Abstract
Thirteen drosophilid species belonging to seven genera and two subfamilies are reported from three coral islands (namely Europa, Juan de Nova and Glorioso) that belong to the Scattered Islands in the Indian Ocean. Five species are cosmopolitan and five are African. Three are endemic to the insular Western Indian Ocean, including a presumably new Scaptodrosophila species. On the island of Juan de Nova, most captured flies had pollinia attached to the bases of their proboscis. DNA analysis using the rbcl gene revealed that these pollinia belong to the genus Leptadenia (Apocynaceae), of which a single species L. madagascariensis, endemic in Madagascar and Comoros, is present in this island. This is the first reported association between this plant and drosophilids.
Keywords: taxonomy, DNA barcoding, island biogeography, myophily, conservation
Introduction
The islands of the Western Indian Ocean harbor a distinctive drosophilid fauna that is mainly dominated by African species.1-3 Among 158 species,3 25 are endemic to Madagascar, 12 to Seychelles, 31 to Mauritius and 2 to La Réunion. However, the fauna of the islands encircling Madagascar that are called Scattered Islands (Îles éparses) by the French administration have not been investigated. The Scattered Islands consist of four small coral islands (Europa, 30 km2; Juan de Nova, 5 km2; Glorioso islands, 7 km2; Tromelin, 1 km2) and an atoll (Bassas da India). All are located in the Mozambique Channel, except Tromelin which is located 470 km east of Madagascar and 560 km west of Mauritius and La Réunion (Fig. 1). The maximum altitude of these islands ranges from 7 to 14 m. Bassas da India is an atoll that becomes totally submerged by water everyday at high tides. Each of the remaining four islands is presently inhabited by a small military camp only.4
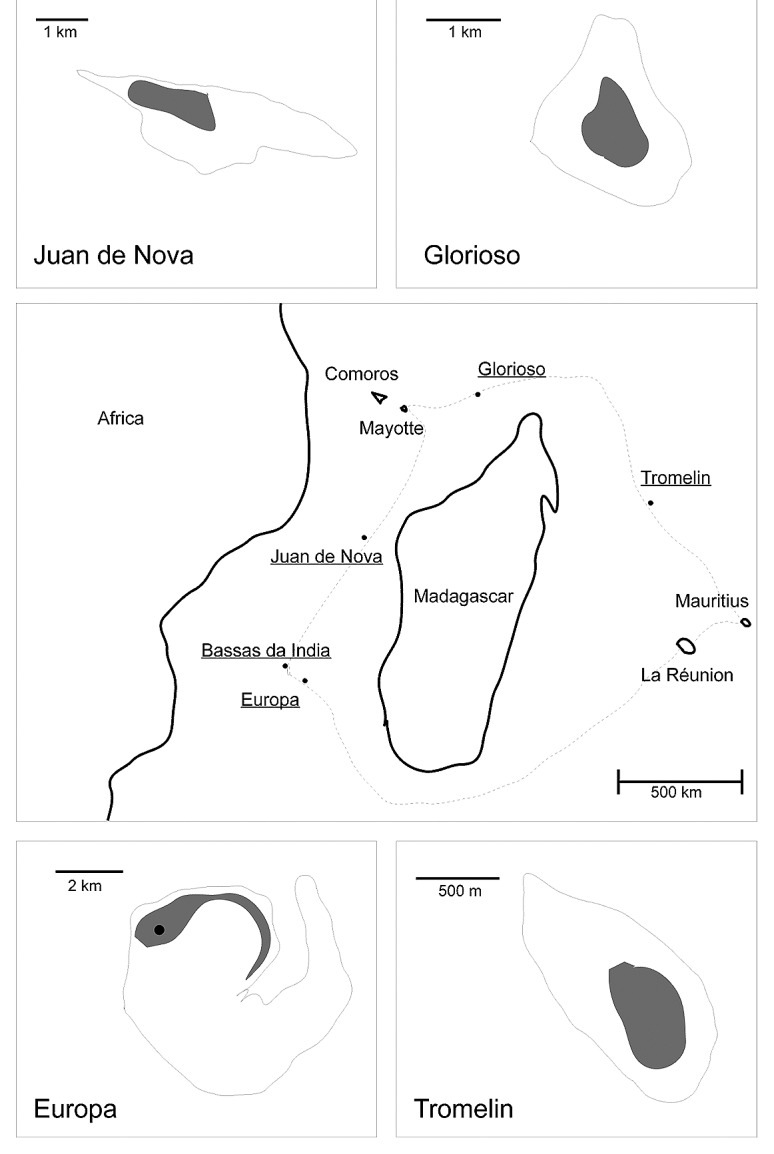
Figure 1. Maps of the Western Indian Ocean showing the geographical position of the Scattered Islands (underlined) and the trajectory of the Marion Dufresne ship (dashed line). Gray areas indicate locations where the traps were placed. The trap where drosophilids were collected in Europa is indicated with a dot.
