Abstract
Background
The mosquito Aedes albopictus is undergoing a worldwide expansion with potential consequences on transmission of various arboviruses. This species has been first detected in Lebanon in 2003.
Methods
We performed a phylogenetic study of Lebanese specimens and assessed their host preference by detecting human, cat, dog and chicken immunoglobulins in mosquito blood-meals. Their capacity to transmit arboviruses was investigated by providing infectious blood-meals using an artificial feeding system followed by detection of viral particles in mosquito saliva.
Results
Our results suggest that Lebanese strains are part of the recent wave of Ae. albopictus expansion and are related to some European, African and North American strains. They exhibited a host preference towards humans and an important capacity to transmit arboviruses. Indeed, we showed that Ae. albopictus was able to transmit chikungunya (CHIKV), dengue (DENV) and West-Nile (WNV) viruses. At day 10 after an infectious blood-meal at a titer of 108 MID50/ml, 30% of mosquitoes delivered an average of 515 ± 781 viral particles of CHIKV in saliva collected using a forced salivation technique and 55% with an average of 245 ± 304 viral particles when infected with WNV. Whereas DENV was not found in saliva at day 10 post-infection (pi), an average of 174 ± 455 viral particles was detected in 38.1% of mosquitoes tested at day 21 after an infectious blood-meal at a higher titer of 109 MID50/ml.
Conclusion
These observations suggest that Ae. albopictus around Beirut is a potential vector of the three tested arboviruses.
Background
Arthropod-borne viruses (arboviruses) are an important cause of human illnesses. They are biologically transmitted to humans mainly by the bite of haematophagous insects, particularly mosquitoes. Arboviruses represent a wide variety of RNA viruses including the families of Flaviviridae and Togaviridae. Their emergence in new areas is correlated with the geographic expansion of vector species, facilitated by increasing trading and touristic activities. Indeed, the recent outbreaks of arboviruses in Southern Europe [1,2] were associated with the introduction of Aedes albopictus in this region. This mosquito species, native to Southeast Asia, has invaded Americas, Africa and Europe during the last 30 years [3]. In Europe, it was recorded for the first time in Albania in 1979 [4], then in Italy [5,6]. It is now present in all European countries around the Mediterranean Sea [7]. Aedes albopictus was also introduced to the Near East. It was first detected in Israel in 2002 [8], then in Lebanon in 2003 and in Syria in 2005 [9]. This area of the Mediterranean basin has experienced in the past several epidemics of arboviral diseases such as dengue [10] and West-Nile fever [11]. The introduction of Ae. albopictus should therefore be considered as a public health threat, especially because this mosquito is considered highly competent in transmitting various arboviruses [12], including chikungunya virus (CHIKV) [13,14], dengue virus (DENV) [15,16] and West-Nile virus (WNV) [17].
Lebanon is currently experiencing important demographic and economical changes with expanding urbanization, mainly around its capital Beirut, providing favourable environment for the proliferation of Ae. albopictus. The establishment of this mosquito, in addition to the important flow of Lebanese expatriates returning to the country during the summer season and of workers coming from Asian and African countries that are endemic for arboviral diseases, increase the risk of local transmission of these arboviruses. Here, we report a study combining a phylogenetic approach, an estimation of host preferences, and an evaluation of vector competence of Lebanese strains of Ae. albopictus for CHIKV, DENV and WNV.
Methods
Collection of specimens
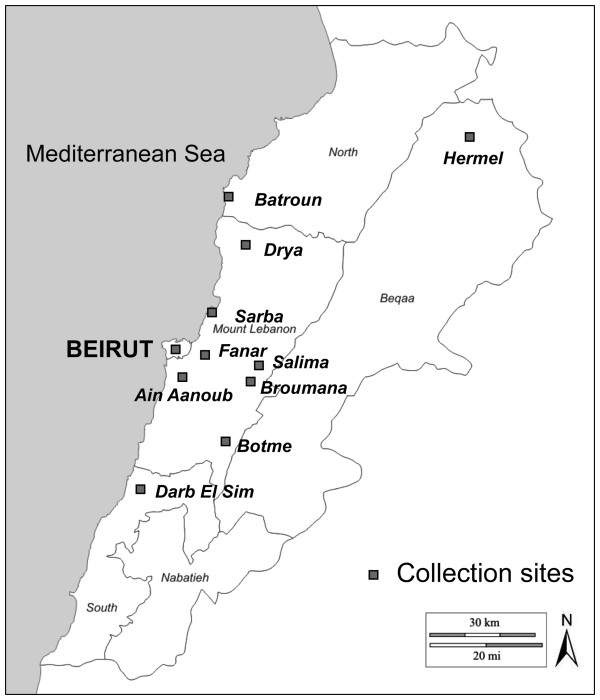
Aedes albopictus specimens were sampled during the months of September and October of 2009 and 2010. Eggs were collected using ovitraps from Sarba, a coastal agglomeration located at 20 km north of Beirut, and from Fanar, an agglomeration at 250 m of altitude on the hills of the Mount Lebanon chain east of Beirut (Figure 1). Wooden strips were removed weekly from ovitraps, then dried for at least 3 days. Eggs were carefully removed and stored in humid chambers before hatching. Resulting F0/F1 adults were used for vector competence studies. Adults were collected by manual aspiration or using BG-sentinel traps or CDC miniature light traps coupled with dry ice as attractant. Traps were left in outdoor microhabitats for 18–24 hours and collected adults were stored at −20°C for further processing (blood-meal identification and phylogenetic analysis).
Figure 1.
Map of Lebanon showing collection localities of Ae. albopictus samples. Specimens included in the phylogenetic study originated from Sarba, Fanar, Mansourieh, Broumana, Ain Anoub and Drya.
Blood-meal identification
Mosquitoes were individually ground in wells of polystyrene plates previously saturated with a blocking buffer (3% fat free milk in PBS). The grinding was performed in 150 μl of PBS containing 3% fat free milk and 0.05% Tween 20, using an electric grinder (Micro Motor Escort®). Aliquots of each mosquito lysate were then stored at −20°C for later use.
