Abstract
Dorsoventral (DV) axis formation in Drosophila begins during oogenesis through the graded activation of the EGF receptor (EGFR)-Ras-MAPK signaling pathway in the follicle cell layer of the egg chamber. EGFR signaling, which is higher in dorsal follicle cells, represses expression of the sulfotransferase-encoding gene pipe, thereby delimiting a ventral domain of Pipe activity that is critical for the subsequent induction of ventral embryonic fates. We have characterized the transcriptional circuit that links EGFR signaling to pipe repression: in dorsal follicle cells, the homeodomain transcription factor Mirror (Mirr), which is induced by EGFR signaling, directly represses pipe transcription, whereas in ventral follicle cells, the HMG-box protein Capicua (Cic) supports pipe expression by repressing mirr. Although Cic is under negative post-transcriptional regulation by Ras-MAPK signaling in different contexts, the relevance of this mechanism for the interpretation of the EGFR signal during DV pattern formation remains unclear. Here, we consider a model where EGFR-mediated downregulation of Cic modulates the spatial distribution of Mirr protein in lateral follicle cells, thereby contributing to define the position at which the pipe expression border is formed.
Keywords: Drosophila, dorsoventral patterning, oogenesis, follicle cells, EGFR signaling, Capicua, Mirror, MAPK, pipe
A remarkable feature of Drosophila development is the highly elaborate maternal control that is required for embryonic patterning. This is perhaps best illustrated by the establishment of embryonic dorsoventral (DV) polarity, which requires multiple maternal functions that regulate the progressive transfer of DV positional information from the ovarian egg chamber to the embryo.1,2 This transfer occurs in two main steps. First, the ventral follicle cells around the oocyte secrete localized determinants that are incorporated in the ventral portion of the vitelline membrane, the innermost eggshell layer, where they remain stored until fertilization takes place. Then, following fertilization, the ventral determinants initiate a secondary ventralizing signal from the vitelline membrane to the embryo. This signal relies on a cascade of serine proteases that cleaves the Spätzle ligand so that it can activate the Toll receptor on the ventral surface of the embryo. When Toll is activated, the Rel-family protein Dorsal is translocated to the nucleus, where it acts as a morphogen to regulate the expression of zygotic genes along the embryonic DV axis.1,3
While many aspects of this inductive process have been well established, the mechanisms regulating its early steps in the follicle cell layer have remained less understood. A key regulator in this context is Pipe, a sulfotransferase that is homologous to vertebrate glycosaminoglycan-modifying enzymes.4 Pipe, which is expressed in ventral, but not dorsal, follicle cells, mediates sulfation of structural proteins that are secreted by the follicle cells and become embedded in the ventral side of the vitelline membrane.4,5 These sulfated components function as the ventral determinants required to activate the proteolytic cascade that triggers Spätzle-Toll signaling, possibly by promoting functional interactions between the Snake and Easter proteases.6
A long-standing issue has been how pipe transcription is initially restricted to the ventral follicle cells. In fact, the first symmetry-breaking event that determines DV polarity in the egg chamber occurs in dorsal positions. By mid-oogenesis (stage 7–8), the oocyte nucleus migrates to a cortical, anterior location within the oocyte, which will become the future dorsal-anterior (DA) region of the egg. The asymmetric positioning of the oocyte nucleus directs the transport of gurken transcripts to the DA corner of the oocyte. By stage 9–10, the Gurken product, a TGF-α-related factor, is secreted form the oocyte and activates the EGF receptor (EGFR) in the overlying DA follicle cells (reviewed in ref. 7). Activated EGFR then signals through the Ras-MAPK pathway and regulates DV polarity by repressing pipe expression in DA and lateral follicle cells.8-10 Genetic analyses have shown that this repression is cell-autonomous, indicating that it occurs in direct response to EGFR activity and does not require other secondary, diffusible signals (see also below). Furthermore, since EGFR signaling forms a dorsal-to-ventral gradient (due to diffusion of Gurken from the dorsal side), whereas pipe exhibits a rather sharp border of expression, the repression of pipe probably represents a switch-like response above a certain threshold of EGFR activity.11
Mechanism of pipe Repression
Recently, work from different laboratories, including ours, has characterized the molecular pathway by which EGFR signaling represses pipe transcription.12-14 The results show that Mirror (Mirr), a homeodomain transcription factor induced by EGFR signaling in DA follicle cells,15,16 represses pipe expression by binding to a cis-regulatory element in the pipe upstream region. This repression occurs cell-autonomously in all dorsal and lateral follicle cells where pipe is normally off, implying that Mirr is responsible for the repressive activity of EGFR signaling on pipe transcription.12 Given that Mirr is distributed forming a gradient with high levels in DA cells and low levels in lateral follicle cells15,16 (see below), the simplest model derived from these studies is that Mirr establishes a lateral repression threshold for pipe transcription that prevents its activation by broadly distributed factors. Ultimately, these opposing inputs would be integrated at the level of pipe cis-regulatory elements, thus generating sharp borders of pipe expression.
