Abstract
As tissues and organs are formed, they acquire a specific shape that plays an integral role in their ability to function properly. A relatively simple system that has been used to examine how tissues and organs are shaped is the formation of an elongated Drosophila egg. While it has been known for some time that Drosophila egg elongation requires interactions between a polarized intracellular basal actin network and a polarized extracellular network of basal lamina proteins, how these interactions contribute to egg elongation remained unclear. Recent studies using live imaging have revealed two novel processes, global tissue rotation and oscillating basal actomyosin contractions, which have provided significant insight into how the two polarized protein networks cooperate to produce an elongated egg. This review summarizes the proteins involved in Drosophila egg elongation and how this recent work has contributed to our current understanding of how egg elongation is achieved.
Keywords: Drosophila, actin, extracellular matrix, basal lamina, actomyosin contraction, global tissue rotation
Introduction
The remarkable transformation that occurs during development as a single cell is transformed into a mature organism not only requires the generation of a large number of differentiated cells, but also the organization of these cells into tissues and organs. To do this, cells must undergo orchestrated cell shape changes, cell migrations and cell rearrangements, which not only allow the tissues and organs to be formed, but also allow them to acquire the appropriate shape. These cell behaviors are dependent upon coordinated reorganization of the actin cytoskeleton, actomyosin contraction and both cell-cell and cell-substrate adhesion. While we have obtained a basic understanding of how each of these contributes to cell behavior, how they work together to influence tissue and organ shape remains poorly understood. The process by which a Drosophila egg acquires its elongated shape provides an excellent model for studying how an organ is shaped. Drosophila egg chamber elongation employs many of the basic cell behaviors seen in other systems as well as novel processes uncovered by recent studies using live imaging techniques,1,2 which provide significant insight into how dynamic interactions between a contractile actomyosin network and the extracellular matrix (ECM) components of a basal lamina cooperate to produce an elongated egg.
Overview of Drosophila oogenesis
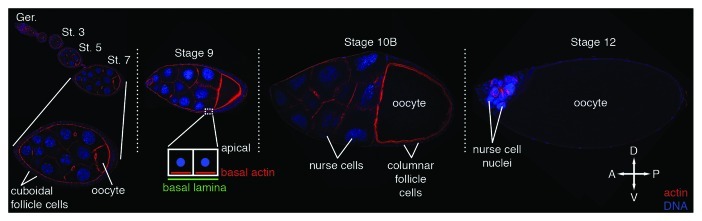
The production of an elongated Drosophila egg takes place in the ovary of the adult female. Each ovary consists of ~16 ovarioles, which are long chains of six to seven progressively older egg chambers (or follicles) separated by a small number of somatic stalk cells (Fig. 1).3 At the anterior tip of each ovariole is a structure called the germarium that contains both germline and somatic stem cells, and is the site of egg chamber assembly. Each egg chamber is composed of a cyst or group of 16 interconnected germline cells, one oocyte and 15 nurse cells, all surrounded by a single layer of somatic follicle cells (Fig. 1). Each germline cyst is derived from a single cell, the cystoblast that undergoes four rounds of cell division with incomplete cytokinesis to give rise to the 16 interconnected germline cells. One of these cells will become the oocyte and initiate meiosis, while the remaining 15 cells will become nurse cells and undergo several rounds of endoreplication. Once the cyst is formed, it becomes encased by a layer of somatic follicle cells and moves out of the germarium as a fully assembled egg chamber. As the egg chamber matures over the next 2.5–3 d, it proceeds through a series of 14 stages, which can be distinguished by a variety of morphological criteria including shape and size of the oocyte, nurse cells and entire egg chamber as well as the movements of specific subsets of follicle cells described below.3
Figure 1. Drosophila oogenesis. Egg chambers from a subset of the stages of oogenesis, stained with phalloidin to visualize F-actin (red) and DAPI to visualize DNA (blue), reveal the major changes in the size and shape of the egg chamber and its component cells over time. A single focal plane through the center of each chamber is shown. Dotted lines separate egg chambers that were obtained from distinct ovarioles. A higher magnification image of the stage 7 egg chamber is included to show how the cuboidal follicle cell layer completely surrounds the germline cells at this stage. A similar organization of the egg chamber is seen from stages 1–8. The anterior/posterior (A/P) and dorsal/ventral (D/V) axes are indicated. Ger, germarium; St, stage.
Throughout oogenesis, the volume of the oocyte increases by approximately four orders of magnitude.4 This increase in oocyte volume is due in large part to the action of the nurse cells. As their name implies, the nurse cells produce many of the macromolecules and organelles that the oocyte needs to initiate development after fertilization. These include maternal mRNAs and proteins, as well as ribosomes and mitochondria,3,5 all of which are transported from the nurse cells into the oocyte through the cytoplasmic bridges (or ring canals), which are formed as a result of the incomplete cytokinesis that creates the germline cyst.6 This transport consists of two phases, slow and fast. Slow transport occurs throughout the early stages of oogenesis (stages 1–10) and, combined with the uptake of yolk proteins from the follicle cells and hemolymph (stages 8–10), is responsible for the increase in the size of the oocyte so that it occupies roughly half of the egg chamber by the beginning of stage 10. Fast transport occurs near the end of oogenesis (stage 11) when all of the nurse cells simultaneously dump the contents of their cytoplasm into the oocyte, allowing the oocyte to achieve its final volume.5,7 After nurse cell dumping is complete, the nurse cells are no longer needed and undergo programmed cell death.8,9
The follicle cells also play important roles in the formation of a mature egg. Interactions between the posterior follicle cells and the oocyte help establish the anterior/posterior (A/P) axis within the follicle cell layer, the oocyte and eventually the embryo.10,11 Near the end of oogenesis, the follicle cells are responsible for producing the vitelline envelope and eggshell that protect the embryo as it develops, allow the embryo to obtain oxygen and facilitate fertilization and the hatching of the mature first instar larvae at the end of embryogenesis. When the egg chamber is first formed, the follicle cells are organized in a simple epithelial monolayer of cuboidal cells that completely surround the germline cells (Fig. 1). By the end of stage 6, the follicle cells have completed four to five rounds of cell division resulting in ~1,000 follicle cells that then undergo several rounds of endoreplication and growth.3 Between stages 9 and 11, the follicle cells undergo a series of morphological changes and cell migrations, which result in the oocyte becoming completely surrounded by follicle cells. At the beginning of stage 9, most of the follicle cells initiate a posterior migration to become concentrated around the oocyte, while a few follicle cells (~50) flatten and stretch out over the nurse cells. During this migration, the follicle cells that will ultimately be in direct contact with the oocyte change shape from cuboidal to columnar (Fig. 1). This change in cell shape, along with the increase in the size of the germline cells, are thought to drive the posterior migration of the follicle cells.12,13 At the same time, a small group of 6–10 follicle cells at the anterior tip of the egg chamber, called border cells, break away from the follicle cell layer and migrate in between the nurse cells to the anterior boundary of the oocyte.14-16 Their arrival at the oocyte boundary marks the beginning of stage 10 and coincides with the completion of the posterior migration of the columnar follicle cells. The border cell cluster will then spread out and ultimately contribute to the formation of the micropyle, the structure through which the sperm gain access to the egg. The beginning of stage 10 also marks the initiation of the movement of the anterior-most columnar cells, the centripetal cells, up and over the anterior end of the oocyte.12,17 The centripetal cells eventually make contact with and form connections to the border cells resulting in the formation of a continuous layer of follicle cells around the oocyte. The centripetal cells will ultimately form the operculum, the region of the eggshell through which the larva will emerge, as well as contribute to the formation of the micropyle. Finally, two groups of follicle cells that cover the dorsal anterior region of the oocyte will undergo a series of well-characterized cell shape changes and cell migrations to form the dorsal appendages,18 the eggshell structures that allow the exchange of gases between the developing embryo and its environment.
In addition to the changes in size and shape seen in the germline and follicle cells, the egg chamber as a whole also undergoes a dramatic change in shape as it is transformed from a sphere into an ellipsoid. This process, referred to as egg chamber elongation, results in an egg chamber that is 2.5 times longer than it is wide.1 The mature egg then maintains this ellipsoid shape, which likely facilitates its movement through the oviduct to be fertilized and eventually laid. Egg chamber elongation starts around stage 5 and continues throughout oogenesis with the most dramatic changes in egg chamber shape occurring between stages 5 and 11 (Fig. 1). During this time, the egg chamber is also undergoing a significant amount of growth, increasing its volume by as much as 100-fold.19 In order for the egg chamber to elongate, this increase in volume must be channeled along the long, or A/P axis, of the egg chamber. This has been proposed to be accomplished through the formation of a “molecular corset” that constrains the central portions of the egg chamber, forcing any increase in volume to preferentially expand the ends of the chamber.20,21 The components of the proposed molecular corset include two polarized protein networks; one that consists of bundles of parallel actin filaments on the basal side of each follicle cell that are uniformly oriented throughout the cell layer (Fig. 2) and one that consists of a parallel array of ECM proteins within the basal lamina that completely surrounds the basal surface of the follicle cell layer of each egg chamber (Fig. 3). Both protein networks are oriented so that their parallel arrays are perpendicular to the long, or A/P axis, of the egg chamber, as would be expected for the proposed molecular corset (Figs. 2 and 3). While the presence of these two polarized protein networks has been known for years,20-23 the relationship between them and how they facilitate egg chamber elongation was unclear. Recent use of live imaging techniques to visualize elongating egg chambers has provided significant insight into this relationship, revealing two novel processes that create or use these networks to mediate egg chamber elongation; global rotation of the egg chamber within the surrounding basal lamina1 and oscillating basal actomyosin contractions in a subset of follicle cells.2 This review will summarize how the elucidation of these novel processes has impacted our current understanding of egg chamber elongation, including how the follicle cell basal actin filaments and ECM components of the basal lamina become polarized as well as how these two polarized arrays interact and how they contribute to the elongation of the egg chamber.
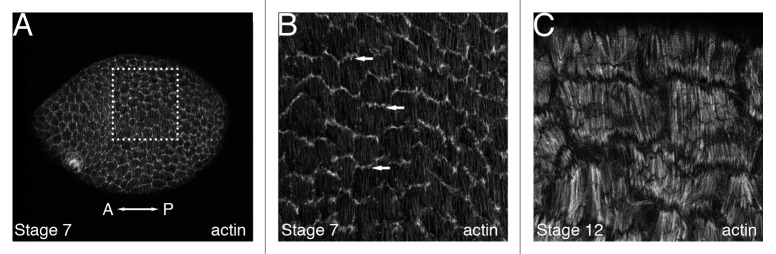
Figure 2. Follicle cell basal actin filaments in wild-type egg chambers. The basal follicle cell surface of either an entire stage 7 egg chamber (A) or high magnification views of a subset of follicle cells from a stage 7 (B) or stage 12 (C) egg chamber that have been stained with phalloidin to visualize F-actin. All panels are oriented so that anterior is to the left. (B) High magnification view of the dotted box in (A) shows that the follicle cells in a stage 7 egg chamber produce actin-based protrusions (arrows) oriented in the same direction from a single-cell edge. These protrusions appear to reach out over the basal surface of the adjacent follicle cell. (C) High magnification view of the basal surface of a stage 12 egg chamber reveals basal actin filaments that are thicker and more pronounced than stage 7, with striations that resemble those seen in stress fibers in cultured cells.
