Abstract
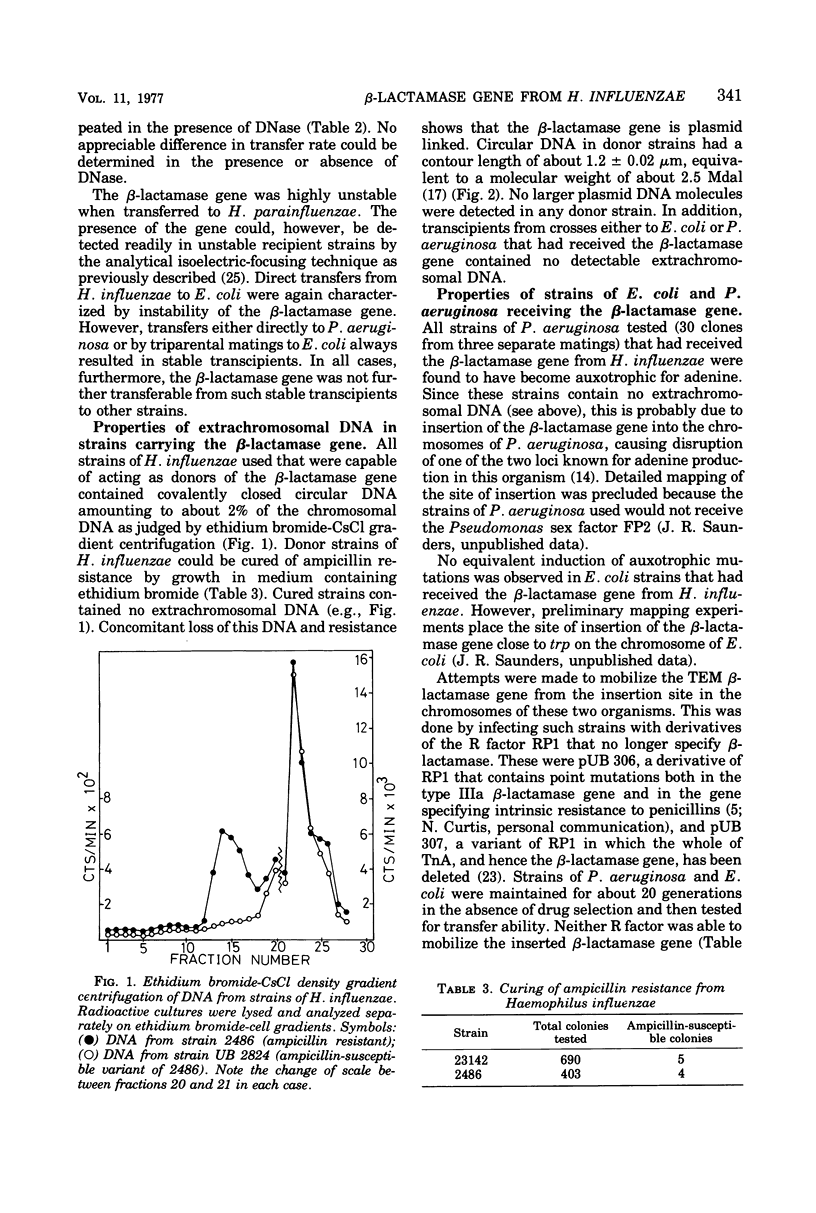
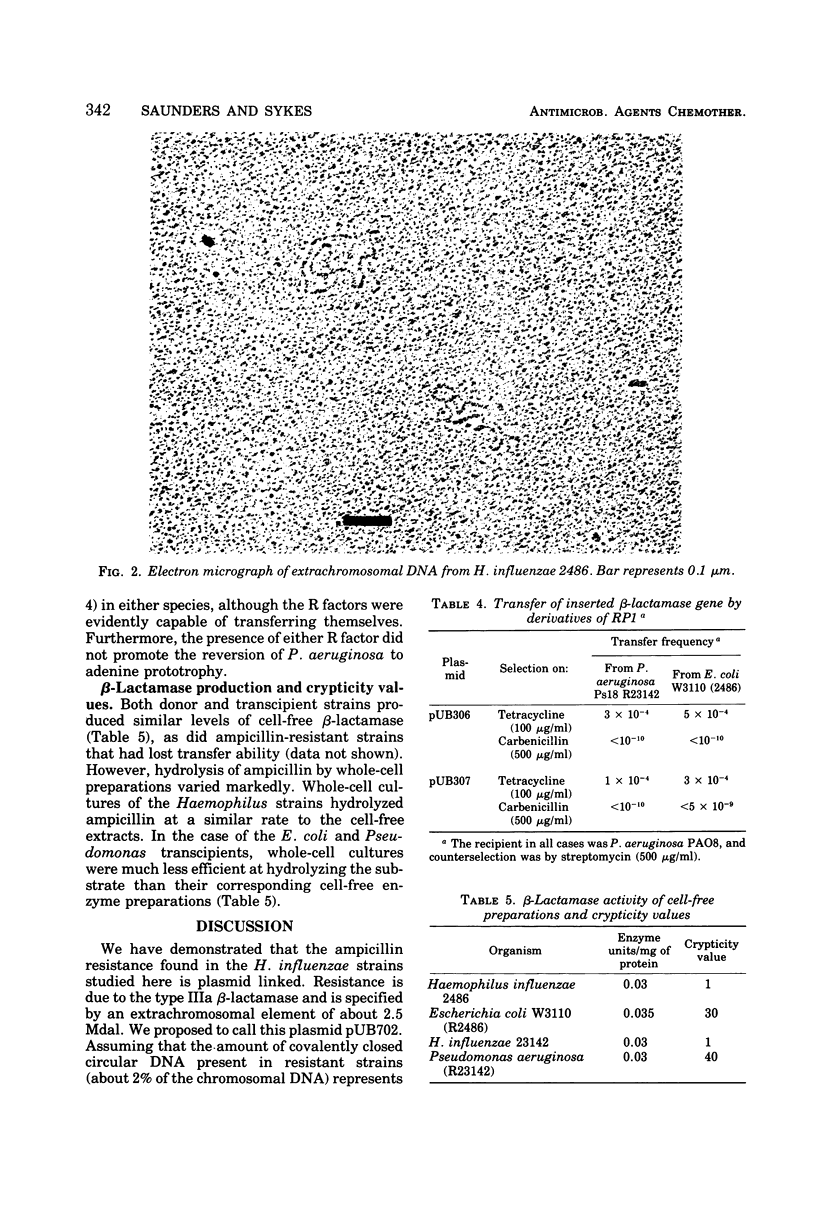
A number of ampicillin-resistant strains of Haemophilus influenzae could donate a gene specifying the type IIIa (TEM) β-lactamase to Haemophilus parainfluenzae, Escherichia coli, and Pseudomonas aeruginosa. Donor strains rapidly lost their ability to transfer ampicillin resistance on storage or subculture. Such strains also apparently contained a single species of covalently closed circular deoxyribonucleic acid of contour length 1.2 μm, equivalent to about 2.5 × 106 daltons. No species of plasmid deoxyribonucleic acid large enough to encode sex factor activity was detected. Despite this, transfer occurred to several bacterial genera in the presence of deoxyribonuclease, suggesting that transmissibility was by conjugation. The β-lactamase gene was generally unstable after transfer and was lost in the absence of selection. Where stable transcipients were found, this was evidently by insertion of the β-lactamase gene into the host chromosome. In P. aeruginosa insertion was always accompanied by induction of auxotrophy for adenine, suggesting insertion at a specific site. It is believed that insertion also occurred at one site on the chromosome of Escherichia coli. Crypticity measurements for β-lactamase activity showed that there was little or no penetration barrier to β-lactam drugs in Haemophilus. This may explain the long delay in the acquisition of ampicillin resistance by this organism.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. S. A rapid screening test for transfer factors in drug-sensitive Enterobacteriaceae. Nature. 1965 Dec 4;208(5014):1016–1017. doi: 10.1038/2081016a0. [DOI] [PubMed] [Google Scholar]
- Barth P. T., Datta N., Hedges R. W., Grinter N. J. Transposition of a deoxyribonucleic acid sequence encoding trimethoprim and streptomycin resistances from R483 to other replicons. J Bacteriol. 1976 Mar;125(3):800–810. doi: 10.1128/jb.125.3.800-810.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett P. M., Richmond M. H. Translocation of a discrete piece of deoxyribonucleic acid carrying an amp gene between replicons in Eschericha coli. J Bacteriol. 1976 Apr;126(1):1–6. doi: 10.1128/jb.126.1.1-6.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes R. C. Molecular structure of bacterial plasmids. Bacteriol Rev. 1972 Sep;36(3):361–405. doi: 10.1128/br.36.3.361-405.