Abstract
Neuropeptides are ubiquitous in both mammals and invertebrates and play essential roles in regulation and modulation of many developmental and physiological processes through activation of G-protein-coupled-receptors (GPCRs). However, the mechanisms by which many of the neuropeptides regulate specific neural function and behaviors remain undefined. Here we investigate the functions of Drosulfakinin (DSK), the Drosophila homolog of vertebrate neuropeptide cholecystokinin (CCK), which is the most abundant neuropeptide in the central nervous system. We provide biochemical evidence that sulfated DSK-1 and DSK-2 activate the CCKLR-17D1 receptor in a cell culture assay. We further examine the role of DSK and CCKLR-17D1 in the regulation of larval locomotion, both in a semi-intact larval preparation and in intact larvae under intense light exposure. Our results suggest that DSK/CCKLR-17D1 signaling promote larval body wall muscle contraction and is necessary for mediating locomotor behavior in stress-induced escape response.
Keywords: Drosulfakinin, CCKLR, neuropeptide, GPCR, larval locomotion
Introduction
Neuropeptides are present ubiquitously in both vertebrates and invertebrates and are involved in regulation of numerous important processes such as development, reproduction, homeostasis and behavior. They are produced by small sets of neurons in the central nervous system, as well as by various neurosecretory organs. Neuropeptides can function as neurotransmitters, neuromodulators, and hormones by activation of cognate G-protein-coupled receptors (GPCRs) localized on the plasma membrane of target cells.1 Despite the importance and prevalence of neuropeptide signaling, our knowledge of how specific neuropeptides regulate specific behaviors is still largely incomplete.
Drosophila has become an attractive model for studying neuropeptides because of its well-characterized nervous system and the wide array of molecular and genetic tools that are available. The Drosophila genome contains about 40 genes encoding neuropeptide precursors and about 50 genes predicted to encode neuropeptide receptors.2,3 Although many of these neuropeptides and neuropeptide receptors share structural and functional homology with vertebrate counterparts,2 functional characterization of neuropeptides and their receptors is still at an early stage.
Drosulfakinin (DSK) is the Drosophila homolog of the vertebrate neurohormone cholecystokinin (CCK), which is the most abundant neuropeptide in the vertebrate central nervous system.4 DSK belongs to the family of FMRFamide-related peptides (FaRPs) that influence various physiological processes in invertebrates, including cardioexcitatory activity,5,6 feeding and egg-laying behaviors,7,8 and learning and memory9,10 similar to the known roles of CCK in vertebrates. In invertebrates, FaRPs are also involved in regulation of body wall muscle activity. Early studies in the locust suggested that FMRF-like peptides enhance synaptic efficacy at the neuromuscular junction (NMJ),11 and studies in crustaceans suggest that these peptides modulate presynaptic Ca2+-channel activity.12 In Drosophila, products from the FMRFa gene can modulate the strength of muscle contraction at the NMJs when perfused onto standard larval nerve-muscle preparations.13 We recently discovered that CCKLR-17D1, which encodes a CCK-like receptor in Drosophila, has a novel role as a positive regulator for growth of the larval NMJ.14 Moreover, mutations of dsk (the gene encoding Drosulfakinin) and CCKLR-17D1 exhibit similar NMJ undergrowth phenotypes.14 Dominant phenotypic interactions between dsk and CCKLR-17D1 mutations in double heterozygotes further suggested that DSK is a ligand for CCKLR-17D1.14
Here we use the β-arrestin2-GFP translocation assay in cultured cells15 to demonstrate that DSK-1 and DSK-2 are indeed functional ligands of CCKLR-17D1 and that sulfation of these peptides is essential for their biological activity. We have also examined the roles of DSK peptides and CCKLR-17D1 in the regulation of larval locomotion, both in a semi-intact preparation and in intact larvae exposed to intense light. Our results suggest that DSK/CCKLR-17D1 signaling promotes larval body wall muscle contraction and is necessary for mediating locomotor behavior in a stress-induced escape response.