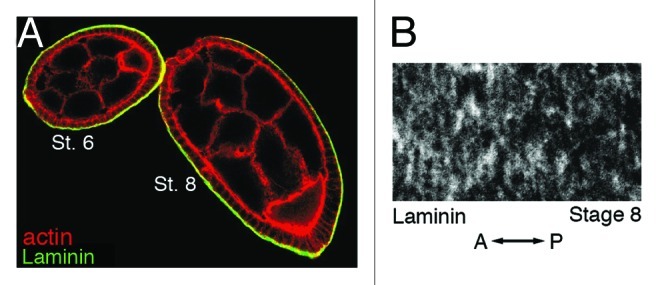
Figure 3. Laminin is found along the outer basal surface of follicle cells and becomes oriented into strands that are perpendicular to the A/P axis of the egg chamber. (A) A stage 6 and stage 8 egg chamber that have been stained with phalloidin to visualize F-actin (red) and an antibody against the laminin α chain (green). (B) High magnification view of a stage 8 egg chamber that has been stained with an antibody against the laminin α chain. Orientation of the A/P axis of the egg chamber is indicated. St., Stage. (A and B) reprinted from reference 29 with permission from Elsevier.
Changes in the Organization of Follicle Cell Basal Actin During Oogenesis
One of the central components of the proposed molecular corset involved in egg chamber elongation is the polarized network of actin filaments present along the basal surface of the follicle cells. The follicle cell layer is arranged around the germline cells so that the apical surfaces of the follicle cells face the germline cells while their basal surfaces face the exterior of the egg chamber (Fig. 1). Before the follicle cell basal actin filaments can be assembled, distinct apical and basal domains of these cells must be established. This occurs in a stepwise manner within the germarium before an egg chamber is assembled.24 Large bundles of actin filaments are first visible along the basal surface of the follicle cells in the posterior half of the germarium.25 These actin filaments are arranged in a parallel array that wraps around the germarium perpendicular to its long axis, foreshadowing their arrangement in later staged egg chambers. However, this global parallel organization is lost as the egg chambers exit the germarium, with follicle cells in early staged egg chambers displaying basal actin filament bundles that appear to “swirl” around the chamber.25 It is interesting to note that the cortical actin that underlies the plasma membrane of each follicle cell is not yet evident in these early egg chambers, making the bundled follicle cell basal actin filaments appear as if they are continuous across cell boundaries. By stage 4, follicle cell cortical actin is evident, providing a clear outline of each cell. While the basal actin filaments within each follicle cell at this stage appear to be organized into roughly parallel arrays, the polarity of the filaments is not coordinated across the basal surface of the follicle cell layer.25 The global polarization of the basal actin filaments once again becomes apparent during stages 5 and 6 when the basal filaments in follicle cells near the anterior and posterior poles become oriented perpendicular to the A/P axis.25 The basal actin filaments in the more central follicle cells gradually take on a similar orientation so that by stage 7 the basal filaments within all follicle cells are uniformly oriented (Fig. 2A). Around this time the follicle cells also extend actin-based protrusions from one of their basal cell edges parallel to the A/P axis of the egg chamber that reach out over the basal surface of the adjacent follicle cell21 (Fig. 2B, arrows). This resembles the formation of actin-based protrusions from the leading edge of a migrating cell26 and suggests that a distinct “leading” and “trailing” edge of each follicle cell has been established. Furthermore, since all of the follicle cells in a given egg chamber produce protrusions along the same basal cell edge, this distinction must somehow be coordinated throughout the cell layer. The next major change in the follicle cell basal actin occurs during stage 9 in the subset of follicle cells that become stretched out over the nurse cells. As these cells lengthen and narrow their basal actin filaments lose the parallel bundled organization and take on a broad, mesh-like distribution.20 The remaining majority of follicle cells retain the parallel organization of their basal actin filaments until stages 10B and 11 when these filaments undergo a transient change in orientation.20,27,28 During stage 10B, the basal actin filaments become thicker and begin to shift their orientation. During stage 11, this shift continues so that the filaments within each follicle cell become organized into a fan-shaped array that is oriented along the long axis of the chamber with the base of the fan positioned at the posterior edge of the cell.27 By stage 12, the fan-shaped orientation has returned back to the parallel circumferential arrays seen in earlier stages, which are maintained for the remainder of oogenesis (Fig. 2C).
Changes in the Organization of ECM Components of the Follicle Cell Basal Lamina During Oogenesis
The second major component of the molecular corset is represented by the polarized organization of several ECM components of the follicle cell basal lamina, including collagen IV, laminin and perlecan. Laminin A is present along the outer basal surface of the follicle cells starting at stage 2 (Fig. 3A).25 During stages 6–9, laminin A is organized into a regular pattern of long parallel strands that encircle the egg chamber perpendicular to its A/P axis (Fig. 3B).22,29 Collagen IV is also present along the outer basal surface of the follicle cells from the time an egg chamber first emerges from the germarium.1 In early chambers, collagen IV is present as puncta or short fibers with no clear polarization. Starting around stage 5, the collagen IV fibers grow in length, increase in density and become oriented perpendicular to the A/P axis of the egg chamber.1 The collagen IV fibers increase in length and density until they reach a maximum at stage 8, after which their density decreases while their length remains roughly the same through stage 12.1 Perlecan can be found in the basal lamina surrounding the basal surface of the follicle cells starting at stage 3.30 Similar to laminin and collagen IV, perlecan fibers are arranged into parallel arrays that are oriented perpendicular to the A/P axis of the egg chamber by stage 7.30 However, detailed studies of when this organization of the perlecan fibers arises have not yet been performed.
The Follicle Cell Basal Actin Network and its Interaction with the ECM Influences Egg Chamber Elongation
The observation that the follicle cell basal actin network and the ECM components of the follicle cell basal lamina come into alignment with each other over roughly the same time frame during oogenesis (stages 5–7) suggests that they may be either coordinately regulated or somehow influence each other as the egg chamber elongates. Support for this first came from the identification and analysis of genes that when mutated, result in the production of eggs that are both shorter and broader than wild-type, a.k.a. round eggs (Fig. 4). These so-called round egg genes encode ECM components of the follicle cell basal lamina, proteins that regulate actin dynamics and/or actomyosin contractility and proteins that mediate cell-cell or cell-ECM interactions. Most of the round egg genes are required during developmental stages prior to adulthood. Therefore, analysis of their role during egg chamber elongation has relied on either the expression of gene-specific RNAi (RNA interference) constructs31 within follicle cells or the generation of clones of homozygous mutant follicle cells within an initially heterozygous population.32 The round egg genes can be placed into four broad classes based on when during the construction of the polarized follicle cell basal actin filament network they appear to be required, including those that are (1) required for the formation and/or maintenance of the basal actin filaments, (2) required to establish and/or maintain the parallel organization of the basal actin filaments, (3) required to establish and/or maintain the coordinated orientation of follicle cell basal actin filaments throughout the follicle cell layer and (4) are not required for the formation, organization or orientation of the follicle cell basal actin filaments (Fig. 5).
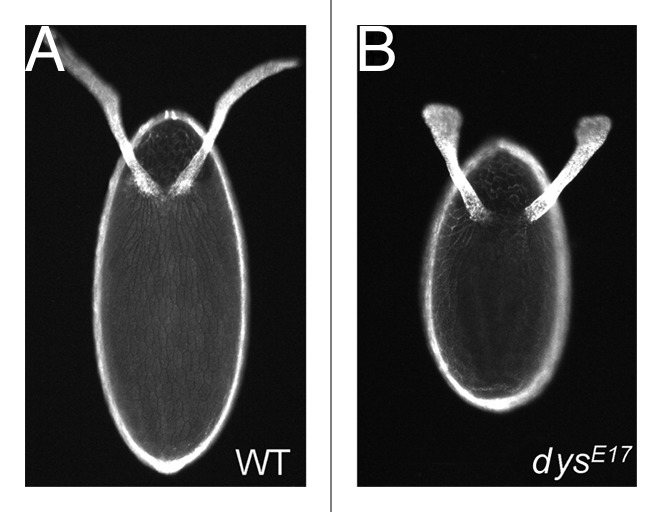
Figure 4. Round eggs are shorter and broader than wild-type. Dorsal view of eggs from wild-type (A) or dys mutant (dysE17/Df(3R)6184) (B) females. dys is a member of the class 3 round egg genes. Similar round eggs are produced when other round egg genes are mutated, irrespective of which class they belong to. Eggs are oriented with anterior at the top of the image. (A and B) reprinted from reference 60 with permission from Elsevier.
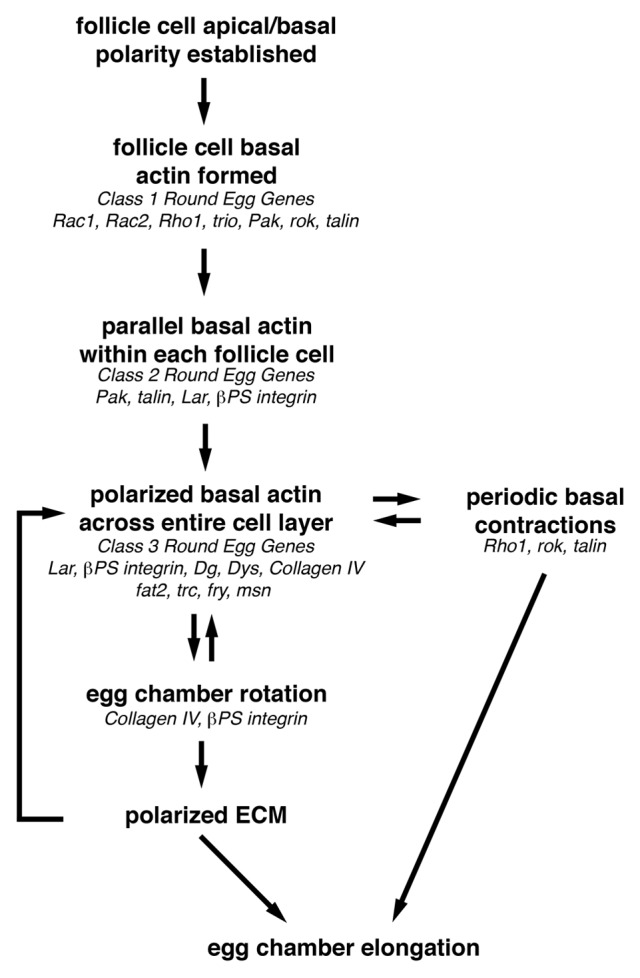
Figure 5. Overview of how egg chamber elongation is achieved. Before egg chamber elongation can begin, a uniformly polarized network of follicle cell basal actin filaments must be established. This requires that the follicle cell basal domain be specified so that the basal actin filaments can be formed, then organized into parallel bundles within each follicle cell that then become oriented perpendicular to the A/P axis of the egg chamber. This uniformly polarized follicle cell basal actin network is then used to provide the force necessary to drive the active migration of the follicle cells over the stationary basal lamina during egg chamber rotation from stage 5–8. As the follicle cells migrate, they organize the ECM components of the basal lamina into parallel arrays that reinforce the orientation of the polarized basal actin filaments and form the molecular corset that contributes to egg chamber elongation. Then during stages 9 and 10, the polarized follicle cell basal actin filaments, together with non-muscle Myosin II, mediate the periodic basal contractions that may maintain the basal actin filaments and contribute to egg chamber elongation by augmenting the molecular corset around the center of the egg chamber. Genes that may function at each step of egg chamber elongation based on their loss-of-function phenotype are indicated in italics.