Previous entomological surveys were conducted on all islands except Juan de Nova and Bassas da India. The presence of 154 insect species, of which 24 were endemic, has been reported.5-8 However, no drosophilid species has ever been recorded from these islands. In April 2011, a CNRS-funded scientific expedition was joined by four of us (JRD, NG, JH and AY). One of us (JH), with Benoît Dumeau, spent 52 d on Europa from October to December 2011. We report here the presence of 13 drosophilid species including a probably new Scaptodrosophila species and we describe a new association between drosophilids and the flowering plant Leptadenia madagascariensis Decne. on the island of Juan de Nova.
Results and Discussion
Taxonomy and biodiversity
Table 1 shows the list of drosophilid species collected and their geographical distribution. In total, 13 species belonging to seven genera and two subfamilies were collected. Of which, 10 species are also found on the African mainland and five outside Africa. Three species are endemic to the insular Western Indian Ocean.
Table 1. Geographical distribution and number of drosophilid species collected in the Sparse Islands.
|
Geographical region |
EUR |
JDN |
GLO |
TRO |
COM |
MAD |
SEY |
MAU |
REU |
AFR |
COS |
|
| Date of collection |
Apr. 2011 |
Nov. 2011 |
Apr. 2011 |
Apr. 2011 |
Apr. 2011 |
|
|
|
|
|
|
|
|
Subfamily Drosophilinae Genus Chymomyza Group procnemis Chymomyza bicolor |
0 |
0 |
0 |
1 |
0 |
|
|
● |
|
|
|
|
| Genus Drosophila Subgenus Sophophora Group ananassae Drosophila malerkotliana |
0 |
0 |
10 |
379 |
0 |
|
|
● |
● |
|
● |
● |
| Group melanogaster Drosophila melanogaster |
0 |
1 |
0 |
0 |
0 |
● |
● |
● |
● |
● |
● |
● |
|
Drosophila simulans |
0 |
0 |
4 |
0 |
0 |
● |
● |
● |
|
● |
● |
● |
| Genus Lissocephala Group sanu Lissocephala sanu |
0 |
0 |
0 |
12 |
0 |
|
|
|
|
● |
● |
|
| Genus Scaptodrosophila Group aterrima Scaptodrosophila caliginosa |
0 |
0 |
1 |
0 |
0 |
|
● |
● |
● |
|
● |
|
| Group brunnea Scaptodrosophila sp |
0 |
0 |
10 |
20 |
0 |
|
|
|
|
|
|
|
| Group latifasciaeformis Scaptodrosophila latifasciaeformis |
0 |
0 |
18 |
55 |
0 |
|
● |
● |
● |
● |
● |
● |
| Group saba Scaptodrosophila bangi |
0 |
0 |
0 |
3 |
0 |
● |
|
|
● |
● |
● |
|
| Genus Zaprionus Group inermis Zaprionus tuberculatus |
0 |
0 |
0 |
21 |
0 |
● |
● |
● |
● |
● |
● |
|
| Group vittiger Zaprionus indianus |
0 |
0 |
50 |
109 |
0 |
● |
● |
● |
● |
● |
● |
● |
|
Subfamily Steganinae Genus Cacoxenus Group perspicax Cacoxenus polyodous |
0 |
5 |
0 |
0 |
0 |
|
|
|
● |
● |
● |
|
| Genus Leucophenga Group mutabilis Leucophenga malgachensis |
0 |
0 |
0 |
2 |
0 |
|
● |
|
|
|
? |
|
| Number of species | 0 | 2 | 6 | 9 | 0 | 5 | 7 | 9 | 8 | 8 | 10 | 6 |
Dots indicate species that have been reported in other Indian Ocean islands or in mainland Africa, as well as cosmopolitan species.3 Question mark indicates probable presence. EUR, Europa; JDN, Juan de Nova; GLO, Glorioso; TRO, Tromelin; COM, Comoros; MAD, Madagascar; SEY, Seychelles; MAU, Mauritius; REU, La Réunion; AFR, mainland Africa; COS, cosmopolitan.
