Abstract
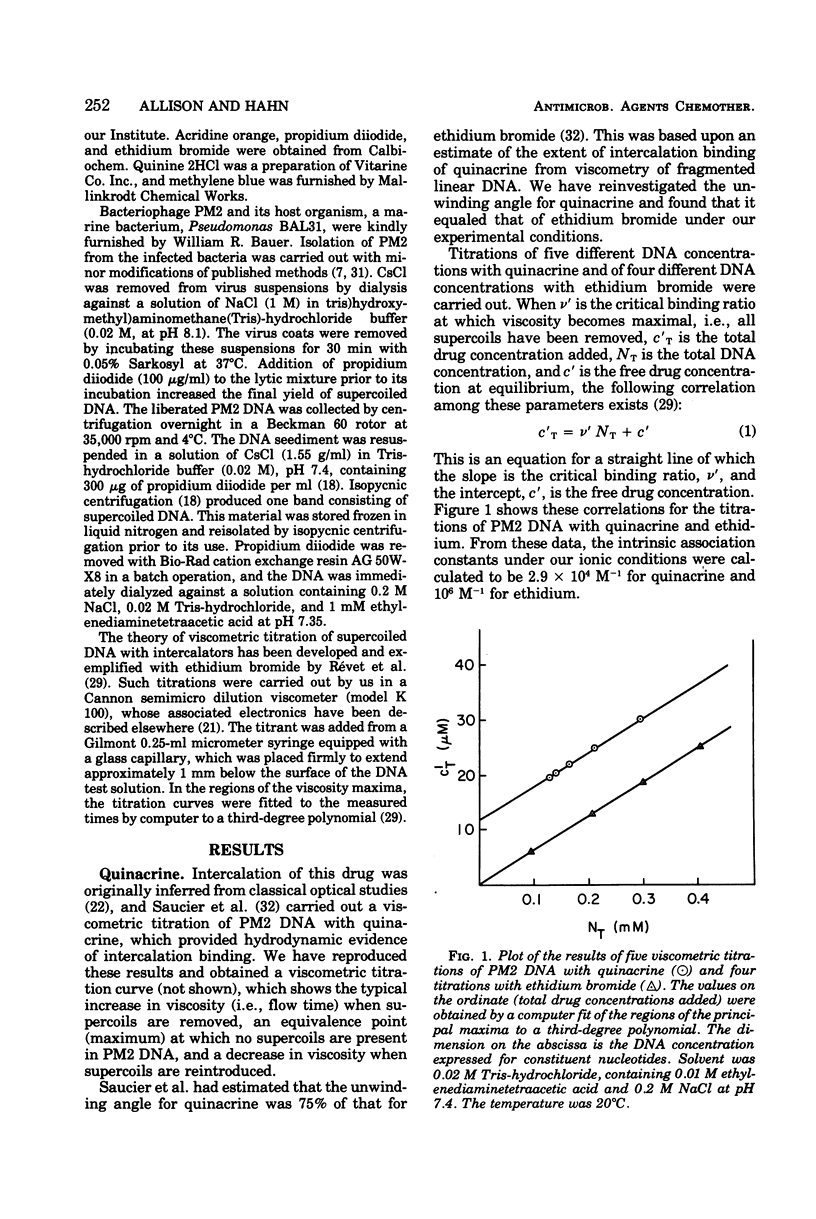
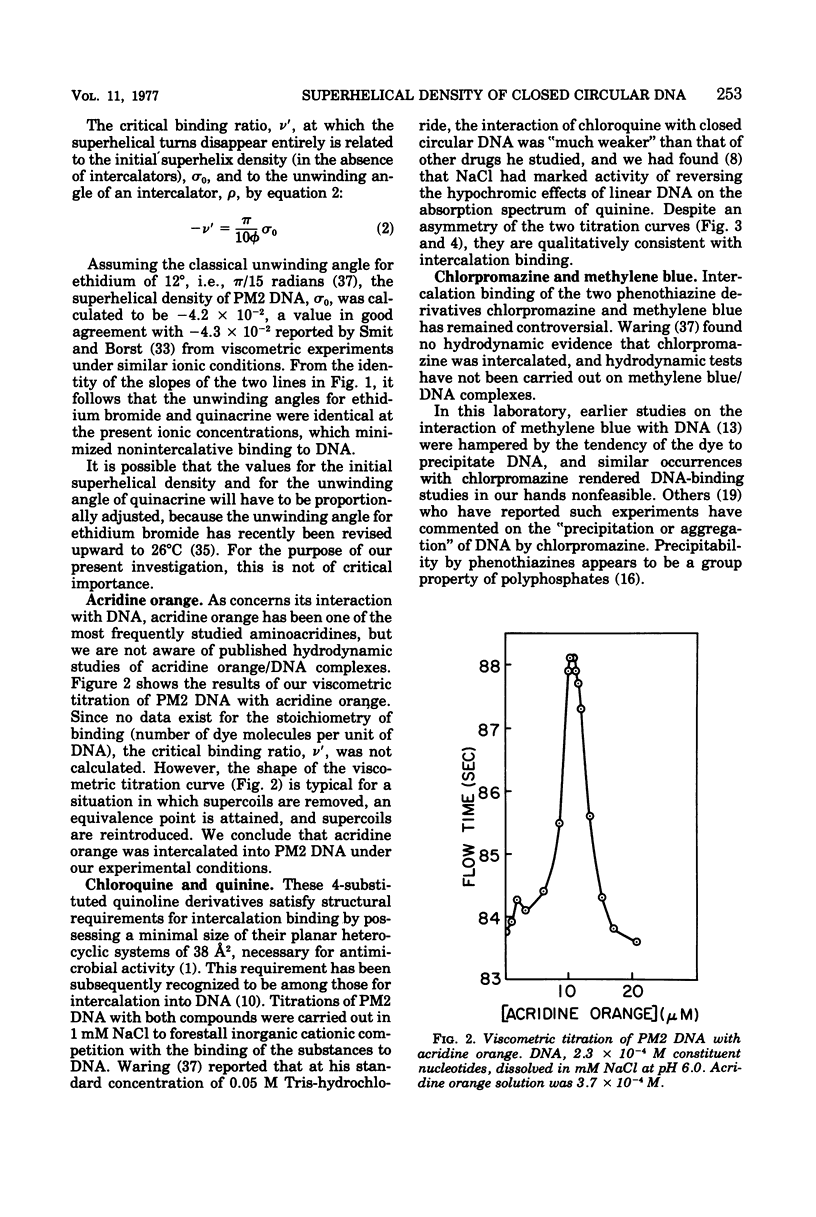
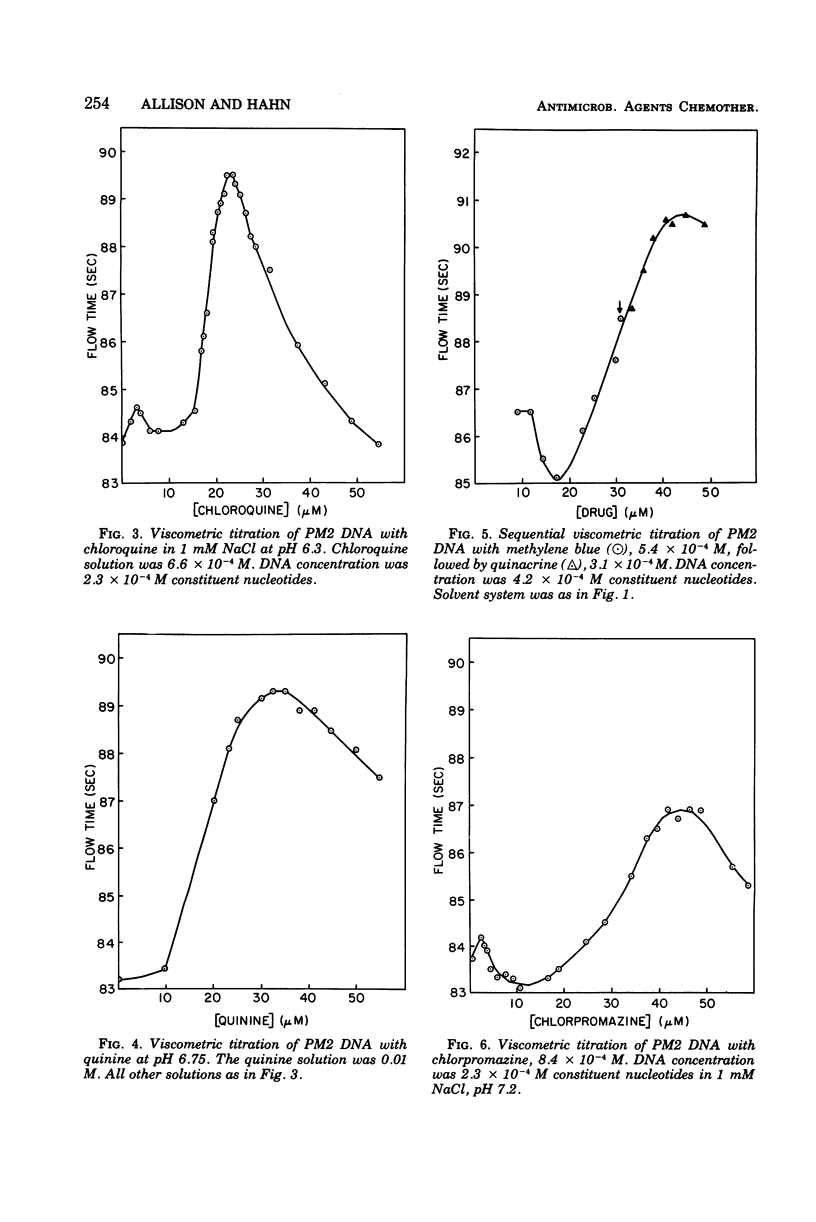
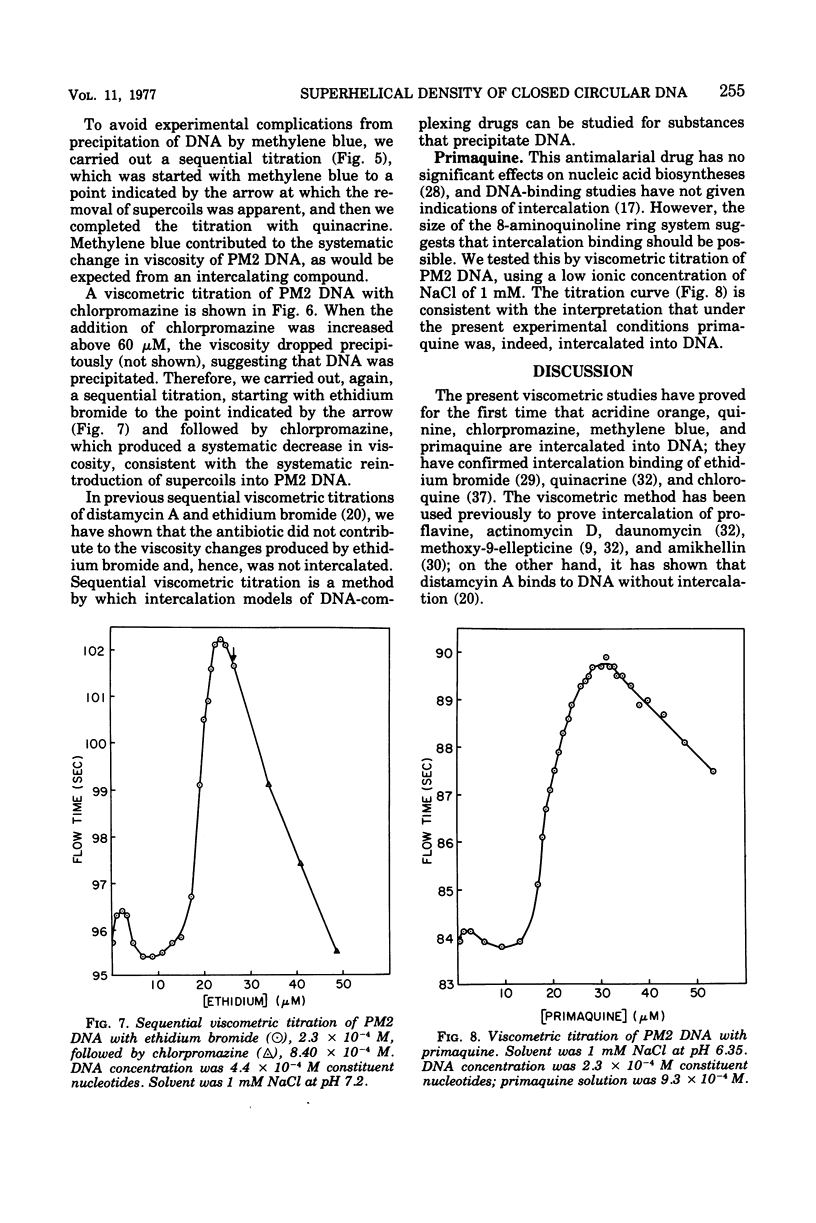
The following compounds, which possess anti-R-plasmid activity (Hahn and Ciak, 1976) in decreasing order, were shown by viscometric titration to change systematically the superhelical density of closed circular PM2 deoxyribonucleic acid in the manner of intercalators: ethidium bromide, quinacrine, acridine orange, quinine, chlorpromazine, chloroquine, and methylene blue. The same effect was caused by the antimalarial drug primaquine, which has not been tested for antiplasmid activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer W., Vinograd J. The interaction of closed circular DNA with intercalative dyes. I. The superhelix density of SV40 DNA in the presence and absence of dye. J Mol Biol. 1968 Apr 14;33(1):141–171. doi: 10.1016/0022-2836(68)90286-6. [DOI] [PubMed] [Google Scholar]
- Blake A., Peacocke A. R. The interaction of aminocridines with nucleic acids. Biopolymers. 1968;6(9):1225–1253. doi: 10.1002/bip.1968.360060902. [DOI] [PubMed] [Google Scholar]
- Bradley D. F., Wolf M. K. AGGREGATION OF DYES BOUND TO POLYANIONS. Proc Natl Acad Sci U S A. 1959 Jul;45(7):944–952. doi: 10.1073/pnas.45.7.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra P., Zunino F., Gaur V. P., Zaccara A., Woltersdorf M., Luoni G., Götz A. Mode of tilorone hydrochloride interaction to DNA and polydeoxyribonucleotides. FEBS Lett. 1972 Nov 15;28(1):5–9. doi: 10.1016/0014-5793(72)80662-8. [DOI] [PubMed] [Google Scholar]