Previous studies had demonstrated a role of Mirr in the specification of DA follicle cells that direct the formation of dorsal respiratory appendages in the eggshell.17,18 This process is also initiated by EGFR signaling, but was considered to be independent of the gene circuit controlling embryonic patterning. However, the current evidence shows that Mirr is a key factor in both processes. Interestingly, Fuchs et al. (ref. 13) have identified related Mirr-responsive elements in pipe and the broad gene, an EGFR-regulated target involved in dorsal appendage morphogenesis. The authors found that Mirr represses both genes in dorsal follicle cells during stage 9–10A, whereas by stage 10B Mirr plays a role in activating a late broad enhancer. This appears consistent with a more complex regulation of eggshell morphogenesis compared with that of embryonic patterning.13,17,19
Role of Capicua in DV Patterning
We have also addressed the role of Capicua (Cic) in DV axis formation.12 Cic is an HMG-box repressor that functions as a sensor of Ras-MAPK signaling pathways.20 In the ovary, Cic activity is essential to maintain pipe expression in all ventral follicle cells; consequently, loss of maternal Cic function leads to severe dorsalization of the embryo.21 However, the mechanism of Cic function in this context was unclear. It turns out that Cic represses mirr expression in ventral follicle cells, thereby supporting pipe transcription in this region.12,17 Cic-mediated repression of mirr is particularly evident in ventral anterior follicle cells: mosaic analyses using cic mutations cause clear derepression of mirr in anterior but not posterior regions of the follicular epithelium.17 The interpretation of this regional effect is that Cic blocks the ventral induction of mirr by anterior positional cues that may include a Decapentaplegic (Dpp)/TGFβ signal.17 These anterior inputs may function cooperatively with EGFR signaling to activate mirr expression in DA cells, whereas they must be counteracted via Cic repression in ventral cells.17,20 Importantly, we have shown that the same repressor circuit operates in ventral-posterior regions, where pipe expression also depends on Cic-mediated repression of mirr.12 Thus, loss of Cic activity in ventral-posterior follicle cells induces low-level activity of Mirr that is nevertheless sufficient to repress pipe.
EGFR-Mediated Downregulation of Cic
In several contexts, Ras-MAPK activation leads to downregulation of Cic nuclear levels.20 In DA follicle cells, MAPK-dependent phosphorylation of Cic causes partial re-localization of Cic protein to the cytoplasm, reducing its nuclear levels by ~50%.22 This may contribute to the induction of mirr transcription through derepression. However, EGFR signaling has a positive input on mirr expression that is independent of Cic: cic mutant egg chambers show high expression of mirr in DA cells and low ectopic expression in ventral cells, whereas gurken; cic double mutant ovaries show low mirr expression throughout the anterior circumference of the epithelium.17 Thus, although Cic is essential for DV axis formation, the contribution of EGFR-dependent downregulation of Cic in this process is unclear.12,17,22
To re-examine this issue, we have tested if EGFR-mediated downregulation of Cic detectably reduces Cic repressor activity in dorsal follicle cells during stage 10. To this end, we analyzed a synthetic enhancer composed of Cic repressor and Gal4 activator binding sites (CUASC; Fig. 1A). This enhancer functions as a sensor of Cic-mediated repression in the presence of Gal4 protein.23 We find that a CUASC-lacZ reporter shows preferential dorsal transcription in egg chambers with uniform Gal4 expression (Fig. 1B), and that this pattern expands ventrally in fs(1)K10 mutant ovaries (Fig. 1C), which display ectopic EGFR activity in ventral regions.24 This indicates that Cic repressor activity is indeed downregulated by EGFR signaling in dorsal follicle cells, either through the observed decrease in Cic nuclear concentration, or through some additional inhibition of Cic function in response to MAPK phosphorylation.