Species diversity differed greatly among islands. No drosophilid was collected on the island of Tromelin as would be expected from the dry and stormy climate of this small island. In Europa and in spite of its floral richness and semi-arid climate, we could not find any drosophilid during our first visit in April 2011, which lasted three days. However, a later 52-d expedition in October–December 2011 yielded two species using fermented fruit traps: many specimens of the steganine Cacoxenus polyodous whose larvae prey on mealy bugs,9 and a single female of Drosophila melanogaster. Cacoxenus adults are usually only accidentally attracted to fruit baits, and the trap was located in a sisal (Agavaceae) field infested by a large colony of undetermined mealy bugs. D. melanogaster is a cosmopolitan, human commensal species that is usually found near human settlements and easily captured using fruit traps. However, the presence of only a single specimen in December near the military camp and its absence in April suggest that D. melanogaster may not be well established on Europa.
The greatest species diversity is encountered on the islands of Juan de Nova and Glorioso. The 11 species collected on these islands can be classified into three categories: four are cosmopolitan (i.e., found on at least three continents including the Americas) and generalists: Drosophila malerkotliana, Drosophila simulans, Scaptodrosophila latifasciaeformis and Zaprionus indianus; four are pan-African: the fig-breeder Lissocephala sanu (bred from Ficus grevei Baill. on Glorioso), the flower-breeder Scaptodrosophila caliginosa, the palm-breeder Scaptodrosophila bangi, and the generalist Zaprionus tuberculatus; and three are endemic to the insular Indian Ocean: Chymomyza bicolor, Scaptodrosophila sp and Leucophenga malgachensis (which may also be present in South Africa10).
Association with Leptadenia
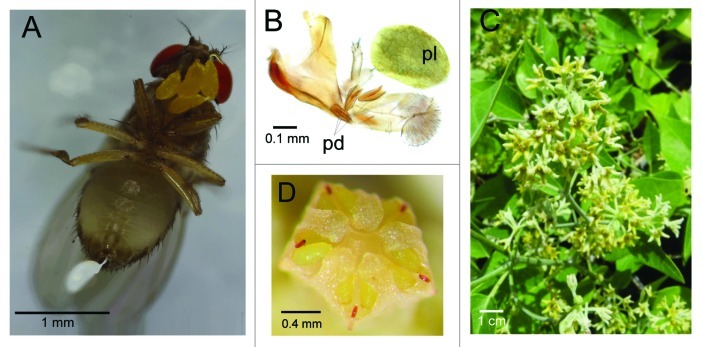
An unexpected phenomenon was observed on flies collected using fermented fruit traps on the island of Juan de Nova. Ninety percent of these flies, belonging to six species (Table 2) harbored large yellow bodies at the lower margin of the head capsule and the proboscis (Fig. 2A). Each yellow body consisted of a pair of cellular aggregates connected and attached to the fly body by a transparent peduncle (Fig. 2B). Microscopic observation suggested that these bodies might be pollinia. In order to determine the origin of these bodies, their DNA was extracted and the chloroplastic gene, ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit (rbcL), which is used in plant DNA barcoding,11 was amplified. PCR amplicons of the correct size were obtained, and a GenBank similarity search using BLAST retrieved one sequence with 100% sequence identity, from the species Leptadenia reticulata (Retz.) Wight and Arn. (Apocynaceae) (accession number EU196282). However, L. reticulata is endemic to India and likely absent in the insular Indian Ocean.12 In Juan de Nova, the only representative of the genus Leptadenia is L. madagascariensis (Fig. 2C; V. Boullet, J. Hivert and M. Lacoste, in lit.; not mentioned by Perrier de la Bâthie in 192113 but cited by Bosser in 195214). Pollinia of L. madagascariensis specimens collected at the same time as the flies (Fig. 2D) were exactly similar to the yellow bodies present on the fly probosces. We also sequenced rbcL from conserved flowers of a L. madagascariensis specimen, and found 100% sequence identity between rbcL from fly-attached pollinia and from L. reticulata. A high similarity in rbcL sequences is frequent in plant molecular taxonomy studies.11 This example shows the utility of DNA barcoding in identifying plants at the genus level by non-specialist, but also rise the concerns of its limitations at the species level.
