Abstract
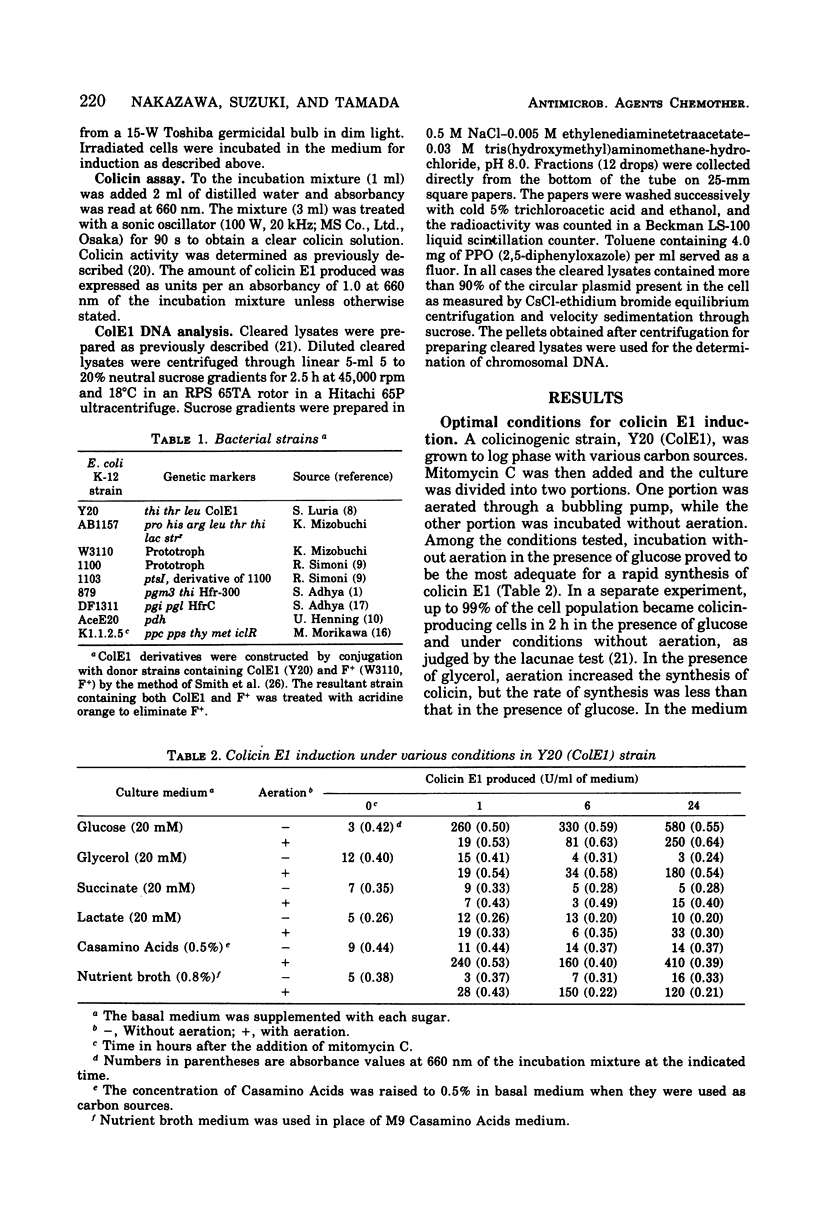
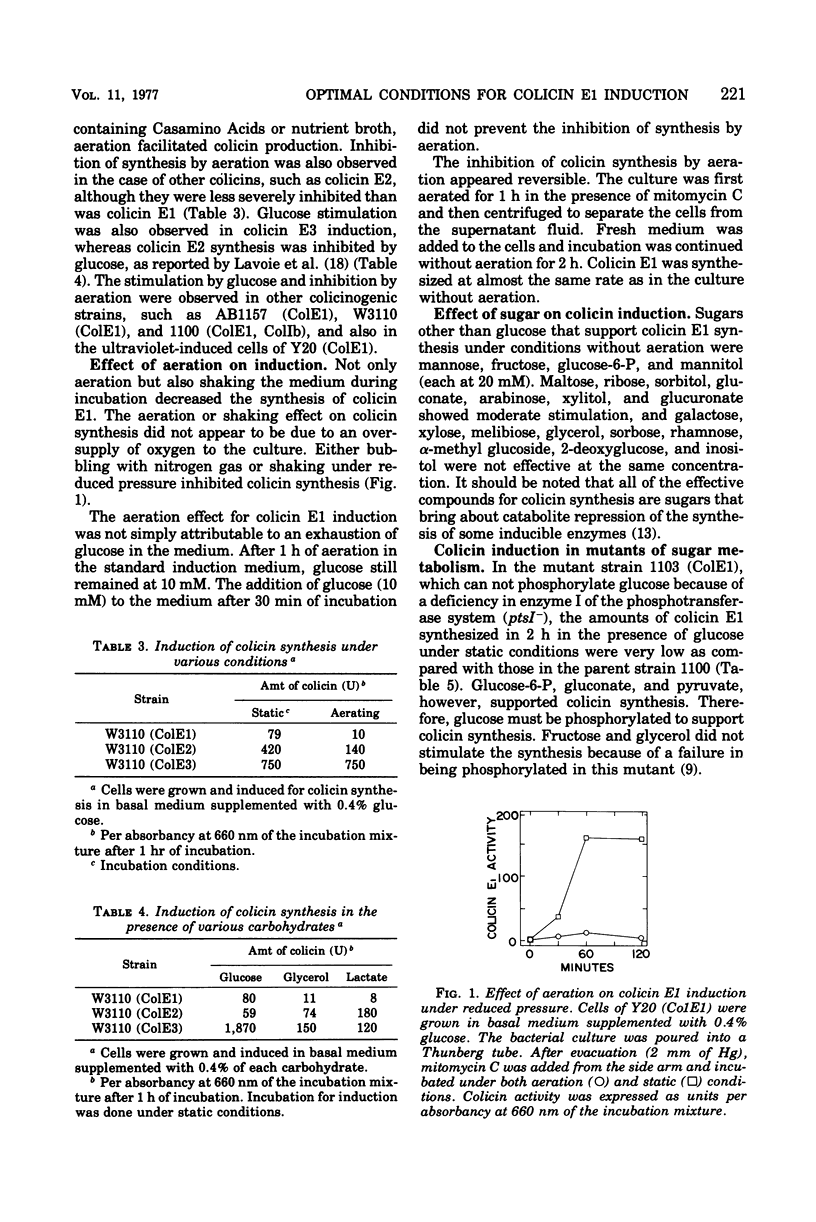
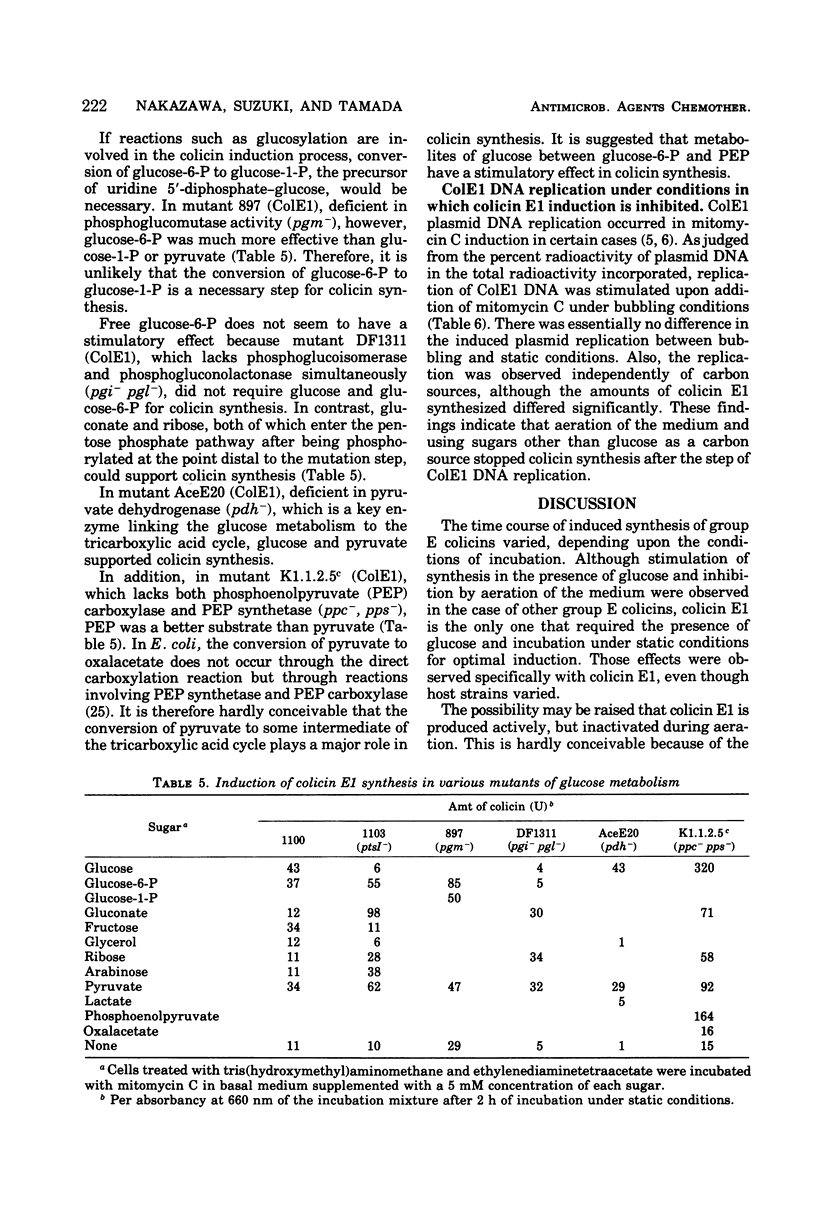
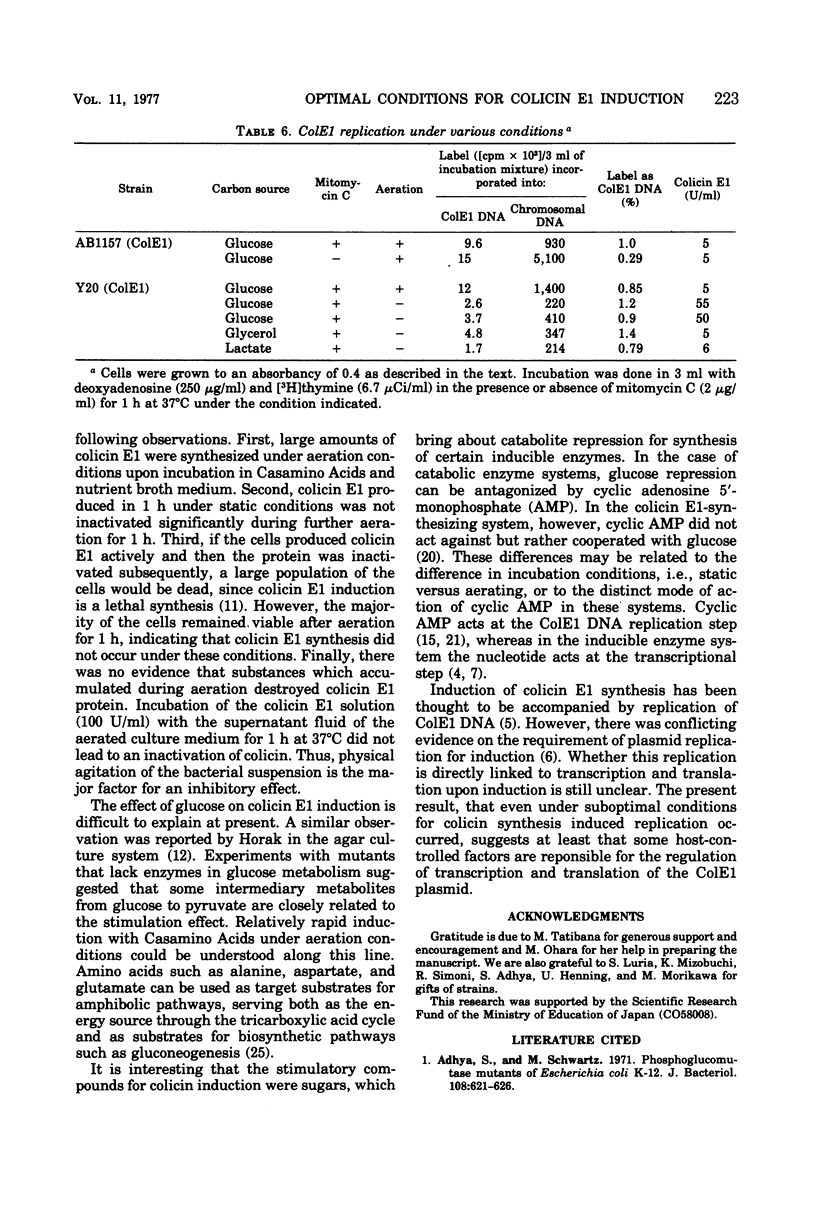
A rapid rate and large amounts of colicin E1 synthesis were induced by incubating colicinogenic cells without aeration in the presence of glucose. The decrease in ability of colicin induction by aeration was not due to an ample supply of oxygen, but to the simple agitation of the bacterial suspension. Sugars other than glucose that support colicin synthesis were mannose, fructose, glucose-6-phosphate, and mannitol. The effect of sugars in mutants deficient in enzymes of glucose metabolism suggested that some intermediary metabolites between glucose and pyruvate are closely related to the stimulation of colicin synthesis under these conditions. Analyses of deoxyribonucleic acid replication indicated that the induced replication of the colicin E1 plasmid deoxyribonucleic acid occurred even under conditions in which colicin was not actively synthesized.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adhya S., Schwartz M. Phosphoglucomutase mutants of Escherichia coli K-12. J Bacteriol. 1971 Nov;108(2):621–626. doi: 10.1128/jb.108.2.621-626.