Abstract
Somatic sexual dimorphisms outside of the nervous system in Drosophila melanogaster are largely controlled by the male- and female-specific Doublesex transcription factors (DSXM and DSXF, respectively). The DSX proteins must act at the right times and places in development to regulate the diverse array of genes that sculpt male and female characteristics across a variety of tissues. To explore how cellular and developmental contexts integrate with doublesex (dsx) gene function, we focused on the sexually dimorphic number of gustatory sense organs (GSOs) in the foreleg. We show that DSXM and DSXF promote and repress GSO formation, respectively, and that their relative contribution to this dimorphism varies along the proximodistal axis of the foreleg. Our results suggest that the DSX proteins impact specification of the gustatory sensory organ precursors (SOPs). DSXF then acts later in the foreleg to regulate gustatory receptor neuron axon guidance. These results suggest that the foreleg provides a unique opportunity for examining the context-dependent functions of DSX.
Introduction
Studies in Drosophila melanogaster have revealed that many complex biological processes, such as the specification of somatic structures [1], [2], segmental identity [3], and sexual differentiation [4], [5], are under the control of master regulatory genes. These genes may act at multiple times in the course of these processes to regulate the expression of distinct sets of target genes. dsx is a unique master regulatory gene in that it functions across a wide variety of tissues to specify nearly all aspects of sexual development and differentiation. To manifest its various functions, dsx is necessarily responsive to three fundamental contexts: 1) the chromosomal sex of the cells in which it is expressed; 2) cell type; and 3) developmental stage of the cells in which it is expressed.
Chromosomal sex impacts dsx function via the sex determination hierarchy, which determines whether dsx transcripts are spliced to encode the transcription factors DSXM in males or DSXF in females [6] (Fig. 1A). DSXM and DSXF contain a shared zinc finger DNA-binding domain but differ in having sex-specific C-termini [7], [8], [9]. They are thought to bind to, and sex-specifically regulate the transcription of, a common set of target genes. For example, both DSXM and DSXF bind to the enhancer region of the genes encoding Yolk Proteins (YP) 1 and 2, but DSXM down-regulates YP production while DSXF up-regulates YP production [8], [10] . Thus, the chromosomal sex of a cell determines the sex-specific functions of dsx via post-transcriptional mechanisms.
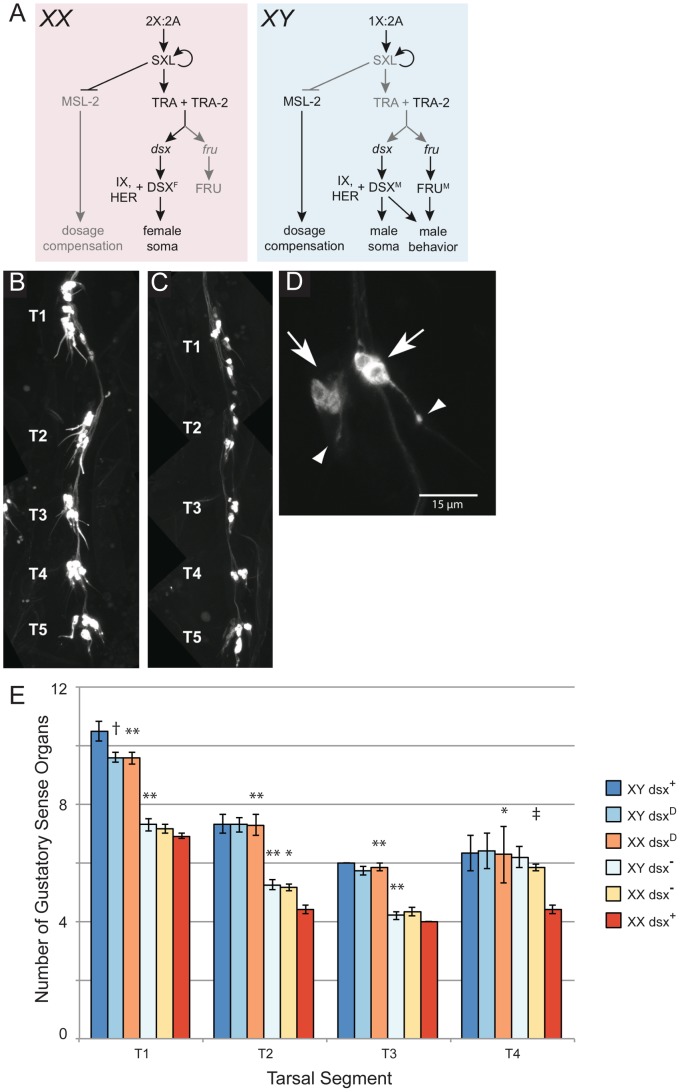
Figure 1. dsx regulates the number of foreleg GSOs.
(A) The sex determination hierarchy directs the generation of sex-specific DSX and FRU isoforms. The 2∶2 ratio of X chromosomes to autosomes in females sets off a female-specific alternative RNA splicing cascade in which TRA directs splicing of dsx and fru transcripts into the female forms. The lack of TRA activity in males results in the production of male forms of these transcripts. (B–D) poxn-GAL4 driving expression of UAS-mCD8::GFP in a (B) male and (C) female foreleg at 48 h APF. Tarsal segments T1–T5 are indicated. Note that there are more clusters of neurons labeled in the male than in the female in T1–T4. (D) Magnified view of two distinct GSOs. The GRNs (arrows) of each GSO project their dendrites into the base of their cognate bristle (arrowheads). (E) Quantitation of foreleg GSOs in T1–T4. 3XP3DsRed was used to distinguish XY flies from XX flies in a dsx-deficient background where chromosomal sex could not otherwise be distinguished. All XY males had a sex chromosome genotype of w/Y. The genotype of the sex chromosomes of dsx-deficient chromosomal females was w/w, 3XP3DsRed, while all other females were w/y w, 3XP3DsRed. Genotype abbreviations: dsx + (UAS-mCD8::GFP; FRT82B dsx1, poxn-GAL4/TM6B). dsx − (UAS-mCD8::GFP; FRT82B dsx1, poxn-GAL4/dsxM+R13). dsxD (UAS-mCD8::GFP; FRT82B dsx1, poxn-GAL4/dsxD). dsx + and dsxD are siblings from the same cross. Error bars indicate SEM. P-values are for comparisons between the indicated dsx mutant and dsx + of the same chromosomal sex. (*p = .07, **p<.0001, † p = .04, ‡ p = .14, Tukey multiple comparisons of means.).
Transcriptional activation of dsx is also under complex spatial regulation [11], [12], such that only specific cells express dsx to produce sexually dimorphic traits. These traits include the genitalia, the male-specific sex combs, abdominal segment number and pigmentation, YP production, pheromone production, and gonadogenesis (reviewed in [6], [13], [14]) [15], [16]. dsx also controls aspects of neurogenesis [17], [18], [19], [20] and sexual behavior, where it works in conjunction with male-specific functions of fruitless (fru) to direct sex-specific aspects of nervous system differentiation (reviewed in [21], [22], [23], [24], [25], [26]). Thus, dsx regulates a diverse array of developmental programs.
dsx also functions in various temporal contexts to regulate developmental programs at the appropriate stage. dsx functions in the somatic gonad from embryogenesis through adulthood, while it regulates transcription of the YP genes in the fat body only during adulthood (reviewed in [13], [27]). dsx can also function at multiple times within the developmental program of a tissue. In the genital imaginal disc, dsx begins functioning early to direct sex-specific patterns of cell proliferation (reviewed in [6], [14]) and then later controls morphogenesis and differentiation of the disc to form sex-appropriate genitalia and analia (reviewed in [6]) [28]. Similarly during foreleg development, dsx functions initially to specify the number of bristles in the male-specific sex comb, then subsequently specifies the morphology of these bristles [29]. Thus temporal context, like cell type, constrains the function of dsx such that it regulates the right genes at the right times in development.
To understand how dsx sculpts sexually dimorphic development, many studies have focused on identifying the genes that dsx directly or indirectly regulates (reviewed in [6], [13]) [28], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41]. These studies have offered insight as to how dsx regulates diverse developmental processes. First, dsx regulates a number of patterning and signaling pathway genes in a sex-specific manner during the development of particular tissues, while these same genes are also expressed independent of dsx during the development of other tissues. Second, different genes are regulated by dsx in different cell types. A major focus will then be to determine how dsx function is integrated with spatiotemporal information such that specific target genes are sex-specifically regulated in appropriate cell types and at appropriate times in development.
Here, we address the function of dsx in the development of the gustatory sense organs (GSOs) of the foreleg. GSOs are a class of bristle sensory organs (sensilla) in which multiple gustatory receptor neurons (GRNs) project their dendrites into the shaft of a bristle to detect tastants [42], [43]. The foreleg GSOs of Drosophila melanogaster are associated with two sexual dimorphisms. First, males have a greater number of GSOs on their forelegs than do females [43], [44], and second, the axons of many of the constituent GRNs project across the midline of the ventral nerve cord (VNC) in males but not in females [45], [46]. dsx has a known role in both of these dimorphisms: it regulates the development of at least some of the GSOs in the first tarsal segment (T1) of the foreleg [44], and represses VNC midline crossing by GRN axons [46].