Results
Sulfated Drosulfakinin peptides activate CCKLR-17D1
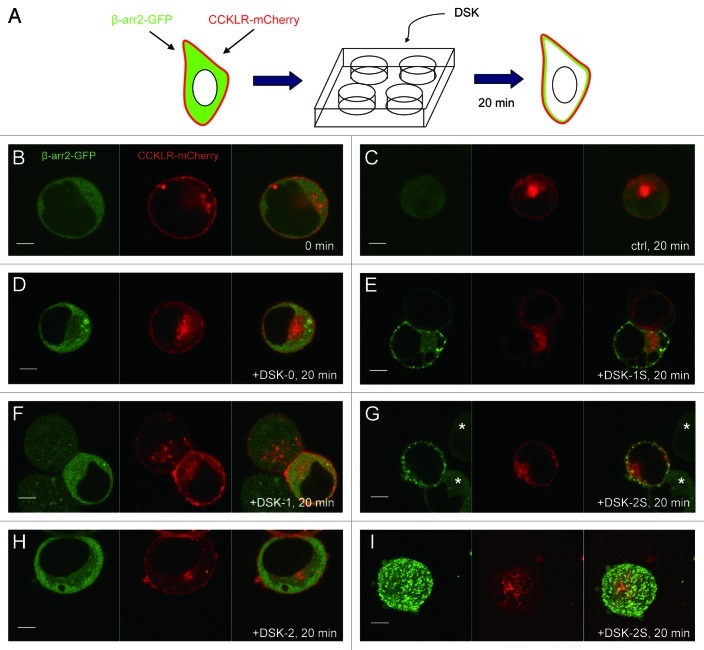
Our previous study provided genetic evidence that DSK (Table 1) is a ligand for CCKLR-17D1.14 In this study, we sought to provide additional biochemical evidence for this conclusion. The intracellular translocation of the β-arrestin2-GFP chimera has been used as an assay to assess desensitization of known mammalian GPCRs and to identify ligands for several orphan Drosophila GPCRs.15 Here, we modified the assay by co-expressing mCherry-tagged CCKLR-17D1 with β-arrestin2-GFP in HEK-293 cells. This procedure enables visualization of both GPCR expression and localization and β-arrestin2-GFP translocation (Fig. 1A). Approximately 40% of HEK-293 cells co-transfected with CCKLR-17D1-mCherry and β-arrestin2-GFP show punctate localization of CCKLR-17D1-mCherry on the cell membrane and diffuse localization of β-arrestin2-GFP in the cytoplasm (Fig. 1B). These are the cells that were scored in the following experiments. Cells with abnormal CCKLR-17D1-mCherry expression or localization were excluded from the subsequent analysis. However these cells did provide a useful negative control to rule out the possibility that any response elicited by addition of neuropeptide was mediated by an endogenous GPCR expressed in HEK-293 cells.
Table 1. Amino acid sequences of Drosulfakinin peptides.
|
Peptide ID |
Peptide sequence |
| DSK-0 a |
N Q K T M S F-NH2 |
| DSK-1 a |
F D D Y G H M R F-NH2 |
| DSK-1S |
F D D Y*G H M R F-NH2 |
| DSK-2 a |
G G D D Q F D D Y G H M R F-NH2 |
| DSK-2S | G G D D Q F D D Y*G H M R F-NH2 |
Y* denotes sulfated tyrosine, Thr(SO3H). a Sequence predicted from the cloned pro-drosulfakinin gene dsk.21
Figure 1. Translocation of βarr2-GFP in response to DSK-1S and DSK-2S, but not DSK-0, in HEK-293 cells expressing CCKLR-17D1. (A) Dual-label system for visualization of βarr2-GFP translocation in GPCR-expressing cells. mCherry-tagged CCKLR-17D1 enables visualization of cells with GPCR expression and proper localization to the cell membrane. (B–H) Confocal images (0.1 μM optical slice) of HEK-293 cells transiently expressing both CCKLR-17D1-mCherry and βarr2-GFP. (B) Before addition of peptide, CCKLR-17D1-mCherry localizes to the cell membrane and βarr2-GFP is diffuse in the cytoplasm. (C) Control cells with addition of peptide-free solution (sham experiment) for 20 min. In cells that are exposed to DSK-0 (D), DSK-1 (F) or DSK-2 (H) for 20 min, βarr2-GFP remains diffuse in the cytoplasm, similar to control cells. When cells are exposed to DSK-1S (E) and DSK-2S (G) for 20 min, βarr2-GFP displays clear translocation to the membrane. Cells marked with asterisks in this panel are examples of cells that express βarr2-GFP but fail to express CCKLR-17D1-mCherry. As shown, these cells do not respond to the addition of DSK-2S or DSK-1S (not shown) peptides indicating that the response is dependent on the presence of CCKLR-17D1-mCherry rather than an endogenous receptor present in HEK-293 cells. (I) Z-stack of confocal images of HEK-293 cells exposed to DSK-2S for 20 min. Peptides were applied at 1 μM.
A previous study of DSK peptides demonstrated that sulfation of the tyrosine residue in DSK-1 and DSK-2 (Table 1) is necessary for their activity.19 Therefore, we tested all three putative peptides (DSK-0, DSK-1 and DSK-2), produced from the DSK precursor (Table 1), and the sulfated forms of DSK-1 and DSK-2 (designated as DSK-1S and DSK-2S) in the translocation assay. Cells exposed to 1 μM DSK-1S or DSK-2S clearly display translocation of the β-arrestin2-GFP to the cell membrane within 20 min (Fig. 1E and G).
