Abstract
Background
While BRCA mutation carriers possess a 20-40% lifetime risk of developing ovarian cancer, knowledge about genetic modifying factors influencing the phenotypic expression remains obscure. We explored the distribution of the MDM2 polymorphisms SNP309T>G and the recently discovered SNP285G>C in Norwegian patients with BRCA related ovarian cancer.
Methods
221 BRCA related ovarian cancer cases (BRCA1; n = 161 and BRCA2; n = 60) were tested for the MDM2 polymorphisms. Results were compared to healthy controls (n = 2,465).
Results
The SNP309G allele was associated with elevated OR for ovarian cancer in BRCA1 mutation carriers (SNP309TG: OR 1.53; CI 1.07-2.19; p = 0.020; SNP309GG: OR 1.92; CI 1.19-3.10; p = 0.009; SNP309TG+GG combined: OR 1.61; CI 1.15-2.27; p = 0.005). In contrast, the SNP285C allele reduced risk of BRCA1 related ovarian cancer in carriers of the SNP309G allele (OR 0.50; CI 0.24-1.04; p = 0.057). Censoring individuals carrying the SNP285C/309G haplotype from the analysis elevated the OR related to the SNP309G allele (OR 1.73; CI 1.23-2.45; p = 0.002). The mean age at disease onset was 3.1 years earlier in carriers of SNP309TG+GG as compared to carriers of SNP309TT (p = 0.068). No such associations were found in BRCA2 related ovarian cancer.
Conclusions
Our results indicate the SNP309G allele to increase and the SNP285C allele to reduce the risk of BRCA1 related ovarian cancer. If confirmed in independent studies, this finding may have implications to counseling and decision-making regarding risk reducing measures in BRCA1 mutation carriers.
Keywords: Ovarian cancer, BRCA, MDM2 SNP285, MDM2 SNP309
Background
The prognosis of ovarian cancer is dismal with a 5 year overall survival of 40-45%, as two thirds are diagnosed with advanced disease, when the success of therapeutic modalities is very limited [1-3]. The average annual incidence rate is 10 per 100,000 women, and 8-13% carries a germline mutation in the BRCA genes [3-7]. Meta-analyses have revealed a wide variance in cumulative ovarian cancer risk for BRCA mutation carriers, on average 40% in BRCA1 versus 11-18% in BRCA2 mutation carriers [1,2]. BRCA1 related ovarian cancer are diagnosed at an earlier age than sporadic ovarian cancer cases, while average age at diagnosis for BRCA2 mutation carriers is similar to sporadic cases [2,3]. This leaves BRCA1 mutation carriers in particular, with a difficult decision regarding risk-reducing salpingo-oophorectomy at young age, with impact on somatic, sexual and mental morbidity [8-12]. Thus, identification of additional predictive factors modifying risk of disease may have implications to genetic counseling and timing of prophylactic surgery.
Among potential risk modifying factors are SNPs altering the function of MDM2, the murine double-minute 2 homolog. MDM2 encodes an ubiquitin protein ligase targeting the tumor protein p53, as well as other proteins involved in cell cycle control like the retinoblastoma-associated protein pRb [13,14]. The BRCA related high grade serous carcinomas are characterized by a high level of genetic instability [15]. Overexpression and/or amplification of MDM2 have also been considered an alternative mechanism of p53 inactivation in cancers with wild-type TP53[16-19].
In 2004, Arnold Levine’s group published a newly discovered polymorphism in the intronic P2 promoter of MDM2, SNP309T>G (rs2279744; NM_002392.3:c.14+309T>G) [20]. The SNP309G allele was shown to enhance MDM2 transcription by extending a Sp1 binding site. Further, they reported SNP309G to be associated with accelerated formation of soft tissue sarcomas in individuals carrying a TP53 germline mutation (Li-Fraumeni syndrome) as well as in sporadic soft tissue sarcomas, lymphomas, colorectal cancer and estrogen receptor-rich breast cancer [20-22]. However, subsequent studies attempting to address the impact of SNP309 status on cancer risk and age at disease onset in various solid malignancies have produced conflicting results [23-27].