Our study addresses two questions regarding the role of dsx in GSO development. First, we asked if dsx is responsible for the sexually dimorphic number of GSOs across all tarsal segments of the foreleg and when this dimorphism is established. Second, we sought to determine if GSO number and GRN axonal projections arise interdependently as the result of a single upstream developmental decision commanded by dsx or if these dimorphisms each result from independent developmental events that are individually controlled by dsx. We found that dsx does control sex-specific GSO numbers early in GSO development and that this function is temporally separable from the previously described role of dsx in regulating GRN axonal projections in the VNC. Thus, dsx regulates two distinct developmental events that impact the GSO at different times in development.
Materials and Methods
Drosophila Stocks
Unless otherwise indicated, crosses were at 25°C under standard conditions. To examine GRNs in dsx mutants and controls, w; UAS-mCD8::GFP; FRT82B dsx1, poxn-GAL4-14-1-7/TM6B females were crossed to w, 3XP3-DsRed; dsxM+R13/TM6B males. To generate dsx-masculinized females, y w, 3XP3-DsRed; dsxD/TM6B males were crossed to w; UAS-mCD8::GFP; FRT82B dsx1, poxn-GAL4-14-1-7/TM6B females. poxn-GAL4-14-1-7 was from M. Noll [47]. Male parents carrying the X-chromosomal transgene 3XP3-DsRed (from O. Schuldiner [48]) were used to distinguish XY (non-fluorescent eyes) and XX (fluorescent eyes) dsx1/dsxM +R13 and dsx1/dsxD progeny, as these genotypes are otherwise indistinguishable. neur-lacZ (A101) and ase-lacZ were from the Bloomington Drosophila Stock Center at Indiana University (IN, USA).
For post-mitotic expression of UAS-DSXF [from K. Burtis (University of California, Davis, CA, USA)] in FRUM-positive neurons, w; UAS-mCD8::GFP; fruGAL4/MKRS flies were crossed to w; UAS-DSXF/SM6. fruGAL4 [49] was from B. Dickson.
Generation of Anti-DSXDBD
The DSXDBD antigen was the generous gift of J. Marvin and L. Looger (Janelia Farm Research Campus, Ashburn, VA, USA). A 60-amino acid polypeptide (MSISPRTPPNCARCRNHGLKITLKGHKRYCKFRYCTCEKCRLTADRQRVMALQTALRRAQ) corresponding to the DNA binding domain (DBD) of the DSX proteins [50] was encoded by a synthetically generated DNA (DNA2.0) and expressed in E. coli BL21(pLysS) as a direct fusion to an N-terminal 6-histidine affinity tag in a modified pRSET A vector (Invitrogen). The fusion protein was affinity purified over a Ni2+-NTA resin (Qiagen) by an imidazole gradient, and conjugated to keyhole limpet hemocyanin. Hybridoma clones from immunized mice were screened by in vitro immunoassay against the DSXDBD polypeptide (RayBiotech, Inc.), and one produced anti-DSXDBD, which stains nuclei of third instar larval tissues in patterns consistent with the expression pattern of dsxGAL4 (Fig. S1 and C. Robinett unpublished data) [11]. Anti-DSXDBD does not stain foreleg discs of dsx nulls (Fig. S2).
Preparation and Examination of Tissues
Tissues were fixed in 4% paraformaldehyde (Electron Microscopy Sciences) in phosphate-buffered saline (PBS). Unless otherwise noted, tissues for immunofluorescence were blocked in 5% normal goat serum in PBS containing 0.1% Triton X-100 (Sigma) (PBST), and Alexa Fluor fluorescently conjugated goat secondary antibodies (Molecular Probes/Invitrogen) were used at 1∶500. Tissues were mounted in Vectashield (Vector Laboratories) with or without DAPI. Sample imaging was performed on Zeiss laser scanning confocal microscopes LSM 510 or 710 (Carl Zeiss), and Z-stacks were manipulated using ImageJ (NIH). Images were cropped, rotated, and adjusted for contrast and brightness using Adobe Photoshop.
To examine poxn-GAL4-driven expression of UAS-mCD8::GFP in 48 h APF forelegs, pupae were removed from puparia in PBS, fixed for 30–45 minutes at 22°C, washed three times in PBS, and examined for native GFP fluorescence. For 2–8 h APF, white pre-pupae were collected at the stage of puparium formation, individually sexed based on the larger size of the male gonads, aged appropriately at 25°C, kept on ice or at 4°C until dissection in PBST, fixed for 25 minutes at 22°C, and washed three times for 15 minutes in PBST. Pupal cuticle secretion around 8 h APF blocks antibody penetration [51], [52], [53], [54], so 8 h APF forelegs were dissected off of fixed pre-pupae by either cutting between T1 and the femur or plucking the entire foreleg away from the pre-pupal carcass. T1 was often damaged or had excessive background staining, preventing assessment of GSO numbers. Dissected legs were blocked in PBST plus 5% normal goat serum (PBSTN) for at least 16 hours at 4°C, incubated in primary mouse monoclonal antibody 22C10 [Developmental Studies Hybridoma Bank at the University of Iowa (DSHB), USA] at 1∶20 for 40–48 hours at 4°C, washed four times in PBST over the course of 10 hours at 22°C or 24 hours at 4°C, and re-blocked in PBSTN overnight at 4°C. Treatment with the secondary antibody, Alexa Fluor 568 anti-mouse was the same as per the primary antibody. Legs were mounted in a circle of nail polish painted onto the slide to prevent flattening of the tissue.
GSO lineage cells were identified based on strong fluorescent signals from both native GFP and 22C10. Inspection of the confocal Z-stack slices determined whether a given cell or cell cluster met our criteria to be a GSO: in T3, a cluster of tightly grouped cells or a large single cell or two large paired cells expressing GFP and stained with 22C10 were classified as GSOs; in T2 and T4, criteria were the same as for T3, but the epithelial expression of poxn-GAL4-driven UAS-mCD8::GFP required close inspection of morphology, size, and clustering of the cells, which were discounted if they did not appear similar to the GSOs identified in T3.
Larval tissues were immunostained as described in [11]. The DSXM and AC time-course used rat anti-DSXM 5528 (from B. Oliver [55]) at 1∶200 and mouse monoclonal anti-AC (DSHB) at 1∶40 followed by Alexa Fluors 568 anti-rat and 488 anti-mouse. In each time-point preparation, male third instar gonads were included as internal controls; successful immunostaining of gonadal somatic cells confirmed reagent competence when DSXM was not detected in foreleg discs (C. Robinett, unpublished data). For each time-point, a foreleg disc pair (identified by the unique physical association of the two discs) from each of three larvae was mounted in a circle of nail polish (described above). DSX and ase-lacZ or neur-lacZ expression were detected with anti-DSXDBD at 1∶100 and rabbit anti-β-galactosidase (Cappel/MP Biomedicals, LLC) (1∶500), respectively, followed by Alexa Fluors 488 anti-mouse and 568 anti-rabbit.
For examination of GRN axon morphology, VNCs were dissected from 1-day-old adults and fixed and stained as per [56] using rabbit anti-GFP (Invitrogen) at 1∶1000 and rat anti-DN-cadherin (DN-EX#8) (DSHB) at 1∶40 followed by Alexa Fluors 488 anti-rabbit and 647 anti-rat.
DSXM and AC Time-course
Canton S larvae were raised at low-density in standard molasses food bottles for ca. 72 hours at 25°C. The food surface was then overlaid with 20% sucrose in H2O at 22°C and gently agitated to “float” the larvae into the liquid. 10–30 large larvae having second instar anterior spiracle morphology [57] were transferred to a 2.5-cm diameter food vial supplemented with Brewer’s yeast paste; multiple such vials were set up over the course of an hour before being transferred to 25°C. After 2 hours, larvae were collected from these vials by the method above. Males having third instar anterior spiracle morphology were designated 0 h 3I and returned to 25°C on fresh food with Brewer’s yeast paste for the duration of the time point, whereupon larvae were collected again and held on ice until dissection. Because of the time taken to handle, sort and stage the larvae, all time points are approximations of ±3 hours.
Results
dsx Specifies the Sexually Dimorphic Number of Foreleg GSOs
Male forelegs have more GSOs than do female forelegs across tarsal segments 1 through 4 (T1–T4) [43], [58], [59]. In T1 (the most proximal tarsal segment), this sexual dimorphism is regulated by dsx [44] (wherein GSOs are referred to as “bractless bristles”). We revisited the T1 dimorphism and additionally asked if dsx regulates the number of GSOs in T2–T4 by examining the forelegs of males and females that are null for dsx function (dsx1/dsxM+R13) and comparing them to those with one wild-type copy of dsx (dsx1/TM6B, control). GSO cells were marked by expression of Pox neuro-GAL4-14 (hereafter, poxn-GAL4) driving UAS-mCD8::GFP [47] and visualized at 48 hours after puparium formation (h APF) as clusters of GRNs whose dendrites converged toward the surface of the leg (Fig. 1B–D). Consistent with previous quantitations of GSOs based on bristle morphology [43], [58], [59], our counts of GSOs on control (dsx+) male and female forelegs showed that males have more GSOs than do females in tarsal segments T1–T4 (Fig. 1E). In contrast, dsx null flies exhibited no significant differences between males and females in the numbers of GSOs in foreleg T1–T4 (Fig. 1E), indicating that sex-specific dsx functions are necessary for this sexual dimorphism. Thus, our results with a transheterozygous allelic combination recapitulated the findings of previous experiments with homozygous dsx1 mutants in T1 [44], and further, we found that dsx regulates GSO numbers across T2–T4.