In contrast, DSK-0, and the unsulfated DSK-1 and DSK-2 fail to trigger the response and the β-arrestin2-GFP remains diffuse in the cytoplasm as also observed in untreated control cells (Fig. 1D, F and H). Importantly, cells that do not express CCKLR-17D1-mCherry fail to trigger β-arrestin2-GFP translocation under the same treatment (Fig. 1G, asterisk), indicating that HEK-293 cells do not express an endogeneous GPCR capable of being activated by DSK. In optical slices, translocated β-arrestin2-GFP appears as discrete puncta forming a continuous halo at the cell periphery (Fig. 1E and G). These puncta cover the entire cell membrane as shown in a 3D Z-stack of the optical slices (Fig. 1I). Many of these puncta colocalize with CCKLR-17D1-mCherry puncta (Fig. 1G), as expected if activation of CCKLR-17D1 recruits β-arrestin2-GFP to the cell membrane. The β-arrestin2-GFP translocation responses were essentially all-or-none (i.e., in each cell β-arrestin2-GFP either translocates to the membrane or remains diffuse in the cytoplasm) so that we were able to readily score each cell as a positive or negative response without intermediate phenotypes. We observed that > 70% of cells expressing both CCKLR-17D1-mCherry and β-arrestin2-GFP displayed translocation of β-arrestin2-GFP in response to DSK-1S and DSK-2S. The response triggered by DSK peptides is specific because other unrelated peptides (such as proctolin) fail to evoke β-arrestin2-GFP translocation (data not shown). These results demonstrate that DSK-1 and DSK-2 but not DSK-0 are functional ligands of CCKLR-17D1 and that sulfation of the Tyrosine residue in DSK-1 and DSK-2 is essential for activity of these peptides.
DSK-1S and DSK-2S promote larval fictive locomotion
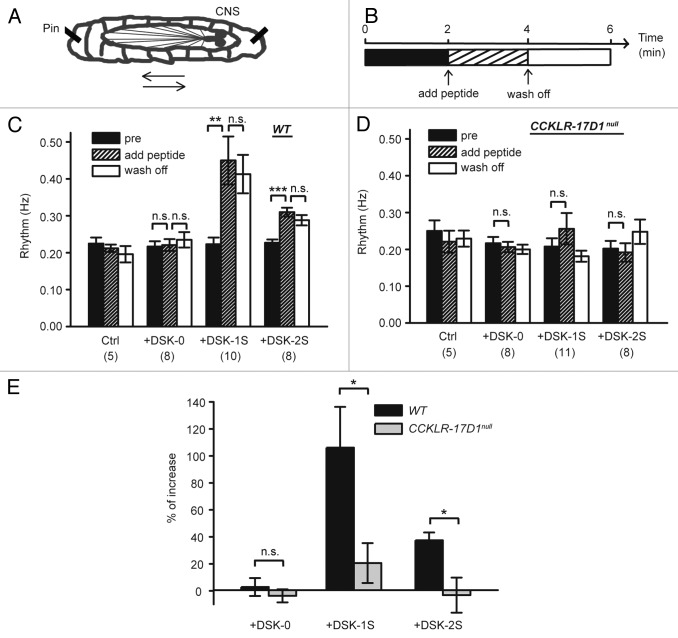
Having found that DSK peptides activate CCKLR-17D1, we examined these peptides for possible effects on larval locomotor activity using a fictive locomotion assay modified from Klose et al.17 We measured the frequency of peristaltic waves in response to DSK-0, DSK-1S and DSK-2S (Fig. 2A) in a semi-intact larval preparation, in which hemolymph is replaced with hemolymph-like HL-3 saline. The initial frequency of fictive locomotion is 0.22 ± 0.014 Hz. After 6 min, the frequency is still 0.22 ± 0.015 Hz but it diminishes significantly over 20 min to 0.03 ± 0.009 Hz. This reduction in locomotor activity is believed to result, at least in part, from exhaustion of endogenous neuromodulators.17 Thus, we performed our experiments during the first 6 min after larval dissection to ensure stability of the fictive locomotion frequency (Fig. 2B). Addition of DSK-0 (5 μM) to the bath does not change the frequency of fictive locomotion compared with addition of peptide-free saline (Fig. 2C). In contrast, addition of 5 μM DSK-1S or DSK-2S to the bath results in a significant increase in the frequency of fictive locomotion over baseline (Fig. 2C). The frequency does not change significantly following washout of the peptide (Fig. 2C), consistent with previous reports on the sustained excitatory effects of neuropeptides on larval NMJs.13,17 These results indicate that DSK-1S and DSK-2S can stimulate larval locomotor behavior. In CCKLR-17D1 null mutant larvae, addition of DSK-0, DSK-1S or DSK-2S (Fig. 2D) does not enhance fictive locomotion. Thus, CCKLR-17D1 is required for DSK-induced enhancement of larval locomotion. The percent increase in frequency of fictive locomotion evoked by DSK peptides is shown in Figure 2E. DSK-1S has the strongest stimulatory effect, increasing the frequency of fictive locomotion by 100%, whereas DSK-2S increases it by 40% (Fig. 2E).