- Ciak J., Hahn F. E. Chloroquine: mode of action. Science. 1966 Jan 21;151(3708):347–349. doi: 10.1126/science.151.3708.347. [DOI] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S., Sinsheimer R. L. DNA of bacteriophage PM2: a closed circular double-stranded molecule. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1164–1168. doi: 10.1073/pnas.63.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estensen R. D., Krey A. K., Hahn F. E. Studies on a deoxyribonucleic acid-quinine complex. Mol Pharmacol. 1969 Sep;5(5):532–541. [PubMed] [Google Scholar]
- Festy B., Poisson J., Paoletti C. A new DNA intercalating drug: Methoxy-9-ellipticine. FEBS Lett. 1971 Oct 1;17(2):321–323. doi: 10.1016/0014-5793(71)80176-x. [DOI] [PubMed] [Google Scholar]
- HELE P. THE INTERACTION OF PHENOTHIAZINE DERIVATIVES AND IMIPRAMINE WITH POLYPHOSPHATES OF BIOLOGICAL INTEREST. Biochim Biophys Acta. 1963 Dec 20;76:647–649. [PubMed] [Google Scholar]
- Hahn F. E., Ciak J. Elimination of resistance determinants from R-factor R1 by intercalative compounds. Antimicrob Agents Chemother. 1976 Jan;9(1):77–80. doi: 10.1128/aac.9.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn F. E., Ciak J. The problems of drug-resistant pathogenic bacteria. Elimination of bacterial episomes by DNA-complexing compounds. Ann N Y Acad Sci. 1971 Jun 11;182:295–304. doi: 10.1111/j.1749-6632.1971.tb30665.x. [DOI] [PubMed] [Google Scholar]