To address whether expression of DSXM in XX chromosomal females that lacked DSXF is sufficient to produce male-like numbers of GSOs, we examined dsxD/dsx1 flies in which only male-specific dsx transcripts are produced regardless of chromosomal sex (see Materials and Methods) [4], [60]. The number of GSOs in XX; dsxD/dsx1 individuals did not significantly differ from that of their XY; dsxD/dsx1 siblings in T1–T4 (Fig. 1E). Thus, in the absence of DSXF, DSXM is sufficient to generate the male number of foreleg GSOs.
The greater number of GSOs in males relative to females could be due to a GSO-promoting action of DSXM in males, a GSO-suppressing action of DSXF in females, or a combination of these two possibilities. When we compared control males and females to dsx null flies, we found evidence for each of these three possibilities amongst the foreleg tarsal segments (Fig. 1E). In T1, dsx null males and dsx null females had an average number of GSOs (7.3±0.2 SEM and 7.2±0.2, respectively) that did not significantly differ from the number of GSOs found in dsx+ control females (6.9±0.1), but were significantly less than the number of GSOs present in dsx+ males (10.5±0.3). Similarly in T3, dsx null males and dsx null females had an average number of GSOs (4.2±0.1 and 4.3±0.1, respectively) that did not significantly differ from the number of GSOs in dsx+ control females (4.0±0.0), but were significantly less than the number of GSOs present in dsx+ males (6.0±0.0). Thus in T1 and T3, DSXM induces the male-specific number of GSOs, whereas DSXF appears to have no effect on the number of GSOs. In T4, the number of GSOs in both dsx null males and dsx null females (6.2±0. and 5.8±0.1, respectively) were roughly equal to the number present in dsx+ control males (6.3±0.6) and higher than the number in dsx+ control females (4.4±0.1). These data indicate that in T4, DSXF represses the development of two GSOs, while DSXM has no effect. In T2, dsx null males and females had numbers of GSOs (5.3±0.2 and 5.2±0.1, respectively) that differed significantly from both control males (7.3±0.3) and females (4.4±0.1). Thus in T2, DSXM promotes GSO formation in males, while DSXF represses GSO formation in females.
We conclude that the wild-type function of dsx ensures that each of the T1–T4 tarsal segments elaborates a greater number of GSOs in males than in females, but that this shared outcome arises by differential use of DSXM and DSXF in the tarsal segments. This distinction implies that positional information is integrated with sexual identity (i.e. dsx expression) on a fine-scale along the proximodistal axis of the developing foreleg.
The sexually dimorphic number of GSOs is established by 8 h APF
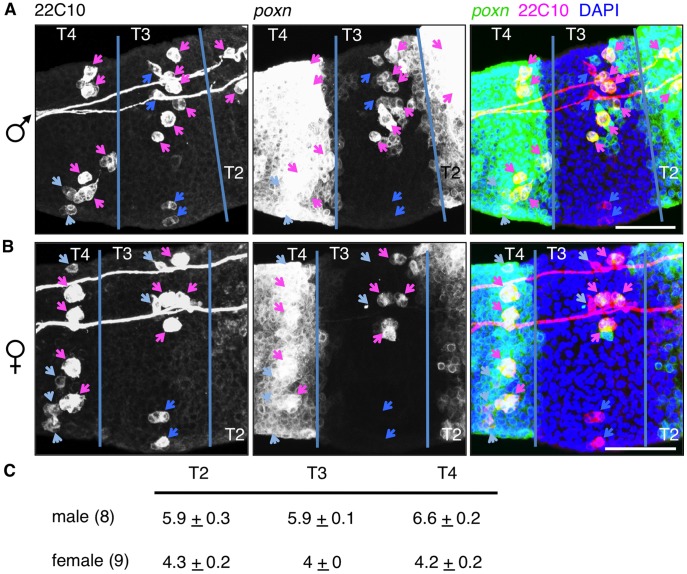
To address how dsx function specifies the sex-specific number of foreleg GSOs, we sought to determine when this sexual dimorphism was first apparent. We found that GSOs were identifiable by morphology and cell clustering as early as 14 h APF using the poxn-GAL4 marker and that the sexual dimorphism in GSO number was already apparent (C. Robinett, unpublished data). Examination of earlier time points was complicated by the expression of poxn-GAL4 across the leg disc epithelium of T2 and T4 from 0–6 h APF (Figs. 2 and S3), regions where poxn is required for formation of the intertarsal joints [61]. Because cells of the GSOs could not be clearly distinguished from epithelial cells using poxn-GAL4 alone, we incorporated staining with the 22C10 monoclonal antibody, which marks both neurons and other cell types of nascent sensory organs [62] and thus allows us to positively identify the GSOs.
Figure 2. The sexually dimorphic number of foreleg GSOs is specified by 8 h APF.
Male (A) and female (B) forelegs with poxn-GAL4 driving UAS-mCD8::GFP (green) were stained for 22C10 (magenta) at 8 h APF. Merged images on right show overlap in yellow and DAPI-stained DNA (blue). Tarsal segment boundaries indicated with blue lines. Cells marked with 22C10 were classified based both on colocalization of poxn-GAL4 and morphology of the cells or cell clusters: GSO lineage cells (magenta arrows); non-GSO cells that lack poxn-GAL4 in T3 (dark blue arrows); non-GSO cells marked by poxn-GAL4 but lacking GSO morphology in T4 (light blue arrows). Scale bars, 50 µm. (C) Averages and SEMs of quantitated GSO numbers in T2, T3, and T4 for both male (n = 8) and female (n = 9) forelegs at 8 h APF.
In 8 h APF foreleg discs, GSOs could be recognized as multi-cell clusters that exhibited strong signals for both GFP and 22C10 (see Materials and Methods) (Figs. 2A,B and S4). There were also single large cells and pairs of large cells exhibiting both GFP and 22C10 signals at strengths similar to what was observed for cells within the GSO cell clusters (Figs. 2A,B, S4 and S5). Given that 22C10 marks nascent sensory organ cells, we consider these large cells to be the single sensory organ precursor cells (SOPs), which each give rise to a single GSO, or to the first pair of daughters of an SOP, respectively. As both of these cell types represent the GSO lineage, they were equated to GSOs in our analysis. We counted GSOs in male and female tarsal segments T2–T4 and found that the numbers were the same as seen in adults (Fig. 2C, compare to Fig. 1E). (T1 was not examined, see Materials and Methods). Therefore, the sexual dimorphism in foreleg GSOs number is established prior to 8 h APF. Because a subset of gustatory SOPs appeared to be undergoing their first cell division around 8 h APF, the developmental mechanism that establishes the sexually dimorphic number of GSOs likely acts prior to the division of gustatory SOPs.
DSX is present in proneural clusters and SOPs
It has been reported that the foreleg gustatory SOPs are specified before or near the time of puparium formation [52]. We therefore examined the distribution of DSX protein in T2–T4 of the foreleg disc before and at 0 h APF using anti-DSXDBD, a monoclonal antibody that recognizes both DSXM and DSXF (see Materials and Methods and Figs. S1 and S2). At this stage of development, foreleg discs of male and female larvae exhibited DSX proteins in a crescent of epithelial cells that occupied the T1 domain as well as in smaller patches of epithelial cells in T2–T4 (Fig. S2A). DSX was not detected across the T5 disc epithelium or in regions of the foreleg disc that were proximal to T1, and there was no staining of the disc epithelium in other leg discs at this stage (Fig. S2D,F,G). This distribution of DSX is in accord with the expression pattern of the dsx promoter [11]. Although the distribution of DSX appeared to be the same in male and female foreleg discs, males showed stronger signal intensity (Fig. S2A,B).
We note two important features in the distribution of DSX. First, the number of epithelial cells marked by DSXM is far greater than the number of GSOs in the adult foreleg, indicating that dsx expression is not restricted to the SOPs that will give rise to the GSOs. Second, the distribution of DSX in broad swaths of epithelial cells is restricted to those leg tissues that produce sexually dimorphic GSO numbers, as foreleg segment T5 and the tarsus of the second leg exhibit sexually monomorphic bristles [43], [59], [63]. These data thus suggest that dsx may regulate GSO numbers by exerting its function broadly across the epithelium of tarsal segments T2–T4 at a time preceding SOP specification.