Figure 2. DSK-1S and DSK-2S promote larval fictive locomotion. (A) Diagram of restrained semi-intact third instar wandering larva (arrow indicates direction of peristaltic wave of locomotor muscle contractions). (B) Diagram of the experimental scheme. (C) Frequency of fictive locomotion in semi-intact wild-type larvae significantly increases following addition of DSK-1S or DSK-2S into hemolymph-like saline HL-3 and remains high after wash out. Frequency of fictive locomotion does not change after addition of DSK-0. In the control (ctrl) experiment, peptide-free solution is added at 2 min. (n) is the number of larvae scored. Error bars denote SEM ***, p < 0.001. **, p < 0.01. n.s., not significant (paired Student’s t-test). (D) Contraction frequency in semi-intact CCKLR-17D1null mutant larvae does not change significantly after addition of DSK-0, DSK-1S, or DSK-2S (paired Student’s t-test). (E) Percent increase in contraction frequency after addition of peptide. Percent increase is calculated for each individual larva as (frqpost – frqpre)/frqpre. Error bars denote SEM *, p < 0.05. n.s., not significant (unpaired Student’s t-test).
DSK/CCKLR signaling modulates stress-induced larval escape behavior
We also sought to determine whether DSK/CCKLR-17D1 signaling affects crawling behavior in intact larvae. Under standard laboratory conditions, the behavior of CCKLR-17D1 and dsk mutant larvae appears normal—they feed and crawl actively and pupariate properly after crawling up the walls of culture vials. Consequently, we examined their behavior under stress to try to uncover more subtle behavioral phenotypes. Drosophila larvae respond to bright light by moving away from the source and respond to adverse elevations in temperature by increasing the rate and amount of crawling.17,20 These adaptive behaviors help protect larvae from desiccation in natural conditions.
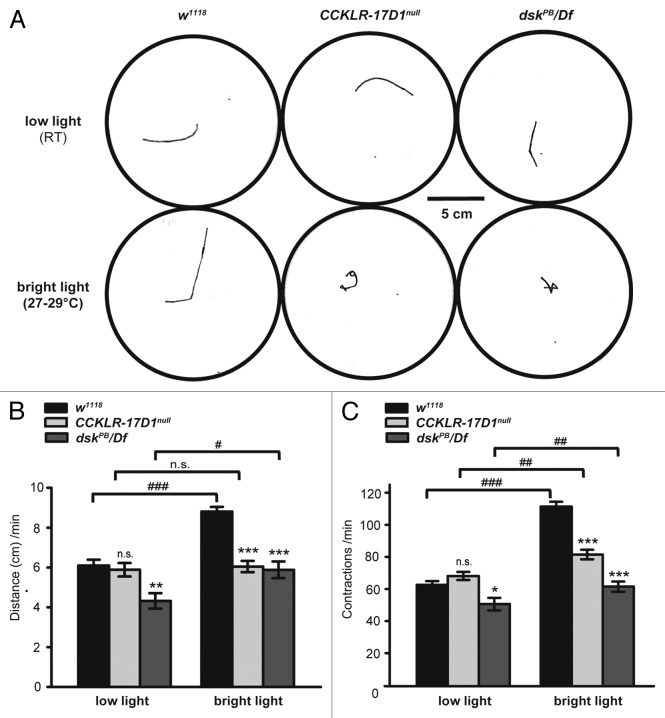
In our assay, wild-type larvae travel 61.0 ± 2.9 mm in one minute under dim light at 20–22°C (Fig. 3A and B). The distance traveled by CCKLR-17D1 mutant larvae does not differ from wild-type (58.9 ± 3.4 mm) but it is reduced in dsk mutants (43.2 ± 3.9 mm) (Fig. 3A and B). Under bright light and elevated temperature (27–30°C), wild-type larvae travel 88.1 ± 2.3 mm in one minute whereas CCKLR-17D1 and dsk mutants travel only 60.5 ± 2.8 mm and 58.8 ± 4.2 mm respectively (Fig. 3A and B). In addition, the frequency of peristaltic contractions increases in wild-type larvae under these conditions and they mostly follow straight travel paths (Fig. 3A and C). In contrast, bright light and elevated temperature elicit only a slight increase in contraction rate in CCKLR-17D1 and dsk mutant larvae (Fig. 3C). Moreover, the condition of bright light and elevated temperature appears to elicit a significant increase in turning and waving behavior in the mutant larvae (Fig. 3A). In response to bright light, the fraction of CCKLR-17D1 mutant larvae following an erratic path (see Materials and Methods) increased from 7% to 67%. Similarly for dsk mutant larvae this fraction increased from 0% to 78%. In contrast, the fraction of wild-type larvae following an erratic path remained uniform in low light vs. bright light (21% vs. 20%). Thus, DSK/CCKLR signaling appears to mediate proper locomotor responses to stresses such as intense light and elevated temperature in vivo.