Table 2. Species list and proportion of flies collected on Juan de Nova bearing yellow pollinia on their probosces.
|
Species |
No. of flies |
Proportion of flies with pollinia |
No. of pollinia per fly |
|
Zaprionus indianus |
50 |
> 90% |
1–6 |
|
Drosophila malerkotliana |
10 |
30% |
1–2 |
|
Scaptodrosophila sp |
10 |
10% |
1 |
|
Scaptodrosophila latifasciaeformis |
18 |
6% |
1 |
|
Drosophila simulans |
4 |
0% |
0 |
| Scaptodrosophila caliginosa | 1 | 0% | 0 |
Figure 2. Association between drosophilids and Leptadenia madagascariensis on the island of Juan de Nova. (A) A Scaptodrosophila latifasciaeformis female carrying several yellow pollinia on its proboscis. (B) Microscopic structure of a pollinium (pl) and three peduncles (pd) attached to a fly proboscis. (C) L. madagascariensis flowers on a shrub. (D) pollinia in a L. madagascariensis flower.
Many drosophilid species are known to breed in flowers,15-17 but plant pollination by flies, so-called myophily, was previously observed for only a few drosophilid species.18 Apocynaceae plants are usually myophilic and known to emit odors that mimic fruit substrates.19 Flies are usually attracted by the flower odor, but once on the flower they do not oviposit. According to the ASCLEPOL database (http://www.bio.uni-bayreuth.de/planta2/research/pollina/as_pol_d.htm), some drosophilid species were observed visiting different Apocynaceae hosts and carrying their pollinia on their probosces, but never from a species belonging to the genus Leptadenia. In South Africa, Agnew20 described in detail the association between several drosophilid species (namely D. melanogaster, D. simulans, D. immigrans and Zaprionus vittiger) and Orbea schweinfurthii (A. Berger) Bruyns (Apocynaceae). Bhatnagar21 recorded D. seguyi on Cosmostigma cordatum (Poir.) Almeida in India, and Yamashiro et al.22 found D. melanogaster on Cynanchum japonicum (Morren and Decaisne) Hemsl. and D. immigrans and Scaptodrosophila coracina on V. katoi (Ohwi) Kitag. in Japan.
It is important to note that L. madagascariensis is also found in different localities in Madagascar and Comoros and that most of the drosophilid species observed to carry its pollinia in Juan de Nova also exist in these places. However, no pollinia have ever been reported on drosophilid probosces in these locations and we did not find any pollinia on drosophilid flies during our previous field trips in Madagascar. Two hypotheses may explain the strong association with L. madagascariensis on the island of Juan de Nova: either the plants on this island belong to a certain variety or subspecies that have evolved attractive smells specifically targeting drosophilids, or that at the time of collection (the beginning of the dry season in Juan de Nova4), only L. madagascariensis flowers presented an appropriate sugar resource for drosophilid flies. Future seasonal surveys of drosophilids on Juan de Nova and genetic and biochemical comparisons of L. madagascariensis in different islands of the Western Indian Ocean will certainly help to elucidate the nature of its association with drosophilids.
A last interesting problem is the role of myophily for the reproduction of Leptadenia. Although this problem should deserve further studies, we would like to point out that Leptadenia fructification success is very low: a single plant usually produces very many flowers but very few fruits (our observations). Allogamy and inefficient pollinia transportation by flies may explain the low fructification rate in Leptadenia.
Materials and Methods
Flies were collected either using fermenting banana traps hanged in shadowed shrubs or by net sweeping on moist litter. To make sure that there is no accidental importation of drosophilids, unripe bananas were bought in La Réunion and conserved in a refrigerator on the boat. Banana traps were prepared several days in advance and left fermenting in a closed box. We then made sure that there were no flies in the traps when we set them up in the field. We checked for flies in the traps for the next three days. Sweeping with a net over rotten fruits and litter was also used. Identification was made by AY and JRD according to Lamb,23 Tsacas et al.,24 Tsacas and Chassagnard,9,25 Bächli et al.,10 Cariou et al.2 and Yassin and David.26 Flies were kept alive on standard laboratory medium or killed in absolute alcohol for subsequent analyses.