Class 1 round egg genes: required for the formation and/or maintenance of the follicle cell basal actin filaments
Mutation of the round egg genes that fall into the first class, results in a loss or severe reduction of follicle cell basal actin filaments, suggesting that they are required for the formation and/or maintenance of these filaments (Fig. 5). Class 1 round egg genes include the small GTPases Rac1, Rac2 and Rho1, the Rac GEF (guanine nucleotide exchange factor) trio, the Rac effector Pak, the Rho1 effector rok and the cell-ECM adhesion component talin. Rho and Rac, along with Cdc42, are members of the Rho family of small GTPases. Like most small GTPases, the Rho family GTPases cycle between an active GTP-bound state and inactive GDP-bound state. This cycle is regulated by three classes of proteins GEFs, GAPs (GTPase-activating proteins) and GDIs (guanine nucleotide dissociation inhibitors).33 In their active state, the Rho family GTPases are capable of interacting with and regulating the activity of a wide variety of target or effector proteins that mediate the cellular functions influenced by these regulatory proteins. The Rho family GTPases have been shown to regulate multiple cellular processes including cell adhesion, cell migration, cell polarity, endocytosis, vesicle trafficking, cell proliferation, oncogenesis and gene transcription.34 However, the most relevant function of Rho family GTPases to egg chamber elongation is their well-established regulation of the actin cytoskeleton.35,36 In migrating cells, Rac regulates actin polymerization along the leading edge and promotes the formation of nascent cell-ECM adhesions in this region, while Rho regulates actomyosin contraction farther back from the leading edge and promotes the formation of more mature cell-ECM adhesions, stress fibers and retraction of the trailing edge of the cell.35,37
Expression of a dominant negative version of Rho1 in a subset of follicle cells, results in a severe reduction of basal actin filaments with some cells appearing to completely lack basal filaments altogether.2 This suggests that Rho1 is required for the formation and/or maintenance of the basal actin filaments. Consistent with this, follicle cell clones that carry a loss of function mutation in Rho1 cause a failure of egg chamber elongation.29 Rok (Rho kinase) is a serine/threonine kinase that is activated by Rho1 and has been shown to function downstream of Rho1 during stress fiber formation and actomyosin contraction in a variety of cell types.38 Expression of Rok-RNAi in follicle cell clones results in a reduction in the levels of basal actin, although not to the same degree as seen when dominant negative Rho1 is expressed.2 Furthermore, the actin filaments that remain appear to retain their parallel organization within each cell as well as their orientation relative to the basal filaments in surrounding wild-type cells.2 Together, these results suggest that actomyosin contraction, mediated by the Rho1/Rok pathway, may play a role in the formation and/or maintenance of follicle cell basal actin filaments, although additional effectors downstream of Rho1 may also be required.
In Drosophila, there are three Rac-like genes, Rac1, Rac2 and Mtl.39 Clones of follicle cells that are mutant for both Rac1 and Rac2 display a complete loss of basal actin filaments.40 This is consistent with the role of Rac in regulating actin polymerization in migrating cells and suggests that Rac1 and Rac2 are also required for the formation and/or maintenance of the basal actin network in follicle cells. Furthermore, the effect of Rac1 and Rac2 on follicle cell basal actin appears to be nonautonomous as some of the wild-type cells bordering a Rac1/Rac2 mutant clone display either a loss of basal actin or mild disruptions in the organization and orientation of the filaments.40 Interestingly, egg chambers that carry large Rac1/Rac2 mutant clones do not appear significantly rounded.40 Although this effect has not been quantified, it may suggest that an egg chamber can elongate even when some of the follicle cells lack basal actin filaments. The Rac GEF Trio promotes the exchange of GDP for GTP for all three Drosophila Rac-like proteins, thereby activating them.41 Although human Trio is also capable of interacting with and activating Rho, evidence of this interaction has not yet been demonstrated in Drosophila.42 While follicle cell clones that are mutant for trio have a significant reduction in the number of basal actin filaments, the small number of filaments that are still visible remain organized into parallel arrays that are oriented in the same manner as the surrounding wild-type cells.40 This suggests that while Trio is required for the formation and/or maintenance of the basal actin filaments, it does not function as the sole GEF for Rac1 and Rac2 in follicle cells. It should be noted that the ultimate effect that loss of Trio has on egg chamber elongation has not yet been examined. Pak (p21-activated kinase) is a serine/threonine kinase that is activated by Rac and Cdc42.43 Clones of Pak mutant follicle cells display a severe reduction of basal actin filaments with most cells completely lacking filaments, especially when the clone contains a large number of cells.40 In those Pak mutant follicle cells that retain some basal actin, the filaments still appear as thick bundles, but these are often no longer organized into parallel arrays and instead appear to clump together and cross over each other forming a dense meshwork over the basal surface of the cell.40 This suggests that Pak may be a key Rac effector that mediates the formation and/or maintenance of the follicle cell basal actin network and may also be required for the organization of the bundled actin filaments into parallel arrays. Furthermore, Pak's regulation of basal actin does not appear to be cell autonomous as wild-type cells bordering the clone occasionally display reduced or disorganized bundles, while mutant cells along the border of the clone occasionally retain at least a few parallel actin bundles.40 Pak mutant egg chambers often also display regions where the follicle cells are arranged into multiple layers rather than the normal single layer, consistent with an additional role for Pak in establishing and/or maintaining apical/basal follicle cell polarity.40 Interestingly, the Pak round egg mutant phenotype has recently been shown to be suppressed by the introduction of a single mutant copy of any one of several different members of the Rho1/Rok pathway suggesting that Pak may function in opposition to Rho1 during egg chamber elongation.44
Talin, encoded by the rhea gene, is a large cytoplasmic protein that links the intracellular domain of integrins to the actin cytoskeleton and plays an essential role in integrin-mediated cell-ECM adhesion.45 Clones of follicle cells mutant for rhea or expressing Talin-RNAi display a reduction in follicle cell basal actin filaments, with the remaining filaments no longer being organized into parallel arrays.2,46 This suggests that Talin is required for the formation and/or maintenance of the follicle cell basal actin network as well as to establish and/or maintain their parallel orientation. It is interesting to note that Talin has also been shown to inhibit transcription of the shotgun gene in follicle cells in an integrin-independent manner.46 shotgun encodes the cell-cell adhesion protein DE-cadherin (Drosophila E-cadherin), suggesting that the effect of loss of Talin in follicle cells could result from an overexpression of DE-cadherin in these cells. However, overexpression of DE-cadherin specifically in follicle cells has no effect on the relative abundance, organization or orientation of the basal actin filaments,2 suggesting that Talin must influence the basal actin filaments through another mechanism.
The round egg genes in this first class appear to be required for the formation and/or maintenance of the follicle cell basal actin filaments. However, in order to distinguish between these two possibilities, it will be necessary to examine basal actin in null-mutant follicle cells prior to egg chamber formation when basal actin first appears, which has not yet been done for any of the genes in this class.
Class 2 round egg genes: required to establish and/or maintain the parallel organization of the follicle cell basal actin filaments
Mutations in round egg genes that fall into class 2 result in follicle cell basal actin filaments that are present but no longer organized into parallel bundles, suggesting that these genes are required to establish and/or maintain the parallel organization of the basal actin filaments (Fig. 5). Interestingly, all of the class 2 round egg genes also display loss-of-function phenotypes that place them into either class 1 or class 3. This suggests that the formation and/or maintenance of the basal actin filaments, their organization into parallel bundles and their orientation perpendicular to the A/P axis of the egg chamber, are linked. Although all class 2 genes could be placed into another class, the definition of a separate class is warranted as not all class 1 and class 3 genes can be placed into class 2 (Fig. 5).
The second class of round egg genes contains four genes; two that are also considered members of class 1, Pak and talin, and two that are also considered members of class 3, Lar (Leukocyte-antigen-related-like) and the integrin subunit βPS. As mentioned above, loss of Pak or talin not only results in a loss or severe reduction of follicle cell basal actin filaments, but the filaments that remain are not organized into parallel bundles within each cell. This suggests that Pak and Talin are required for both the formation and/or maintenance of the follicle cell basal actin filaments as well as to establish and/or maintain their parallel organization within each cell.
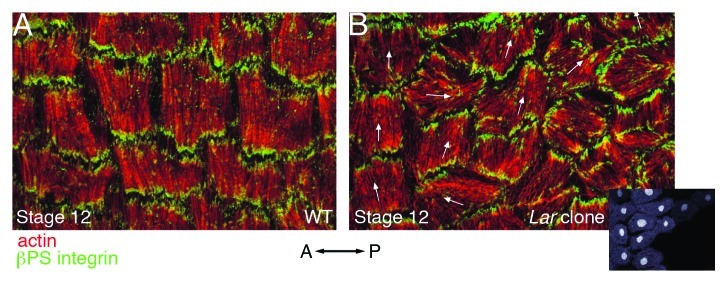
Lar encodes a transmembrane receptor tyrosine phosphatase that was initially identified as being required for axon guidance in the Drosophila nervous system.47 In vitro binding and cell culture assays using the human Lar protein indicate that its extracellular domain binds laminin,48 suggesting that Drosophila Lar may also interact with this ECM protein. Furthermore, the intracellular domain of Drosophila Lar has been shown to bind to and dephosphorylate the actin-binding protein Ena (Enabled) in vitro.49 Since the binding of mammalian Ena/VASP proteins to actin has been shown to promote its continued polymerization,50 Lar may provide a link between the actin cytoskeleton and the ECM while also contributing to actin dynamics at these sites. Although clones of Lar mutant follicle cells retain roughly equivalent levels of basal actin filaments compared with wild-type cells,25 the organization or orientation of these filaments is disrupted. In some Lar mutant cells, the basal actin filaments are disorganized, forming centrally located aggregates or accumulating around the periphery of the cell.29 In other Lar mutant cells, the basal actin filaments remain organized in parallel bundles within each cell, however their orientation perpendicular to the A/P axis of the egg chamber is lost (Fig. 6).25,29 No matter which actin phenotype is seen in the Lar mutant cells, some of the surrounding wild-type cells display parallel basal actin filaments that are not uniformly oriented perpendicular to the A/P axis of the egg chamber (Fig. 6).25,29 Taken together, these results suggest that Lar is required to initiate and/or maintain the parallel organization of follicle cell basal actin filaments within each follicle cell as well as coordinate their orientation throughout the follicle cell layer.
Figure 6. Loss of the round egg gene Lar results in follicle cell basal actin filaments that remain parallel within each cell but are no longer uniformly oriented across the follicle cell layer. High magnification views of a subset of follicle cells from a stage 12 wild-type egg chamber (A) or a stage 12 egg chamber that contains a clone of Lar mutant follicle cells (B). Both egg chambers have been stained with phalloidin to visualize F-actin (red) and an antibody against βPS integrin (green). Inset in (B) shows GFP marking wild-type cells at one-third scale. The mean orientation of actin filaments within wild-type cells in (B) is indicated with arrows. While some wild-type cells retain the proper orientation (far left cells), those closest to the mutant clone display defects in filament orientation, which resemble those in the Lar mutant cells (far right, without arrows). Orientation of the A/P axis of the egg chamber is indicated. (A and B) reprinted from reference 29 with permission from Elsevier.