Recently, we reported a second polymorphism in the MDM2 P2 promoter, SNP285G>C (rs117039649; NM_002392.3:c.14+285G>C), located 24 bp upstream of SNP309 [28]. Among cancer patients and controls tested (n > 7,000), the SNP285C allele was not detected in any individuals carrying the SNP309TT genotype. Thus, we found the SNP285C variant in complete linkage disequilibrium with the SNP309G allele, creating the distinct SNP285C/309G haplotype (p < 1.0 x 10-10) [28]. SNP285C antagonizes the biological effect of SNP309G by reducing the Sp1 ligand binding and reduces the risk of several cancer types [28,29]. Interestingly, the SNP285C/309G haplotype was detected in West European Caucasians, accounting for 11.7% of the SNP309G alleles, but was absent in a Chinese population. Thus, SNP285C may be a confounding factor providing ethnic disparity in evaluating potential impact of SNP309 on cancer risk [30].
In our recent study, we reported an elevated risk of sporadic ovarian cancer in Caucasians carrying the SNP309G allele [28]. In contrast, we found the SNP285C allele to reduce ovarian cancer risk by 37% in carriers of the SNP285GC/309TG genotype versus carriers of the SNP285GG/309TG genotype [28]. Here we report the distribution of the MDM2 SNP285G>C and SNP309T>G polymorphisms in 221 Norwegian ovarian cancer patients diagnosed with germline mutations in BRCA1 (n = 161) and BRCA2 (n = 60). We compared the distribution of the MDM2 polymorphisms SNP285G>C and SNP309T>G in these patients to a group of 2,465 healthy controls [28].
Results
Effect of MDM2 SNP status on ovarian cancer risk in BRCA mutation carriers
Among the 221 mutation carriers, 161 carried a germline mutation in BRCA1 and 60 in BRCA2. Distribution of the MDM2 promoter SNPs in BRCA carriers and healthy controls are presented in Table 1, together with previously published data of these SNPs in sporadic ovarian cancer patients [28].
Table 1.
Genotypic and allelic frequencies of the MDM2 SNP285 and SNP309 in BRCA related ovarian cancer, sporadic ovarian cancer and healthy controls
| |
SNP285 |
BRCA1 related |
BRCA2 related |
Sporadic disease* |
Healthy controls* |
||||
|---|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | n | (%) | ||
| SNP285 |
GG |
153 |
(95.0) |
58 |
(96.7) |
1260 |
(93.7) |
2 274 |
(92.3) |
| |
GC |
8 |
(5.0) |
2 |
(3.3) |
82 |
(6.1) |
183 |
(7.4) |
| |
CC |
0 |
(0.0) |
0 |
(0.0) |
3 |
(0.2) |
8 |
(0.3) |
| |
Total |
161 |
(100) |
60 |
(100) |
1 345 |
(100) |
2 465 |
(100) |
| |
G |
314 |
(97.5) |
118 |
(98.3) |
2 602 |
(96.7) |
4 731 |
(96.0) |
| |
C ** |
8 |
(2.5) |
2 |
(1.7) |
88 |
(3.3) |
199 |
(4.0) |
| SNP309 |
TT |
52 |
(32.3) |
22 |
(36.7) |
515 |
(38.3) |
1 072 |
(43.5) |
| |
TG |
81 |
(50.3) |
32 |
(53.3) |
661 |
(49.1) |
1 093 |
(44.3) |
| |
GG |
28 |
(17.4) |
6 |
(10.0) |
169 |
(12.6) |
300 |
(12.2) |
| |
Total |
161 |
(100) |
60 |
(100) |
1 345 |
(100) |
2 465 |
(100) |
| |
T |
185 |
(57.5) |
76 |
(63.3) |
1 691 |
(62.9) |
3 237 |
(65.7) |
| G ** | 137 | (42.5) | 44 | (36.7) | 999 | (37.1) | 1 693 | (34.3) | |
* Previously published data [28].