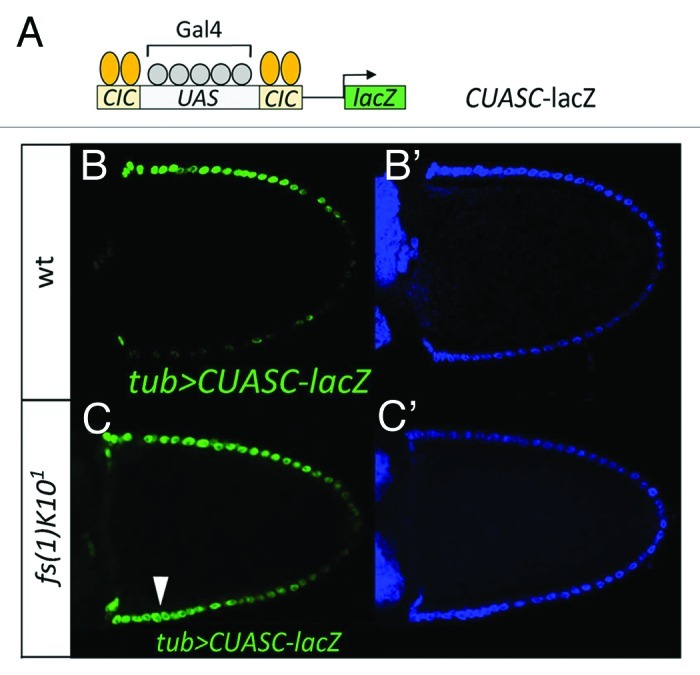
Figure 1. EGFR signaling downregulates Cic repressor activity in dorsal follicle cells. (A) Diagram of the CUASC-lacZ reporter construct containing five Gal4/UAS binding sites flanked by two Cic binding sites on either side. (B and C) Lateral views of stage-10 wild-type (B and B’) and fs(1)K101 (C and C’) egg chambers carrying tubulin-Gal4 and CUASC-lacZ insertions. Double stainings with anti−β−galactosidase antibody (B and C, green) and DAPI (B’ and C’, blue) are shown. Dorsal is up as indicated by the dorsal position of the oocyte nucleus under Nomarski optics (not shown). CUASC-lacZ expression shows preferential dorsal expression in a wild-type background, and is ectopically expressed in ventral regions of the fs(1)K101 egg chamber (arrowhead, C).
We have also analyzed a Cic mutant derivative, CicΔC2, which lacks a MAPK docking site that is essential for EGFR-mediated downregulation of Cic.22 We previously reported that CicΔC2, when expressed at physiological levels using genomic transgenes, does not cause overt repression of mirr in DA follicle cells22 (see also Fig. 2A and B). This is consistent with the notion that EGFR-induced activation of mirr is not just a consequence of Cic downregulation, but involves other unknown mechanism(s)17,22 (see above). However, CicΔC2 does cause moderate expansion of pipe mRNA expression toward the dorsal side of the egg chamber.22 Using a pipe-lacZ reporter, we also see modest expansion of lacZ expression in cicΔC2 egg chambers (Fig. 2E and F). This suggests that EGFR-mediated downregulation of Cic plays a role in setting up the precise position at which the pipe expression border is formed. Below, we argue that this effect on pipe expression is a consequence of Cic acting as a repressor of mirr.