Integrins are transmembrane proteins that mediate adhesion between a cell and the ECM. The extracellular domain of integrins binds to ECM proteins, while their intracellular domain binds to a multiprotein complex that links them to the actin cytoskelton.51 Integrins are also capable of mediating intracellular signaling following ECM binding that can influence a variety of cellular processes.52 Each functional integrin protein is a heterodimer that consists of one α and one β subunit,53 and the Drosophila genome contains five α and two β subunits.54 The βv subunit is only expressed in the midgut,55 making βPS, encoded by the myospheroid (mys) gene, the primary β subunit used in Drosophila. βPS, along with all five of the α subunits, appear to affect egg chamber elongation, although not to the same degree. Egg chambers with large clones of βPS (mys), αPS1 (encoded by multiple edematous wings, mew) or αPS2 (encoded by inflated, if) mutant follicle cells all produce significantly rounded eggs, while egg chambers with large clones of αP3 (encoded by scab, scb) and egg chambers expressing αPS4-RNAi or αPS5-RNAi produce eggs that are 5–10% shorter in length.1,29,56,57 To determine the effect of removing all integrin dimers on basal actin, clones of mys (βPS integrin) mutant follicle cells were generated.1,27,29 mys (βPS integrin) mutant follicle cells display basal actin filaments that are either concentrated at the periphery of the cell,1,27 or maintain their normal parallel organization within each cell but are no longer oriented perpendicular to the A/P axis of the egg chamber.29 In both cases, some of the wild-type cells surrounding the clone display parallel basal actin filaments that also lose their uniform orientation perpendicular to the A/P axis of the egg chamber.1,29 This suggests that, like Lar, βPS integrin is required to initiate and/or maintain the parallel organization of follicle cell basal actin filaments within each follicle cell as well as coordinate their orientation throughout the follicle cell layer. In addition to the effects on the organization and orientation of the basal actin filaments, Delon and Brown27 also found that mys (βPS integrin) mutant follicle cells had ~33% more basal actin than adjacent wild-type cells, suggesting that integrins are also required to limit the amount of follicle cell basal actin.
Class 3 round egg genes: required to establish and/or maintain the coordinated orientation of follicle cell basal actin filaments throughout the follicle cell layer
Mutation of the round egg genes that fall into class 3 result in basal actin filaments that are organized into parallel bundles, but are not oriented perpendicular to the A/P axis of the egg chamber, suggesting that they are required to establish and/or maintain the coordinated orientation of these filaments throughout the follicle cell layer (Fig. 5). In addition to Lar and βPS integrin, the third class of round egg genes include the ECM receptor Dystroglycan (Dg) and its intracellular binding partner Dystrophin (Dys), the ECM component collagen IV α2, the Cadherin superfamily member kugelei (a.k.a. fat2) and the cytoplasmic signaling proteins tricornered (trc), furry (fry) and misshapen (msn). Loss of Lar or βPS integrin in follicle cells results in basal actin filaments that are either no longer organized into parallel arrays or are present in parallel bundles that are not properly oriented perpendicular to the A/P axis of the egg chamber in both mutant and adjacent wild-type cells. This suggests that Lar and integrins are required to initiate and/or maintain the parallel organization of follicle cell basal actin filaments within each follicle cell as well as establish and/or maintain the coordinated orientation of these filaments throughout the follicle cell layer.
Dg and Dys are central components of the dystrophin-glycoprotein complex (a.k.a. dystrophin-associated protein complex).58 The dystrophin-glycoprotein complex was initially identified as being required in muscle cells to allow the plasma membrane to withstand mechanical stress generated by contraction. As a result, loss of many of the components of this complex has been linked to muscular dystrophy.58 Dg is a transmembrane protein that binds to ECM components including laminin and perlecan through its extracellular domain. Although in follicle cells Dg may primarily interact with perlecan as the localization of Dg to the follicle cell basal membrane is dependent on perlecan but not laminin.30 The intracellular domain of Dg binds Dys, which in turn binds actin, effectively linking the ECM and the actin cytoskeleton. Clones of follicle cells that lack either Dg or Dys display basal actin filaments that are organized into parallel arrays within each cell, but are not properly oriented perpendicular to the A/P axis of the egg chamber.59,60 Interestingly, the defect in basal actin orientation is restricted to the Dg or Dys mutant cells as nearby wild-type cells display normal basal actin filament orientation.60 This suggests that Dg and Dys are required to initiate and/or maintain the proper orientation of the follicle cell basal actin filaments, but only within a given cell and not throughout the cell layer as is the case for the other round egg ECM-interacting proteins Lar and integrins.
Collagen IV is an ECM protein component of the follicle cell basal lamina. Each collagen IV molecule is comprised of three α-chains organized into a triple helix that undergoes further assembly to form the collagen matrix.61 There are two type IV collagen genes in Drosophila, α1(IV) encoded by the Cg25C gene62,63 and α2(IV) encoded by the viking gene,64 both of which are thought to contribute to basal lamina throughout the animal.65 Visualization of collagen IV using a viking-GFP (green fluorescent protein) reporter reveals the presence of collagen IV fibers starting around stage 1, which become oriented perpendicular to the A/P axis of the egg chamber starting around stage 5, mirroring the orientation of the follicle cell basal actin filaments.1 Egg chambers with follicle cell layers that consist entirely of viking mutant cells display basal actin filaments that are initially organized into parallel arrays oriented perpendicular to the A/P axis of the egg chamber. However, around stage 7, this uniform orientation of the basal actin filaments across the cell layer is lost resulting in basal filaments whose orientation often varies from cell to cell.1 This suggests that while collagen IV is required to maintain the proper orientation of the basal actin filaments, it is not required for the initial establishment of this orientation.
Fat2, encoded by the kugelei (a.k.a. kugel) gene, is a member of the cadherin superfamily of calcium-dependent transmembrane proteins that mediate adhesion between adjacent cells. kugelei (fat2) mutant adult females are viable but produce round eggs.22,66 When egg chambers from kugelei (fat2) mutant females are examined, the follicle cell basal actin filaments are still arranged in parallel bundles within each cell, however, the bundles are no longer oriented perpendicular to the A/P axis of the egg chamber.22,66 A similar disruption of basal actin filament orientation is seen when clones of kugelei (fat2) mutant follicle cells are generated and this disruption extends to wild-type cells that lie outside the clone. However, in order to see this phenotype, at least 60% of the follicle cells in a given egg chamber must be mutant.66 Taken together, these results suggest that Fat2 is required to initiate and/or maintain the coordinated orientation of the follicle cell basal actin filaments across the follicle cell layer. Interestingly, the Fat2 protein has a restricted localization along the basal surface of follicle cells such that it is only found along one of the two cell edges where the basal actin filaments terminate.66 Therefore, Fat2 may influence basal actin filament orientation by specifying differences between the basal follicle cell edges that are parallel to the A/P axis of the egg chamber.
Trc is a NDR (nuclear DBF2-related) kinase family member that has been shown to function with the large HEAT/Armadillo repeat-containing protein Fry during the morphogenesis of epidermal hairs and bristles67-71 as well as the tiling and branching of sensory neuron dendrites in Drosophila.72 Trc and Fry not only physically interact,69,71 but this interaction has a positive effect on the kinase activity of Trc,72 which is essential for its in vivo functions.71,72 Egg chambers that are comprised predominantly of trc or fry mutant follicle cells display similar phenotypes, where the basal actin filaments are still organized into parallel arrays within each follicle cell, but most of these arrays are no longer oriented perpendicular to the A/P axis of the egg chamber.73 This suggests that Trc and Fry are required for the initiation and/or maintenance of basal actin filament orientation, although additional experiments will need to be performed to address whether they function in an autonomous or non-autonomous manner. Consistent with this proposed role, the Trc protein shows a polarized distribution along the basal follicle cell surface with punctae that are concentrated along the cell edges where the basal actin filaments terminate.73 In addition to the essential interaction with HEAT/Armadillo repeat-containing proteins, NDR kinases must also be phosphorylated by a Ste20-like protein kinase in order to be fully activated.74 A Drosophila Ste20-like protein kinase, Msn, has also been shown to be required for egg chamber elongation raising the possibility that it may regulate Trc activity in follicle cells.73 Egg chambers that are comprised predominantly of msn mutant follicle cells display basal actin filaments that are organized into parallel arrays within each cell, but are not properly oriented perpendicular to the A/P axis of the egg chamber.73 This resembles the phenotype seen in trc and fry mutant follicle cells, although Horne-Badovinac et al.73 note that the msn mutant phenotype is more severe, but do not examine this difference in detail. Taken together, these results suggest that Msn is also required for the initiation and/or maintenance of basal actin filament orientation, although additional experiments will need to be performed to further examine its potential roles independent of the NDR kinase pathway as well as to address whether it functions in an autonomous or nonautonomous manner.
Class 4 round egg genes: not required for the formation, organization or orientation of the follicle cell basal actin filaments
The fourth class of round egg genes contains a single gene, tensin.27,75 Tensin is an adaptor protein that links integrins to the actin cytoskeleton. In follicle cells, Tensin has been shown to localize to the ends of the polarized basal actin filaments along with integrins.27 However, Tensin does not become strongly concentrated at these sites until stage 12,27 well after the basal actin filaments have achieved their polarized organization, which may explain why loss of Tensin has no apparent effect on the abundance, organization or orientation of these filaments. This suggests that Tensin and integrin-based adhesion may contribute to egg chamber elongation after the follicle cell basal actin filaments have been formed and properly oriented.
Insights Into the Mechanism of Egg Chamber Elongation
The basal actin phenotypes associated with loss of the round egg genes described above clearly demonstrate that egg chamber elongation requires a properly constructed array of parallel basal actin filaments that are uniformly oriented across the follicle cell layer. Furthermore, the proteins encoded by these round egg genes indicate the importance of not only regulating actin dynamics and actomyosin contractility, but also cell-cell and cell-ECM interactions in the formation and/or maintenance of the polarized basal actin network. However, until recently, it was not clear how actomyosin contractility, cell-cell and cell-ECM interactions worked together to influence the basal actin network and achieve egg chamber elongation. Significant insight into these relationships came with the recent identification of two processes that contribute to egg chamber elongation; global egg chamber rotation1 and oscillating contractions of a follicle cell basal actomyosin network.2
Egg chamber rotation contributes to egg chamber elongation by mediating the formation of the molecular corset
Haigo and Bilder1 recently used live-cell imaging to uncover a novel morphogenetic movement that appears to drive the earliest stages of egg chamber elongation. From the beginning of stage 5 through the beginning of stage 9, the entire egg chamber, both germline and follicle cells, rotates around its A/P axis while the surrounding basal lamina remains stationary. This rotation can be either clockwise or counterclockwise around the A/P axis, and although each egg chamber will only rotate in a single direction, multiple egg chambers within a given ovariole or chain of egg chambers can rotate simultaneously in different directions (Fig. 7). Over the approximately 20 h that egg chamber rotation occurs, each chamber completes roughly three revolutions with an average velocity of ~0.5 μm/min. One way to visualize egg chamber rotation is to think of the layer of connected follicle cells actively migrating in a single, unified direction over the stationary basal lamina with the attached germline cells coming along for the ride. In this model, the active migration of the follicle cells over the basal lamina generate the force necessary to power rotation, while ECM-interacting transmembrane proteins on the follicle cell basal surface simultaneously direct the organization of basal lamina ECM components into the parallel arrays of the molecular corset (Fig. 7). The reader is encouraged to view an amazing movie of egg chamber rotation that is available as part of the supporting online material for the Haigo and Bilder1 paper (www.sciencemag.org/content/suppl/2011/01/03/science.1199424.DC1/1199424S2.mov).