** Minor allele.
For SNP309, we found the frequency of the minor allele, SNP309G, to be significantly higher in BRCA1 mutation carriers (42.5%) than in healthy controls (34.3%; p = 0.003). Notably, censoring individuals harboring the SNP285C allele (previously shown to counteract the effect of SNP309G) strengthened this association (p < 0.001). No such difference was observed for BRCA2 mutation carriers (p > 0.5).
In BRCA1 mutation carriers we found an elevated OR for ovarian cancer related to the SNP309TG and SNP309GG genotypes (SNP309TG; OR 1.53; CI 1.07-2.19; p = 0.020; SNP309GG; OR 1.92; CI 1.19-3.10; p = 0.009; SNP309TG + GG combined: OR 1.61; CI 1.15-2.27; p = 0.005) (Figure 1). Censoring individuals harboring the SNP285C allele strengthened the association between SNP309G status and increased ovarian cancer risk (SNP309TG: 1.62; CI 1.12-2.33; p = 0.010; SNP309GG: OR 2.18; CI 1.33-3.56; p = 0.003; SNP309TG + GG combined: OR 1.73; CI 1.23-2.45; p = 0.002).
Figure 1.
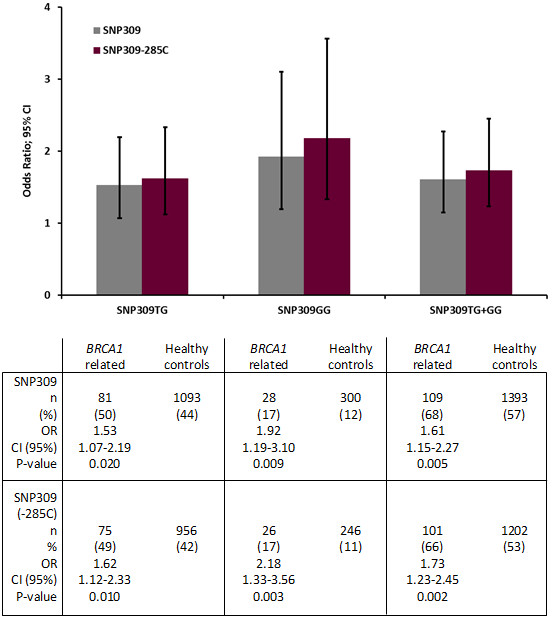
Impact of the MDM2 SNP309G and SNP285C on BRCA1 related ovarian cancer versus healthy controls. Bars indicate odds ratio (OR) for ovarian cancer depending on MDM2 SNP status, error bars indicate 95% CI and p-values are calculated by Fisher exact test.
Regarding SNP285 status, the SNP285C/309G haplotype was detected in 8 out of 161 (5%) individuals carrying a BRCA1 mutation and 2 out of 60 (3.3%) with a BRCA2 mutation (Table 1). Although numbers are small, the frequency of the SNP285C/309G haplotype among carriers of the SNP309G was lower in BRCA1 mutation carriers as compared to the frequency in healthy individuals (OR 0.50; CI 0.24-1.04; p = 0.057). The minor allele frequency in this subgroup being 3.7% for BRCA1 mutation carriers and 7.1% for controls (p = 0.051).
Effect of MDM2 SNP status on age at ovarian cancer onset
We further examined the effect of MDM2 SNP status with respect to age at onset of ovarian cancer in BRCA mutation carriers. The average age at diagnosis in patients harboring the SNP309 genotypes TT, TG and GG were 54.2, 51.2 and 51.8 years, respectively. When censoring patients harboring the SNP285C/309G haplotype, the mean age at onset for the TT, TG and GG genotypes were 54.2, 51.1 and 51.0 years, respectively. Thus, carriers of the SNP309TG and GG genotypes were 3.1 years younger as compared to carriers of the SNP309TT genotype (p = 0.068; Figure 2). The mean age at onset in BRCA2 mutation carriers did not differ from sporadic ovarian cancer cases (59.6 versus 61.2 years), and we observed no differences in age at onset related to SNP309 status.
Figure 2.