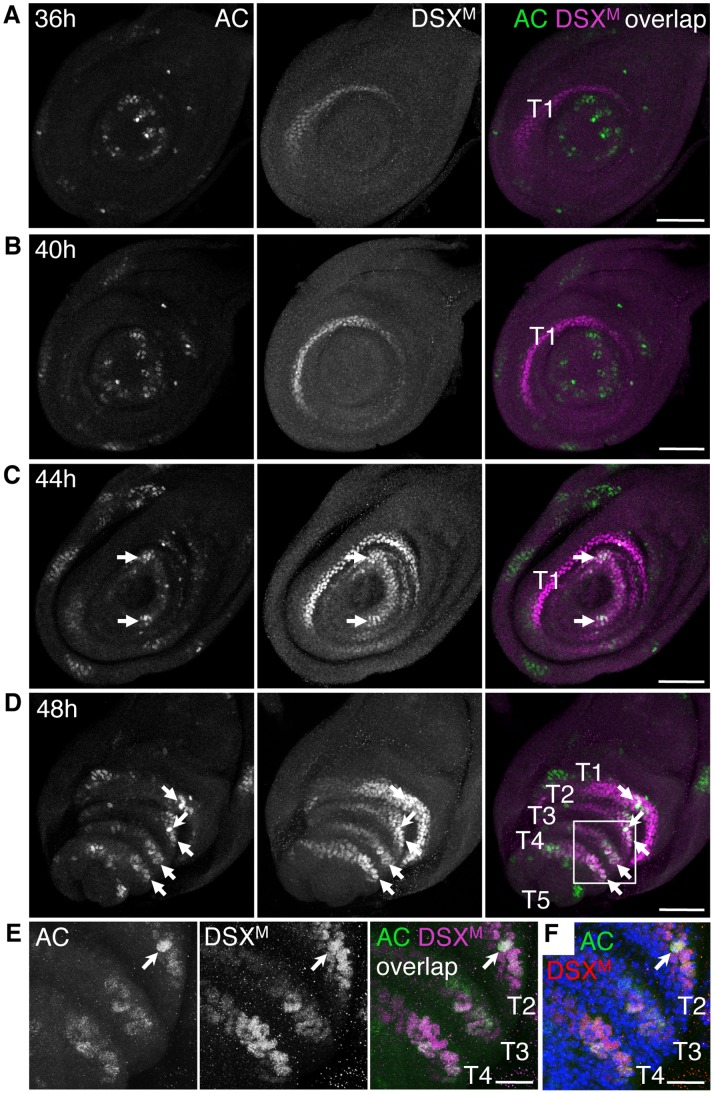
In the wing imaginal disc, specification of the thoracic mechanosensory organ SOPs depends on the expression of the proneural genes achaete (ac) and scute (sc), which confer neural potential to patches of epithelial cells called proneural clusters from which the SOP cell is selected (reviewed in [64], [65], [66]). To ascertain whether dsx might function in proneural clusters of the foreleg disc, we determined the pattern of DSXM with respect to Achaete (AC) in foreleg discs of male late third instar larvae. Nascent third instar larvae (0 h 3I) were isolated, raised at 25°C, and then analyzed at 4-hour increments from 24 h 3I until the time of puparium formation at 48 h 3I, which is equivalent to 0 h APF. At 24 h 3I, AC was detected only in a few cells of T5, while DSXM was absent from the disc (Fig. S7A). AC-positive cells were observed at a greater number in T5 at 28 h 3I (Fig. S7B), as well as in more proximal domains at 32 h 3I (Fig. S7C). However, DSXM was not detected in the foreleg disc until 36 h 3I, when it weakly marked a crescent of epithelial cells in T1 (Fig. 3A). This staining signal intensified at 40 h 3I, and by 44 h 3I DSXM appeared in swaths of epithelial cells in the more distal tarsal segments, T2–T4 (Fig. 3B,C). The pattern of AC also became more complex during 36–44 h 3I as multiple patches of AC-positive cells were distributed across the proximal and distal regions of the foreleg disc (Fig. 3A–C). Importantly, there was significant overlap between DSXM and AC in patches of cells distal to T1 at 44 h 3I, and this pattern became more pronounced in T2–T4 at 48 h 3I (Fig. 3C,D). Because AC marks cells with proneural potential, we consider these co-expressing patches of cells to be nascent proneural clusters. Further, because leg mechanosensory sense organs are not specified until around 8 h APF [51], [52], we assume that these proneural clusters will give rise to gustatory SOPs.
Figure 3. DSXM is present in the foreleg disc epithelium when AC accumulates in proneural clusters.
(A–E) Male foreleg discs from the indicated time points of third instar larval development were stained for AC (green) and DSXM (magenta). Merged images on right show overlap in white. (A and B) From 36–40 h 3I, DSXM is present in a crescent within T1 and there is no overlap with AC. (C) At 44 h 3I, DSXM signal increases across the epithelium of tarsal segments distal to T1 (i.e. toward disc center) and is present in some clusters of AC-positive cells (arrows). (D) At 48 h 3I, DSXM is present in swaths of epithelial cells in T1–T4 and overlaps in these segments with subsets of the AC-positive cells that are proneural clusters (arrows). A candidate SOP with high levels of AC and DSXM (barbed arrow) is seen in T2. (E) Magnified view of boxed region in (D). Candidate SOP in T2 (barbed arrow). (F) Same image as (E) with AC (green), DSXM (red), and stained with DAPI (blue) to visualize all nuclei in the focal planes shown. All images are projections of only those focal planes that encompass the majority of DSXM signal within the disc. Scale bars (A–D) 50 µm and (E and F) 10 µm.
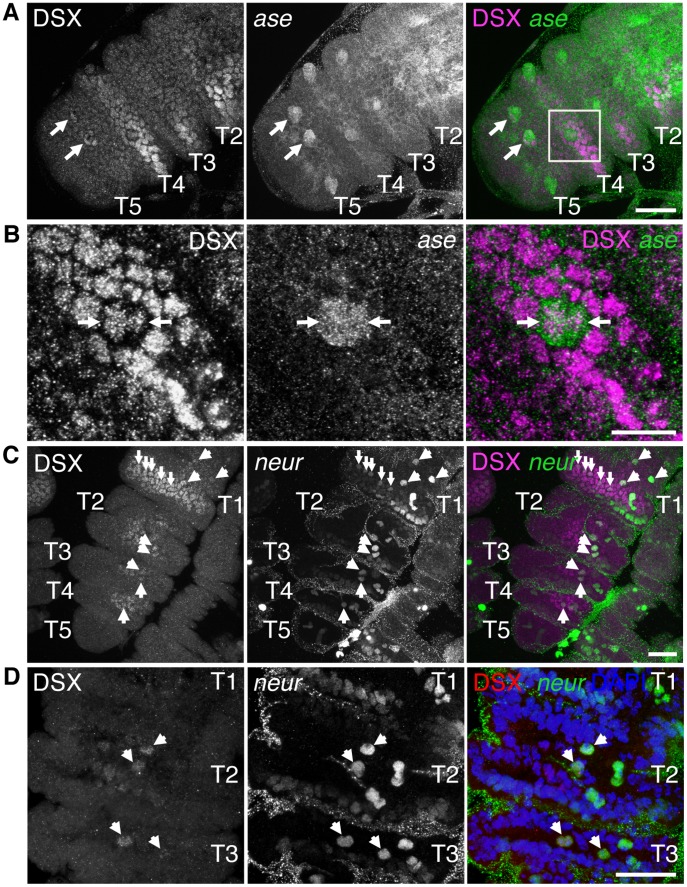
Once an SOP is specified within the proneural cluster, it accumulates high levels of AC and Scute (SC) and concomitantly initiates expression of the neural precursor gene asense (ase), which in the embryonic nervous system and wing imaginal disc promotes the SOP fate and ensures proper development of sensory organs [67], [68], [69], [70]. To determine if DSXM is present early in cells of the GSO lineage following SOP specification, we examined the distribution of DSXM with respect to ase-lacZ, a cytoplasmic reporter of ase expression that is expressed in SOPs and their first daughter cells [68]. In male foreleg discs at 0 h APF, DSXM was present in many of the disc epithelial cells within T1–T4, as well as in the previously mentioned clusters of cells in T5 (Fig. 4, see also Fig. 2). In all tarsal segments, ase-lacZ was expressed in distinct clusters of cells that generally resembled the pattern seen with poxn-GAL4 at later time-points, and some of these cells also contained DSXM (Fig. 4A,B), as indicated by the nuclear-localized DSXM signal surrounded by cytoplasmic β-galactosidase immunoreactivity. In T5, the cells containing DSXM were part of a cluster of cells that expressed ase-lacZ (Fig. 4A). In T4, DSXM was seen in a pair of ase-lacZ cells whose cytoplasm occupied a larger volume than the surrounding epithelial cells (Fig. 4B). That the ase-lacZ cytoplasmic staining corresponded to only two cells was confirmed by nuclear staining (Fig. S6). This pair of tightly associated cells expressing ase-lacZ is assumed to be the immediate daughters of a recently divided SOP. Thus, DSXM is present in the immediate progeny of at least a subset of SOPs in the foreleg tarsal segments.
Figure 4. DSX is present in SOP daughters of the foreleg disc.
(A and B) DSX (magenta) is present across the tarsal segment epithelium in male discs at 0 h APF as well as in subsets of cells expressing ase-lacZ (green) in T5 (arrows) and T4 (boxed area). (B) Magnified view of boxed region in (A) shown as a partial projection. Daughters of a recently divided SOP (arrows). (C) DSX (magenta) is present in the tarsal segment epithelium in male discs at 6 h APF. DSX overlaps with neur-lacZ expression (green) in several cells across T1–T5 (arrowheads) and in a transverse row of cells in T1 that likely correspond to the sex comb bristle lineages (small arrows). (D) T2–T3 from a separate male leg disc at 6 h APF marked as per (C) with DSX (red) in right panel and DAPI-stained DNA (blue). For A–D, images on right are a merge of the left and middle images. Projection of multiple focal planes shown. Projection of multiple focal planes shown. Scale bars (A, C, and D) 25 µm and (B) 10 µm.
We also examined the distribution of DSXM with respect to neuralized-lacZ (neur-lacZ), a marker of SOPs and their progeny [71], [72], at the later time-point of 6 h APF. DSXM was present in patches of cells on the anterior surface of T1–T4, with broader expression in cells of distal T1 (Fig. 4C,D). Across T1–T4, DSXM colocalized with the expression of neur-lacZ in a few large cells that are likely to be gustatory SOPs (Fig. 4C,D), which is consistent with the presence of dividing poxn-GAL4-expressing cells that were positive for 22C10 at 6 h APF (Fig. S8). Whether the presence of DSXM in a subset of gustatory SOPS results from either continued expression of dsx in these cells or perdurance of the protein from expression in cells of the proneural clusters, we conclude that dsx is expressed at a time and place that would allow it to regulate the specification of the gustatory SOP fate.