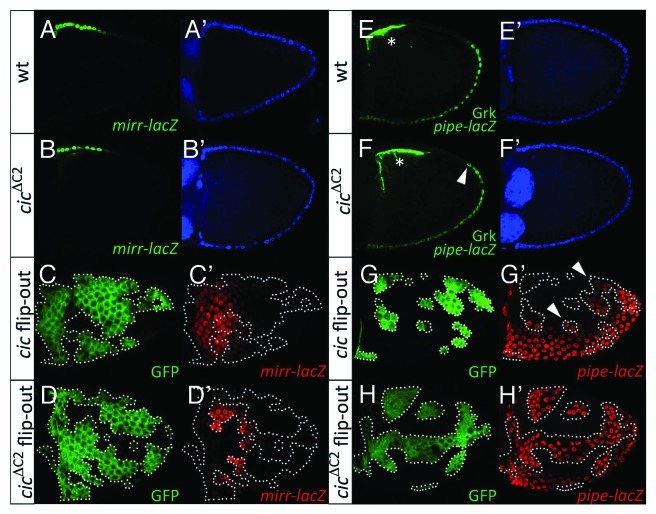
Figure 2. EGFR signaling modulates Cic-mediated repression of mirr. (A and B) Lateral views of stage-10 wild-type (A and A’) and cicΔC2 (B and B’) egg chambers carrying the mirrP2 (mirr-lacZ; ref. 33) enhancer trap. Double stainings with anti-β-galactosidase (A and B, green) and DAPI (A’ and B’, blue) are shown. (C and D) Dorsal views of stage-10 mosaic egg chambers expressing Cic (C and C’) and CicΔC2 (D and D’) proteins in clones marked by expression of GFP (C and D, green). (C’ and D’) show anti-β-galactosidase stainings of mirr-lacZ expression (red). Only CicΔC2 causes full repression of mirr-lacZ. (E and F) Lateral views of stage-10 wild-type (E and E’) and cicΔC2 (F and F’) egg chambers carrying the M2-lacZ (pipe-lacZ; ref. 12) reporter. Stainings with anti-β-galactosidase and anti-Gurken antibodies (E and F, green) and DAPI (E’ and F’, blue) are shown; the Gurken signals are indicated with asterisks. A single genomic cicΔC2 transgene (see ref. 22) leads to expanded pipe-lacZ expression by an average of 1.3 cells (n = 20) in the dorsal-posterior region (arrowhead in panel F). (G and H) Lateral views of stage-10 mosaic egg chambers expressing Cic (G and G’) and CicΔC2 (H and H’) proteins in clones marked by expression of GFP (G and H, green). G’ and H’ show anti-β-galactosidase stainings of M1-lacZ (pipe-lacZ; ref. 12) expression (red). Note that Cic-expressing clones show partial derepression of pipe-lacZ close to the endogenous pipe domain (arrowheads, G’), whereas CicΔC2 causes robust derepression of the reporter in all positions.
We have seen that Mirr directly controls the pipe expression boundary in lateral and dorsal-posterior follicle cells. In these cells, the levels of Mirr are very low or undetectable, albeit sufficient to mediate repression of pipe;12 see also Figure 2A and E showing that detectable mirr expression does not reach the pipe domain. Therefore, we reasoned that downregulation of Cic might influence the pattern of Mirr distribution within this region of low Mirr expression, thereby affecting pipe transcription. To further test this idea, we have assayed the effects of expressing Cic and CicΔC2 proteins in follicle cell clones using the GAL4-UAS system. We found that overexpression of Cic does not cause detectable changes in mirr expression, irrespective of the position where the clone is formed (Fig. 2C). However, Cic overexpression does induce ectopic pipe transcription in lateral regions close to the endogenous pipe domain, although such ectopic expression did not occur in more dorsal positions (Fig. 2G). In contrast, high-level expression of CicΔC2 produced full repression of mirr, and the corresponding derepression of pipe, in all lateral and dorsal clones (Fig. 2D and H). This stronger effect of CicΔC2 is likely attributable to its ability to escape EGFR-mediated downregulation in dorsal cells, so that high Cic activity in the CicΔC2-expressing clone can effectively repress mirr. Thus, changes in Cic repressor activity can affect the expression of mirr throughout the entire follicular epithelium, causing either ectopic ventral expression when Cic is absent or complete dorsal repression when Cic activity is increased (refs. 12, 17 and this work). Altogether, the results suggest a competition mechanism between Cic-mediated repression and EGFR-dependent and -independent activation of mirr, which leads to graded expression of mirr in dorsal and lateral follicle cells. Additionally, this is consistent with a role of Cic downregulation in facilitating mirr expression in lateral and dorsal-posterior follicle cells, where low, but functional Mirr activity defines the pipe expression border.
Conclusion
In summary, we propose a model where EGFR signaling regulates the size of the pipe expression domain through both Cic-dependent and –independent mechanisms (Fig. 3). In DA cells, EGFR signaling induces Mirr expression, which then represses pipe; in this position, downregulation of Cic may contribute to Mirr induction but other EGFR-dependent mechanisms are also involved.17,22 In lateral and dorsal-posterior cells, declining levels of EGFR signaling and Mirr reach a threshold where pipe is no longer repressed and its border is formed. The precise position of this border is influenced by EGFR-dependent downregulation of Cic, which potentiates mirr expression and places the pipe border more ventrally. Lastly, in ventral follicle cells, very low EGFR signaling activity and other positive anterior inputs that act on mirr (see above) are counteracted by Cic, so that mirr is repressed and pipe can be transcribed.