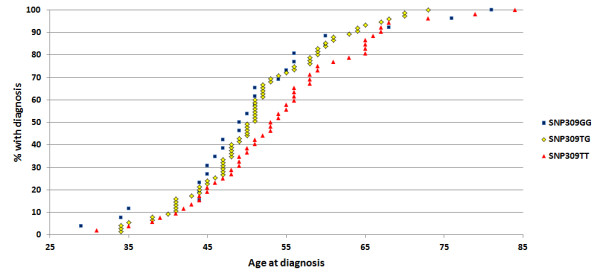
Impact of the MDM2 SNP309 genotypes on age at ovarian cancer diagnosis in BRCA1 mutation carriers. The cumulative percentage of individuals with the SNP309TT genotype (red triangles), the SNP309TG genotype (yellow diamonds) and the SNP309GG genotype (blue squares) plotted against age at ovarian cancer diagnosis. The SNP285C/309G haplotype is censored in the dataset.
Discussion
Currently, little is known about factors acting as genetic modifiers to ovarian cancer tumorigenesis. While several studies have explored the effect of genetic variants on cancer risk in BRCA mutation carriers [31-33], no distinct genetic variant that strongly predicts cancer risk in these subgroups have been identified.
Studies addressing the impact of the MDM2 SNP309 status on risk of tumor development and age at onset in different types of cancers have provided conflicting results, with a trend for positive associations in Asians but a lack of correlation in Caucasians [23,25-27]. While the MDM2 SNP309G is an ancient polymorphism detected in all ethnic groups examined so far, its frequency display distinct ethnic variation [26], accounting for 12.4% of the alleles in African Americans [32] contrasting about 34% in Caucasians [28,34] and 50-55% in Asians [30-37]. In contrast, SNP285C is a more recent polymorphism arising on the SNP309G allele and with a distribution so far restricted to Caucasian populations [28,30]. Previously, we found the SNP285C/309G haplotype to account for 11.7% of all the SNP309G alleles in Western Europeans (British, Dutch and Norwegian populations) but in less than 2% of Finns and absent in healthy Chinese individuals [28]. In general, this distribution has been confirmed by The 1000 Genomes Project Consortium [38]. Thus, we hypothesize that the occurrence of the SNP285C/309G haplotype may have contributed to the conflicting results from studies addressing the impact of SNP309 status on ovarian cancer risk in Caucasian populations.
Only a few studies have addressed the risk of ovarian cancer with respect to SNP309 status. Three studies (two Caucasian populations and one Japanese) reported no association between SNP309G status and elevated risk of ovarian cancer [37,39,40]. In contrast, the SNP309G allele was found to be a potential protective factor, associated with a reduced risk of ovarian cancer, in a Chinese population [36]. However, these studies included a limited numbers of patients (302 or less) without defined BRCA mutation status. In our previous study on Caucasians evaluating nearly 2,000 sporadic ovarian cancer cases and > 3,500 healthy controls, we found an elevated risk of ovarian cancer in carriers of the SNP309TG and SNP309GG genotypes [28]. However, we found a reduced cancer risk in carriers of the SNP309TG genotype harboring the SNP285C/309G haplotype.
To the best of our knowledge, only two studies have addressed the impact of SNP309 on ovarian cancer risk in BRCA mutation carriers. Yarden et al. [41] found the SNP309GG genotype to be significantly associated with risk of BRCA1 related ovarian cancer in Ashkenazi Jews diagnosed before 51 years of age, while Copson et al. [42] detected a non-significant trend for increased incidence of ovarian cancer in British BRCA1 mutation carriers harboring the SNP309GG genotype. While the distribution of the SNP285C allele in Ashkenazi Jews is unknown, it occurs in the British population [28], thus, it may have influenced the result reported by Copson et al. [42], partly masking an effect of the SNP309G status on ovarian cancer risk in this population.
Here, we report an elevated OR for ovarian cancer in BRCA1 mutation carriers harboring a MDM2 SNP309TG or SNP309GG genotype. While the exact OR values presented should be interpreted carefully due to a limited number of observations, the results indicate that SNP309 status may influence ovarian cancer risk with OR to a similar extent in BRCA1 mutation carriers as observed in sporadic ovarian cancer [28].