DSXF can Repress Midline Crossing by GRN Axons Independent of its Regulation of Neurogenesis
We previously showed that DSXF in females represses VNC midline crossing by GRN axonal projections [46]. Two possibilities were proposed to account for this phenomenon: 1) DSXF regulates axon guidance in foreleg GRNs to prevent midline crossing in females; or 2) DSXF prevents midline crossing by preventing the birth of male-specific neurons that would send axonal projections across the VNC midline. If DSXF regulates axon guidance independent of its role in regulating GSO formation, then it should be possible to perturb dsx function in such a way as to preserve the wild-type number of male-specific GSOs but alter the axonal morphology of their GRNs. Alternatively, if DSXF acts only to regulate GSO numbers, then perturbing dsx function in males after GSO number has been specified should not affect GRN axonal morphology.
We tested these hypotheses by expressing DSXF in nascent male GRNs during axonal development. Specifically, we drove UAS-DSXF with fruGAL4 [49] which initiates expression in postmitotic GRNs before their nascent axons encounter the VNC midline (D. Mellert, unpublished data). To assess behavior of GRN axons at the VNC midline, fruGAL4 expression was visualized by simultaneously driving UAS-mCD8::GFP while the sensory neuropil was highlighted by counterstaining DN-cadherin [73]. Surprisingly, DN-cadherin staining alone was sufficient to assess midline crossing (see Fig. 5D’,E’,F’), and corroborated the findings for fruGAL4-driven expression of UAS-mCD8::GFP. We crossed w; UAS-mCD8::GFP; fruGAL4 females to w; UAS-DSXF/SM6 males and examined three classes of progeny: control males and females that carried the SM6 chromosome, and males that expressed UAS-DSXF under the control of fruGAL4. As expected, control males (Fig. 5A) had more fruGAL4-expressing GRNs in their forelegs than control females (Fig. 5C), and only in control males were GRNs observed to have crossed the VNC midline (Fig. 5D, F). In contrast, when DSXF was expressed in the fruGAL4-expressing GRNs, midline crossing was eliminated (Fig. 5E), even though the number of GSOs appeared the same (Fig. 5B). This indicates that the roles of DSXF are temporally separable during development of the female foreleg: DSXF first acts early to regulate neurogenesis then acts in the terminally differentiated neuron to regulate axon guidance. Thus, dsx functions in two distinct developmental contexts within the GSO lineage.
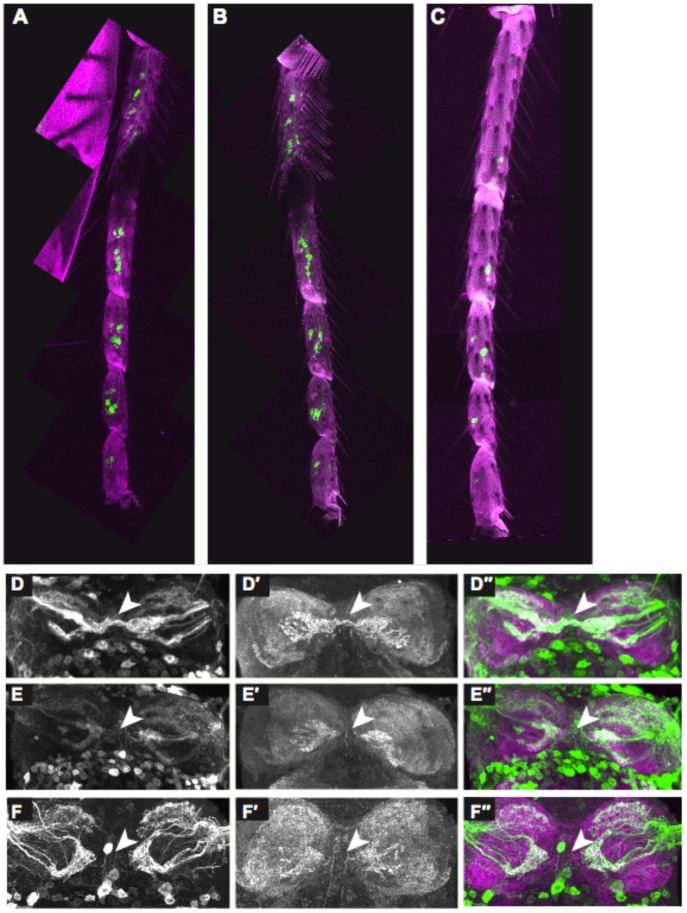
Figure 5. dsx regulates axonal morphology independent of GSO number.
(A–F”) fruGAL4 driving UAS-mCD8::GFP (green) labels GRN cell bodies and axons. Forelegs of (A) control male (UAS-mCD8::GFP/SM6; fruGAL4/+ ), (B) male with feminized GRNs (UAS-DSXF/UAS-mCD8::GFP; fruGAL4/+), and (C) control female (UAS-mCD8::GFP/SM6; fruGAL4/+). Cuticular autofluorescence (magenta). There is no difference in the number of FRUM-positive GRN clusters between forelegs of the two male genotypes, while both have more than the female. (D–F”) VNC prothoracic neuromeres with labeled GRN projections (D, E, F; green in D”, E”, F”) and counterstained for DN-cadherin (D’, E’, F’; magenta in D”, E”, F”). Arrowheads indicate the VNC midline. (D–D”) GRNs cross the midline in control males, but feminized male GRNs do not cross (E–E”). (F–F”) GRNs also do not cross the midline in control females.
Discussion
We report that dsx regulates the sexually dimorphic number of GSOs across all tarsal segments of the foreleg: DSXM promotes and DSXF represses the development of certain GSOs. The effects of this regulation are apparent by 8 h APF, when the GSOs are first identified, and the spatiotemporal pattern of DSX implies that dsx determines the number of gustatory SOPs. dsx exhibits a surprising degree of context sensitivity: the relative importance of DSXM and DSXF varies along the proximodistal axis of the foreleg and, during the course of GSO development, DSXF progresses from regulating cell fate to regulating axon guidance.
Given that dsx controls the formation of the other sexually dimorphic cuticular structures of the fly, as well as the number of GSOs in segment T1 of the foreleg [44], we anticipated that dsx would regulate the sex-specific GSO numbers in segments T2–T4 of the foreleg. However, the manner in which this regulation is achieved across the tarsal segments was surprising. Although each of the T1–T4 foreleg tarsal segments produces more GSOs in males than in females, in two segments this difference is achieved by promoting formation of several GSOs in males (via the action of DSXM), in one segment it is achieved by repressing the formation of several GSOs in females (via the action of DSXF), and in another segment, both DSXM and DSXF act to regulate GSO number. This is more complicated than the simpler a priori expectation that the function of dsx would be the same across the T1–T4 foreleg segments.
That DSXM and DSXF can be utilized differentially has been previously established. In the fat body, female-specific expression of Yp1 and Yp2 depends on up-regulation by DSXF in females and down-regulation by DSXM in males [8], [10], [74]. Thus, in dsx null flies, both sexes express these genes at equivalent levels. Similarly, DSXF activates and DSXM represses expression at the bric-a-brac locus to generate sex-specific pigmentation in the abdominal epithelium [75]. In these two cases, both DSX proteins contribute to regulation of a single trait, similar to the regulation of GSO number in T2. In contrast, desatF is activated by DSXF in oenocytes to produce female-specific pheromones without influence from DSXM [31]. This single isoform-mediated regulation bears similarity to the regulation of foreleg GSOs in T1, T3 and T4. Whereas the previous studies found that DSXM or DSXF were differentially utilized to sculpt sexually dimorphic traits arising from developmentally distinct tissues, we have found that these transcription factors can be differentially utilized across a single developmental field–the epithelium of the foreleg disc. Moreover, the differential roles of DSXM and DSXF in different tarsal segments suggest that each segment may have independently evolved a molecular mechanism for integrating sexual and proximodistal axis information within the foreleg disc to produce more GSOs in the male.
We also sought to determine when the function of dsx impacts neurogenesis to generate the numbers of GSOs. Although the details of foreleg GSO development have not been specifically reported, studies of the mechanosensory macrochaete lineages of the notum provide a basic framework for the multi-step process of sensory organ neurogenesis (reviewed in [64], [65], [76], [77]). The initiating event is patterned expression of the proneural genes ac and sc, which imparts the potential to produce SOPs to specific clusters of epithelial cells across the disc epithelium. Subsequent cell-cell interactions within the cluster typically specify a single SOP (see also [78], [79], [80]). The nascent SOP must then sustain its fate and undergo a series of stereotyped cell divisions to produce all of the cells of the sensory organ (reviewed in [76], [77]). Any of the molecular processes that underlie these stages could be influenced by the functions of dsx.
We were struck by the broad distribution of the DSX proteins across the T1–T4 foreleg disc epithelium before and at 0 h APF, a time when the gustatory SOPs are specified [52]. Because the number of DSX-positive cells far exceeded the number of gustatory SOPs necessary to give rise to the GSOs, we infer that dsx is acting prior to or during SOP formation. This is consistent with the frequent colocalization of DSX with AC in proneural clusters, which suggests that dsx might act within these cells to determine whether the SOP fate is promoted in males or repressed in females. The broad distribution of the DSX proteins could ensure that sexual information is available for integration with positional information across the foreleg disc epithelium to guide sexually dimorphic development.