To determine the animal species from which blood-meals originated, serum immunoglobulins specific to the most common urban hosts (poultry, dog, cat or human), were detected using a standard sandwich ELISA [18]. Briefly, plates were coated overnight at +4°C with 50 μl of capture antibodies, then saturated with 200 μl PBS containing 3% fat free milk. Mosquito lysates, diluted at 1/100, were added in duplicates and incubated for 1 hour at room temperature, followed by conjugated antibodies. Mosquito lysates and secondary antibodies were diluted in PBS buffer containing 3% fat free milk and 0.05% Tween 20. The immune complex was revealed by adding TMB substrate. The reaction was stopped after 15 min by adding 3 N HCl and the optical density was measured at 450 nm. All incubation steps were separated by 4 washes with PBS containing 0.05% Tween 20. Lysates obtained from males and females artificially fed on blood of the four host species tested were used as negative and positive controls respectively.
Capture antibodies were used at the following optimal concentrations: 4 μg/ml for rabbit anti-chicken IgY and goat anti-human IgG, and 2 μg/ml for rabbit anti-dog IgG and goat anti-cat IgG. Secondary peroxidase-conjugated antibodies were used at the following optimal concentrations: 0.05 μg/ml for goat anti-human IgG and rabbit anti-dog IgG and 0.2 μg/ml for goat anti-cat IgG, and rabbit anti-chicken IgY. All captures and secondary antibodies were affinity-pure and Fc fragment specific (Jackson ImmunoResearch Laboratories, Inc).
DNA extraction and phylogenetic analysis
DNA was extracted from adults using the CTAB protocol [19]. Briefly, each specimen was ground in 200 μl CTAB lysis buffer (2% CTAB, 1.4 M NaCl, 10 mM EDTA, 100 mM Tris pH 8). The homogenate was incubated at 65°C for 5 min, then 200 μl chloroform were added and the mixture was centrifuged at 12,000 rpm for 5 min. The upper phase containing the DNA suspension was transferred into another tube, mixed with 200 μl isopropanol and centrifuged again at 12,000 rpm for 15 min. The DNA pellet was mixed with 200 μl of ethanol 70% and centrifuged at 12,000 rpm for 5 min. The supernatant was discarded and the pellet was dried for 30 min, then resuspended in 20 μl double distilled (dd) H2O. Extracted DNA was used as template for amplification of three mtDNA genes: a 360 bp fragment for cytochrome b (cytb), a 600 bp fragment for cytochrome oxidase I (COI) and a 450 bp fragment for NADH deshydrogenase 5 (ND5). Three sets of primers were used: for cytb, L14841 (5’-AAAAAGCTTCCATCCAACATCTCAGCATGATGAAA-3’) and H15149 (5’-AAACTGCAGCCCCTCAGAATGATATTTGTCCTCA-3’) [20]; for COI, CI-J-1632 (5’-TGATCAAATTTATAAT-3’) and CI-N-2191 (5’-GGTAAAATTAAAATATAAACTTC-3’) [21] and for ND5, ND5FOR (5’-TCCTTAGAATAAAATCCCGC-3’) and ND5REV (5’-GTTTCTGCTTTAGTTCATTCTTC-3’) [22]. Each reaction was performed in a DNA Engine Peltier thermal cycler (Bio-Rad®), in a final volume of 30 μl. The PCR mixture contained 5U Promega Taq polymerase, 3 μl Taq polymerase buffer (10X), 0.5 μl of dNTPs (25 μM), 1 μl of each primer (10 μM), 1 μl of DNA, and 22.5 μl of water-free nuclease. The amplification reaction was performed under the following conditions: denaturation at 95°C for 4 min followed by heating at 95°C for 40 seconds; annealing for 1 min, at 46°C for cytb, 40°C for COI and 44°C for ND5; elongation for 1 min, at 66°C for cytb and COI, and at 70°C for ND5. The mixture was submitted to 35 cycles and to a final extension step of 7 min, at 66°C for cytb and COI and at 70°C for ND5. PCR products were separated by electrophoresis in a 1% agarose gel and purified using the Illustra GFX PCR DNA and gel band purification kit (GE Healthcare®). Genes were sequenced in an automated DNA sequence (ABI PRISM® 3130). Sequences were read using ChromasPro software.
Gene sequences were aligned using ClustalW software. A phylogenetic tree based on combined analysis of the three genes was constructed using Lebanese specimens as well as 13 other Ae. albopictus specimens described in [23]. A Maximum Likelihood tree was built using PHYML [24]. The significance of internal branches was evaluated using 1000 bootstrap replicates. Aedes aegypti from Hawai was used as outgroup.
Vector competence studies
Virus preparation
The three tested viruses were produced on C6/36 cells of Ae. albopictus. The CHIKV strain provided by the French National Reference Center for Arboviruses was isolated in 2005 from a human case in La Reunion; it presents an amino acid substitution (A226V) in the envelope glycoprotein E1 [25]. The DENV-2 strain provided by Prof. Leon Rosen was isolated from a human serum collected in Bangkok (Thailand) in 1974. The WNV strain was isolated from an infected horse in the Camargue (France) in 2000 [26]. Virus titers were calculated by the 50% endpoint method [27] and expressed as mosquito infectious doses (MID50) per ml. The titer of blood-meals was 108 MID50/ml, comparable to viremias estimated in patients [28-30].
Artificial feeding
Mosquitoes were exposed to an infectious blood-meal using an artificial feeding system. Three ml of infectious blood-meal were prepared by mixing 2 ml washed rabbit red blood cells with 1 ml viral suspension and 5 mM ATP solution. One-week-old females were starved 24 hours before feeding, then allowed to feed on the infectious blood-meal through a chicken skin membrane covering the base of a glass feeder. Batches of 60 mosquitoes isolated in plastic boxes were placed under the glass feeders that were maintained at 37°C. Engorged females were selected, maintained in cardboard boxes and supplied with 10% sucrose at 28°C until use.