While the SNP285C/309G haplotype was observed in 13.1% of the SNP309G allele carriers in healthy controls [28], the percentage in BRCA1 mutation carriers was only 7.3% (OR 0.50; CI 0.24-1.04). The lower incidence of the SNP285C/309G haplotype found in BRCA1 mutation carriers with ovarian cancer may indicate a risk reducing effect of the SNP285C allele. However, this finding needs confirmation by independent studies.
A key feature in BRCA related breast and ovarian cancer is earlier age at onset as compared to sporadic disease, with the most distinct differences related to BRCA1 mutations. When censoring patients harboring the SNP285C/309G haplotype from our analyses, we found BRCA1 related cancer cases carrying the SNP309TG and SNP309GG genotypes to be on average 3.1 year younger at time of diagnoses as compared to carriers of the SNP309TT genotype. Although this difference was of borderline statistical significance, it supports the finding that the SNP309G allele promotes ovarian tumorigenesis in BRCA1 mutation carriers. If confirmed in other studies, this information may be useful for timing of risk reducing surgery in healthy BRCA1 mutation carriers, as the age at onset in index cancer cases has been found to predict individual risk [1].
Conclusions
In conclusion, we found the MDM2 SNP309 to increase and SNP285C to reduce the risk of ovarian cancer in BRCA1 related ovarian cancer. These findings may have potential implications to genetic counseling and prophylactic strategies in BRCA1 mutation carriers.
Methods
Ovarian cancer patients
In Norway, ovarian cancer treatment is centralized, and The Norwegian Radium Hospital, covers 50% of the population. A total of 221 BRCA mutation carriers were identified among 1,566 patients diagnosed and treated for invasive epithelial ovarian cancer; 161 carrying a mutation in BRCA1 and 60 in BRCA2. All patients were offered counseling and genetic testing irrespective of family history of cancer, and participants signed a written informed consent before testing. Pathology reports were reviewed in all cases. Family members of mutation carriers were offered genetic counseling, testing and medical follow-up.
Controls
The 1,345 sporadic ovarian cancer patients (mutation carriers excluded) and the 2,465 healthy controls used for comparisons are previously described [28].
BRCA mutation analysis
All included patients were subjected to genetic testing in the BRCA genes. Lymphocyte DNA was prepared from peripheral blood by standard procedures (MagAttract DNA Blood M48 Kit, Qiagen), amplified by polymerase chain reaction (HotMaster Taq DNA Polymerase, 5 PRIME) and subjected to subsequent analysis. Mutation analysis was performed with the application of different screening techniques (fragment length analysis with fluorescent primers, MLPA analysis (SALSA MLPA kits, MRC Holland) and direct sequencing (BigDye Terminator Cycling Sequencing Kit, Applied Biosystems) to detect genetic variations in the BRCA genes. All detected mutations were confirmed by independent DNA extractions, PCRs and sequencing reactions.
MDM2 promoter genotyping
A region of the MDM2 P2 promoter covering SNP285 and SNP309 was amplified by PCR and sequenced as previously described [28].
Statistical analysis
Statistical analyses were performed using the SPSS software package (version 15.0.1). Differences regarding SNP frequency were evaluated by Chi square and Fisher exact tests, and MDM2 SNP distribution among ovarian cancer patients and healthy controls presented as odds ratio (OR). Differences in age at onset were evaluated by Kruskal-Wallis and Mann–Whitney rank tests. All p-values are given as two-sided and confidence intervals (CI) for odds ratios are given as 95%.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MB performed the genetic testing in the BRCA genes and parts of the MDM2 SNP testing, audited the clinical data together with AD and wrote the paper; SK performed the majority of the MDM2 SNP testing, performed the statistical analysis and co-authored the paper; PEL supervised data analysis and co-authored the paper; AD audited the clinical data and co-authored the paper. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Merete Bjørnslett, Email: meretebj@ulrik.uio.no.
Stian Knappskog, Email: stian.knappskog@med.uib.no.