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis N. A., Richmond M. H. Effect of R-factor-mediated genes on some surface properties of Escherichia coli. Antimicrob Agents Chemother. 1974 Dec;6(6):666–671. doi: 10.1128/aac.6.6.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Graaff J., Elwell L. P., Falkow S. Molecular nature of two beta-lactamase-specifying plasmids isolated from Haemophilus influenzae type b. J Bacteriol. 1976 Apr;126(1):439–446. doi: 10.1128/jb.126.1.439-446.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwell L. P., De Graaff J., Seibert D., Falkow S. Plasmid-linked ampicillin resistance in haempohilus influenza type b. Infect Immun. 1975 Aug;12(2):404–410. doi: 10.1128/iai.12.2.404-410.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar W. E., Jr, O'Dell N. M. Beta-lactamase activity in ampicillin-resistant Haemophilus influenzae. Antimicrob Agents Chemother. 1974 Nov;6(5):625–629. doi: 10.1128/aac.6.5.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinsted J., Saunders J. R., Ingram L. C., Sykes R. B., Richmond M. H. Properties of a R factor which originated in Pseudomonas aeruginosa 1822. J Bacteriol. 1972 May;110(2):529–537. doi: 10.1128/jb.110.2.529-537.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Jacob A. E. Transposition of ampicillin resistance from RP4 to other replicons. Mol Gen Genet. 1974;132(1):31–40. doi: 10.1007/BF00268228. [DOI] [PubMed] [Google Scholar]
- Heffron F., Rubens C., Falkow S. Translocation of a plasmid DNA sequence which mediates ampicillin resistance: molecular nature and specificity of insertion. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3623–3627. doi: 10.1073/pnas.72.9.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffron F., Sublett R., Hedges R. W., Jacob A., Falkow S. Origin of the TEM-beta-lactamase gene found on plasmids. J Bacteriol. 1975 Apr;122(1):250–256. doi: 10.1128/jb.122.1.250-256.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan W., Ross S., Rodriguez W., Controni G., Saz A. K. Haemophilus influenzae type B resistant to ampicillin. A report of two cases. JAMA. 1974 Jul 15;229(3):298–301. [PubMed] [Google Scholar]
- Kleckner N., Chan R. K., Tye B. K., Botstein D. Mutagenesis by insertion of a drug-resistance element carrying an inverted repetition. J Mol Biol. 1975 Oct 5;97(4):561–575. doi: 10.1016/s0022-2836(75)80059-3. [DOI] [PubMed] [Google Scholar]