Indirect immunofluorescence assay (IFA) on head squashes
On day 14 post-infection (pi), virus presence in mosquito heads was investigated by indirect IFA. Heads were separated from the thorax, transferred on a microscope slide, and squashed under another slide. After 20 min fixation in cold acetone and air-drying at room temperature, anti-virus polyclonal antibody and fluorescein-conjugated secondary antibody were sequentially applied on head squashes [31]. Samples were examined under a fluorescence microscope.
qRT-PCR for assessing viral load
At different days pi, total nucleic acids were extracted from individual mosquitoes and from dissected organs (midguts, wings, and salivary glands) and analyzed as follows: material was ground in 350 μl of RA1 solution and RNA was extracted using NucleoSpin® RNA II kit. Total RNA was resuspended in 40 μl dd H2O, then a volume of 1 to 5 μl was used in a one-step RT-PCR reaction performed with a Power SYBR® Green RNA-to-CT™ one step kit (Applied Biosystem). The reaction in a volume of 25 μl contained: 1 to 5 RNA templates, 12.5 μl 2X Power SYBR® Green I RT-PCR Mix, 100–250 nM sense primer, 100–250 nM anti-sense primer, 0.2 μl RT enzyme mix and ddH2O. Primers were selected in an encoding region (Table 1). The PCR program was: 48°C for 30 min, 95°C for 10 min; 40 cycles of 95°C for 15 s, 60°C for 1 min, 72°C for 30 s and 90°C for 15 s. A standard curve was generated using duplicates, from 102 to 108 copies of RNA synthetic transcripts per reaction. Quantification of viral RNA was achieved by comparing the threshold cycle (Ct) values of samples to those of standards, according to the ΔCt analysis.
Table 1.
Primer pairs used to quantify CHIKV, DENV, and WNV by qRT-PCR
| Virus | Viral gene | Sense primer (5’-3’) | Anti-sense primer (5’-3’) | Fragment size (bp) | Reference |
|---|---|---|---|---|---|
| CHIKV |
E2 |
9018 – CAC CGC CGC AAC TAC CG |
9235 - GAT TGG TGA CCG CGG CA |
217 |
[25] |
| DENV-2 |
C |
153 - GAG AAA CCG CGT GTC AAC TG |
266- GGA AAC GAA GGA ATG CCA CC). |
113 |
modified from [32] |
| WNV | C/M | 175 - GTG TTG GCT CTC TTG GCG TT | 279 - AGG TGT TTC ATC GCT GTT TG | 104 | [33] |
Transmission assay
After exposure to an infectious blood-meal, mosquitoes were tested for viral transmission potential at days 10 and 21 pi, by collecting saliva using the forced salivation technique [14]. Briefly, mosquitoes were anesthetized on ice and legs and wings were removed. The proboscis was then inserted into a pipette tip containing 5 μl of fetal bovine serum (FBS). After 45 min, the tip content was transferred in 45 μl of L15 medium.
Plaque assay on C6/36 cells
Saliva suspensions were titrated by focus fluorescent assay on C6/36 cells of Aedes albopictus[34]. Samples were serially diluted and inoculated into C6/36 cells in 96-well plates. After an incubation of 3 days for CHIKV or 5 days for WNV and DENV-2 at 28°C, cells were stained using hyper-immune ascetic fluid specific to each virus as the primary antibody and conjugated goat anti-mouse as the secondary antibody.
Statistical analysis
The Fisher’s exact test was used for comparisons of rates (DIR and TR) and the Kruskall-Wallis test for comparisons of means from the STATA software (StataCorp LP, Texas, USA).
Results
Host preferences
A total of 333 Ae. albopictus specimens were collected, including 136 females. Among those, 32 were obviously engorged (Table 2). The origin of the blood-meal was detected in 71.9% of them and was as follows: 46.8% of Ae. albopictus fed on humans, 15.7% on poultry, 6.3% on cats and 3.1% on dogs, suggesting that Ae. albopictus is preferentially anthropophilic (Fisher’s exact test: p < 0.05). All tested blood-meals came from a single host i.e., no mixed blood-meal was detected.
Table 2.
Origin of Aedes albopictus blood-meals identified by ELISA (sandwich) technique
| Collection method | Number ofAe. albopictus | Number of females | Number of engorged females |
Blood-meal origin |
||||
|---|---|---|---|---|---|---|---|---|
| Human | Cat | Dog | Poultry | Unidentified | ||||
| Manual Aspiration |
100 |
40 |
18 |
12 |
1 |
1 |
1 |
3 |
| CDC trap with CO2 |
53 |
10 |
2 |
0 |
1 |
0 |
1 |
0 |
| BG sentinel |
180 |
86 |
12 |
3 |
0 |
0 |
3 |
6 |
| Total | 333 | 136 | 32 | 15 (46.8%) | 2 (6.3%) | 1 (3.1%) | 5 (15.7%) | 9 (28.1%) |
Phylogenetic analysis
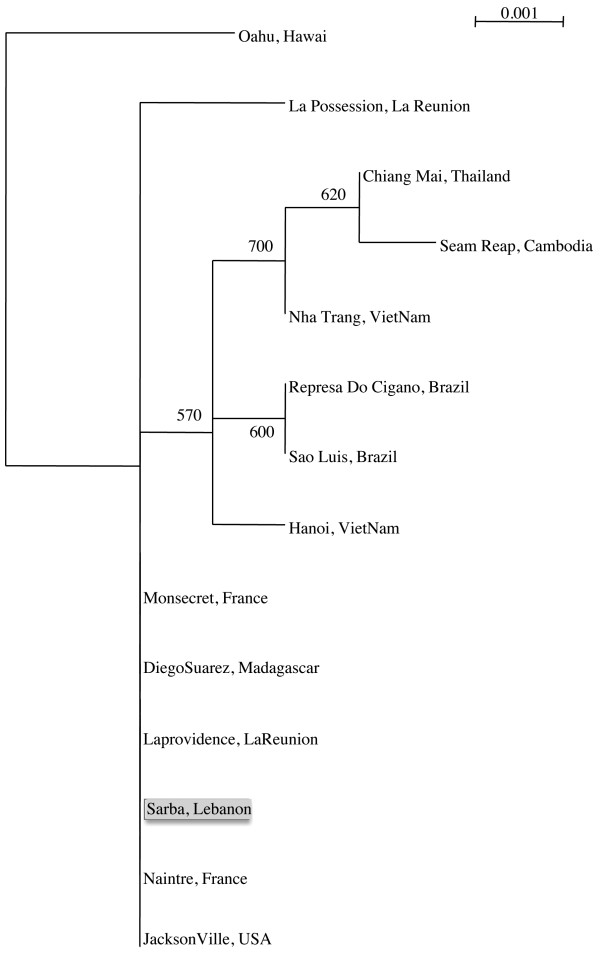
The alignment of the sequences corresponding to the three mitochondrial genes cytb, COI and ND5 from two specimens originating from each of the 6 localities sampled revealed no sequence polymorphism. Consequently, only specimens from one locality, Sarba, were considered for the phylogenetic analysis. The Lebanese Ae. albopictus sequences are identical to sequences obtained from specimens circulating in France, Madagascar and USA and La Providence (La Reunion). They were distantly related to the Asian and Brazilian specimens included in this study, which formed a distinct subgroup on the phylogenetic tree (Figure 2).