Per Eystein Lønning, Email: per.lonning@helse-bergen.no.
Anne Dørum, Email: anne.dorum@medisin.uio.no.
Acknowledgements
We are grateful to MSc Britt Dahl at Oslo University Hospital Norwegian Radium Hospital for excellent technical assistance. This work was supported by grants from the Norwegian Cancer Society, the Norwegian Research Council, the Western Norway Regional Health Authorities and the Inger and John Fredriksen Foundation for Ovarian Cancer Research.
References
- Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL. et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25:1329–1333. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Registry of Norway. Cancer in Norway 2008 – Cancer incidence, mortality, survival and prevalence in Norway. Cancer Registry of Norway. 2009.
- Pal T, Permuth-Wey J, Betts JA, Krischer JP, Fiorica J, Arango H. et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104:2807–2816. doi: 10.1002/cncr.21536. [DOI] [PubMed] [Google Scholar]
- Risch HA, McLaughlin JR, Cole DE, Rosen B, Bradley L, Kwan E. et al. Prevalence and penetrance of germline BRCA1 and BRCA2 mutations in a population series of 649 women with ovarian cancer. Am J Hum Genet. 2001;68:700–710. doi: 10.1086/318787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ACOG. Hereditary breast and ovarian cancer syndrome. Gynecol Oncol. 2009;113:6–11. doi: 10.1016/j.ygyno.2009.02.017. [DOI] [PubMed] [Google Scholar]
- King MC, Marks JH, Mandell JB. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643–646. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- Michelsen TM, Pripp AH, Tonstad S, Trope CG, Dorum A. Metabolic syndrome after risk-reducing salpingo-oophorectomy in women at high risk for hereditary breast ovarian cancer: a controlled observational study. Eur J Cancer. 2009;45:82–89. doi: 10.1016/j.ejca.2008.09.028. [DOI] [PubMed] [Google Scholar]
- Laughlin GA, Barrett-Connor E, Kritz-Silverstein D, von MD. Hysterectomy, oophorectomy, and endogenous sex hormone levels in older women: the Rancho Bernardo Study. J Clin Endocrinol Metab. 2000;85:645–651. doi: 10.1210/jc.85.2.645. [DOI] [PubMed] [Google Scholar]
- Atsma F, Bartelink ML, Grobbee DE, van der Schouw YT. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause. 2006;13:265–279. doi: 10.1097/01.gme.0000218683.97338.ea. [DOI] [PubMed] [Google Scholar]
- Rocca WA, Grossardt BR, de AM, Malkasian GD, Melton LJ III. Survival patterns after oophorectomy in premenopausal women: a population-based cohort study. Lancet Oncol. 2006;7:821–828. doi: 10.1016/S1470-2045(06)70869-5. [DOI] [PubMed] [Google Scholar]
- Gallagher JC. Effect of early menopause on bone mineral density and fractures. Menopause. 2007;14:567–571. doi: 10.1097/gme.0b013e31804c793d. [DOI] [PubMed] [Google Scholar]
- Chen J, Wu X, Lin J, Levine AJ. mdm-2 inhibits the G1 arrest and apoptosis functions of the p53 tumor suppressor protein. Mol Cell Biol. 1996;16:2445–2452. doi: 10.1128/mcb.16.5.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- Kurman RJ, Visvanathan K, Roden R, Wu TC, Shih I. Early detection and treatment of ovarian cancer: shifting from early stage to minimal volume of disease based on a new model of carcinogenesis. Am J Obstet Gynecol. 2008;198:351–356. doi: 10.1016/j.ajog.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach FS, Tokino T, Meltzer P, Burrell M, Oliner JD, Smith S. et al. p53 Mutation and MDM2 amplification in human soft tissue sarcomas. Cancer Res. 1993;53:2231–2234. [PubMed] [Google Scholar]