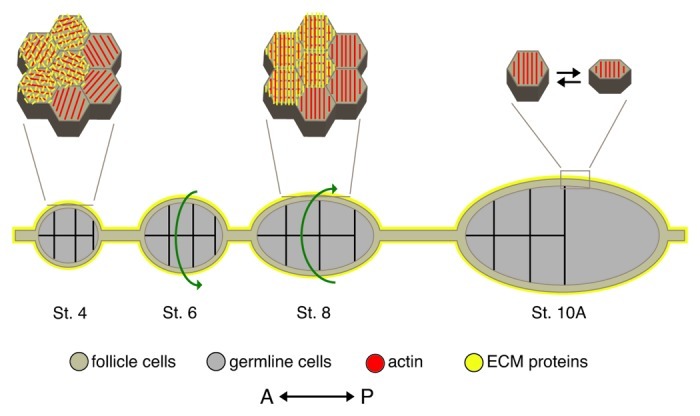
Figure 7. Model of egg chamber elongation. During oogenesis, egg chambers undergo a dramatic change in shape from a sphere (St. 4) to an ellipse (St. 10A). This is achieved through global rotation of the egg chamber within a static basal lamina (St. 5–8) and periodic contractions of a polarized basal actomyosin network (St. 9–10). Diagram depicts a cross section of egg chambers along with a high magnification view of the follicle cell basal surface for a subset of stages. Prior to the onset of egg chamber rotation, the follicle cell basal actin filaments (red) are parallel within each cell but are not uniformly oriented across the follicle cell layer and the ECM protein components of the follicle cell basal lamina (yellow) are unorganized. As the egg chamber rotates (green arrows indicate direction of rotation), the follicle cell basal actin filaments and the ECM fibers become aligned perpendicular to the A/P axis of the egg chamber forming the molecular corset. After the egg chamber stops rotating, a subset of follicle cells, concentrated around the widest point of the egg chamber, undergo periodic contractions of the polarized basal actin filaments, which are mediated by non-muscle Myosin II. These contractions result in a temporary change in the follicle cell basal surface and the generation of an inward force that reinforces the broader molecular corset. St., stage. Adapted from reference 88,and reprinted with permission from Elsevier.
As the egg chamber rotates, the follicle cells secrete collagen IV α2 fibers, which subsequently increase in length and density and become organized into parallel arrays that are oriented perpendicular to the A/P axis of the egg chamber, mirroring the orientation of the follicle cell basal actin filaments.1 This collagen IV array is required for both egg chamber elongation and rotation as most egg chambers with follicle cell layers entirely mutant for viking (collagen IV α2) are not only significantly rounder than wild-type starting at stage 8, but also either fail to rotate or display abnormal, off-axis rotation around this stage.1 Similar failures to elongate and rotate starting slightly earlier, around stage 5, are also seen in the majority of egg chambers that carry clones of mys (βPS integrin) mutant follicle cells,1 suggesting that interactions between the follicle cells and ECM components are also required to facilitate egg chamber rotation and elongation. Importantly, these follicle cell-ECM interactions appear to influence the organization of the collagen IV array during rotation, as egg chambers in which rotation is blocked due to the presence of mys (βPS integrin) mutant follicle cells display collagen IV α2 fibers which are shorter and wider than wild-type and are no longer uniformly oriented perpendicular to the A/P axis.1
However, the role of the polarized follicle cell basal actin network in egg chamber rotation cannot be overlooked as this network is also disrupted in egg chambers carrying either mys (βPS integrin) or viking (collagen IV α2) mutant follicle cells.1,27,29 Furthermore, there is a correlation between the onset of the rotation defect and when disruptions of the basal actin filament network become apparent. This is most clearly seen in egg chambers with follicle cell layers entirely mutant for viking (collagen IV α2).1 At stage 7, when most of the egg chambers containing viking (collagen IV α2) mutant follicle cells have a shape indistinguishable from wild-type and are rotating normally, the follicle cell basal actin filaments are uniformly oriented across the follicle cell layer. However, at stage 8, when most of the egg chambers containing viking (collagen IV α2) mutant follicle cells first appear rounder than wild-type and fail to rotate, the follicle cell basal actin filaments no longer display a uniform orientation across the follicle cell layer, although the filaments within each cell are still organized into parallel arrays. This suggests that a uniformly polarized follicle cell basal actin network may contribute to egg chamber elongation by facilitating normal egg chamber rotation. Furthermore, the polarized network of collagen IV fibers is not required for the initiation of egg chamber rotation, but is required for rotation to continue, potentially by helping to maintain the uniform orientation of the follicle cell basal actin filaments throughout the follicle cell layer. After the initiation of rotation, the follicle cells will migrate over collagen IV fibers whose orientation has already been at least somewhat influenced by other follicle cells. The interaction of these oriented collagen IV fibers with ECM-interacting proteins that also interact with the basal actin filaments could then reinforce the orientation of these filaments.
So, both of the polarized protein networks that have been proposed to comprise the molecular corset, the follicle cell basal actin and the ECM collagen IV fibers, are required for egg chamber rotation and ultimately egg chamber elongation. However, their relative contributions to the function of the molecular corset do not appear to be equivalent. Treatment of fully elongated stage 12 egg chambers with collagenase, which disrupts the collagen IV and follicle cell basal actin networks, causes the chambers to lose their ellipsoid shape and become rounded. In contrast, treatment of stage 12 egg chambers with latrunculin A, which only disrupts the follicle cell basal actin network, has essentially no effect on the shape of the chambers.1 This has led to a model in which the polarized basal actin network is responsible for initiating and maintaining the active migration of the follicle cells over the basal lamina that drives egg chamber rotation. As they migrate, the follicle cells then organize the collagen IV fibers into a parallel array through ECM-interacting proteins, like integrins. The parallel collagen IV fiber network then, in turn, reinforces the polarized orientation of the basal actin network and ultimately provides lasting structural support, which limits the expansion of the egg chamber along its short or dorsal/ventral axis forcing any increase in volume to result in an expansion along the A/P axis (Fig. 5).1
While the description of egg chamber rotation in Drosophila was made only recently,1 rotation of insect egg chambers (follicles) was first observed by D.F. Went in 1977 in cultured egg chambers from the gall midge, Heteropeza pygmaea.76 The overall organization of an individual gall midge egg chamber is similar to that of Drosophila. The gall midge egg chamber consists of an oocyte connected to a syncytial nurse chamber, which are both surrounded by a layer of follicle cells.76 Interestingly, egg chamber rotation in these two insects is remarkably similar with rotation involving the movement of the entire egg chamber and occurring around its longitudinal axis.1,76 While Went and colleagues were unable to discern the basal lamina in cultured gall midge egg chambers, the failure of rotating egg chambers to move when placed on a coverslip suggests that the basal lamina remains stationary while the gall midge egg chamber rotates within it.77 The follicle cell layer also appears to play a central role in gall midge egg chamber rotation, as cultured gall midge egg chambers that lack a follicle cell layer do not rotate.76 A connection between egg chamber rotation and elongation is also shared between these insects as gall midge egg chambers that display off-axis rotation stop rotating prematurely and result in the production of spherical eggs.76 While Went and colleagues were unable to examine the follicle cell basal actin network, they did find actin-containing microvilli along the basal surface of the follicle cells using electron microscopy, which they proposed might interact with the basal lamina during rotation.77 These microvilli may be analogous to the actin-based protrusions produced along the basal surface of the follicle cells in Drosophila egg chambers.21 Together, these similarities suggest that rotation may facilitate egg chamber elongation in multiple insects and supports a role for global tissue rotation as a more general mechanism for influencing tissue shape during development.
Rotating follicle cells share characteristics with migrating cells
During egg chamber rotation, the migrating follicle cells display many of the features of migrating cells in other systems including the production of actin-based protrusions from a single cell edge, the presence of an oriented contractile actomyosin network and the ability to influence and be influenced by the ECM over which they are migrating. Before a cell can start migrating, it needs to define the leading edge that will produce actin-based protrusions in the direction of migration. In follicle cells, this is likely achieved at least in part by Fat2. The Fat2 protein localizes to one of the basal cell edges parallel to the A/P axis of the egg chamber just prior to the appearance of actin-based protrusions.66 Interestingly, some kugelei (fat2) mutant follicle cells appear to produce protrusions from multiple cell edges,66 which further supports a role for Fat2 in specifying the leading edge of these cells. A notable difference between follicle cells and most other migrating cells is that none of the follicle cells have a truly free leading edge, which is a cell edge within the plane of migration that is not connected to another follicle cell. Even those cells in other systems that migrate as an interconnected group or sheet typically have a subset of cells with a free leading edge that is not attached to another migrating cell. However, in these cases, the purpose of migration is to move cells toward a final destination, while the follicle cells have no true final destination, but are moving along as if on a treadmill with the purpose of organizing the ECM to generate the molecular corset. Thus far, imaging of the actin-based protrusions produced by follicle cells during egg chamber rotation has been limited to fixed samples. In order to determine whether the follicle cell protrusions are analogous to those produced by other migrating cells, it will be necessary to use live imaging to examine their behavior during egg chamber rotation as well as whether this behavior is influenced by the same proteins that influence the behavior of protrusions in other cell types and the consequences of their loss.
Another common feature of many migrating cells is the presence of stress fibers. While there are three major types of stress fibers in cultured cells,78 ventral stress fibers are most similar to follicle cell basal actin filaments. Ventral stress fibers are bundled actin filaments present along the side of the cell that contacts the surface over which it migrates. They are oriented parallel to the direction of migration with integrin-containing focal adhesions present at both ends that connect these fibers to the ECM.78 Similarly, starting around stages 5 and 6, the existing follicle cell basal actin filaments become oriented parallel to the direction they will migrate during egg chamber rotation, and βPS integrin, along with several other known components of focal adhesions, become localized to the ends of these filaments.27 For most cultured migrating cells, the force required to produce forward movement is generated, at least in part, by contraction of the ventral stress fibers mediated by an association with non-muscle Myosin II motor proteins. Since these fibers are anchored at either end by focal adhesions that connect them to the ECM, their contraction results in the generation of traction forces which help pull the cell forward.79 In follicle cells, the activated form of non-muscle Myosin II is present at the basal surface starting at stage 7.44,80 This suggests that contraction of the basal actin filaments may be involved in generating the force necessary to mediate follicle cell migration during egg chamber rotation. Although rotation begins roughly 8 h earlier, around stage 5,1 which raises the possibility that the initiation of follicle cell migration does not involve non-muscle Myosin II based contraction. However, the observation that the follicle cell basal actin filaments initially become oriented at the anterior and posterior-most ends of the egg chamber during stages 5 and 6,25 suggests that these cells may be the first to initiate migration. If this is true, then the localization of active non-muscle Myosin II would be largely restricted to small subsets of cells at either end of the egg chamber, which could be difficult to see when examining fixed-egg chambers.