Figure 2.
Phylogenetic relationships among Ae. albopictus based on the combined analysis of the three mtDNA genes: cytb, COI and NADH5. The analysis was performed using PhyML (Guindon & Gascuel, 2003). The significance of internal branches was evaluated using 1000 bootstrap replications. The tree includes one Lebanese specimen (Sarba) and 13 others derived from Mousson et al. (2003) which have the following accession numbers for the cytb, COI and ND5 genes: Sarba JX912501, JX912500, JX912502; Represa do Cigano AJ970990, AJ971003, AJ971016; Hanoi AJ970991, AJ971004, AJ971017; Jacksonville AJ970992, AJ971005, AJ971018; Seam Reap AJ970993, AJ971006, AJ971019; Diego Suarez AJ970994, AJ971007, AJ971020; Montsecret AJ970995, AJ971008, AJ971021; Naintre AJ970996, AJ971009, AJ971022; Nha Trang AJ970997, AJ971010, AJ971023; La Possession AJ970999, AJ971012, AJ971025; La Providence AJ971000, AJ971013, AJ971026; Sao Luis AJ971001, AJ971014, AJ971027; Chiang Mai AJ971002, AJ971015, AJ971028 and Oahu AJ970998, AJ971011, AJ971024, used as outgroup.
Vector competence studies
Susceptibility to infection
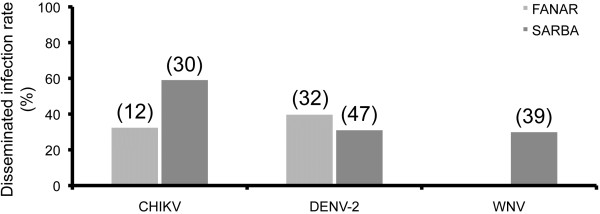
When mosquitoes from Sarba and Fanar were exposed to an infectious blood-meal at a titer of 108 MID50/ml, disseminated infection rates estimated by IFA on head squashes reached 60% for CHIKV and 30-40% for WNV and DENV-2 (Figure 3). When comparing mosquito populations from both localities after their exposure to these viruses, no significant difference in dissemination was detected (Fisher’s exact test: p > 0.05). Therefore, only the population from Sarba locality was considered for further analysis.
Figure 3.
Disseminated infection rates of Ae. albopictus at day 14 post-infection. We exposed two mosquito populations, originating respectively from Fanar and Sarba, to an infectious blood-meal containing CHIKV, DENV-2 or WNV at a titer of 108 MID50/ml. At day 14 pi, the virus was detected on head squashes of surviving females by immuno-fluorescence assay. Disseminated infection rate corresponds to the proportion of females with infected head squashes (i.e. virus has disseminated beyond the midgut) among tested females. In brackets, the number of mosquitoes analyzed.
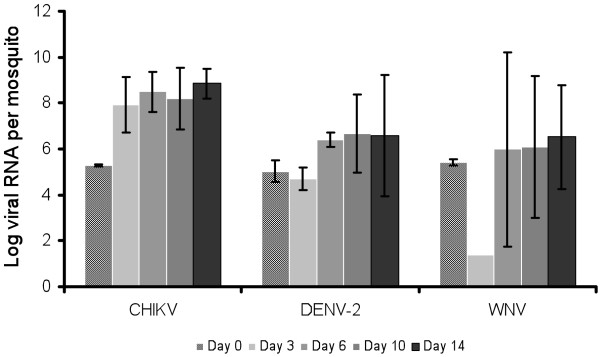
Viral replication
The number of viral RNA copies in mosquitoes was determined at days 0, 3, 6, 10 and 14 after exposure to a blood-meal at 108 MID50/ml using qRT-PCR. The results indicated that this number increased gradually from day 0 to day 14 (Figure 4). Indeed, mosquito specimens ingested 105.3±0.05 CHIKV particles and the maximum of viral replication was reached at day 6 pi with 108.5±0.9 followed by a plateau around 108 viral RNA per mosquito. With DENV-2, mosquitoes ingested 105.0±0.5 particles, and viral replication reached its maximum at day 10 pi attaining 106.7±1.7 copies per mosquito. By contrast, WNV presented a different profile: the ingestion of 105.4±0.1 of viral RNA particles was followed by a drastic decrease to 101.4 and then, an increase to reach a maximum of 106.5±2.3 viral RNA at day 14 pi. Therefore, day 10 after ingestion of the infectious blood-meal was chosen for further analysis, for all three viruses. When considering each virus, no significant difference was detected according to day pi (Kruskall-Wallis test: p > 0.05).
Figure 4.
Viral replication in Ae. albopictus SARBA after infection. We exposed mosquitoes to an infectious blood-meal containing CHIKV, DENV-2 or WNV at a titer of 108 MID50/ml. At days 0, 3, 6, 10 and 14 pi, RNA was extracted from 5 individual mosquitoes and the number of viral RNA copies was assessed by quantitative RT-PCR.