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazaral M., Helinski D. R. Circular DNA forms of colicinogenic factors E1, E2 and E3 from Escherichia coli. J Mol Biol. 1968 Sep 14;36(2):185–194. doi: 10.1016/0022-2836(68)90374-4. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- De Crombrugghe B., Chen B., Anderson W., Nissley P., Gottesman M., Pastan I., Perlman R. Lac DNA, RNA polymerase and cyclic AMP receptor protein, cyclic AMP, lac repressor and inducer are the essential elements for controlled lac transcription. Nat New Biol. 1971 Jun 2;231(22):139–142. doi: 10.1038/newbio231139a0. [DOI] [PubMed] [Google Scholar]
- Durkacz B. W., Kennedy C. K., Sherratt D. J. Plasmid replication and the induced synthesis of colicins E1 and E2 in Escherichia coli. J Bacteriol. 1974 Mar;117(3):940–946. doi: 10.1128/jb.117.3.940-946.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eron L., Arditti R., Zubay G., Connaway S., Beckwith J. R. An adenosine 3':5'-cyclic monophosphate-binding protein that acts on the transcription process. Proc Natl Acad Sci U S A. 1971 Jan;68(1):215–218. doi: 10.1073/pnas.68.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields K. L., Luria S. E. Effects of colicins E1 and K on transport systems. J Bacteriol. 