Figure 3.CCKLR-17D1 and dsk mutants exhibit deficits in stress-induced escape behavior. (A) Representative traces of tracks made by crawling larvae of the indicated genotype during a 1 min recording period under dim light (20–22°C) and bright light (27–30°C). (B) Distance traveled during a 1 min recording period under low light and bright light. The distance traveled by control larvae is significantly increased under bright light (p < 0.001). In contrast, dskPB/Df mutants exhibit a relatively smaller increase (p < 0.05) and CCKLR-17D1null mutants exhibit no significant increase under bright light. Compared with control larvae, both CCKLR-17D1null and dskPB/Df mutants travel significantly shorter distance under bright light conditions (p < 0.001). (C) Number of body wall contractions during a 1 min recording period under low light and bright light. The number of contractions in control larvae is significantly increased under bright light (p < 0.001). Both CCKLR-17D1null and dskPB/Df mutant larvae also show a significant increase in the number of contractions under bright light but not to the same extent as control larvae and the number of contractions in both mutants differs significantly from controls (p < 0.001). Number of larvae assayed: w1118 dark (28), CCKLR-17D1null dark (27), dskPB/Df dark (11), w1118 light (34), CCKLR-17D1null light (27), dskPB/Df light (10). Error bars denote SEM *** or ###, p < 0.001. ** or ##, p < 0.01. * or #, p < 0.05. n.s, not significant (unpaired Student’s t-test was used in all cases since each larva was tested only once. Pairwise comparisons are either with w1118 control under the same condition or as indicated by brackets).
Discussion
We previously inferred from genetic studies on NMJ development that DSK peptides are ligands for CCKLR-17D1. Using a modified version of the β-arrestin2-GFP translocation assay developed by Johnson et al.,15 we have now shown that DSK peptides can activate CCKLR-17D1 in living cells. We tested all three predicted DSK peptides encoded by dsk21 and found that sulfated DSK-1 and sulfated DSK-2 were active, whereas DSK-0 and unsulfated DSK-1 and DSK-2 were inactive. The inactivity of DSK-0 may be related to the fact that it lacks a sulfatable tyrosine residue. Our modified assay in which CCKLR-17D1 was tagged with mCherry was advantageous because it enabled us to score β-arrestin2 translocation only in those cells with proper expression and membrane localization of the GPCR thereby avoiding false-negative results. Moreover, the cells that did not express CCKLR-17D1-mCherry and that did not respond to addition of sulfated-DSK peptides provided an important negative control to rule out the presence of endogenous receptors for DSK in HEK cells. This modified assay may prove similarly useful in the analysis of other ligand/GPCR pairs in the future. It should be noted that our results do not rule out the possibility that DSK can activate receptors other than CCKLR-17D1. In fact, there is evidence that DSK can also activate CCKLR-17D3,19 which might serve different functions. Likewise, neuropeptides other than DSK could possibly activate CCKLR-17D1 in vivo with similar or different effects as DSK. Further investigation is required to explore those possibilities.
Bath application of DSK peptides to a semi-intact larval preparation stimulated fictive larval locomotion by increasing the frequency of peristaltic contractions. Consistent with the translocation assay, only DSK-1S and DSK-2S elicited this response, which was also dependent on CCKLR-17D1. In our previous study,14 we found that CCKLR-17D1 expression in motor neurons was required to promote NMJ growth. However, the effect of DSK/CCKLR signaling on larval locomotion most likely occurs via a distinct mechanism because increased contraction frequency presumably reflects an increase in motor firing patterns governed by neural circuits in the central nervous system upstream of the NMJ. Together with our previous study, these results suggest that DSK/CCKLR signaling in Drosophila affects at least two different aspects of the larval locomotor pathway: a long-term effect on NMJ growth during development and a short-term effect on modulation of motor firing patterns. The neurons in which CCKLR-17D1 must be expressed to mediate the latter phenotype remain to be determined.
Klose et al., observed effects on fictive larval behavior similar to those described here that were dependent on two other GPCRs (FR and DmsR) activated by DPKQDFMRFamide.17 It will thus be of interest to investigate whether there is any functional overlap, direct or indirect, between these two neuropeptide signaling systems in the regulation of larval locomotion.