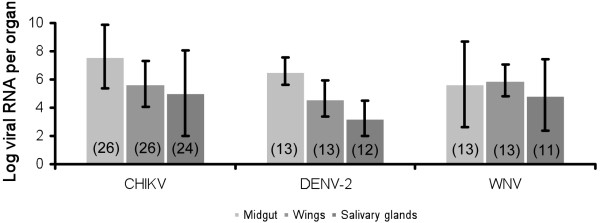
Viral dissemination
Ten days after administration of an infected blood-meal at a titer of 108 MID50/ml, we analyzed the number of viral RNA copies in different mosquito organs, namely the midgut, wings and salivary glands. This allowed us to estimate the virus efficiency in disseminating from the midgut to secondary organs (Figure 5). With CHIKV, the number of viral RNA copies was 107.6±2.2 in the midgut, it decreased in the wings (105.7±0.9) and in the salivary glands (105.0±3.0), indicating a slight limitation of viral dissemination. This was also observed with DENV-2: 106.6±1.6 RNA copies in the midgut, 104.6±1.3 in the wings and 103.3±1.1 in the salivary glands. By contrast, WNV was not limited during its route of dissemination inside the mosquito as all three organs hosted comparable loads of viral RNA: 105.7±3.0 copies in the midgut, 105.9±1.2 in the wings and 104.9±2.5 in the salivary glands. Except for WNV, a significant difference was found between the three organs analyzed (Kruskall-Wallis test: p < 0.05) with the highest viral load detected in midguts.
Figure 5.
Mean number of virus in different organs of Ae. albopictus SARBA at day 10 post-infection. We exposed mosquitoes to an infectious blood-meal with CHIKV, DENV-2 or WNV at a titer of 108 MID50/ml. Mosquitoes were dissected to isolate organs (midgut, wings and salivary glands) for RNA extraction and virus quantification by quantitative RT-PCR. In brackets, the number of mosquitoes analyzed.
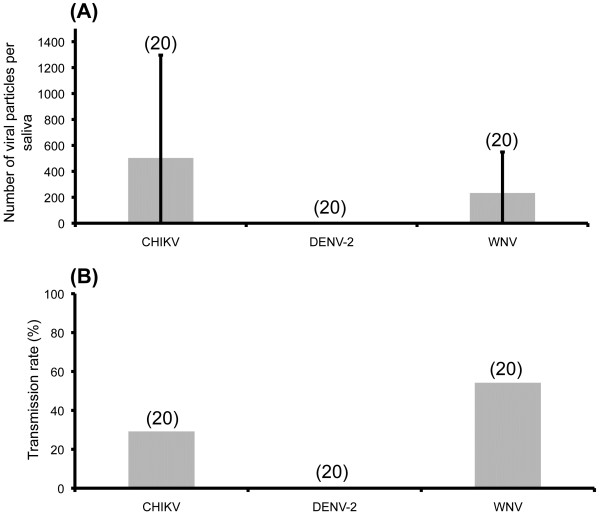
Viral transmission
Ten days after exposure to an infectious blood-meal at 108 MID50/ml, saliva was collected to estimate the potential of mosquitoes to transmit the virus. Results (Figure 6) show that 30% of mosquitoes delivered an average of 515 ± 781 viral particles of CHIKV in saliva, while 55% delivered an average of 245 ± 304 viral particles of WNV. However, no DENV-2 was detected in mosquito saliva at day 10 pi. Therefore, we estimated the number of viral particles in saliva after an infectious blood containing DENV-2 at 109 MID50/ml. In agreement with the previous experiment, no virus was detected in saliva at day 10 pi. However, at day 21 pi, the saliva of 38.1% of the mosquitoes contained DENV-2 viral particles with an average of 174 ± 455 viral load, suggesting that a longer time of incubation at 28°C was needed to lead to an efficient transmission of DENV-2.
Figure 6.
Number of viral particles (A) and transmission rate (B) of Ae. albopictus SARBA at day 10 post-infection. We exposed mosquitoes to an infectious blood-meal containing CHIKV, DENV-2 or WNV at a titer of 108 MID50/ml. At day 10 pi, wings and legs of 20 mosquitoes were removed and the proboscis inserted into a capillary tube filled with L15 medium supplemented with 10% FBS. After 45 min, saliva was collected and titrated by fluorescent foci method on C6/36 cells. Transmission rate corresponds to the number of infected saliva among tested ones. In brackets, the number of mosquitoes analyzed.
Discussion
Our results show that the tested Lebanese specimens of Ae. albopictus are mostly anthropophilic and can efficiently transmit the three arboviruses, CHIKV, DENV and WNV. Based on a phylogenetic analysis, we corroborate that this mosquito is likely to be part of the last worldwide wave of Ae. albopictus expansion.
Ae. albopictus is known to have an opportunistic feeding behaviour among birds, reptiles, amphibians and mammals [35,36]. In our study, this mosquito shows a marked preference for human blood was found by Delatte et al. (2010) [37] and Ponlawat & Harrington (2005) [38]. Such anthropophily was observed in areas where Ae. albopictus was involved as primary vector in the transmission of arboviruses [37,39].
Our findings show an absence of genetic diversity within the tested Ae. albopictus populations when examining the three mitochondrial genes, which suggests that Ae. albopictus has expanded to new geographic areas established from a few founder females [22,40]. Other genetic markers such as microsatellites should be tested [41,42]. This mosquito was observed for the first time in Lebanon in 2003. Our field observations confirm that this invasive species succeeded to establish in the coastal and middle altitude areas of the Mount Lebanon chain (Figure 1) where it represents a source of nuisance because of its aggressive biting behavior. On the other hand, vector competence study shows that under laboratory conditions, the tested Lebanese strains of Ae. albopictus were able to disseminate efficiently the three viruses CHIKV, DENV and WNV. The role of Ae. albopictus as a field vector of WNV seems to be less probable but should not be neglected. Indeed, Ae. albopictus has been found naturally infected with WNV [43], is able to feed on avian hosts [36] and to transmit the virus to horses [44]. Following infection with DENV-2, disseminated infection rates were roughly similar to values found in previous studies, lower than 50% [16,45-47]. However, disseminated infection rates following exposure to CHIKV were lower than those reported from Corsica [47] and North Italy [16], as those ranged from 75% to 100%. When examining the profile of replication (Figure 4), viral RNA loads were found to peak more rapidly for CHIKV (day 6) than for the other two viruses (day 10 for DENV-2 and day 14 for WNV). As in other studies [16,47], the salivary glands of Ae. albopictus can contain around 104 CHIKV at day 10 pi.