- Reifenberger G, Liu L, Ichimura K, Schmidt EE, Collins VP. Amplification and overexpression of the MDM2 gene in a subset of human malignant gliomas without p53 mutations. Cancer Res. 1993;53:2736–2739. [PubMed] [Google Scholar]
- Bartel F, Meye A, Wurl P, Kappler M, Bache M, Lautenschlager C. et al. Amplification of the MDM2 gene, but not expression of splice variants of MDM2 MRNA, is associated with prognosis in soft tissue sarcoma. Int J Cancer. 2001;95:168–175. doi: 10.1002/1097-0215(20010520)95:3<168::AID-IJC1029>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Biernat W, Kleihues P, Yonekawa Y, Ohgaki H. Amplification and overexpression of MDM2 in primary (de novo) glioblastomas. J Neuropathol Exp Neurol. 1997;56:180–185. doi: 10.1097/00005072-199702000-00009. [DOI] [PubMed] [Google Scholar]
- Bond GL, Hu W, Bond EE, Robins H, Lutzker SG, Arva NC. et al. A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell. 2004;119:591–602. doi: 10.1016/j.cell.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Bond GL, Hirshfield KM, Kirchhoff T, Alexe G, Bond EE, Robins H. et al. MDM2 SNP309 accelerates tumor formation in a gender-specific and hormone-dependent manner. Cancer Res. 2006;66:5104–5110. doi: 10.1158/0008-5472.CAN-06-0180. [DOI] [PubMed] [Google Scholar]
- Bond GL, Menin C, Bertorelle R, Alhopuro P, Aaltonen LA, Levine AJ. MDM2 SNP309 accelerates colorectal tumour formation in women. J Med Genet. 2006;43:950–952. doi: 10.1136/jmg.2006.043539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MK, Reincke S, Broeks A, Braaf LM, Hogervorst FB, Tollenaar RA. et al. Do MDM2 SNP309 and TP53 R72P interact in breast cancer susceptibility? A large pooled series from the breast cancer association consortium. Cancer Res. 2007;67:9584–9590. doi: 10.1158/0008-5472.CAN-07-0738. [DOI] [PubMed] [Google Scholar]
- Wilkening S, Bermejo JL, Hemminki K. MDM2 SNP309 and cancer risk: a combined analysis. Carcinogenesis. 2007;28:2262–2267. doi: 10.1093/carcin/bgm191. [DOI] [PubMed] [Google Scholar]
- Hu Z, Jin G, Wang L, Chen F, Wang X, Shen H. MDM2 promoter polymorphism SNP309 contributes to tumor susceptibility: evidence from 21 case–control studies. Cancer Epidemiol Biomarkers Prev. 2007;16:2717–2723. doi: 10.1158/1055-9965.EPI-07-0634. [DOI] [PubMed] [Google Scholar]
- Economopoulos KP, Sergentanis TN. Differential effects of MDM2 SNP309 polymorphism on breast cancer risk along with race: a meta-analysis. Breast Cancer Res Treat. 2009;120:211–216. doi: 10.1007/s10549-009-0467-1. [DOI] [PubMed] [Google Scholar]
- Gui XH, Qiu LX, Zhang HF, Zhang DP, Zhong WZ, Li J. et al. MDM2 309 T/G polymorphism is associated with lung cancer risk among Asians. Eur J Cancer. 2009;45:2023–2026. doi: 10.1016/j.ejca.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Knappskog S, Bjornslett M, Myklebust LM, Huijts PE, Vreeswijk MP, Edvardsen H. et al. The MDM2 Promoter SNP285C/309G Haplotype Diminishes Sp1 Transcription Factor Binding and Reduces Risk for Breast and Ovarian Cancer in Caucasians. Cancer Cell. 2011;19:273–282. doi: 10.1016/j.ccr.2010.12.019. [DOI] [PubMed] [Google Scholar]
- Knappskog S, Trovik J, Marcickiewicz J, Tingulstad S, Staff AC, Romundstad P. et al. SNP285C modulates oestrogen receptor/Sp1 binding to the MDM2 promoter and reduces the risk of endometrial but not prostatic cancer. Eur J Cancer. 2012;48:1988–1996. doi: 10.1016/j.ejca.2011.10.024. [DOI] [PubMed] [Google Scholar]
