsia V, Neumann DM, Lukiw WJ, Thompson HW. Rabbit and mouse models of HSV-1 latency, reactivation, and recurrent eye disease. J Biomed Biotech. 2012. in press. [DOI] [PMC free article] [PubMed]
- Chentoufi AA, Dasgupta G, Christensen ND, Hu J, Choudhury ZS, Azeem A, Jester JV, Nesburn AB, Wechsler SL, BenMohamed L. A novel HLA (HLA-A*0201) transgenic rabbit model for preclinical evaluation of human CD8+ T cell epitope-based vaccines against ocular herpes. J Immunol. 2010;184:2561–2571. doi: 10.4049/jimmunol.0902322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesburn AB, Slanina S, Burke RL, Ghiasi H, Bahri S, Wechsler SL. Local periocular vaccination protects against eye disease more effectively than systemic vaccination following primary ocular herpes simplex virus infection in rabbits. J Virol. 1998;72:7715–7721. doi: 10.1128/jvi.72.10.7715-7721.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesburn AB, Ghiasi H, Wechsler SL. Ocular safety and efficacy of an HSV-1 gD vaccine during primary and latent infection. Invest Ophthalmol Vis Sci. 1990;31:1497–1502. [PubMed] [Google Scholar]
- Nesburn AB, Burke RL, Ghiasi H, Slanina SM, Wechsler SL. Therapeutic periocular vaccination with a subunit vaccine induces higher levels of herpes simplex virus-specific tear secretory immunoglobulin A than systemic vaccination and provides protection against recurrent spontaneous ocular shedding of virus in latently infected rabbits. Virology. 1998;252:200–209. doi: 10.1006/viro.1998.9454. [DOI] [PubMed] [Google Scholar]
- Caselli E, Balboni PG, Incorvaia C, Argnani R, Parmeggiani F, Cassai E, Manservigi R. Local and systemic inoculation of DNA or protein gB1s-based vaccines induce a protective immunity against rabbit ocular HSV-1 infection. Vaccine. 2000;19:1225–1231. doi: 10.1016/S0264-410X(00)00242-5. [DOI] [PubMed] [Google Scholar]
- Hill JM, Ball MJ, Neumann DM, Azcuy AM, Bhattacharjee PS, Bouhanik S, Clement C, Lukiw WJ, Foster TP, Kumar M, Kaufman HE, Thompson HW. The high prevalence of herpes simplex virus type 1 DNA in human trigeminal ganglia is not a function of age or gender. J Virol. 2008;82:8230–8234. doi: 10.1128/JVI.00686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]