- Hahn F. E., O'Brien R. L., Ciak J., Allison J. L., Olenick J. G. Studies on modes of action of chloroquine, quinacrine, and quinine and on chloroquine resistance. Mil Med. 1966 Sep;131(9 Suppl):1071–1089. [PubMed] [Google Scholar]
- Hahn F. E. Strategy and tactics of chemotherapeutic drug development. Naturwissenschaften. 1975 Oct;62(10):449–458. doi: 10.1007/BF00600499. [DOI] [PubMed] [Google Scholar]
- Hudson B., Upholt W. B., Devinny J., Vinograd J. The use of an ethidium analogue in the dye-buoyant density procedure for the isolation of closed circular DNA: the variation of the superhelix density of mitochondrial DNA. Proc Natl Acad Sci U S A. 1969 Mar;62(3):813–820. doi: 10.1073/pnas.62.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantesaria P., Marfey P. The effect of chlorpromazine on some properties DNA in solution. Physiol Chem Phys. 1975;7(1):53–67. [PubMed] [Google Scholar]
- Krey A. K., Allison R. G., Hahn F. E. Interactions of the antibiotic, distamycin A, with native DNA and with synthetic duplex polydeoxyribonucleotides. FEBS Lett. 1973 Jan 1;29(1):58–62. doi: 10.1016/0014-5793(73)80015-8. [DOI] [PubMed] [Google Scholar]
- Krey A. K., Hahn F. E. Studies on the Methyl Green-DNA complex and its dissociation by drugs. Biochemistry. 1975 Nov 18;14(23):5061–5067. doi: 10.1021/bi00694a005. [DOI] [PubMed] [Google Scholar]