Yellow bodies carried by flies collected on the island of Juan de Nova were investigated and photographed using a Leica binocular stereoscope. They were carefully separated from fly heads and preserved in absolute ethanol. DNA was extracted using DNeasy Blood and Tissue Kit (QIAGEN) and universal primers (rbcLa_f and rbcLa_rev) of the chloroplast gene rbcl11 were used. PCR amplification conditions were: 5 min at 95°C, followed by 5 cycles of 30 sec at 94°C, 1 min at 55°C and 1 min at 72°C, then 30 cycles of 30 sec at 94°C, 1 min at 54°C and 1 min at 72°C, followed by 10 min at 72°C. Amplicons were sequenced by Beckman-Coulter Genomics and sequence similarity was searched in GenBank using BLAST (blast.ncbi.nlm.nih.gov/Blast.cgi).
Acknowledgments
The Marion Dufresne 2011 expedition was funded by the CNRS-INEE 'Iles Eparses' project to VD. Morphological and molecular taxonomy was funded by an ATIP-AVENIR to VO and IDEV grant to BA. We are also grateful to the Terres Australes et Antarctiques Françaises (TAAF) for the logistic organization of the expedition. Amélie Rast provided valuable assistance in the lab.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/fly/article/21583
References
- 1.Lachaise D, Harry M, Solignac M. Affinités biogéographiques des Drosophilidae de Madagascar et des îles de l'Océan Indien. In: Lourenco WR (ed), Biogéographie de Madagascar, 1996, pp. 467-78, Orstom, Paris. [Google Scholar]
- 2.Cariou ML, Lachaise D, Matyot P, Gerlach J, Montchamp C, Legrand D, et al. Family Drosophilidae. Faunistica. 2009;85:355–85. [Google Scholar]
- 3.Yassin A, David JR. Phylogenetic biogeography of Afrotropical Drosophilidae. In: Gailis M, Kalniņš (eds), Biogeography, 2010, pp. 105-136, Nova Science Publishers, New York. [Google Scholar]
- 4.Caceres S. Etude préalable pour le classement en réserve naturelle des Iles Eparses. Mémoire de DESS Sciences et Gestion de l'Environnement Tropical de l'Université de la Réunion 2003; http://etic.univ-reunion.fr/get/documents/Iles%20Eparses/IlesEparses_SarahCACERES_2002.pdf
- 5.Paulian R. Observations sur la faune terrestre de l'île Tromelin. Le Naturaliste Malgache. 1955;7:1–7. [Google Scholar]
- 6.Paulian R. Les Insectes des Iles Glorieuses. Entomologiste. 1989;45:203–8. [Google Scholar]
- 7.Vieitte P. L'entomofaune de l'île Europa. Editions du Muséum. 1966;91:191–210. [Google Scholar]
- 8.Staub F. Geography and ecology of Tromelin Island. Atoll Res Bull. 1970;136:197–209. [Google Scholar]
- 9.Tsacas L, Chassagnard MT. Les espèces afrotropicales du sous-genre Gitonides Knab du genre Cacoxenus Loew à larves prédatrices de cochenilles (Diptera: Drosophilidae) Ann Soc Entomol Fr. 1999;35:91–121. [Google Scholar]
- 10.Bächli G, Vilela CR, McEvey SF. Nine new species of Afrotropical Leucophenga (Diptera: Drosophilidae) Mitt Schweiz Entomol Ges. 2005;78:23–57. [Google Scholar]
- 11.Kress WJ, Erickson DL. A two-locus global DNA barcode for land plants: the coding rbcL gene complements the non-coding trnH-psbA spacer region. PLoS One. 2007;2:e508. doi: 10.1371/journal.pone.0000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panwar J, Tarafdar JC. Distribution of three endangered medicinal plant species and their colonization with arbuscular mycorrhizal fungi. J Arid Environ. 2006;65:337–50. doi: 10.1016/j.jaridenv.2005.07.008. [DOI] [Google Scholar]