While the ECM was once thought to merely provide a roadway over which a cell migrates, recent work has revealed an increasing number of interactions between a cell and the ECM that influence both cell behavior and the organization of the ECM.81,82 During egg chamber rotation, reciprocal interactions between the ECM components of the basal lamina and the follicle cell basal actin filaments are mediated by several transmembrane proteins including integrins, Lar and Dg. αPS1βPS is the only integrin heterodimer expressed during egg chamber rotation, with the other integrin heterodimers being expressed only after stage 10.27,56 αPS1βPS integrin has been shown to bind laminin A,83 suggesting that it may facilitate the organization of this ECM component of the follicle cell basal lamina into parallel arrays during egg chamber rotation (Fig. 3). However, Lar most likely also binds to laminin A48 and may therefore also contribute to the polarization of the laminin A network during rotation. Like laminin A, perlecan and collagen IV are also present in the follicle cell basal lamina and become organized into parallel arrays during egg chamber rotation. The organization of the perlecan network does not appear to rely on collagen IV, as egg chambers with follicle cell layers that consist entirely of viking (collagen IV α2) mutant cells display a properly oriented perlecan network until these chambers stop rotating at stage 8.1 Although the contribution of perlecan to the molecular corset has not been directly examined, egg chambers with follicle cells that lack Dg, which binds perlecan, produce round eggs.59,60 While a transmembrane protein that binds to collagen IV during egg chamber rotation has not yet been identified, it is possible that the orientation of the collagen IV network is not achieved through a direct connection with the migrating follicle cells, but rather through interactions with either laminin A or perlecan, which could indirectly link collagen IV to the migrating follicle cells. Additional experiments will need to be performed to provide further insight into how these ECM components interact with each other and with the follicle cells in the assembly of the molecular corset, as well as to clarify their respective contributions to its function.
Oscillating basal actomyosin contractions contribute to egg chamber elongation by reinforcing the molecular corset
While egg chamber rotation mediates the early stages of egg chamber elongation, it stops prior to the point at which the egg chamber reaches its final elongated shape.1 Furthermore, egg chambers carrying either mys (βPS integrin) or viking (collagen IV α2) mutant follicle cells that fail to rotate, still undergo some elongation.1 Therefore, there must be other mechanisms that mediate this later phase of elongation. Using live-cell imaging, He et al.2 recently identified one such mechanism in the form of oscillating contractions of the polarized follicle cell basal actin network that are facilitated by association with the motor protein non-muscle Myosin II. These oscillating basal contractions follow egg chamber rotation, occurring during stages 9 and 10, making them an excellent candidate to mediate the later phase of egg chamber elongation.
During stages 9 and 10, non-muscle Myosin II accumulates at the basal surface of a subset of follicle cells, mediates contraction and then disappears, reappearing a short time later to initiate another cycle.2 While this cycle is seen in multiple follicle cells at any given time point, it does not appear to be coordinated across the cell layer, with each follicle cell acting essentially autonomously with regard to when the cycle is initiated. The follicle cells do, however, appear to share a common cycle length and period following initiation.2 Since non-muscle Myosin II is mediating contraction of actin filaments that are organized in parallel arrays, this contraction has a polarized effect on the basal surface area of the follicle cells. The follicle cells shorten along the D/V axis, parallel to the basal actin filaments, but display only a minimal change in length along the A/P axis (Fig. 7).2 In contrast to other periodic actomyosin contractions that mediate apical cell shape changes during Drosophila gastrulation and dorsal closure,84 this change in basal surface area is transient with the follicle cells returning to their original shape between contractions.2
Interestingly, not all follicle cells within the layer participate in this contraction cycle. Recall that during stage 9, the majority of follicle cells are changing shape from cuboidal to columnar and migrating toward the posterior end of the egg chamber, while a small number of follicle cells become stretched out over the nurse cells. During stage 9 and the first half of stage 10, contraction is predominantly seen in the columnar follicle cells adjacent to those that are being stretched over the nurse cells.2 While this group of contracting follicle cells starts out in the anterior region of the egg chamber, it shifts relatively quickly to the center of the egg chamber where, due to volume changes in the germline cells, they remain through the end of stage 10. Finally, by late stage 10, nearly all follicle cells that overlie the oocyte show periodic accumulations of non-muscle Myosin II and periodic contractions.2 While this may suggest a role for periodic basal contraction in mediating the posterior migration of the columnar follicle cells, this has not yet been examined. However, there is experimental evidence to support a role for these contractions in egg chamber elongation.2 Treatment of late stage 9 or early stage 10 egg chambers with cytochalasin D, which disrupts actin filaments and inhibits actin polymerization, not only blocks the periodic contractions, but also results in an increase in the width (i.e., along the D/V axis) of the egg chamber. On the other hand, treatment with the calcium ionophore ionomycin, which promotes the contraction of actomyosin networks in smooth muscle cells, increases the amount of basal non-muscle Myosin II present and results in a simultaneous decrease in width and increase in length of the egg chamber. Therefore, too much or too little follicle cell basal contraction can influence egg chamber shape. In contrast, contraction of the apical actomyosin network and the resulting apical surface constriction is required during earlier stages of oogenesis (~prior to stage 7) to maintain follicle cell height and the integrity of the follicle cell layer presumably by counteracting the outward force of the growing germline cells.80 Although during stages 9 and 10, when periodic basal contractions are occurring, the apical follicle cell surface shows only small, random fluctuations in cell shape with minimal changes in non-muscle Myosin II concentration, suggesting that apical actomyosin contraction does not contribute significantly to egg chamber shape during later stages of oogenesis.2
The periodic basal accumulation of non-muscle Myosin II and subsequent contractions appear to be regulated by the Rho1/Rok pathway. Recall that Rho regulates stress fiber formation and actomyosin contraction, often by activating its downstream effector Rok. Expression of either a dominant negative form of Rho1 or Rok-RNAi in clones of follicle cells results in a failure to accumulate non-muscle Myosin II at the basal surface, whereas expression of a constitutively active form of Rho1 results in a constant high level of basal non-muscle Myosin II.2 Cell-ECM interactions also appear to influence basal non-muscle Myosin II accumulation. Expression of Talin-RNAi in clones of follicle cells results in a 50% reduction in basal non-muscle Myosin II levels relative to adjacent wild-type cells, while overexpression of the focal adhesion adaptor protein Paxillin85 results in a 60% increase in basal non-muscle Myosin II levels and egg chambers that are longer and narrower than wild-type.2 However, caution must be used in interpreting the decreases in basal non-muscle Myosin II accumulation seen in follicle cells expressing a dominant negative version of Rho1, Talin-RNAi or Rok-RNAi, as these cells also display a decrease in basal actin levels.2 While this reduction in basal actin filaments could be due to a requirement for actomyosin contraction in the maintenance of the basal actin filaments, as is the case for ventral stress fibers in other cell types,86 it could also indicate a role for Rho1 and Rok in the formation of these filaments. In addition to its ability to regulate actomyosin contraction, the Rho1/Rok pathway is also capable of regulating actin polymerization by activating the formin Diaphanous as well as stabilizing existing actin filaments by inactivating the filament-severing protein Cofilin,36 both of which could mediate the formation of the follicle cell basal actin filaments (Fig. 5).
Together, these results have led to a model where the periodic basal contractions mediate egg chamber elongation, not by inducing a permanent change in follicle cell shape, but rather by providing an inward propagating force that can counteract the outward force produced by the increase in volume of the germline cells. Given that the majority of cells undergoing periodic basal contraction are centrally positioned around the egg chamber, the inward force generated by the periodic basal contractions could contribute to the molecular corset2 by reinforcing the previously established polarized ECM array around the widest portion of the egg chamber and assist in directing increases in volume toward the anterior and posterior ends of the egg chamber. In this way, the periodic basal contractions could be thought of as contributing to a “molecular belt” that augments the broader molecular corset of the oriented ECM proteins along the region where the greatest force would be needed to be generated in order for the egg chamber to elongate (Fig. 5).
Actomyosin contraction contributes to shape changes in multiple Drosophila tissues
Periodic actomyosin contraction is used during a variety of developmental processes to mediate changes in tissue shape through changes in cell shape and cell rearrangements. In Drosophila, these include the invagination of the mesoderm and lengthening of the germband during gastrulation as well as the convergence of two edges of an epithelial sheet during dorsal closure.84,87 While periodic actomyosin contraction is required for all of these processes, the periodic contractions of the follicle cell molecular belt differ from those that mediate the other processes in two critical ways. First, the periodic contractions during egg chamber elongation occur at the basal rather than the apical surface. The organization of the actomyosin network differs significantly between these two surfaces, with the apical actomyosin network consisting of a seemingly unorganized meshwork of actin filaments and associated non-muscle Myosin II that crisscross the apical cell surface and are often anchored at the cell-cell adherens junctions. Contraction of this apical actomyosin meshwork can either be uniform, resulting in apical constriction as is seen during mesoderm invagination and dorsal closure, or polarized toward an interface with a single neighboring cell as is seen during germband extension. On the other hand, the follicle cell basal actomyosin network consists of a highly organized array of parallel actin filaments and associated non-muscle Myosin II that are anchored at cell-ECM focal adhesions. Contraction of this basal actomyosin network results in a polarized change in cell shape so that only the basal cell edges that run parallel to the basal actin filaments decrease in length. This not only influences cell shape in an alternate way, but may also result in the production of force in directions that differ from those produced by contraction of the apical actomyosin network. The second way the periodic follicle cell basal contractions differ from the other actomyosin contractions is that the change in the basal cell surface is temporary, with the cell returning to its pre-contraction dimensions shortly after each contraction. While the apical actomyosin contractions seen during mesoderm invagination, germband extension and dorsal closure are also periodic, they result in a lasting change in cell shape so that the cell experiences intermittent changes in shape that are maintained between contractions. This has been proposed to result from a ratchet mechanism that may be mediated by the cell-cell adherens junctions associated with these apical actomyosin networks. The periodic basal contractions therefore represent a novel way that periodic actomyosin contraction can be used to influence tissue shape. Future work to further examine how the periodic basal contractions are regulated as well as how the force generated contributes to egg chamber elongation will provide additional insight into the variety of ways that actomyosin contraction contributes to changes in tissue shape during development.