1969 Jan;97(1):57–63. doi: 10.1128/jb.97.1.57-63.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. F., Wilson G. The role of a phosphoenolpyruvate-dependent kinase system in beta-glucoside catabolism in Escherichia coli. Proc Natl Acad Sci U S A. 1968 Mar;59(3):988–995. doi: 10.1073/pnas.59.3.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschman H. R., Helinski D. R. Comparative study of the events associated with colicin induction. J Bacteriol. 1967 Sep;94(3):691–699. doi: 10.1128/jb.94.3.691-699.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horák V. Glucose repression of the synthesis of some colicins. Folia Microbiol (Praha) 1973;18(1):75–76. doi: 10.1007/BF02884251. [DOI] [PubMed] [Google Scholar]
- Hsie A. W., Rickenberg H. V. Catabolite repression in Escherichia coli: the role of glucose 6-phosphate. Biochem Biophys Res Commun. 1967 Nov 17;29(3):303–310. doi: 10.1016/0006-291x(67)90453-6. [DOI] [PubMed] [Google Scholar]
- Katz L., Kingsbury D. T., Helinski D. R. Stimulation by cyclic adenosine monophosphate of plasmid deoxyribonucleic acid replication and catabolite repression of the plasmid deoxyribonucleic acid-protein relaxation complex. J Bacteriol. 