Exposure of wild-type larvae to bright, high-temperature light stimulates crawling with significant increases in distance traveled and in the frequency of body wall contractions. As the bright light condition is thought to mimic exposure to the sun in natural environments, the observed responses may represent adaptive behaviors to avoid stressful environments. We found that dsk and CCKLR-17D1 mutant larvae were significantly impaired in this escape response compared with normal controls. Notably, not only were distance traveled and frequency of contractions under bright light significantly reduced in mutant larvae compared with controls, but the crawling behavior was qualitatively different as well. Control larvae traveled in mostly straight paths with few turns under bright light, whereas both dsk and CCKLR-17D1 mutant larvae displayed considerable turning and waving resulting in meandering paths. Thus, DSK/CCKLR-17D1 signaling is required in vivo to mediate an effective escape response under stress. These results are consistent with recent work in nematodes showing that FMRFamide-related peptides play a role in stress-induced behavior.20 Together with the results of the fictive locomotion assay, these studies further suggest that DSK/CCKLR signaling is important for regulating larval locomotion. It will now be of interest to further unravel the biological functions of DSK/CCKLR signaling in vivo and to determine how these functions are integrated with those of other neuropeptide signaling systems in Drosophila larvae.
Materials and Methods
Fly stocks
w1118 was used as the wild-type control for genetic background. Df(1)Exel9051 (CCKLR-17D1null) (FBst0007762) and Df(3R)2–2 (FBab0002471) were obtained from the Bloomington Stock Center. dskf02468 was obtained from Exelixis at Harvard Medical School. All stocks were raised on conventional fly medium and maintained at room temperature (RT, 21–23°C).
Molecular cloning
A full-length CCKLR-17D1 cDNA was generated by PCR amplification using two templates: cDNA clone pOT2-IP18747 (Berkeley Drosophila Genome Project, for bp 731–2022,) and wild-type genomic DNA (for bp 1–730), followed by ligation of the two PCR fragments. CCKLR-17D1 cDNA was cloned into pUAS-C5 attB-C-term mCherry vector.14 A full-length CCKLR-17D1 construct tagged with mCherry was generated by PCR amplification from the UAS-CCKLR-17D1::mCherry construct.14 Primers flanking the predicted open reading frame were synthesized with incorporated restriction sites to facilitate directional cloning into the pcDNA5/FRT vector (Invitrogen). Sequences of the primers are as follows: 5′-AAAGCTAGCATGTTGCCGCGCCTGTG-3′, 5′-AAAAGCGGCCGCTTCAGAGTCGCGGACT-3′. Accuracy of all receptor constructs was confirmed by sequencing.
Peptides
Procedures for synthesis of DSK-1 (FDDYGHMRF-NH2) and DSK-2 (GGDDQFDDYGHMRF-NH2) peptides and sulfation of the tyrosine residue (DSK-1S and DSK-2S) were previously described.8,16 The identity of the samples was confirmed via MALDI-TOF mass spectrometry and quantified via amino acid analysis.8,16 DSK-0 (NQKTMSF-NH2) was purchased from GenScript. Peptides were dissolved in 80% aqueous acetonitrile (made to 0.01% TFA) and stored at -20°C.
Transfections and cell culture
HEK-293 cells were transiently transfected using Effectene Transfection Reagent (Qiagen) using 0.3 μg DNA for a 12-well plate. Cells were transfected with a 5:1 ratio of CCKLR-17D1-mCherry DNA and βarr2-GFP DNAs. Cells were cultured in a humidified incubator at 37°C under 5% CO2 atmosphere and were split every 3 d 1:10. Growth medium was MEM (Invitrogen) supplemented with 10% fetal bovine serum and Pen Strip antibiotics.
β-arrestin translocation assays
HEK-293 cells were transfected as described above in a 12-well plate. Translocation assays were performed as described by Johnson et al., 2003.15 Briefly, growth medium was replaced with MEM α without phenol red 30 min prior to assays. Peptides were dissolved in the same medium to reach a final concentration of 1 μM. 100 μl of peptide solution was added at room temperature to each 1 ml well of culture medium (1:10 volume of culture medium). To avoid disturbing the cells, the peptide solution was added dropwise and then mixed by gently shaking the plate. After 20 min, the cells were suspended by pipetting and dropped onto glass slides for imaging. Images were collected using 488 nm and 561 nm excitation on a laser scanning confocal microscope (LSM 510; Carl Zeiss, Inc.) equipped with EC Plan-Neofluar 40 × NA 1.3 oil differential interference contrast or Plan-Apochromat 63 × NA 1.4 oil differential interference contrast objectives and the accompanying software. Images were processed in the LSM image browser (Carl Zeiss, Inc.) Brightness and contrast were optimized for each image independently in Adobe Photoshop. All images were captured at room temperature.