- LERMAN L. S. The structure of the DNA-acridine complex. Proc Natl Acad Sci U S A. 1963 Jan 15;49:94–102. doi: 10.1073/pnas.49.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W., Crothers D. M. Interactions of heteroaromatic compounds with nucleic acids. 1. The influence of heteroatoms and polarizability on the base specificity of intercalating ligands. Eur J Biochem. 1975 May;54(1):267–277. doi: 10.1111/j.1432-1033.1975.tb04137.x. [DOI] [PubMed] [Google Scholar]
- O'Brien R. L., Allison J. L., Hahn F. E. Evidence for intercalation of chloroquine into DNA. Biochim Biophys Acta. 1966 Dec 21;129(3):622–624. doi: 10.1016/0005-2787(66)90078-5. [DOI] [PubMed] [Google Scholar]
- OHNISHI S. I., MCCONNELL H. M. INTERACTION OF THE RADICAL ION OF CHLORPROMAZINE WITH DEOXYRIBONUCLEIC ACID. J Am Chem Soc. 1965 May 20;87:2293–2293. doi: 10.1021/ja01088a040. [DOI] [PubMed] [Google Scholar]
- Olenick J. G. Antibacterial action of primaquine: effects in vitro on polypeptide synthesis and in vivo on ribosomes and ribosomal ribonucleic acid. Antimicrob Agents Chemother. 1975 Dec;8(6):754–756. doi: 10.1128/aac.8.6.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olenick J. G., Hahn F. E. Mode of action of primaquine: preferential inhibition of protein biosynthesis in Bacillus megaterium. Antimicrob Agents Chemother. 1972 Mar;1(3):259–262. doi: 10.1128/aac.1.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rucheton M., Jeanteur P. Studies on amikhellin. I. Intercalative binding to double-stranded DNA. Biochimie. 1973;55(11):1415–1420. doi: 10.1016/s0300-9084(74)80548-1. [DOI] [PubMed] [Google Scholar]