1973 May;114(2):577–591. doi: 10.1128/jb.114.2.577-591.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg H. L., Smith J. Genetic control of hexose phosphate uptake by Escherichia coli. Nature. 1969 Dec 27;224(5226):1261–1262. doi: 10.1038/2241261a0. [DOI] [PubMed] [Google Scholar]
- Kupor S. R., Fraenkel D. G. 6-phosphogluconolactonase mutants of Escherichia coli and a maltose blue gene. J Bacteriol. 1969 Dec;100(3):1296–1301. doi: 10.1128/jb.100.3.1296-1301.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie M., Mathieu L. G., Charron-Allie L. Inhibition of colicin production by fermentable sugars. Can J Microbiol. 1974 Feb;20(2):269–272. doi: 10.1139/m74-044. [DOI] [PubMed] [Google Scholar]
- Luria S. E. Phage, colicins, and macroregulatory phenomena. Science. 1970 Jun 5;168(3936):1166–1170. doi: 10.1126/science.168.3936.1166. [DOI] [PubMed] [Google Scholar]
- Nakazawa A., Tamada T. Induction of colicin E1 synthesis by neocarzinostatin and bleomycin. J Antibiot (Tokyo) 1974 Dec;27(12):984–986. doi: 10.7164/antibiotics.27.984. [DOI] [PubMed] [Google Scholar]