In contrast to T2–T4, DSX was not apparent in the foreleg epithelium of T5, which produces a sexually monomorphic GSO number. Thus, the presence of DSX in the epithelium correlates with the adult sexual dimorphism in GSO number, consistent with the notion that dsx is expressed at the right time and place to impact SOP selection in the foreleg. However, at 0 h APF we observed DSX in two nascent sensory organs expressing ase-lacZ (Fig. 4A, and C. Robinett, unpublished data) [67], [68]. We speculate that these sensory organs correspond to the GSOs containing GRNs that express pickpocket 25 (ppk25), which is enriched in males and required for their normal response to female pheromones [81], [82]. Thus, the presence of DSX in the nascent GSOs may forecast sexually dimorphic gene expression in the adult GSO.
In addition to specifying foreleg GSO numbers, we observed a temporally distinct function for dsx in the GRNs. During pupal development, GRN axons project proximally along the leg nerves and into the VNC, and here the behavior of the axon depends on the activities of FRUM or DSXF. In males, FRUM promotes crossing of the VNC midline by the axons, but in females, DSXF represses this behavior [46]. This dual regulation causes GRN axons to project across the VNC midline only in males [45]. We previously proposed two competing hypotheses to explain the action of DSXF: 1) DSXF directly affects axon guidance in differentiating GRNs; or 2) only male-specific GRNs are competent to cross the VNC midline and DSXF indirectly affects midline crossing by repressing formation of the male-specific GSOs. Having shown that post-mitotic expression of DSXF in FruM-expressing GRNs subsequent to the establishment of GSO number prevents midline crossing, we now reject the second hypothesis. Moreover, the early sexual information that impacts GSO number does not irreversibly determine sex-specific development of the GRNs as they continue to be sensitive to the action of DSXF (and presumably FRUM). Because dsx and fru are classically thought of as acting in parallel, we were intrigued to find both genes regulating the same phenotype in a common set of GRNs. Determining whether they coregulate a common set of target genes or independently regulate distinct targets will be of great interest.
Because DSXM and DSXF differentially impact GSO numbers in different tarsal segments, and DSXF regulates the later process of axon guidance, the identity of the genes directly regulated by dsx during foreleg development likely changes with spatiotemporal context. Although we currently do not know which genes are directly regulated by dsx in the foreleg epithelium or the GSO lineage, the available data on in vivo DSX binding sites [41] may reveal genes that are known to be involved in peripheral neurogenesis or axon guidance. The challenge will then be to determine if such candidates exhibit sexually dimorphic expression in the different tarsal segments at different developmental time points. In this way, development of the foreleg GSOs presents a unique opportunity for investigating how dsx function is integrated with spatiotemporal context across a changing developmental landscape.
Supporting Information
Immunoreactivity of anti-DSXDBD. Late third instar larval tissues stained with anti-DSXDBD (white in A–F; green in A’–F’) and shown as partial projections of a confocal stack. Images A’–F’ are merged with DAPI-stained DNA (blue). (A, A’) Low resolution dorsal view of brain and VNC. (B, B’) Clusters of labeled nuclei (arrows) in posterior of brain. (C, C’) Labeled nuclei in the abdominal ganglion of the VNC. (D, D’) Labeled epithelial cell nuclei in tarsal segments of the foreleg imaginal disc. (E, E’) Labeled fat body nuclei. (F, F’) Labeled somatic cell nuclei of the male gonad include cyst cells (arrows) and the posterior cells (bracket). Scale bars (A,B) 50 µm and (C–F) 25 µm.
(TIF)
Immunoreactivity of anti-DSXDBD is specific to DSX. (A–B, E–G) Tissue from mature third instar larvae stained with anti-DSXDBD (white in A–G, red in A’ and B’, green in E’–G’) and merged with DAPI-stained DNA (blue in A’–G’). (A, A’) Wild-type male foreleg disc. (B, B’) Wild-type female foreleg disc, shown in lower magnification than male. (C and D) Wild-type male foreleg disc (C) and second leg disc (D) at 0 h APF showing distribution of immunoreactivity across foreleg tarsal segments. Tibia (tib). Note clusters of DSX-positive cells in T5 (arrows). (E,E’) dsx mutant foreleg disc homozygous for the dsx deficiency Df(3R)f00683-d07058, which was generated by FLP/FRT-mediated deletion of the native chromosomal sequence between piggyBac insertions f00683 and d07058, as per the methods of Parks et al. [Nat. Gen. 36(3):288-92. 2004]. Note loss of immunoreactivity. (F, F’) Wild-type male foreleg discs, second leg disc, and partial ventral view of CNS. Note that the second leg disc lacks immunoreactivity. (G, G’) Magnifed view of second leg disc from (F, F’). Scale bars (A, B, E) 50 µm, (C, D, G) 25 µm and (F) 100 µm.
(TIF)
Expression of poxn-GAL4 in the larval leg disc after puparium formation. 22C10 labels cells of the nascent gustatory sensillum, while poxn-GAL4 driving UAS- mCD8::GFP is expressed across the epithelium of T4 and T2. (A) 0 h APF. (B) 2 h APF. (C) 6 h APF. Each sample is compressed ot a different degree. Scale bars, 25 µm.
(TIF)
View of whole 8-h APF forelegs shown in Fig. 2 . Male (A) and female (B). The left panels show 22C10 staining, while the right panels are a merge of 22C10 (magenta), poxn-GAL4 driving UAS-mCD8::GFP (green), and DNA stained with DAPI (blue). Tarsal segments boundaries are indicated with light blue lines in left panels. Cells marked with 22C10 were classified based on both colocalization of poxn-GAL4 and morphology of the cells or cell clusters: GSO lineage cells (magenta arrows); non-GSO cells that lack poxn-GAL4 in T1 and T3 (dark blue arrows); non-GSO cells marked by poxn-GAL4 but lacking GSO morphology in T2 and T4 (light blue arrows). In panel (A), the row of 22C10-positive cells (bracket) in T1 are likely the sex comb SOPs. Scale bars, 50 µm.
(TIF)
Gustatory SOPs and daughter cells at 8 h APF. Male foreleg disc at 8 h APF with poxn-GAL4 driving UAS-mcd8::GFP (green), stained with 22C10 (red) and DAPI (blue). (A) Whole foreleg disc. Cells marked with the barbed arrowhead or arrowhead are enlarged in (B) and (C), respectively. (B) A large single cell in T3 is likely to be a pre- mitotic SOP. (C) Pair of large cells in T2 in which the lower cell has metaphase chromosomes (arrow). Note that poxn-GAL4 is expressed strongly in all cells of the T2 epithelium. Projections of confocal slices shown. Scale bars, (A) 50 µm and (B,C) 10 µm.
(TIF)
DSX is present in the daughters of a recently divided SOP. Shown is DAPI staining of the ase-lacZ–expressing, anti-DSXDBD-stained cells from Fig. 4B. Two masses of DNA can be distinguished (green arrows), indicating separate nuclei. This pair of tightly associated cells expressing ase-lacZ is assumed to be the immediate daughters of a recently divided SOP.
(TIF)
DSXM is not present in the male foreleg disc epithelium at 32 h 3I or preceding time points. (A–C) Male foreleg discs from the indicated time points of third instar larval development were stained for AC (left panels) and DSXM (middle panels). Right panels show merged images of DSXM (magenta), AC (green) and DAPI-stained DNA (blue). From 24–32 h 3I, DSXM is not detected in the foreleg disc, while AC is present in single cells and cell clusters mostly in T5 at the center of the discs. The number of AC-positive cells increases over time. All images are projections of only those confocal sections that encompass the majority of AC signal from a given disc as no DSXM signal was detected. Scale bars, 50 µm.
(TIF)
Some gustatory SOPs are dividing at 6 h APF. (A and B) T4–T2 region of male foreleg disc with poxn-GAL4 driving UAS-mCD8::GFP (green in middle and right panels) stained with DAPI (white in left panel, blue in middle and right panels) and 22C10 (red in right panel). (A) Several 22C10-positive cells expressing poxn-GAL4 have mitotic figures indicating cell division (arrows). (B) Enlargement of the dividing cell in the T3 region of (A) (arrow). Projection of only a few confocal sections shown. Scale bars (A) 10 µm and (B) 5 µm.
(TIF)
Acknowledgments
We thank Audrey E. Christiansen, Troy Shirangi and members of the Baker laboratory for discussion of the project. We greatly appreciate the contribution of Jonathan Marvin and Loren Looger in generating the DSX immunogen. We thank: Crystal Sullivan and Alison Howard for administrative support; Guennet Bohm, Karen Hibbard, Monti Mercer, and Todd Laverty for fly husbandry support; Brian Oliver and the Developmental Studies Hybridoma Bank for antibodies; Bloomington Stock Center at Indiana University for fly lines; and FlyBase for technical support.