Fictive locomotion assay
Larval fictive locomotion assays were performed by slight modification of the protocol described by Klose et al., 2010.17 Wandering third instar larvae were restrained by pins at both the anterior and posterior ends in a dissecting chamber and dissected along the dorsal midline to allow replacement of hemolymph with hemolymph–like saline, HL-3.18 HL-3 contained the following (in mM): CaCl2 (1.8), MgCl2 (20.0), KCl (5.0), NaCl (70.0), NaHCO3 (10.0), N,N-bis (2-hydroxyethyl)-2-aminoethanesulfonic acid (5.0), trehalose-2H2O (5.0), sucrose (115.0). Ca2+- and divalent-cation free HL-3 was used during dissection and was replaced with HL-3 containing 1.8 mM Ca2+ for the locomotion assays. The nervous system and the body wall muscles remained intact. The frequency of fictive locomotion was measured by counting the number of peristaltic waves over a 2 min period. Recordings were initiated within ~2 min of the start of dissection, after regular contractions began. Peptides were dissolved in HL-3 saline at 5 μM working concentration. Peptide solutions were applied by exchanging the peptide-free HL-3 saline with peptide containing HL-3 saline.
Stress-induced escape locomotion assay
Light-induced larval locomotor behavior was examined by modifying the protocol described by Klose et al., 2010.17 Briefly, third instar wandering larvae were washed in saline and then dipped for 1 sec in food color solution before being placed at the center of a 14 cm agar plate. Each individual larva was observed for 1 min under minimal light conditions at room temperature (22–23°C) and the number of peristaltic contractions was recorded. After one minute, the larva was removed and the wandering path was traced onto a clear plastic sheet for analysis. A new, independent set of larvae was tested under bright light, to avoid any possible effects of fatigue. A bright, full spectrum light was shined directly onto each larva through the plastic dish. The distance between the light and the larvae was kept constant. The surface temperature as measured with a microprobe was 27–30°C. Wandering paths were digitized and measured using ImageJ (National Institutes of Health; rsb.info.nih.gov/ij/). In addition to quantifying path distance, we also determined whether each path was straight or “erratic.” A path was considered erratic if it contained at least one turn sharper than 90 degrees.
Statistical analyses
Student’s t-tests, either paired or unpaired as appropriate, were used to analyze data for statistical significance in pairwise comparisons. The particular test used and the level of significance are provided in the figure legends.
Acknowledgments
We are grateful to Paul Taghert for generously sharing reagents for GPCR-β-arrestin2 translocation assay and for providing many helpful suggestions. We thank Allen Laughon for providing access to cell culture facility and reagents, and Bloomington Stock Center for providing fly stocks used in these experiments. We thank all members of the Ganetzky laboratory for helpful discussion and critical comments on the project. This research was supported by a grant from the National Institutes of Health (NS15390) to B. G. and a grant from the USDA/DoD DWFP Initiative (#0500-32000-001-01R) to R. J. N.
Glossary
Abbreviations:
- CCK
cholecystokinin
- CCKLR
cholecystokinin-like receptor
- Df
deficiency
- DSK
Drosulfakinin
- FaRPs
FMRFamide-related peptides
- GPCR
G-protein coupled receptor
- NMJ
neuromuscular junction
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/fly/article/21534
References
- 1.Nässel DR. Neuropeptides in the nervous system of Drosophila and other insects: multiple roles as neuromodulators and neurohormones. Prog Neurobiol. 2002;68:1–84. doi: 10.1016/S0301-0082(02)00057-6. [DOI] [PubMed] [Google Scholar]
- 2.Hewes RS, Taghert PH. Neuropeptides and neuropeptide receptors in the Drosophila melanogaster genome. Genome Res. 2001;11:1126–42. doi: 10.1101/gr.169901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hauser F, Williamson M, Cazzamali G, Grimmelikhuijzen CJ. Identifying neuropeptide and protein hormone receptors in Drosophila melanogaster by exploiting genomic data. Brief Funct Genomic Proteomic. 2006;4:321–30. doi: 10.1093/bfgp/eli003. [DOI] [PubMed] [Google Scholar]
- 4.Fink H, Rex A, Voits M, Voigt JP. Major biological actions of CCK--a critical evaluation of research findings. Exp Brain Res. 1998;123:77–83. doi: 10.1007/s002210050546. [DOI] [PubMed] [Google Scholar]