- Nakazawa A., Tamada T. Involvement of cyclic 3',5'-adenosine monophosphate in replication of colicinogenic factor E 1 DNA. Biochem Biophys Res Commun. 1972 Nov 15;49(4):977–983. doi: 10.1016/0006-291x(72)90308-7. [DOI] [PubMed] [Google Scholar]
- Nakazawa A., Tamada T. Stimulation of colicin E 1 synthesis by cyclic 3', 5'-adenosine monophosphate in mitomycin C-induced Escherichia coli. Biochem Biophys Res Commun. 1972 Jan 31;46(2):1004–1010. doi: 10.1016/s0006-291x(72)80241-9. [DOI] [PubMed] [Google Scholar]
- OZEKI H., STOCKER B. A., DE MARGERIE H. Production of colicine by single bacteria. Nature. 1959 Aug 1;184:337–339. doi: 10.1038/184337a0. [DOI] [PubMed] [Google Scholar]
- Pastan I., Perlman R. L. Stimulation of tryptophanase synthesis in Escherichia coli by cyclic 3',5'-adenosine monophosphate. J Biol Chem. 1969 Apr 25;244(8):2226–2232. [PubMed] [Google Scholar]
- SMITH S. M., OZEKI H., STOCKER B. A. TRANSFER OF COLE1 AND COLE2 DURING HIGH-FREQUENCY TRANSMISSION OF COLI IN SALMONELLA TYPHIMURIUM. J Gen Microbiol. 1963 Nov;33:231–242. doi: 10.1099/00221287-33-2-231. [DOI] [PubMed] [Google Scholar]
- Sanwal B. D. Allosteric controls of amphilbolic pathways in bacteria. Bacteriol Rev. 1970 Mar;34(1):20–39. doi: 10.1128/br.34.1.20-39.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]