- Knappskog S, Lonning PE. MDM2 promoter SNP285 and SNP309; phylogeny and impact on cancer risk. Oncotarget. 2011;2:251–258. doi: 10.18632/oncotarget.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebbeck TR, Mitra N, Domchek SM, Wan F, Chuai S, Friebel TM. et al. Modification of ovarian cancer risk by BRCA1/2-interacting genes in a multicenter cohort of BRCA1/2 mutation carriers. Cancer Res. 2009;69:5801–5810. doi: 10.1158/0008-5472.CAN-09-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel C, Versmold B, Wappenschmidt B, Simard J, Easton DF, Peock S. et al. Association of the variants CASP8 D302H and CASP10 V410I with breast and ovarian cancer risk in BRCA1 and BRCA2 mutation carriers. Cancer Epidemiol Biomarkers Prev. 2010;19:2859–2868. doi: 10.1158/1055-9965.EPI-10-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramus SJ, Kartsonaki C, Gayther SA, Pharoah PD, Sinilnikova OM, Beesley J. et al. Genetic variation at 9p22.2 and ovarian cancer risk for BRCA1 and BRCA2 mutation carriers. J Natl Cancer Inst. 2011;103:105–116. doi: 10.1093/jnci/djq494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersma BJ, Howe TM, Goodman JE, Yfantis HG, Lee DH, Chanock SJ. et al. Association of breast cancer outcome with status of p53 and MDM2 SNP309. J Natl Cancer Inst. 2006;98:911–919. doi: 10.1093/jnci/djj245. [DOI] [PubMed] [Google Scholar]
- Ma H, Hu Z, Zhai X, Wang S, Wang X, Qin J. et al. Polymorphisms in the MDM2 promoter and risk of breast cancer: a case–control analysis in a Chinese population. Cancer Lett. 2006;240:261–267. doi: 10.1016/j.canlet.2005.09.019. [DOI] [PubMed] [Google Scholar]
- Kang S, Wang DJ, Li WS, Wang N, Zhou RM, Sun DL. et al. Association of p73 and MDM2 polymorphisms with the risk of epithelial ovarian cancer in Chinese women. Int J Gynecol Cancer. 2009;19:572–577. doi: 10.1111/IGC.0b013e3181a130ab. [DOI] [PubMed] [Google Scholar]
- Ueda M, Yamamoto M, Nunobiki O, Toji E, Sato N, Izuma S. et al. Murine double-minute 2 homolog single nucleotide polymorphism 309 and the risk of gynecologic cancer. Hum Cell. 2009;22:49–54. doi: 10.1111/j.1749-0774.2009.00068.x. [DOI] [PubMed] [Google Scholar]
- 1000 Genomes Project Consortium. A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–1073. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IG, Eccles DM, Choong DY. No association of the MDM2 SNP309 polymorphism with risk of breast or ovarian cancer. Cancer Lett. 2006;240:195–197. doi: 10.1016/j.canlet.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Galic V, Willner J, Wollan M, Garg R, Garcia R, Goff BA. et al. Common polymorphisms in TP53 and MDM2 and the relationship to TP53 mutations and clinical outcomes in women with ovarian and peritoneal carcinomas. Genes Chromosomes Cancer. 2007;46:239–247. doi: 10.1002/gcc.20407. [DOI] [PubMed] [Google Scholar]
- Yarden RI, Friedman E, Metsuyanim S, Olender T, Ben-Asher E, Papa MZ. MDM2 SNP309 accelerates breast and ovarian carcinogenesis in BRCA1 and BRCA2 carriers of Jewish-Ashkenazi descent. Breast Cancer Res Treat. 2008;111:497–504. doi: 10.1007/s10549-007-9797-z. [DOI] [PubMed] [Google Scholar]
- Copson ER, White HE, Blaydes JP, Robinson DO, Johnson PW, Eccles DM. Influence of the MDM2 single nucleotide polymorphism SNP309 on tumour development in BRCA1 mutation carriers. BMC Cancer. 2006;6:80. doi: 10.1186/1471-2407-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]