To be transmitted, viruses must confront a series of “barriers” in the mosquito, that limit dissemination and/or transmission of the virus. The efficiency of these barriers determines the level of mosquito competence. Barriers include the midgut and the salivary glands (reviewed in [48]). In our study, we found that the salivary glands of most tested mosquitoes were infected with virus (84.6% with CHIKV, 96.4% with DENV-2 and 92.3% with WNV, data not shown) but less than 55% were capable of transmission at day 10 pi (30% with CHIKV, 55% with WNV and 0% with DENV-2). It is worth noting that at this time point, and for equal administered infectious doses, WNV was delivered at a lower number than CHIKV. However, this was compensated by a higher proportion of infected mosquitoes. DENV behaves differently; viral particles were detected in the secreted saliva only at day 21 after ingestion of a blood-meal at a titer 10 times higher (i.e., 109 MID50/mL). These restricted conditions argue for a low vector competence of Ae. albopictus for DENV-2. However, a poorly competent vector may drive intense transmission of arboviruses if other conditions are suitable, such as vector density, daily mosquito survival, biting rate… [49]. Also, the midgut barrier could be overwhelmed by increasing virus titers, whether in artificial blood-meals or when mosquitoes are exposed to humans with high viremia [50,51].
Conclusion
Our study confirms the capacity of Ae. albopictus from Sarba (North of Beirut) to transmit, in laboratory conditions, the three viruses, CHIKV, DENV, and WNV. Considering its high preference for human blood, this mosquito should be considered as a serious public health threat for Lebanon and the region where climatic conditions and demographic dynamics are suitable for an active transmission of arboviruses. These findings are particularly important for the Near-East region; the introduced Ae. albopictus appears to be competent candidate to take over the role of Ae. aegypti that used to be the main vector of DENV before its eradication in the 1950s [10]. Thus, surveillance should be reinforced to allow a rapid implementation of control measures.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
NH carried out collections of mosquitoes, identification of blood-meals and sequencing. LM carried out experimental infections of mosquitoes. MV participated in titration assays. SC helped to draft the manuscript. JT helped to draft the manuscript. MAO participated in sequencing and helped to draft the manuscript. ABF conceived the study and drafted the manuscript. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Nabil Haddad, Email: haddad-N@cyberia.net.lb.
Laurence Mousson, Email: laurence.mousson@pasteur.fr.
Marie Vazeille, Email: marie.vazeille@pasteur.fr.
Soulaima Chamat, Email: schamat@ul.edu.lb.
Joelle Tayeh, Email: joelletayeh@hotmail.com.
Mike Abboud Osta, Email: mo07@aub.edu.lb.
Anna-Bella Failloux, Email: anna-bella.failloux@pasteur.fr.
Acknowledgements
We would like to thank Catherine Dauga for her invaluable contribution in the phylogenetic analysis, Dany Azar for his critical advices, Marc Grandadam for providing reagents for viral detection, and Jean-François Charles for improving figures. LM was supported by the ACIP A-10-2009 (Institut Pasteur) and this work was funded by the CEDRE Program (Cooperation for Evaluation and Development of Research), the Lebanese National Center for Scientific Research (grant number 020409) and the Doctorate School of Science and Technology of the Lebanese University.
References
- Rezza G, Nicoletti L, Angelini R, Romi R, Finarelli AC, Panning M, Cordioli P, Fortuna C, Boros S, Magurano F, Silvi G, Angelini P, Dottori M, Ciufolini MG, Majori GC, Cassone A. CHIKV study group. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007;370:1840–1846. doi: 10.1016/S0140-6736(07)61779-6. [DOI] [PubMed] [Google Scholar]
- Grandadam M, Caro V, Plumet S, Thiberge JM, Souarès Y, Failloux AB, Tolou HJ, Budelot M, Cosserat D, Leparc-Goffart I, Desprès P. Chikungunya virus, southeastern France. Emerg Infect Dis. 2011;17:910–913. doi: 10.3201/eid1705.101873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paupy C, Delatte H, Bagny L, Corbel V, Fontenille D. Aedes albopictus, an arbovirus vector: from the darkness to the light. Microbes Infect. 2009;11:1177–1185. doi: 10.1016/j.micinf.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Adhami J, Reiter P. Introduction and establishment of Aedes (Stegomyia) albopictus skuse (Diptera: Culicidae) in Albania. J Am Mosq Control Assoc. 1998;14:340–343. [PubMed] [Google Scholar]
- Sabatini A, Raineri V, Trovato G, Coluzzi M. Aedes albopictus in Italy and possible diffusion of the species into the Mediterranean area. Parassitologia. 1990;32:301–304. [PubMed] [Google Scholar]
- Dalla Pozza G, Majori G. First record of Aedes albopictus establishment in Italy. J Am Mosq Control Assoc. 1992;8:18–20. [PubMed] [Google Scholar]
- Schaffner F, Van Bortel W. Current status of invasive mosquitoes in Europe. ECDC, VBORNET Newsl. 2010;2:6–8. [Google Scholar]
- Pener H, Wilamowski A, Schnur H, Orshan L, Shalom U, Bear A. Letter to the editors. Eur Mosq Bull. 2003;14:32. [Google Scholar]