- Révet B. M., Schmir M., Vinograd J. Direct determination of the superhelix density of closed circular DNA by viscometric titration. Nat New Biol. 1971 Jan 6;229(1):10–13. doi: 10.1038/newbio229010a0. [DOI] [PubMed] [Google Scholar]
- Salditt M., Braunstein S. N., Camerini-Otero R. D., Franklin R. M. Structure and synthesis of a lipid-containing bacteriophage. X. Improved techniques for the purification of bacteriophage PM2. Virology. 1972 Apr;48(1):259–262. doi: 10.1016/0042-6822(72)90133-x. [DOI] [PubMed] [Google Scholar]
- Saucier J. M., Festy B., Le Pecq J. B. The change of the torsion of the DNA helix caused by intercalation. II. Measurement of the relative change of torsion induced by various intercalating drugs. Biochimie. 1971;53(9):973–980. doi: 10.1016/s0300-9084(71)80065-2. [DOI] [PubMed] [Google Scholar]
- Smit E. M., Borst P. The superhelix density of bacteriophage PM2 DNA, determined by a viscometric method. FEBS Lett. 1971 Apr;14(2):125–129. doi: 10.1016/0014-5793(71)80117-5. [DOI] [PubMed] [Google Scholar]
- Takesada H., Saito E., Fujita H., Suzuki K., Wada A. Study on the binding nature of acridine orange to DNA by means of flow dichroism. Bull Chem Soc Jpn. 1970 Jan;43(1):181–187. doi: 10.1246/bcsj.43.181. [DOI] [PubMed] [Google Scholar]
- Wang J. C. The degree of unwinding of the DNA helix by ethidium. I. Titration of twisted PM2 DNA molecules in alkaline cesium chloride density gradients. J Mol Biol. 1974 Nov 15;89(4):783–801. doi: 10.1016/0022-2836(74)90053-9. [DOI] [PubMed] [Google Scholar]