- Lang D. Molecular weights of coliphages and coliphage DNA. 3. Contour length and molecular weight of DNA from bacteriophages T4, T5 and T7, and from bovine papilloma virus. J Mol Biol. 1970 Dec 28;54(3):557–565. doi: 10.1016/0022-2836(70)90126-9. [DOI] [PubMed] [Google Scholar]
- Medeiros A. A., O'Brien T. F. Ampicillin-resistant Haemophilus influenzae type B possessing a TEM-type beta-lactamase but little permeability barrier to ampicillin. Lancet. 1975 Mar 29;1(7909):716–719. doi: 10.1016/s0140-6736(75)91630-x. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P. Staphylococcal penicillinase and the new penicillins. Biochem J. 1962 May;83:229–235. doi: 10.1042/bj0830229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRET C. J. Iodometric assay of penicillinase. Nature. 1954 Nov 27;174(4439):1012–1013. doi: 10.1038/1741012a0. [DOI] [PubMed] [Google Scholar]
- Richmond M. H., Sykes R. B. The beta-lactamases of gram-negative bacteria and their possible physiological role. Adv Microb Physiol. 1973;9:31–88. doi: 10.1016/s0065-2911(08)60376-8. [DOI] [PubMed] [Google Scholar]
- Richmond M. H., Sykes R. B. The chromosomal integration of a -lactamase gene derived from the P-type R-factor RP1 in Escherichia coli. Genet Res. 1972 Oct;20(2):231–237. doi: 10.1017/s0016672300013732. [DOI] [PubMed] [Google Scholar]
- Robinson M. K., Bennett P. M., Richmond M. H. Inhibition of TnA translocation by TnA. J Bacteriol. 1977 Jan;129(1):407–414. doi: 10.1128/jb.129.1.407-414.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes R. B., Matthew M., O'Callaghan C. H. R-factor mediated beta-lactamase production by Haemophilus influenzae. J Med Microbiol. 1975 Aug;8(3):437–441. doi: 10.1099/00222615-8-3-437. [DOI] [PubMed] [Google Scholar]
- Sykes R. B., Matthew M. The beta-lactamases of gram-negative bacteria and their role in resistance to beta-lactam antibiotics. J Antimicrob Chemother. 1976 Jun;2(2):115–157. doi: 10.1093/jac/2.2.115. [DOI] [PubMed] [Google Scholar]
- Thorne G. M., Farrar W. E., Jr Transfer of ampicillin resistance between strains of Haemophilus influenzae type B. J Infect Dis. 1975 Sep;132(3):276–281. doi: 10.1093/infdis/132.3.276. [DOI] [PubMed] [Google Scholar]
- Vega R., Sadoff H. L., Patterson M. J. Mechanisms of ampicillin resistance in Haemophilus influenzae type B. Antimicrob Agents Chemother. 1976 Jan;9(1):164–168. doi: 10.1128/aac.9.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]