Funding Statement
This work was funded by the National Institutes of Health and the Howard Hughes Medical Institute. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Gehring WJ (1996) The master control gene for morphogenesis and evolution of the eye. Genes Cells 1: 11–15. [DOI] [PubMed] [Google Scholar]
- 2. Kim J, Sebring A, Esch JJ, Kraus ME, Vorwerk K, et al. (1996) Integration of positional signals and regulation of wing formation and identity by Drosophila vestigial gene. Nature 382: 133–138. [DOI] [PubMed] [Google Scholar]
- 3. Nusslein-Volhard C, Wieschaus E (1980) Mutations affecting segment number and polarity in Drosophila. Nature 287: 795–801. [DOI] [PubMed] [Google Scholar]
- 4. Baker BS, Ridge KA (1980) Sex and the single cell. I. On the action of major loci affecting sex determination in Drosophila melanogaster. Genetics 94: 383–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cline TW, Meyer BJ (1996) Vive la difference: males vs females in flies vs worms. Annu Rev Genet 30: 637–702. [DOI] [PubMed] [Google Scholar]
- 6. Christiansen AE, Keisman EL, Ahmad SM, Baker BS (2002) Sex comes in from the cold: the integration of sex and pattern. Trends Genet 18: 510–516. [DOI] [PubMed] [Google Scholar]
- 7. Burtis KC, Baker BS (1989) Drosophila doublesex gene controls somatic sexual differentiation by producing alternatively spliced mRNAs encoding related sex-specific polypeptides. Cell 56: 997–1010. [DOI] [PubMed] [Google Scholar]
- 8. Burtis KC, Coschigano KT, Baker BS, Wensink PC (1991) The doublesex proteins of Drosophila melanogaster bind directly to a sex-specific yolk protein gene enhancer. EMBO J 10: 2577–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Erdman SE, Burtis KC (1993) The Drosophila doublesex proteins share a novel zinc finger related DNA binding domain. EMBO J 12: 527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coschigano KT, Wensink PC (1993) Sex-specific transcriptional regulation by the male and female doublesex proteins of Drosophila. Genes Dev 7: 42–54. [DOI] [PubMed] [Google Scholar]
- 11. Robinett CC, Vaughan AG, Knapp JM, Baker BS (2010) Sex and the single cell. II. There is a time and place for sex. PLoS Biol 8: e1000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rideout EJ, Dornan AJ, Neville MC, Eadie S, Goodwin SF (2010) Control of sexual differentiation and behavior by the doublesex gene in Drosophila melanogaster. Nat Neurosci 13: 458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Camara N, Whitworth C, Van Doren M (2008) The creation of sexual dimorphism in the Drosophila soma. Curr Top Dev Biol 83: 65–107. [DOI] [PubMed] [Google Scholar]
- 14. Sanchez L, Guerrero I (2001) The development of the Drosophila genital disc. Bioessays 23: 698–707. [DOI] [PubMed] [Google Scholar]
- 15. Wang W, Kidd BJ, Carroll SB, Yoder JH (2011) Sexually dimorphic regulation of the Wingless morphogen controls sex-specific segment number in Drosophila. Proc Natl Acad Sci U S A 108: 11139–11144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jallon JM, Lauge G, Orssaud L, Antony C (1988) Drosophila melanogaster female pheromones controlled by the doublesex locus. Genet Res 51: 17–22. [Google Scholar]
- 17. Taylor BJ, Truman JW (1992) Commitment of abdominal neuroblasts in Drosophila to a male or female fate is dependent on genes of the sex-determining hierarchy. Development 114: 625–642. [DOI] [PubMed] [Google Scholar]
- 18. Sanders LE, Arbeitman MN (2008) Doublesex establishes sexual dimorphism in the Drosophila central nervous system in an isoform-dependent manner by directing cell number. Dev Biol 320: 378–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kimura K, Hachiya T, Koganezawa M, Tazawa T, Yamamoto D (2008) Fruitless and doublesex coordinate to generate male-specific neurons that can initiate courtship. Neuron 59: 759–769. [DOI] [PubMed] [Google Scholar]
- 20. Rideout EJ, Billeter JC, Goodwin SF (2007) The sex-determination genes fruitless and doublesex specify a neural substrate required for courtship song. Curr Biol 17: 1473–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Manoli DS, Meissner GW, Baker BS (2006) Blueprints for behavior: genetic specification of neural circuitry for innate behaviors. Trends Neurosci 29: 444–451. [DOI] [PubMed] [Google Scholar]
- 22. Billeter JC, Rideout EJ, Dornan AJ, Goodwin SF (2006) Control of male sexual behavior in Drosophila by the sex determination pathway. Curr Biol 16: R766–776. [DOI] [PubMed] [Google Scholar]
- 23. Yamamoto D (2007) The neural and genetic substrates of sexual behavior in Drosophila. Adv Genet 59: 39–66. [DOI] [PubMed] [Google Scholar]
- 24. Dickson BJ (2008) Wired for sex: the neurobiology of Drosophila mating decisions. Science 322: 904–909. [DOI] [PubMed] [Google Scholar]
- 25. Shirangi TR, McKeown M (2007) Sex in flies: what ‘body–mind’ dichotomy? Dev Biol 306: 10–19. [DOI] [PubMed] [Google Scholar]
- 26. Taylor BJ, Villella A, Ryner LC, Baker BS, Hall JC (1994) Behavioral and neurobiological implications of sex-determining factors in Drosophila. Dev Genet 15: 275–296. [DOI] [PubMed] [Google Scholar]
- 27. Bownes M (1994) The regulation of the yolk protein genes, a family of sex differentiation genes in Drosophila melanogaster. Bioessays 16: 745–752. [DOI] [PubMed] [Google Scholar]
- 28. Chatterjee SS, Uppendahl LD, Chowdhury MA, Ip PL, Siegal ML (2011) The female-specific doublesex isoform regulates pleiotropic transcription factors to pattern genital development in Drosophila. Development 138: 1099–1109. [DOI] [PubMed] [Google Scholar]
- 29. Belote JM, Baker BS (1982) Sex determination in Drosophila melanogaster: analysis of transformer-2, a sex-transforming locus. Proc Natl Acad Sci U S A 79: 1568–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tanaka K, Barmina O, Sanders LE, Arbeitman MN, Kopp A (2011) Evolution of sex-specific traits through changes in HOX-dependent doublesex expression. PLoS Biol 9: e1001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shirangi TR, Dufour HD, Williams TM, Carroll SB (2009) Rapid evolution of sex pheromone-producing enzyme expression in Drosophila. PLoS Biol 7: e1000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bray S, Amrein H (2003) A putative Drosophila pheromone receptor expressed in male-specific taste neurons is required for efficient courtship. Neuron 39: 1019–1029. [DOI] [PubMed] [Google Scholar]
- 33. Bownes M, Nothiger R (1981) Sex determining genes and vitellogenin synthesis in Drosophila melanogaster. Mol Gen Genet 182: 222–228. [DOI] [PubMed] [Google Scholar]
- 34. Barmina O, Gonzalo M, McIntyre LM, Kopp A (2005) Sex- and segment-specific modulation of gene expression profiles in Drosophila. Dev Biol 288: 528–544. [DOI] [PubMed] [Google Scholar]
- 35. Lebo MS, Sanders LE, Sun F, Arbeitman MN (2009) Somatic, germline and sex hierarchy regulated gene expression during Drosophila metamorphosis. BMC Genomics 10: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Goldman TD, Arbeitman MN (2007) Genomic and functional studies of Drosophila sex hierarchy regulated gene expression in adult head and nervous system tissues. PLoS Genet 3: e216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Parisi M, Nuttall R, Edwards P, Minor J, Naiman D, et al. (2004) A survey of ovary-, testis-, and soma-biased gene expression in Drosophila melanogaster adults. Genome Biol 5: R40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fujii S, Amrein H (2002) Genes expressed in the Drosophila head reveal a role for fat cells in sex-specific physiology. EMBO J 21: 5353–5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dauwalder B, Tsujimoto S, Moss J, Mattox W (2002) The Drosophila takeout gene is regulated by the somatic sex-determination pathway and affects male courtship behavior. Genes Dev 16: 2879–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Arbeitman MN, Fleming AA, Siegal ML, Null BH, Baker BS (2004) A genomic analysis of Drosophila somatic sexual differentiation and its regulation. Development 131: 2007–2021. [DOI] [PubMed] [Google Scholar]
- 41. Luo SD, Shi GW, Baker BS (2011) Direct targets of the D. melanogaster DSXF protein and the evolution of sexual development. Development 138: 2761–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Grabowski CT, Dethier VG (1954) The structure of the tarsal chemoreceptors of the blowfly, Phormia regina Meigen. J Morphol 94: 1–19. [Google Scholar]
- 43. Nayak SV, Singh RN (1983) Sensilla on the tarsal segments and mouthparts of adult Drosophila melanogaster Meigen (Diptera: Drosophilidae). Int J Insect Morphol Embryol 12: 273–291. [Google Scholar]
- 44. Hildreth PE (1965) Doublesex, Recessive Gene That Transforms Both Males and Females of Drosophila into Intersexes. Genetics 51: 659–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Possidente DR, Murphey RK (1989) Genetic control of sexually dimorphic axon morphology in Drosophila sensory neurons. Dev Biol 132: 448–457. [DOI] [PubMed] [Google Scholar]
- 46. Mellert DJ, Knapp JM, Manoli DS, Meissner GW, Baker BS (2010) Midline crossing by gustatory receptor neuron axons is regulated by fruitless, doublesex and the Roundabout receptors. Development 137: 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Boll W, Noll M (2002) The Drosophila Pox neuro gene: control of male courtship behavior and fertility as revealed by a complete dissection of all enhancers. Development 129: 5667–5681. [DOI] [PubMed] [Google Scholar]
- 48. Schuldiner O, Berdnik D, Levy JM, Wu JS, Luginbuhl D, et al. (2008) piggyBac-based mosaic screen identifies a postmitotic function for cohesin in regulating developmental axon pruning. Dev Cell 14: 227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stockinger P, Kvitsiani D, Rotkopf S, Tirian L, Dickson BJ (2005) Neural circuitry that governs Drosophila male courtship behavior. Cell 121: 795–807. [DOI] [PubMed] [Google Scholar]
- 50. Erdman SE, Chen HJ, Burtis KC (1996) Functional and genetic characterization of the oligomerization and DNA binding properties of the Drosophila doublesex proteins. Genetics 144: 1639–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Orenic TV, Held LI Jr, Paddock SW, Carroll SB (1993) The spatial organization of epidermal structures: hairy establishes the geometrical pattern of Drosophila leg bristles by delimiting the domains of achaete expression. Development 118: 9–20. [DOI] [PubMed] [Google Scholar]
- 52. Nottebohm E, Ramaekers A, Dambly-Chaudiere C, Ghysen A (1994) The leg of Drosophila as a model system for the analysis of neuronal diversity. J Physiol Paris 88: 141–151. [DOI] [PubMed] [Google Scholar]
- 53. Awasaki T, Kimura K (1997) pox-neuro is required for development of chemosensory bristles in Drosophila. J Neurobiol 32: 707–721. [DOI] [PubMed] [Google Scholar]
- 54. Ray K, Hartenstein V, Rodrigues V (1993) Development of the taste bristles on the labellum of Drosophila melanogaster. Dev Biol 155: 26–37. [DOI] [PubMed] [Google Scholar]
- 55. Hempel LU, Oliver B (2007) Sex-specific DoublesexM expression in subsets of Drosophila somatic gonad cells. BMC Dev Biol 7: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Truman JW, Schuppe H, Shepherd D, Williams DW (2004) Developmental architecture of adult-specific lineages in the ventral CNS of Drosophila. Development 131: 5167–5184. [DOI] [PubMed] [Google Scholar]
- 57.Demerec M (1950) Biology of Drosophila. New York: John Wiley & Sons.