- Warhurst D. C., Williamson J. Ribonucleic acid from Plasmodium knowlesi before and after chloroquine treatment. Chem Biol Interact. 1970 Aug;2(2):89–106. doi: 10.1016/0009-2797(70)90042-6. [DOI] [PubMed] [Google Scholar]
- Waring M. Variation of the supercoils in closed circular DNA by binding of antibiotics and drugs: evidence for molecular models involving intercalation. J Mol Biol. 1970 Dec 14;54(2):247–279. doi: 10.1016/0022-2836(70)90429-8. [DOI] [PubMed] [Google Scholar]
- Wolfe A. D., Cook T. M., Hahn F. E. Antibacterial nitroacridine, Nitroakridin 3582: binding to nucleic acids in vitro and effects on selected cell-free model systems of macromolecular biosynthesis. J Bacteriol. 1971 Dec;108(3):1026–1033. doi: 10.1128/jb.108.3.1026-1033.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamabe S. A fluorospectrophotometric study on the binding of acridine orange with DNA and its bases. Arch Biochem Biophys. 1969 Mar;130(1):148–155. doi: 10.1016/0003-9861(69)90020-4. [DOI] [PubMed] [Google Scholar]
- Yamabe S. Further fluorospectrophotometric studies on the binding of acridine orange with DNA. Effects of thermal denaturation of DNA and additions of spermine, kanamycin, dihydrostreptomycin, methylene blue and chlorpromazine. Arch Biochem Biophys. 1973 Jan;154(1):19–27. doi: 10.1016/0003-9861(73)90030-1. [DOI] [PubMed] [Google Scholar]