- 13.Perrier de la Bâthie H. Note sur la constitution géologique et la flore des îles Chesterfield, Juan de Nova, Europa et Nosy-Trozona. Bulletin Economique de Madagascar 1921; 170-176. [Google Scholar]
- 14.Bosser J. Note sur la végétation des îles Europa et Juan de Nova. Le Naturaliste Malgache. 1952;4:41–2. [Google Scholar]
- 15.McEvey SF, Aulard S, Ralisoa-Randrianasolo O. An australian drosophilid (Diptera) on Eucalyptus and Eugenis (Myrtaceae) flowers in Madagascar. J Aust Entomol Soc. 1989;28:53–4. doi: 10.1111/j.1440-6055.1989.tb01193.x. [DOI] [Google Scholar]
- 16.Markow TA, O'Grady PM. Reproductive ecology of Drosophila. Funct Ecol. 2008;22:747–59. doi: 10.1111/j.1365-2435.2008.01457.x. [DOI] [Google Scholar]
- 17.David JR, Yassin A, Rasamizafi LA, Raveloson Ravaomanarivo LH, Debat V. Scratching for food: An original feeding behavior in an African flower breeding Drosophila. Fly (Austin) 2011;5:285–90. doi: 10.4161/fly.5.4.18109. [DOI] [PubMed] [Google Scholar]
- 18.Sultana F, Hu YG, Toda MJ, Takenaka K, Yafuso M. Phylogeny and classification of Colocasiomyia (Diptera, Drosophilidae), and its evolution of pollination mutualism with aroid plants. Syst Entomol. 2006;31:684–702. doi: 10.1111/j.1365-3113.2006.00344.x. [DOI] [Google Scholar]
- 19.Ollerton J, Masinde S, Meve U, Picker M, Whittington A. Fly pollination in Ceropegia (Apocynaceae: Asclepiadoideae): biogeographic and phylogenetic perspectives. Ann Bot. 2009;103:1501–14. doi: 10.1093/aob/mcp072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agnew JD. A case of myophily involving Drosophilidae (Diptera) Journal of South African Botany. 1976;42:85–95. [Google Scholar]
- 21.Bhatnagar S. On insect adaptations for pollination in some asclepiads on Central India. In Kapil RP (ed) Pollination Biology – an Analysis 1986, pp. 37-57, Inter-India Publs, New Delhi. [Google Scholar]
- 22.Yamashiro T, Yamashiro A, Yokoyama J, Maki M. Morphological aspects and phylogenetic analyses of pollination systems in the Tylophora-Vincetoxicum complex (Apocynaceae-Asclepiadoideae) in Japan. Biol J Linn Soc Lond. 2008;93:325–41. doi: 10.1111/j.1095-8312.2007.00896.x. [DOI] [Google Scholar]
- 23.Lamb CG. The Percy Sladen Trust expedition to the Indian Ocean in 1905. XV. Diptera: Heteroneuridae, Ortalidae, Trypetidae, Sepsidae, Micropezidae, Drosophilidae, Geomyzidae, Milichidae. Transactions of the Linnean Society of London. 1914;16:307–72. doi: 10.1111/j.1096-3642.1913.tb00152.x. [DOI] [Google Scholar]
- 24.Tsacas L, Chassagnard MT, David JR. Un nouveau groupe d'espèces afrotropicales anthophiles dans le sous-genre Scaptodrosophila du genre Drosophila (Diptera: Drosophilidae) Ann Soc Entomol Fr. 1988;24:181–202. [Google Scholar]
- 25.Tsacas L, Chassagnard MT. Identité de Drosophila brunnea de Meijere et description de nouvelles espèces orientales et africaines à pointe du scutellum blanche (Diptera, Drosophilidae) Bulletin Zoologisch Museum Universiteit van Amsterdam. 1976;5:89–105. [Google Scholar]
- 26.Yassin A, David JR. Revision of the afrotropical species of Zaprionus (Diptera, Drosophilidae), with descriptions of two new species and notes on internal reproductive structures and immature stages. ZooKeys. 2010;51:33–72. doi: 10.3897/zookeys.51.380. [DOI] [PMC free article] [PubMed] [Google Scholar]