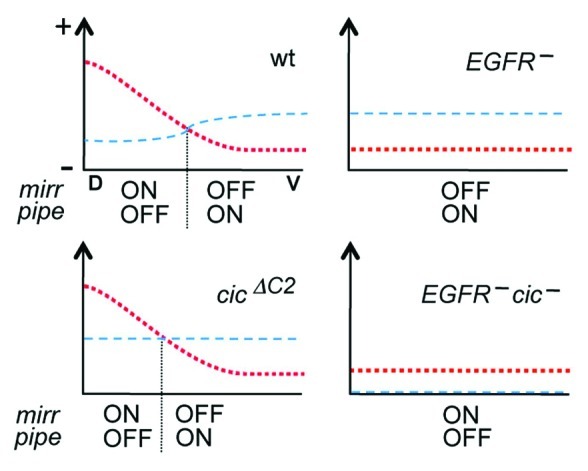
Figure 3. Model for the regulation of mirr and pipe expression in response to EGFR and Cic activities. Each diagram represents the regulatory inputs that control mirr expression in four different genetic backgrounds. For simplicity, only the anterior region of the egg chamber is represented along the DV axis (D, dorsal; V, ventral). The red dotted line represents the combined activation inputs from EGFR signaling and anterior positional cues that may depend on Dpp. The blue dashed line indicates the level of Cic-mediated repression. These inputs are proposed to define the position at which Mirr levels are sufficient to repress pipe (vertical dotted line), thereby establishing its border of expression. In cicΔC2 egg chambers, this position is shifted dorsally as a result of uniform Cic activity throughout the follicular epithelium. Note also that EGFR mutant backgrounds retain a weak activation input from anterior positional cues; this input is insufficient to drive mirr expression unless Cic repressor activity is absent.
In addition, we have noted that this patterning network resembles the mechanism by which the antagonistic activities of Torso-Ras-MAPK signaling and Cic coordinate the subdivision of the early embryo along the anteroposterior axis.12,20 In this case, Torso signaling at the embryonic poles downregulates Cic activity to induce gap repressors such as Tailless (Tll) and Huckebein (Hkb), which are kept repressed by Cic in middle regions of the embryo. Cic-mediated repression of tll and hkb is required for the normal patterning of central body structures, just as Cic-mediated repression of mirr is essential for patterning of ventral embryonic fates. However, downregulation of Cic plays a more critical role in inducing tll expression than it does during EGFR-dependent activation of mirr.20 Understanding these differential responses will require further analyses of the effector mechanism(s) by which EGFR signaling induces mirr expression, and a detailed characterization of other transcriptional inputs that activate mirr and tll expression in each context.
Finally, there are other cases where positional signals are translated into opposing gradients of repressor factors, which in turn regulate spatially restricted expression of downstream targets. For example, Dpp signaling in the Drosophila wing disc generates a complementary gradient of Brinker activity that represses Dpp targets such as optomotor blind and spalt.25-28 Moreover, activity of the Bicoid morphogen in the early embryo establishes opposing gradients of repressor factors (including Runt) that antagonize Bcd-mediated activation;29 additionally, the Torso-regulated gradient of Cic activity at the anterior pole also represses Bcd targets.30 Similarly, interpretation of the Sonic Hedgehog signaling gradient in the vertebrate neural tube depends on cross-repressive interactions among downstream transcription factors (see for example refs. 31 and 32). Thus, the establishment of antagonistic repressor gradients in response to morphogenetic signals may represent a general regulatory principle in development.
Acknowledgments
This work was funded by grants from the Spanish MICINN (BFU2008-01875 and BFU2011-23611 to G.J., and BFU2008-03762, BFU2011-22617 and CONSOLIDER CDS2007-00008 to S.C.), an institutional grant from Fundación Ramón Areces to the CBMSO, and the Generalitat de Catalunya (2009SGR-1075). G.J. is an ICREA investigator.
