Abstract
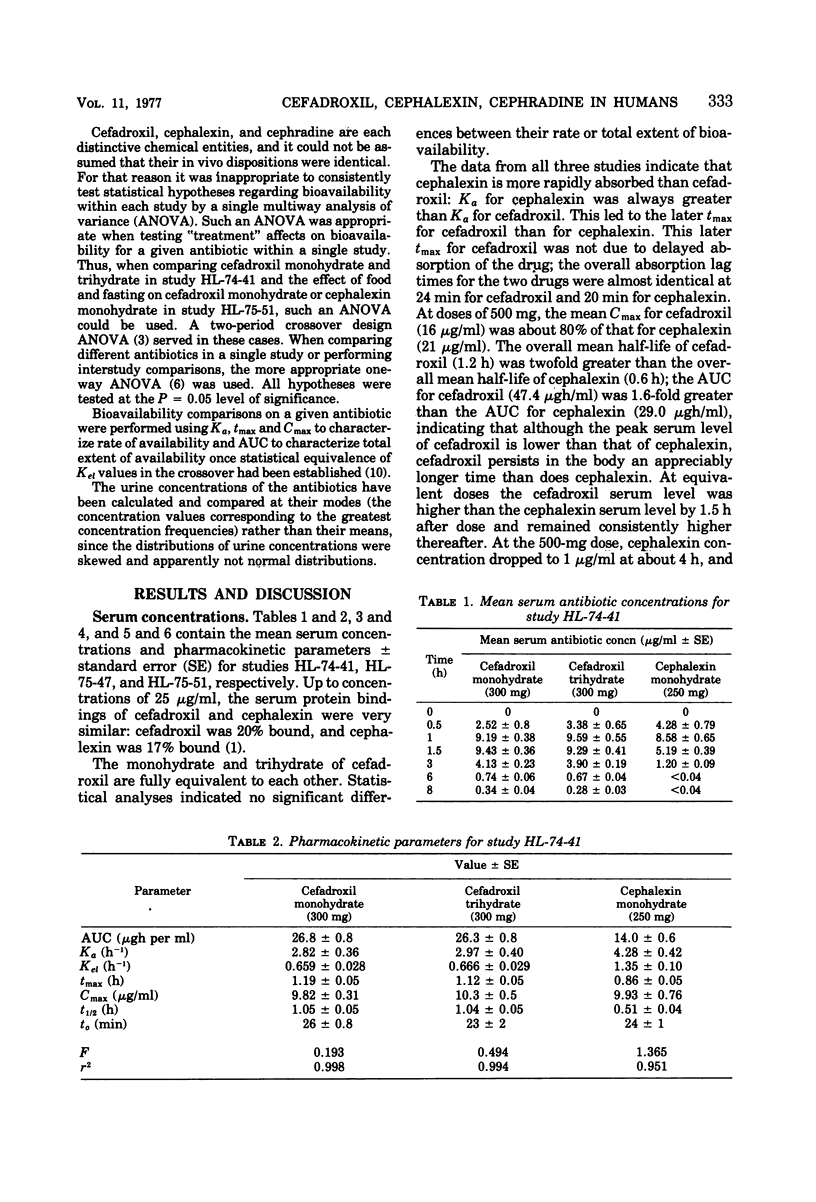
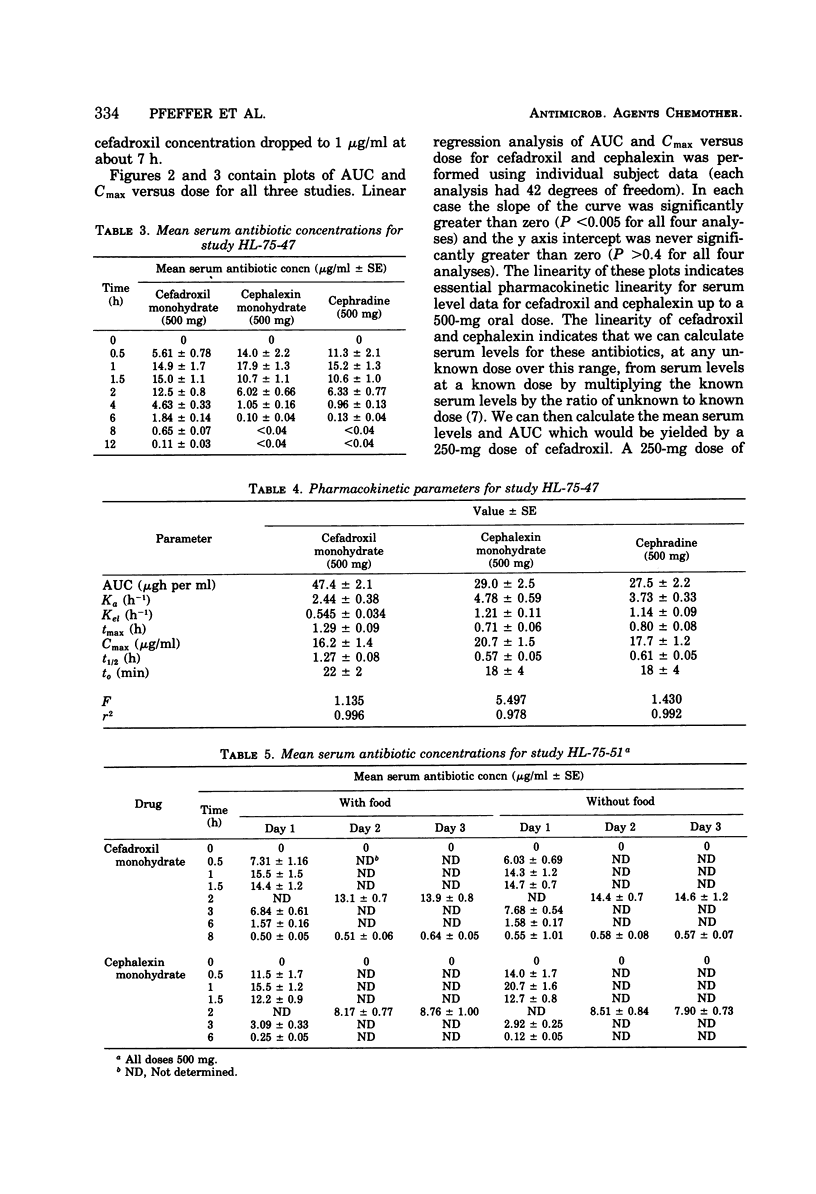
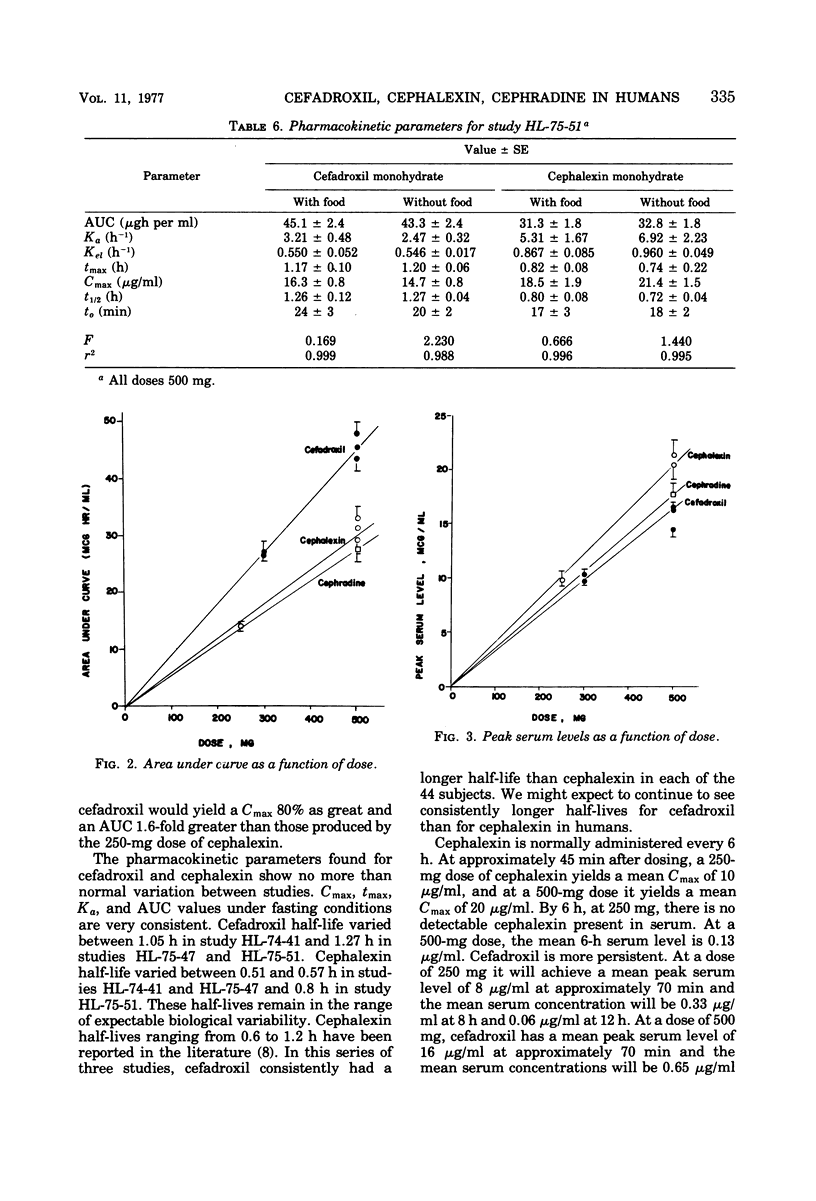
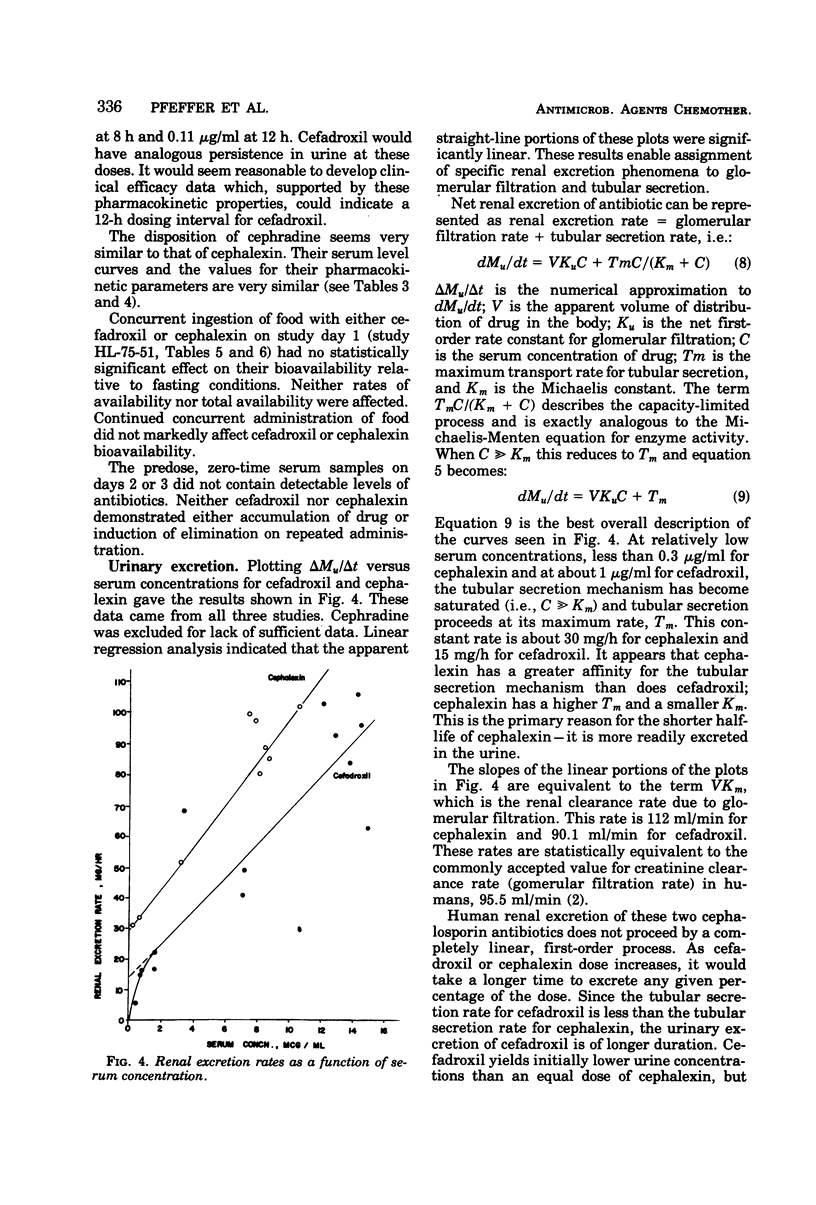
At equivalent oral doses, cefadroxil has a longer serum half-life, slower urinary excretion rate, greater area under the serum level versus time curve than cephalexin or cephradine, and peak serum concentrations that are 75 to 80% those of cephalexin. The calculated, apparent in vivo volume of distribution of cefadroxil is greater than that of cephalexin. These properties infer greater persistence of cefadroxil in serum and urine and more prolonged in vivo bacterial exposure to cefadroxil than to cephalexin or cephradine. Neither cefadroxil nor cephalexin demonstrates drug accumulation on repeated administration. The serum levels achieved by cefadroxil are unaffected by food. The pharmacokinetic properties of cefadroxil are supportive of the development of clinical efficacy data which could indicate that cefadroxil could be administered at 12-h intervals.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buck R. E., Price K. E. Cefadroxil, a new broad-spectrum cephalosporin. Antimicrob Agents Chemother. 1977 Feb;11(2):324–330. doi: 10.1128/aac.11.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIZZLE J. E. THE TWO-PERIOD CHANGE-OVER DESIGN AN ITS USE IN CLINICAL TRIALS. Biometrics. 1965 Jun;21:467–480. [PubMed] [Google Scholar]
- Wagner J. G. An overview of the analysis and interpretation of bioavailability studies in man. Pharmacology. 1972;8(1):102–117. doi: 10.1159/000136328. [DOI] [PubMed] [Google Scholar]