- Haddad N, Harbach RE, Chamat S, Bouharoun-Tayoun H. Presence of Aedes albopictus in Lebanon and Syria. J Am Mosq Control Assoc. 2007;23:226–228. doi: 10.2987/8756-971X(2007)23[226:POAAIL]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Saad B. La dengue au Liban. Sem Hop Paris. 1948;78:2496–2498. [Google Scholar]
- Bin H, Grossman Z, Pokamunski S, Malkinson M, Weiss L, Duvdevani P, Banet C, Weisman Y, Annis E, Gandaku D, Yahalom V, Hindyieh M, Shulman L, Mendelson E. West Nile fever in Israel 1999–2000: from geese to humans. Ann N Y Acad Sci. 2001;951:127–142. doi: 10.1111/j.1749-6632.2001.tb02691.x. [DOI] [PubMed] [Google Scholar]
- Gratz NG. Critical review of the vector status of Aedes albopictus. Med Vet Entomol. 2004;18:215–227. doi: 10.1111/j.0269-283X.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- Tesh RB, Gubler DJ, Rosen L. Variation among goegraphic strains of Aedes albopictus in susceptibility to infection with chikungunya virus. AmJTrop Med Hyg. 1976;25:326–335. doi: 10.4269/ajtmh.1976.25.326. [DOI] [PubMed] [Google Scholar]
- Dubrulle M, Mousson L, Moutailler S, Vazeille M, Failloux AB. Chikungunya virus and Aedes mosquitoes: saliva is infectious as soon as two days after oral infection. PLoS One. 2009;4:e5895. doi: 10.1371/journal.pone.0005895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler DJ, Rosen L. A simple technique for demonstrating transmission of dengue virus by mosquitoes without the use of vertebrate hosts. AmJTrop Med Hyg. 1976;25:146–150. doi: 10.4269/ajtmh.1976.25.146. [DOI] [PubMed] [Google Scholar]
- Talbalaghi A, Moutailler S, Vazeille M, Failloux AB. Is Aedes albopictus for Italy competent enough to sustain new arboviral outbreaks? Med Vet Entomol. 2010;24:83–87. doi: 10.1111/j.1365-2915.2009.00853.x. [DOI] [PubMed] [Google Scholar]
- Sardelis MR, Turell MJ, O’Guinn ML, Andre RG, Roberts DR. Vector competence of three North American strains of Aedes albopictus for West Nile virus. J Am Mosq Control Assoc. 2002;18:284–289. [PubMed] [Google Scholar]
- Chow E, Wirtz RA, Scott TW. Identification of blood meals in Aedes aegypti by antibody sandwich enzyme-linked immunosorbent assay. J Am Mosq Control Assoc. 1993;9:196–205. [PubMed] [Google Scholar]
- Rogers SO, Bendich AJ. Extraction of DNA from plant tissues. Boston, MA: Kluwer Academic Publishers; 1988. pp. l–10. (Plant Molecular Biology Manual). [Google Scholar]
- Kocher TD, Thomas WK, Meyer A, Edwards SV, Pääbo S, Villablanca FX, Wilson AC. Dynamics of mitochondrial DNA evolution in animals: amplification and sequencing with conserved primers. Proc Natl Acad Sci USA. 1989;86:6196–6200. doi: 10.1073/pnas.86.16.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambhampati S, Smith PT. PCR primers for the amplification of four insect mitochondrial gene fragments. Insect Mol Biol. 1995;4:233–236. doi: 10.1111/j.1365-2583.1995.tb00028.x. [DOI] [PubMed] [Google Scholar]
- Birungi J, Munstermann LE. Genetic structure of Aedes albopictus (Diptera: Culicidae) populations based on mitochondrial ND5 sequences: evidence for an independent invasion into Brazil and United States. Ann Entomol Soc Am. 2002;95:125–132. doi: 10.1603/0013-8746(2002)095[0125:GSOAAD]2.0.CO;2. [DOI] [Google Scholar]
- Mousson L, Vazeille M, Chawprom S, Prajakwong S, Rodhain F, Failloux AB. Genetic structure of Aedes aegypti populations in Chiang Mai (Thailand) in relation with dengue transmission. Trop Med Int Hlth. 2002;7:865–872. doi: 10.1046/j.1365-3156.2002.00939.x. [DOI] [PubMed] [Google Scholar]
- Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- Schuffenecker I, Iteman I, Michault A, Murri S, Frangeul L, Vaney MC, Lavenir R, Pardigon N, Reynes JM, Pettinelli F, Biscornet L, Diancourt L, Michel S, Duquerroy S, Guigon G, Frenkiel MP, Bréhin AC, Cubito N, Desprès P, Kunst F, Rey FA, Zeller H, Brisse S. Genome microevolution of Chikungunya viruses causing the Indian Ocean outbreak. PLoS Med. 2006;3:e263. doi: 10.1371/journal.pmed.0030263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgue B, Murri S, Zientara S, Durand B, Durand JP, Zeller H. West Nile outbreak in horses in southern France, 2000: the return after 35 years. Emerg Infect Dis. 2001;7:692–696. doi: 10.3201/eid0704.010417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- Lanciotti RS, Kerst AJ, Nasci RS, Godsey MS, Mitchell CJ, Savage HM, Komar N, Panella NA, Allen BC, Volpe KE, Davis BS, Roehrig JT. Rapid detection of west nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol. 2000;38:4066–4071. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parola P, de Lamballerie X, Jourdan J, Rovery C, Vaillant V, Minodier P, Brouqui P, Flahault A, Raoult D, Charrel RN. Novel chikungunya virus variant in travelers returning from Indian Ocean islands. Emerg Infect Dis. 2006;12:1493–1499. doi: 10.3201/eid1210.060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WK, Chen HL, Yang CF, Hsieh SC, Juan CC, Chang SM, Yu CC, Lin LH, Huang JH, King CC. Slower rates of clearance of viral load and virus-containing immune complexes in patients with dengue hemorrhagic fever. Clin Infect Dis. 2006;43:1023–1030. doi: 10.1086/507635. [DOI] [PubMed] [Google Scholar]
- Kuberski TT, Rosen L. A simple technique for the detection of dengue antigen in mosquitoes by immunofluorescence. AmJTrop Med Hyg. 1977;26:533–537. doi: 10.4269/ajtmh.1977.26.533. [DOI] [PubMed] [Google Scholar]