- 5.Price DA, Greenberg MJ. Purification and characterization of a cardioexcitatory neuropeptide from the central ganglia of a bivalve mollusc. Prep Biochem. 1977;7:261–81. doi: 10.1080/00327487708061643. [DOI] [PubMed] [Google Scholar]
- 6.Nichols R, Manoogian B, Walling E, Mispelon M. Plasticity in the effects of sulfated and nonsulfated sulfakinin on heart contractions. Front Biosci. 2009;14:4035–43. doi: 10.2741/3510. [DOI] [PubMed] [Google Scholar]
- 7.Lange AB, Orchard I. The effects of SchistoFLRFamide on contractions of locust midgut. Peptides. 1998;19:459–67. doi: 10.1016/S0196-9781(97)00465-8. [DOI] [PubMed] [Google Scholar]
- 8.Nachman RJ, Holman GM, Haddon WF, Ling N. Leucosulfakinin, a sulfated insect neuropeptide with homology to gastrin and cholecystokinin. Science. 1986;234:71–3. doi: 10.1126/science.3749893. [DOI] [PubMed] [Google Scholar]
- 9.Guan Z, Giustetto M, Lomvardas S, Kim JH, Miniaci MC, Schwartz JH, et al. Integration of long-term-memory-related synaptic plasticity involves bidirectional regulation of gene expression and chromatin structure. Cell. 2002;111:483–93. doi: 10.1016/S0092-8674(02)01074-7. [DOI] [PubMed] [Google Scholar]
- 10.Mackey SL, Glanzman DL, Small SA, Dyke AM, Kandel ER, Hawkins RD. Tail shock produces inhibition as well as sensitization of the siphon-withdrawal reflex of Aplysia: possible behavioral role for presynaptic inhibition mediated by the peptide Phe-Met-Arg-Phe-NH2. Proc Natl Acad Sci U S A. 1987;84:8730–4. doi: 10.1073/pnas.84.23.8730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robb S, Evans P. THE MODULATORY EFFECT OF SCHISTOFLRFamide ON HEART AND SKELETAL MUSCLE IN THE LOCUST SCHISTOCERCA GREGARIA. J Exp Biol. 1994;197:437–42. doi: 10.1242/jeb.197.1.437. [DOI] [PubMed] [Google Scholar]
- 12.Rathmayer W, Djokaj S, Gaydukov A, Kreissl S. The neuromuscular junctions of the slow and the fast excitatory axon in the closer of the crab Eriphia spinifrons are endowed with different Ca2+ channel types and allow neuron-specific modulation of transmitter release by two neuropeptides. J Neurosci. 2002;22:708–17. doi: 10.1523/JNEUROSCI.22-03-00708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hewes RS, Snowdeal EC, 3rd, Saitoe M, Taghert PH. Functional redundancy of FMRFamide-related peptides at the Drosophila larval neuromuscular junction. J Neurosci. 1998;18:7138–51. doi: 10.1523/JNEUROSCI.18-18-07138.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X, Ganetzky B. A neuropeptide signaling pathway regulates synaptic growth in Drosophila. J Cell Biol. 2012;196:529–43. doi: 10.1083/jcb.201109044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson EC, Bohn LM, Barak LS, Birse RT, Nässel DR, Caron MG, et al. Identification of Drosophila neuropeptide receptors by G protein-coupled receptors-beta-arrestin2 interactions. J Biol Chem. 2003;278:52172–8. doi: 10.1074/jbc.M306756200. [DOI] [PubMed] [Google Scholar]
- 16.Downer KE, Haselton AT, Nachman RJ, Stoffolano JG., Jr. Insect satiety: sulfakinin localization and the effect of drosulfakinin on protein and carbohydrate ingestion in the blow fly, Phormia regina (Diptera: Calliphoridae) J Insect Physiol. 2007;53:106–12. doi: 10.1016/j.jinsphys.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Klose MK, Dason JS, Atwood HL, Boulianne GL, Mercier AJ. Peptide-induced modulation of synaptic transmission and escape response in Drosophila requires two G-protein-coupled receptors. J Neurosci. 2010;30:14724–34. doi: 10.1523/JNEUROSCI.3612-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart BA, Atwood HL, Renger JJ, Wang J, Wu CF. Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J Comp Physiol A. 1994;175:179–91. doi: 10.1007/BF00215114. [DOI] [PubMed] [Google Scholar]
- 19.Kubiak TM, Larsen MJ, Burton KJ, Bannow CA, Martin RA, Zantello MR, et al. Cloning and functional expression of the first Drosophila melanogaster sulfakinin receptor DSK-R1. Biochem Biophys Res Commun. 2002;291:313–20. doi: 10.1006/bbrc.2002.6459. [DOI] [PubMed] [Google Scholar]
- 20.Caldwell JC, Miller MM, Wing S, Soll DR, Eberl DF. Dynamic analysis of larval locomotion in Drosophila chordotonal organ mutants. Proc Natl Acad Sci U S A. 2003;100:16053–8. doi: 10.1073/pnas.2535546100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nichols R, Schneuwly SA, Dixon JE. Identification and characterization of a Drosophila homologue to the vertebrate neuropeptide cholecystokinin. J Biol Chem. 1988;263:12167–70. [PubMed] [Google Scholar]