Footnotes
Previously published online: www.landesbioscience.com/journals/fly/article/21160
References
- 1.Moussian B, Roth S. Dorsoventral axis formation in the Drosophila embryo--shaping and transducing a morphogen gradient. Curr Biol. 2005;15:R887–99. doi: 10.1016/j.cub.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 2.Nilson LA, Schüpbach T. EGF receptor signaling in Drosophila oogenesis. Curr Top Dev Biol. 1999;44:203–43. doi: 10.1016/S0070-2153(08)60471-8. [DOI] [PubMed] [Google Scholar]
- 3.Hong JW, Hendrix DA, Papatsenko D, Levine MS. How the Dorsal gradient works: insights from postgenome technologies. Proc Natl Acad Sci U S A. 2008;105:20072–6. doi: 10.1073/pnas.0806476105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sen J, Goltz JS, Stevens L, Stein D. Spatially restricted expression of pipe in the Drosophila egg chamber defines embryonic dorsal-ventral polarity. Cell. 1998;95:471–81. doi: 10.1016/S0092-8674(00)81615-3. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Z, Stevens LM, Stein D. Sulfation of eggshell components by Pipe defines dorsal-ventral polarity in the Drosophila embryo. Curr Biol. 2009;19:1200–5. doi: 10.1016/j.cub.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho YS, Stevens LM, Stein D. Pipe-dependent ventral processing of Easter by Snake is the defining step in Drosophila embryo DV axis formation. Curr Biol. 2010;20:1133–7. doi: 10.1016/j.cub.2010.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung LS, Schüpbach T, Shvartsman SY. Pattern formation by receptor tyrosine kinases: analysis of the Gurken gradient in Drosophila oogenesis. Curr Opin Genet Dev. 2011;21:719–25. doi: 10.1016/j.gde.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.James KE, Dorman JB, Berg CA. Mosaic analyses reveal the function of Drosophila Ras in embryonic dorsoventral patterning and dorsal follicle cell morphogenesis. Development. 2002;129:2209–22. doi: 10.1242/dev.129.9.2209. [DOI] [PubMed] [Google Scholar]
- 9.Pai LM, Barcelo G, Schüpbach T. D-cbl, a negative regulator of the Egfr pathway, is required for dorsoventral patterning in Drosophila oogenesis. Cell. 2000;103:51–61. doi: 10.1016/S0092-8674(00)00104-5. [DOI] [PubMed] [Google Scholar]
- 10.Peri F, Technau M, Roth S. Mechanisms of Gurken-dependent pipe regulation and the robustness of dorsoventral patterning in Drosophila. Development. 2002;129:2965–75. doi: 10.1242/dev.129.12.2965. [DOI] [PubMed] [Google Scholar]
- 11.Goentoro LA, Reeves GT, Kowal CP, Martinelli L, Schüpbach T, Shvartsman SY. Quantifying the Gurken morphogen gradient in Drosophila oogenesis. Dev Cell. 2006;11:263–72. doi: 10.1016/j.devcel.2006.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andreu MJ, González-Pérez E, Ajuria L, Samper N, González-Crespo S, Campuzano S, et al. Mirror represses pipe expression in follicle cells to initiate dorsoventral axis formation in Drosophila. Development. 2012;139:1110–4. doi: 10.1242/dev.076562. [DOI] [PubMed] [Google Scholar]
- 13.Fuchs A, Cheung LS, Charbonnier E, Shvartsman SY, Pyrowolakis G. Transcriptional interpretation of the EGF receptor signaling gradient. Proc Natl Acad Sci U S A. 2012;109:1572–7. doi: 10.1073/pnas.1115190109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Technau M, Knispel M, Roth S. Molecular mechanisms of EGF signaling-dependent regulation of pipe, a gene crucial for dorsoventral axis formation in Drosophila. Dev Genes Evol. 2012;222:1–17. doi: 10.1007/s00427-011-0384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jordan KC, Clegg NJ, Blasi JA, Morimoto AM, Sen J, Stein D, et al. The homeobox gene mirror links EGF signalling to embryonic dorso-ventral axis formation through notch activation. Nat Genet. 2000;24:429–33. doi: 10.1038/74294. [DOI] [PubMed] [Google Scholar]
- 16.Zhao D, Woolner S, Bownes M. The Mirror transcription factor links signalling pathways in Drosophila oogenesis. Dev Genes Evol. 2000;210:449–57. doi: 10.1007/s004270000081. [DOI] [PubMed] [Google Scholar]