- Lanciotti RS, Calisher CH, Gubler DJ, Chang GJ, Vorndam AV. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol. 1992;30:545–551. doi: 10.1128/jcm.30.3.545-551.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti RS, Roehrig JT, Deubel V, Smith J, Parker M, Steele K, Crise B, Volpe KE, Crabtree MB, Scherret JH, Hall RA, MacKenzie JS, Cropp CB, Panigrahy B, Ostlund E, Schmitt B, Malkinson M, Banet C, Weissman J, Komar N, Savage HM, Stone W, McNamara T, Gubler DJ. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science. 1999;286:2333–2337. doi: 10.1126/science.286.5448.2333. [DOI] [PubMed] [Google Scholar]
- Igarashi A. Isolation of a Singh’s Aedes albopictus cell clone sensitive to dengue and Chikungunya viruses. J Gen Virol. 1978;40:531–544. doi: 10.1099/0022-1317-40-3-531. [DOI] [PubMed] [Google Scholar]
- Savage HM, Niebylski ML, Smith GC, Mitchell CJ, Craig GB Jr. Host-feeding patterns of Aedes albopictus (Diptera: Culicidae) at a temperate North American site. J Med Entomol. 1993;30:27–34. doi: 10.1093/jmedent/30.1.27. [DOI] [PubMed] [Google Scholar]
- Richards SL, Ponnusamy L, Unnasch TR, Hassan HK, Apperson CS. Host-feeding patterns of Aedes albopictus (Diptera: Culicidae) in relation to availability of human and domestic animals in suburban landscapes of Central North Carolina. J Med Entomol. 2006;43:543–551. doi: 10.1603/0022-2585(2006)43[543:HPOAAD]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delatte H, Desvars A, Bouétard A, Bord S, Gimonneau G, Vourc’h G, Fontenille D. Blood-feeding behaviour of Aedes albopictus, a vector of chikungunya on La Réunion. Vector-Borne Zoonotic Dis. 2010;10:249–258. doi: 10.1089/vbz.2009.0026. [DOI] [PubMed] [Google Scholar]
- Ponlawat A, Harrington LC. Blood feeding patterns of Aedes aegypti and Aedes albopictus in Thailand. J Med Entomol. 2005;42:844–849. doi: 10.1603/0022-2585(2005)042[0844:BFPOAA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Valerio L, Marini F, Bongiorno G, Facchinelli L, Pombi M, Caputo B, Maroli M, Della Torre A. Host-Feeding Patterns of Aedes albopictus (Diptera: Culicidae) in Urban and Rural Contexts within Rome Province, Italy. Vector-Borne Zoonotic Dis. 2010;10:291–294. doi: 10.1089/vbz.2009.0007. [DOI] [PubMed] [Google Scholar]
- Delatte H, Bagny L, Brengue C, Bouetard A, Paupy C, Fontenille D. The invaders: phylogeography of dengue and chikungunya viruses Aedes vectors, on the South West islands of the Indian Ocean. Infect Genet Evol. 2011;11:1769–1781. doi: 10.1016/j.meegid.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Porreta D, Gargani M, Bellini R, Calviti M, Urbanelli S. Isolation of microsatellite markers in the tiger mosquito Aedes albopictus (Skuse) Mol Ecol Notes. 2006;6:880–881. doi: 10.1111/j.1471-8286.2006.01384.x. [DOI] [Google Scholar]
- Kamgang B, Brengues C, Fontenille D, Njiokou F, Simard F, Paupy C. Genetic structure of the tiger mosquito, Aedes albopictus, in Cameroon (Central Africa) PLoS One. 2011;6:e20257. doi: 10.1371/journal.pone.0020257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick J, Kyle A, Ferraro W, Delaney RR, Iwaseczko M. Discovery of Aedes albopictus infected with west nile virus in southeastern Pennsylvania. J Am Mosq Control Assoc. 2002;18:131. [PubMed] [Google Scholar]
- Bunning ML, Bowen RA, Cropp CB, Sullivan KG, Davis BS, Komar N, Godsey MS, Baker D, Hettler DL, Holmes DA, Biggerstaff BJ, Mitchell CJ. Experimental infection of horses with West Nile virus. Emerg Infect Dis. 2002;8:380–386. doi: 10.3201/eid0804.010239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazeille-Falcoz M, Mousson L, Rakatoarivony I, Villeret R, Rodhain F, Duchemin JB, Failloux AB. Population genetic structure and vector competence towards dengue 2 virus of Aedes aegypti and Aedes albopictus from Madagascar. AmJTrop Med Hyg. 2001;65:491–497. doi: 10.4269/ajtmh.2001.65.491. [DOI] [PubMed] [Google Scholar]
- Lourenço-de-Oliveira R, Vazeille M, de Bispo Filippis AM, Failloux AB. Large genetic differentiation and low variation in vector competence for dengue and yellow fever viruses of Aedes albopictus from Brazil, the United States, and the Cayman Islands. AmJTrop Med Hyg. 2003;69:105–114. [PubMed] [Google Scholar]
- Moutailler M, Barré H, Vazeille M, Failloux AB. Recently introduced Aedes albopictus in Corsica is competent to Chikungunya virus and in a lesser extent to dengue virus. Trop Med Int Hlth. 2009;14:1105–1109. doi: 10.1111/j.1365-3156.2009.02320.x. [DOI] [PubMed] [Google Scholar]
- Kramer LD, Ebel GD. Dynamics of flavivirus infection in mosquitoes. Adv Virus Res. 2003;60:187–232. doi: 10.1016/s0065-3527(03)60006-0. [DOI] [PubMed] [Google Scholar]
- Turell MJ, O’Guinn ML, Dohm DJ, Jones JW. Vector competence of North American mosquitoes (Diptera: Culicidae) for West Nile virus. J Med Entomol. 2001;38:130–134. doi: 10.1603/0022-2585-38.2.130. [DOI] [PubMed] [Google Scholar]
- Focks DA, Daniels E, Haile DG, Keesling JE. A simulation model of the epidemiology of urban dengue fever: literature analysis, model development, preliminary validation, and samples of simulation results. AmJTrop Med Hyg. 1995;53:489–506. doi: 10.4269/ajtmh.1995.53.489. [DOI] [PubMed] [Google Scholar]
- Woodring JL, Higgs S, Beaty BJ. Natural cycles of vector borne pathogens. CO, USA: Boulder, University Press of Colorado; 1996. pp. 51–72. (Biology of disease vectors). [Google Scholar]