Conclusions and Future Directions
The recent work of Haigo and Bilder1 and He et al.2 has provided novel insights into the process of egg chamber elongation. Prior to this work, it was not clear how the polarized networks of actin and ECM proteins along the basal surface of the follicle cells mediated egg chamber elongation. Through the use of live-imaging techniques, their work revealed two unexpected behaviors that contribute to egg chamber elongation; global rotation of the egg chamber and periodic basal actomyosin contractions. These behaviors utilize the polarized follicle cell basal actin filaments to either generate the polarized ECM protein network via global egg chamber rotation, or provide inward force around the center of the egg chamber via periodic basal actomyosin contractions. While this work has advanced our understanding of how an egg chamber elongates, significant gaps still remain. The next challenges for researchers in this field include clarifying the role of each of the round egg genes and how they interact during egg chamber elongation. In general, this will require a more thorough analysis of the effects that loss of each of these proteins has on the organization of the basal actin filaments and the ECM protein arrays. Ideally, this will not only involve analysis of fixed images from multiple developmental time points, but also the extension of the live-imaging techniques used by the Bilder and Montell labs to directly visualize both networks during the duration of egg chamber elongation in these mutants and thereby determine how egg chamber elongation is compromised in each. Researchers will also need to identify additional proteins that contribute to egg chamber elongation using either a candidate gene approach or genetic screens. The candidate gene approach could be used to examine the role of proteins involved in actin polymerization, actin bundling, actomyosin contraction or cell migration in other systems during egg chamber elongation. Genetic screens, on the other hand, could be used to either identify genes, which when mutated in follicle cells, lead to round eggs, or genes, which when mutated in combination with a previously identified round egg gene, lead to the suppression or enhancement of the round egg mutant phenotype. Both of these genetic screen approaches have been recently used to identify additional proteins that contribute to egg chamber elongation, although detailed characterization of the roles of most of these genes has not yet been performed.44,73 Even with the completion of these recent screens, it is unlikely that all of the genes that contribute to egg chamber elongation have been identified. Therefore, it will be important to continue to develop novel screening methods to reveal additional genes required for this process. Continued work toward uncovering the mechanisms by which egg chambers elongate will not only allow us to gain a more complete understanding of this process, but will also provide insight into the variety of mechanisms that are used to shape organs and tissues during development.
Acknowledgments
I would like to thank Lindsay Regruto for technical assistance in obtaining the images for Figures 1 and 2, as well as Mark Haussmann, Matthew Heintzelman, Traci Stevens and the reviewers for insightful comments on the manuscript.
Glossary
Abbreviations:
- A/P
anterior/posterior
- DE-cadherin
Drosophila E-cadherin
- Dg
Dystroglycan
- Dys
Dystrophin
- ECM
extracellular matrix
- ena
enabled
- fry
furry
- GAP
GTPase-activating protein
- GDI
guanine nucleotide dissociation inhibitor
- GEF
guanine nucleotide exchange factor
- Ger.
Germarium
- GFP
green fluorescent protein
- if
inflated
- Lar
Leukocyte-antigen-related-like
- mew
multiple edematous wings
- msn
misshapen
- mys
myospheroid
- NDR
nuclear DBF2-related
- Pak
p21-activated kinase
- RNAi
RNA interference
- Rok
Rho kinase
- scb
scab
- St.
Stage
- trc
tricornered
- WT
wild type
Footnotes
Previously published online: www.landesbioscience.com/journals/fly/article/21969
References
- 1.Haigo SL, Bilder D. Global tissue revolutions in a morphogenetic movement controlling elongation. Science. 2011;331:1071–4. doi: 10.1126/science.1199424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He L, Wang X, Tang HL, Montell DJ. Tissue elongation requires oscillating contractions of a basal actomyosin network. Nat Cell Biol. 2010;12:1133–42. doi: 10.1038/ncb2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spradling A. Developmental Genetics of Oogenesis. In: Bate M, Martinez-Arias A, eds. The Development of Drosophila melanogaster Plainview, NY: Cold Spring Harbor Laboratory Press, 1993:1-70. [Google Scholar]
- 4.Roth S, Lynch JA. Symmetry breaking during Drosophila oogenesis. Cold Spring Harb Perspect Biol. 2009;1:a001891. doi: 10.1101/cshperspect.a001891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahajan-Miklos S, Cooley L. Intercellular cytoplasm transport during Drosophila oogenesis. Dev Biol. 1994;165:336–51. doi: 10.1006/dbio.1994.1257. [DOI] [PubMed] [Google Scholar]
- 6.Hudson AM, Cooley L. Understanding the function of actin-binding proteins through genetic analysis of Drosophila oogenesis. Annu Rev Genet. 2002;36:455–88. doi: 10.1146/annurev.genet.36.052802.114101. [DOI] [PubMed] [Google Scholar]
- 7.Robinson DN, Cooley L. Genetic analysis of the actin cytoskeleton in the Drosophila ovary. Annu Rev Cell Dev Biol. 1997;13:147–70. doi: 10.1146/annurev.cellbio.13.1.147. [DOI] [PubMed] [Google Scholar]
- 8.Buszczak M, Cooley L. Eggs to die for: cell death during Drosophila oogenesis. Cell Death Differ. 2000;7:1071–4. doi: 10.1038/sj.cdd.4400755. [DOI] [PubMed] [Google Scholar]
- 9.McCall K. Eggs over easy: cell death in the Drosophila ovary. Dev Biol. 2004;274:3–14. doi: 10.1016/j.ydbio.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 10.Poulton JS, Deng WM. Cell-cell communication and axis specification in the Drosophila oocyte. Dev Biol. 2007;311:1–10. doi: 10.1016/j.ydbio.2007.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinhauer J, Kalderon D. Microtubule polarity and axis formation in the Drosophila oocyte. Dev Dyn. 2006;235:1455–68. doi: 10.1002/dvdy.20770. [DOI] [PubMed] [Google Scholar]
- 12.Horne-Badovinac S, Bilder D. Mass transit: epithelial morphogenesis in the Drosophila egg chamber. Dev Dyn. 2005;232:559–74. doi: 10.1002/dvdy.20286. [DOI] [PubMed] [Google Scholar]
- 13.Kolahi KS, White PF, Shreter DM, Classen AK, Bilder D, Mofrad MR. Quantitative analysis of epithelial morphogenesis in Drosophila oogenesis: New insights based on morphometric analysis and mechanical modeling. Dev Biol. 2009;331:129–39. doi: 10.1016/j.ydbio.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aman A, Piotrowski T. Cell migration during morphogenesis. Dev Biol. 2010;341:20–33. doi: 10.1016/j.ydbio.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 15.Montell DJ. Border-cell migration: the race is on. Nat Rev Mol Cell Biol. 2003;4:13–24. doi: 10.1038/nrm1006. [DOI] [PubMed] [Google Scholar]
- 16.Rørth P. Initiating and guiding migration: lessons from border cells. Trends Cell Biol. 2002;12:325–31. doi: 10.1016/S0962-8924(02)02311-5. [DOI] [PubMed] [Google Scholar]
- 17.Wu X, Tanwar PS, Raftery LA. Drosophila follicle cells: morphogenesis in an eggshell. Semin Cell Dev Biol. 2008;19:271–82. doi: 10.1016/j.semcdb.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berg CA. Tube formation in Drosophila egg chambers. Tissue Eng Part A. 2008;14:1479–88. doi: 10.1089/ten.tea.2008.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tourmente S, Lecher P, Degroote F, Renaud M. Mitochondrial development during Drosophila oogenesis: distribution, density and in situ RNA hybridizations. Biol Cell. 1990;68:119–27. doi: 10.1016/0248-4900(90)90296-F. [DOI] [PubMed] [Google Scholar]
- 20.Gutzeit HO. The microfilament pattern in the somatic follicle cells of mid-vitellogenic ovarian follicles of Drosophila. Eur J Cell Biol. 1990;53:349–56. [PubMed] [Google Scholar]
- 21.Gutzeit HO. Organization and in vitro activity of microfilament bundles associated with the basement membrane of Drosophila follicles. Acta Histochem Suppl. 1991;41:201–10. [PubMed] [Google Scholar]
- 22.Gutzeit HO, Eberhardt W, Gratwohl E. Laminin and basement membrane-associated microfilaments in wild-type and mutant Drosophila ovarian follicles. J Cell Sci. 1991;100:781–8. doi: 10.1242/jcs.100.4.781. [DOI] [PubMed] [Google Scholar]
- 23.Gutzeit HO, Haas-Assenbaum A. The somatic envelopes around the germ-line cells of polytrophic insect follicles: structural and functional aspects. Tissue Cell. 1991;23:853–65. doi: 10.1016/0040-8166(91)90035-R. [DOI] [PubMed] [Google Scholar]
- 24.Franz A, Riechmann V. Stepwise polarisation of the Drosophila follicular epithelium. Dev Biol. 2010;338:136–47. doi: 10.1016/j.ydbio.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 25.Frydman HM, Spradling AC. The receptor-like tyrosine phosphatase lar is required for epithelial planar polarity and for axis determination within drosophila ovarian follicles. Development. 2001;128:3209–20. doi: 10.1242/dev.128.16.3209. [DOI] [PubMed] [Google Scholar]
- 26.Ridley AJ. Life at the leading edge. Cell. 2011;145:1012–22. doi: 10.1016/j.cell.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Delon I, Brown NH. The integrin adhesion complex changes its composition and function during morphogenesis of an epithelium. J Cell Sci. 2009;122:4363–74. doi: 10.1242/jcs.055996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wahlström G, Norokorpi HL, Heino TI. Drosophila alpha-actinin in ovarian follicle cells is regulated by EGFR and Dpp signalling and required for cytoskeletal remodelling. Mech Dev. 2006;123:801–18. doi: 10.1016/j.mod.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 29.Bateman J, Reddy RS, Saito H, Van Vactor D. The receptor tyrosine phosphatase Dlar and integrins organize actin filaments in the Drosophila follicular epithelium. Curr Biol. 2001;11:1317–27. doi: 10.1016/S0960-9822(01)00420-1. [DOI] [PubMed] [Google Scholar]
- 30.Schneider M, Khalil AA, Poulton J, Castillejo-Lopez C, Egger-Adam D, Wodarz A, et al. Perlecan and Dystroglycan act at the basal side of the Drosophila follicular epithelium to maintain epithelial organization. Development. 2006;133:3805–15. doi: 10.1242/dev.02549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–6. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- 32.Blair SS. Genetic mosaic techniques for studying Drosophila development. Development. 2003;130:5065–72. doi: 10.1242/dev.00774. [DOI] [PubMed] [Google Scholar]
- 33.Csépányi-Kömi R, Lévay M, Ligeti E. Small G proteins and their regulators in cellular signalling. Mol Cell Endocrinol. 2012;353:10–20. doi: 10.1016/j.mce.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 34.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–35. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 35.Guilluy C, Garcia-Mata R, Burridge K. Rho protein crosstalk: another social network? Trends Cell Biol. 2011;21:718–26. doi: 10.1016/j.tcb.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spiering D, Hodgson L. Dynamics of the Rho-family small GTPases in actin regulation and motility. Cell Adh Migr. 2011;5:170–80. doi: 10.4161/cam.5.2.14403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–79. doi: 10.1016/S0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 38.Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken) 2010;67:545–54. doi: 10.1002/cm.20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hakeda-Suzuki S, Ng J, Tzu J, Dietzl G, Sun Y, Harms M, et al. Rac function and regulation during Drosophila development. Nature. 2002;416:438–42. doi: 10.1038/416438a. [DOI] [PubMed] [Google Scholar]