- 17.Atkey MR, Lachance JF, Walczak M, Rebello T, Nilson LA. Capicua regulates follicle cell fate in the Drosophila ovary through repression of mirror. Development. 2006;133:2115–23. doi: 10.1242/dev.02369. [DOI] [PubMed] [Google Scholar]
- 18.Berg CA. The Drosophila shell game: patterning genes and morphological change. Trends Genet. 2005;21:346–55. doi: 10.1016/j.tig.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Van Buskirk C, Schüpbach T. Versatility in signalling: multiple responses to EGF receptor activation during Drosophila oogenesis. Trends Cell Biol. 1999;9:1–4. doi: 10.1016/S0962-8924(98)01413-5. [DOI] [PubMed] [Google Scholar]
- 20.Jiménez G, Shvartsman SY, Paroush Z. The Capicua repressor - a general sensor of RTK signaling in development and disease. J Cell Sci. 2012;125:1383–91. doi: 10.1242/jcs.092965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goff DJ, Nilson LA, Morisato D. Establishment of dorsal-ventral polarity of the Drosophila egg requires capicua action in ovarian follicle cells. Development. 2001;128:4553–62. doi: 10.1242/dev.128.22.4553. [DOI] [PubMed] [Google Scholar]
- 22.Astigarraga S, Grossman R, Díaz-Delfín J, Caelles C, Paroush Z, Jiménez G. A MAPK docking site is critical for downregulation of Capicua by Torso and EGFR RTK signaling. EMBO J. 2007;26:668–77. doi: 10.1038/sj.emboj.7601532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ajuria L, Nieva C, Winkler C, Kuo D, Samper N, Andreu MJ, et al. Capicua DNA-binding sites are general response elements for RTK signaling in Drosophila. Development. 2011;138:915–24. doi: 10.1242/dev.057729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neuman-Silberberg FS, Schüpbach T. The Drosophila dorsoventral patterning gene gurken produces a dorsally localized RNA and encodes a TGF α-like protein. Cell. 1993;75:165–74. [PubMed] [Google Scholar]
- 25.Campbell G, Tomlinson A. Transducing the Dpp morphogen gradient in the wing of Drosophila: regulation of Dpp targets by brinker. Cell. 1999;96:553–62. doi: 10.1016/S0092-8674(00)80659-5. [DOI] [PubMed] [Google Scholar]
- 26.Jaźwińska A, Kirov N, Wieschaus E, Roth S, Rushlow C. The Drosophila gene brinker reveals a novel mechanism of Dpp target gene regulation. Cell. 1999;96:563–73. doi: 10.1016/S0092-8674(00)80660-1. [DOI] [PubMed] [Google Scholar]
- 27.Minami M, Kinoshita N, Kamoshida Y, Tanimoto H, Tabata T. brinker is a target of Dpp in Drosophila that negatively regulates Dpp-dependent genes. Nature. 1999;398:242–6. doi: 10.1038/18451. [DOI] [PubMed] [Google Scholar]
- 28.Müller B, Hartmann B, Pyrowolakis G, Affolter M, Basler K. Conversion of an extracellular Dpp/BMP morphogen gradient into an inverse transcriptional gradient. Cell. 2003;113:221–33. doi: 10.1016/S0092-8674(03)00241-1. [DOI] [PubMed] [Google Scholar]
- 29.Chen H, Xu Z, Mei C, Yu D, Small S. A system of repressor gradients spatially organizes the boundaries of Bicoid-dependent target genes. Cell. 2012;149:618–29. doi: 10.1016/j.cell.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Löhr U, Chung HR, Beller M, Jäckle H. Antagonistic action of Bicoid and the repressor Capicua determines the spatial limits of Drosophila head gene expression domains. Proc Natl Acad Sci U S A. 2009;106:21695–700. doi: 10.1073/pnas.0910225106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balaskas N, Ribeiro A, Panovska J, Dessaud E, Sasai N, Page KM, et al. Gene regulatory logic for reading the Sonic Hedgehog signaling gradient in the vertebrate neural tube. Cell. 2012;148:273–84. doi: 10.1016/j.cell.2011.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Briscoe J, Pierani A, Jessell TM, Ericson J. A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell. 2000;101:435–45. doi: 10.1016/S0092-8674(00)80853-3. [DOI] [PubMed] [Google Scholar]
- 33.McNeill H, Yang CH, Brodsky M, Ungos J, Simon MA. mirror encodes a novel PBX-class homeoprotein that functions in the definition of the dorsal-ventral border in the Drosophila eye. Genes Dev. 1997;11:1073–82. doi: 10.1101/gad.11.8.1073. [DOI] [PubMed] [Google Scholar]