- 40.Conder R, Yu H, Zahedi B, Harden N. The serine/threonine kinase dPak is required for polarized assembly of F-actin bundles and apical-basal polarity in the Drosophila follicular epithelium. Dev Biol. 2007;305:470–82. doi: 10.1016/j.ydbio.2007.02.034. [DOI] [PubMed] [Google Scholar]
- 41.Newsome TP, Schmidt S, Dietzl G, Keleman K, Asling B, Debant A, et al. Trio combines with dock to regulate Pak activity during photoreceptor axon pathfinding in Drosophila. Cell. 2000;101:283–94. doi: 10.1016/S0092-8674(00)80838-7. [DOI] [PubMed] [Google Scholar]
- 42.Shivalkar M, Giniger E. Control of dendritic morphogenesis by Trio in Drosophila melanogaster. PLoS One. 2012;7:e33737. doi: 10.1371/journal.pone.0033737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harden N, Lee J, Loh HY, Ong YM, Tan I, Leung T, et al. A Drosophila homolog of the Rac- and Cdc42-activated serine/threonine kinase PAK is a potential focal adhesion and focal complex protein that colocalizes with dynamic actin structures. Mol Cell Biol. 1996;16:1896–908. doi: 10.1128/mcb.16.5.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vlachos S, Harden N. Genetic evidence for antagonism between Pak protein kinase and Rho1 small GTPase signaling in regulation of the actin cytoskeleton during Drosophila oogenesis. Genetics. 2011;187:501–12. doi: 10.1534/genetics.110.120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown NH, Gregory SL, Rickoll WL, Fessler LI, Prout M, White RAH, et al. Talin is essential for integrin function in Drosophila. Dev Cell. 2002;3:569–79. doi: 10.1016/S1534-5807(02)00290-3. [DOI] [PubMed] [Google Scholar]
- 46.Bécam IE, Tanentzapf G, Lepesant JA, Brown NH, Huynh JR. Integrin-independent repression of cadherin transcription by talin during axis formation in Drosophila. Nat Cell Biol. 2005;7:510–6. doi: 10.1038/ncb1253. [DOI] [PubMed] [Google Scholar]
- 47.Krueger NX, Van Vactor D, Wan HI, Gelbart WM, Goodman CS, Saito H. The transmembrane tyrosine phosphatase DLAR controls motor axon guidance in Drosophila. Cell. 1996;84:611–22. doi: 10.1016/S0092-8674(00)81036-3. [DOI] [PubMed] [Google Scholar]
- 48.O’Grady P, Thai TC, Saito H. The laminin-nidogen complex is a ligand for a specific splice isoform of the transmembrane protein tyrosine phosphatase LAR. J Cell Biol. 1998;141:1675–84. doi: 10.1083/jcb.141.7.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wills Z, Bateman J, Korey CA, Comer A, Van Vactor D. The tyrosine kinase Abl and its substrate enabled collaborate with the receptor phosphatase Dlar to control motor axon guidance. Neuron. 1999;22:301–12. doi: 10.1016/S0896-6273(00)81091-0. [DOI] [PubMed] [Google Scholar]
- 50.Bear JE, Gertler FB. Ena/VASP: towards resolving a pointed controversy at the barbed end. J Cell Sci. 2009;122:1947–53. doi: 10.1242/jcs.038125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zaidel-Bar R, Geiger B. The switchable integrin adhesome. J Cell Sci. 2010;123:1385–8. doi: 10.1242/jcs.066183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wickström SA, Radovanac K, Fässler R. Genetic analyses of integrin signaling. Cold Spring Harb Perspect Biol. 2011;3:a005116. doi: 10.1101/cshperspect.a005116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-S. [DOI] [PubMed] [Google Scholar]
- 54.Brower DL. Platelets with wings: the maturation of Drosophila integrin biology. Curr Opin Cell Biol. 2003;15:607–13. doi: 10.1016/S0955-0674(03)00102-9. [DOI] [PubMed] [Google Scholar]
- 55.Yee GH, Hynes ROA. A novel, tissue-specific integrin subunit, beta nu, expressed in the midgut of Drosophila melanogaster. Development. 1993;118:845–58. doi: 10.1242/dev.118.3.845. [DOI] [PubMed] [Google Scholar]
- 56.Dinkins MB, Fratto VM, Lemosy EK. Integrin alpha chains exhibit distinct temporal and spatial localization patterns in epithelial cells of the Drosophila ovary. Dev Dyn. 2008;237:3927–39. doi: 10.1002/dvdy.21802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duffy JB, Harrison DA, Perrimon N. Identifying loci required for follicular patterning using directed mosaics. Development. 1998;125:2263–71. doi: 10.1242/dev.125.12.2263. [DOI] [PubMed] [Google Scholar]
- 58.Ervasti JM, Sonnemann KJ. Biology of the striated muscle dystrophin-glycoprotein complex. Int Rev Cytol. 2008;265:191–225. doi: 10.1016/S0074-7696(07)65005-0. [DOI] [PubMed] [Google Scholar]
- 59.Deng WM, Schneider M, Frock R, Castillejo-Lopez C, Gaman EA, Baumgartner S, et al. Dystroglycan is required for polarizing the epithelial cells and the oocyte in Drosophila. Development. 2003;130:173–84. doi: 10.1242/dev.00199. [DOI] [PubMed] [Google Scholar]
- 60.Mirouse V, Christoforou CP, Fritsch C, St Johnston D, Ray RP. Dystroglycan and perlecan provide a basal cue required for epithelial polarity during energetic stress. Dev Cell. 2009;16:83–92. doi: 10.1016/j.devcel.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3:422–33. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 62.Monson JM, Natzle J, Friedman J, McCarthy BJ. Expression and novel structure of a collagen gene in Drosophila. Proc Natl Acad Sci USA. 1982;79:1761–5. doi: 10.1073/pnas.79.6.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Natzle JE, Monson JM, McCarthy BJ. Cytogenetic location and expression of collagen-like genes in Drosophila. Nature. 1982;296:368–71. doi: 10.1038/296368a0. [DOI] [PubMed] [Google Scholar]
- 64.Yasothornsrikul S, Davis WJ, Cramer G, Kimbrell DA, Dearolf CR. viking: identification and characterization of a second type IV collagen in Drosophila. Gene. 1997;198:17–25. doi: 10.1016/S0378-1119(97)00274-6. [DOI] [PubMed] [Google Scholar]
- 65.Broadie K, Baumgartner S, Prokop A. Extracellular matrix and its receptors in Drosophila neural development. Dev Neurobiol. 2011;71:1102–30. doi: 10.1002/dneu.20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Viktorinová I, König T, Schlichting K, Dahmann C. The cadherin Fat2 is required for planar cell polarity in the Drosophila ovary. Development. 2009;136:4123–32. doi: 10.1242/dev.039099. [DOI] [PubMed] [Google Scholar]
- 67.Cong JL, Geng W, He B, Liu JC, Charlton J, Adler PN. The furry gene of Drosophila is important for maintaining the integrity of cellular extensions during morphogenesis. Development. 2001;128:2793–802. doi: 10.1242/dev.128.14.2793. [DOI] [PubMed] [Google Scholar]
- 68.Fang XL, Adler PN. Regulation of cell shape, wing hair initiation and the actin cytoskeleton by Trc/Fry and Wts/Mats complexes. Dev Biol. 2010;341:360–74. doi: 10.1016/j.ydbio.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fang XL, Lu QH, Emoto K, Adler PN. The Drosophila Fry protein interacts with Trc and is highly mobile in vivo. BMC Dev Biol. 2010;10:40. doi: 10.1186/1471-213X-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.He B, Adler PN. The genetic control of arista lateral morphogenesis in Drosophila. Dev Genes Evol. 2002;212:218–29. doi: 10.1007/s00427-002-0229-0. [DOI] [PubMed] [Google Scholar]
- 71.He Y, Emoto K, Fang XL, Ren N, Tian XJ, Jan YN, et al. Drosophila Mob family proteins interact with the related tricornered (Trc) and warts (Wts) kinases. Mol Biol Cell. 2005;16:4139–52. doi: 10.1091/mbc.E05-01-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Emoto K, He Y, Ye B, Grueber WB, Adler PN, Jan LY, et al. Control of dendritic branching and tiling by the Tricornered-kinase/Furry signaling pathway in Drosophila sensory neurons. Cell. 2004;119:245–56. doi: 10.1016/j.cell.2004.09.036. [DOI] [PubMed] [Google Scholar]
- 73.Horne-Badovinac S, Hill J, Gerlach G, 2nd, Menegas W, Bilder D. A screen for round egg mutants in Drosophila identifies tricornered, furry, and misshapen as regulators of egg chamber elongation. G3 (Bethesda) 2012;2:371–8. doi: 10.1534/g3.111.001677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hergovich A, Stegert MR, Schmitz D, Hemmings BA. NDR kinases regulate essential cell processes from yeast to humans. Nat Rev Mol Cell Biol. 2006;7:253–64. doi: 10.1038/nrm1891. [DOI] [PubMed] [Google Scholar]
- 75.Lee SB, Cho KS, Kim E, Chung J. blistery encodes Drosophila tensin protein and interacts with integrin and the JNK signaling pathway during wing development. Development. 2003;130:4001–10. doi: 10.1242/dev.00595. [DOI] [PubMed] [Google Scholar]
- 76.Went DF. Pulsating oocytes and rotating follicles in an insect ovary. Dev Biol. 1977;55:392–6. doi: 10.1016/0012-1606(77)90182-8. [DOI] [PubMed] [Google Scholar]
- 77.Fux T, Went DF, Camenzind R. Movement Pattern and Ultrastructure of Rotating Follicles of the Pedogenetic Gall Midge, Heteropeza-Pygmaea Winnertz (Diptera-Cecidomyiidae) Int J Insect Morphol. 1978;7:415–26. doi: 10.1016/S0020-7322(78)80003-8. [DOI] [Google Scholar]
- 78.Tojkander S, Gateva G, Lappalainen P. Actin stress fibers--assembly, dynamics and biological roles. J Cell Sci. 2012;125:1855–64. doi: 10.1242/jcs.098087. [DOI] [PubMed] [Google Scholar]
- 79.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–9. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 80.Wang Y, Riechmann V. The role of the actomyosin cytoskeleton in coordination of tissue growth during Drosophila oogenesis. Curr Biol. 2007;17:1349–55. doi: 10.1016/j.cub.2007.06.067. [DOI] [PubMed] [Google Scholar]
- 81.Petrie RJ, Doyle AD, Yamada KM. Random versus directionally persistent cell migration. Nat Rev Mol Cell Biol. 2009;10:538–49. doi: 10.1038/nrm2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rozario T, DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol. 2010;341:126–40. doi: 10.1016/j.ydbio.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gotwals PJ, Fessler LI, Wehrli M, Hynes RO. Drosophila PS1 integrin is a laminin receptor and differs in ligand specificity from PS2. Proc Natl Acad Sci USA. 1994;91:11447–51. doi: 10.1073/pnas.91.24.11447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martin AC. Pulsation and stabilization: contractile forces that underlie morphogenesis. Dev Biol. 2010;341:114–25. doi: 10.1016/j.ydbio.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 85.Schaller MD. Paxillin: a focal adhesion-associated adaptor protein. Oncogene. 2001;20:6459–72. doi: 10.1038/sj.onc.1204786. [DOI] [PubMed] [Google Scholar]
- 86.Naumanen P, Lappalainen P, Hotulainen P. Mechanisms of actin stress fibre assembly. J Microsc. 2008;231:446–54. doi: 10.1111/j.1365-2818.2008.02057.x. [DOI] [PubMed] [Google Scholar]
- 87.Gorfinkiel N, Blanchard GB. Dynamics of actomyosin contractile activity during epithelial morphogenesis. Curr Opin Cell Biol. 2011;23:531–9. doi: 10.1016/j.ceb.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 88.Bastock R, St Johnston D. Oogenesis: matrix revolutions. Curr Biol. 2011;21:R231–3. doi: 10.1016/j.cub.2011.01.071. [DOI] [PubMed] [Google Scholar]