- 58. Meunier N, Ferveur JF, Marion-Poll F (2000) Sex-specific non-pheromonal taste receptors in Drosophila. Curr Biol 10: 1583–1586. [DOI] [PubMed] [Google Scholar]
- 59.Venard R, Antony C, Jallon J-M (1989) Drosophila chemoreceptors; Singh RN, Strausfeld NJ, editors. London: Plenum Press.
- 60. Nagoshi RN, Baker BS (1990) Regulation of sex-specific RNA splicing at the Drosophila doublesex gene: cis-acting mutations in exon sequences alter sex-specific RNA splicing patterns. Genes Dev 4: 89–97. [DOI] [PubMed] [Google Scholar]
- 61. Awasaki T, Kimura K (2001) Multiple function of poxn gene in larval PNS development and in adult appendage formation of Drosophila. Dev Genes Evol 211: 20–29. [DOI] [PubMed] [Google Scholar]
- 62. Hartenstein V, Posakony JW (1989) Development of adult sensilla on the wing and notum of Drosophila melanogaster. Development 107: 389–405. [DOI] [PubMed] [Google Scholar]
- 63. Hannah-Alava A (1958) Morphology and chaetotaxy of the legs of Drosophila melanogaster. J Morphol 103: 281–310. [Google Scholar]
- 64. Gomez-Skarmeta JL, Campuzano S, Modolell J (2003) Half a century of neural prepatterning: the story of a few bristles and many genes. Nat Rev Neurosci 4: 587–598. [DOI] [PubMed] [Google Scholar]
- 65. Calleja M, Renaud O, Usui K, Pistillo D, Morata G, et al. (2002) How to pattern an epithelium: lessons from achaete-scute regulation on the notum of Drosophila. Gene 292: 1–12. [DOI] [PubMed] [Google Scholar]
- 66. Simpson P, Woehl R, Usui K (1999) The development and evolution of bristle patterns in Diptera. Development 126: 1349–1364. [DOI] [PubMed] [Google Scholar]
- 67. Brand M, Jarman AP, Jan LY, Jan YN (1993) asense is a Drosophila neural precursor gene and is capable of initiating sense organ formation. Development 119: 1–17. [DOI] [PubMed] [Google Scholar]
- 68. Jarman AP, Brand M, Jan LY, Jan YN (1993) The regulation and function of the helix-loop-helix gene, asense, in Drosophila neural precursors. Development 119: 19–29. [DOI] [PubMed] [Google Scholar]
- 69. Skeath JB, Carroll SB (1991) Regulation of achaete-scute gene expression and sensory organ pattern formation in the Drosophila wing. Genes Dev 5: 984–995. [DOI] [PubMed] [Google Scholar]
- 70. Culi J, Modolell J (1998) Proneural gene self-stimulation in neural precursors: an essential mechanism for sense organ development that is regulated by Notch signaling. Genes Dev 12: 2036–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Huang F, Dambly-Chaudiere C, Ghysen A (1991) The emergence of sense organs in the wing disc of Drosophila. Development 111: 1087–1095. [DOI] [PubMed] [Google Scholar]
- 72. Boulianne GL, de la Concha A, Campos-Ortega JA, Jan LY, Jan YN (1991) The Drosophila neurogenic gene neuralized encodes a novel protein and is expressed in precursors of larval and adult neurons. EMBO J 10: 2975–2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Iwai Y, Hirota Y, Ozaki K, Okano H, Takeichi M, et al. (2002) DN-cadherin is required for spatial arrangement of nerve terminals and ultrastructural organization of synapses. Mol Cell Neurosci 19: 375–388. [DOI] [PubMed] [Google Scholar]
- 74. An W, Wensink PC (1995) Integrating sex- and tissue-specific regulation within a single Drosophila enhancer. Genes Dev 9: 256–266. [DOI] [PubMed] [Google Scholar]
- 75. Williams TM, Selegue JE, Werner T, Gompel N, Kopp A, et al. (2008) The regulation and evolution of a genetic switch controlling sexually dimorphic traits in Drosophila. Cell 134: 610–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lai EC, Orgogozo V (2004) A hidden program in Drosophila peripheral neurogenesis revealed: fundamental principles underlying sensory organ diversity. Dev Biol 269: 1–17. [DOI] [PubMed] [Google Scholar]
- 77. Furman DP, Bukharina TA (2008) How Drosophila melanogaster Forms its Mechanoreceptors. Curr Genomics 9: 312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Campuzano S (2001) Emc, a negative HLH regulator with multiple functions in Drosophila development. Oncogene 20: 8299–8307. [DOI] [PubMed] [Google Scholar]
- 79. Ghysen A, Dambly-Chaudiere C, Jan LY, Jan YN (1993) Cell interactions and gene interactions in peripheral neurogenesis. Genes Dev 7: 723–733. [DOI] [PubMed] [Google Scholar]
- 80. Culi J, Martin-Blanco E, Modolell J (2001) The EGF receptor and N signalling pathways act antagonistically in Drosophila mesothorax bristle patterning. Development 128: 299–308. [DOI] [PubMed] [Google Scholar]
- 81. Lin H, Mann KJ, Starostina E, Kinser RD, Pikielny CW (2005) A Drosophila DEG/ENaC channel subunit is required for male response to female pheromones. Proc Natl Acad Sci U S A 102: 12831–12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Starostina E, Liu T, Vijayan V, Zheng Z, Siwicki KK, et al. (2012) A Drosophila DEG/ENaC subunit functions specifically in gustatory neurons required for male courtship behavior. J Neurosci 32: 4665–4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Immunoreactivity of anti-DSXDBD. Late third instar larval tissues stained with anti-DSXDBD (white in A–F; green in A’–F’) and shown as partial projections of a confocal stack. Images A’–F’ are merged with DAPI-stained DNA (blue). (A, A’) Low resolution dorsal view of brain and VNC. (B, B’) Clusters of labeled nuclei (arrows) in posterior of brain. (C, C’) Labeled nuclei in the abdominal ganglion of the VNC. (D, D’) Labeled epithelial cell nuclei in tarsal segments of the foreleg imaginal disc. (E, E’) Labeled fat body nuclei. (F, F’) Labeled somatic cell nuclei of the male gonad include cyst cells (arrows) and the posterior cells (bracket). Scale bars (A,B) 50 µm and (C–F) 25 µm.
(TIF)
Immunoreactivity of anti-DSXDBD is specific to DSX. (A–B, E–G) Tissue from mature third instar larvae stained with anti-DSXDBD (white in A–G, red in A’ and B’, green in E’–G’) and merged with DAPI-stained DNA (blue in A’–G’). (A, A’) Wild-type male foreleg disc. (B, B’) Wild-type female foreleg disc, shown in lower magnification than male. (C and D) Wild-type male foreleg disc (C) and second leg disc (D) at 0 h APF showing distribution of immunoreactivity across foreleg tarsal segments. Tibia (tib). Note clusters of DSX-positive cells in T5 (arrows). (E,E’) dsx mutant foreleg disc homozygous for the dsx deficiency Df(3R)f00683-d07058, which was generated by FLP/FRT-mediated deletion of the native chromosomal sequence between piggyBac insertions f00683 and d07058, as per the methods of Parks et al. [Nat. Gen. 36(3):288-92. 2004]. Note loss of immunoreactivity. (F, F’) Wild-type male foreleg discs, second leg disc, and partial ventral view of CNS. Note that the second leg disc lacks immunoreactivity. (G, G’) Magnifed view of second leg disc from (F, F’). Scale bars (A, B, E) 50 µm, (C, D, G) 25 µm and (F) 100 µm.
(TIF)
Expression of poxn-GAL4 in the larval leg disc after puparium formation. 22C10 labels cells of the nascent gustatory sensillum, while poxn-GAL4 driving UAS- mCD8::GFP is expressed across the epithelium of T4 and T2. (A) 0 h APF. (B) 2 h APF. (C) 6 h APF. Each sample is compressed ot a different degree. Scale bars, 25 µm.
(TIF)
View of whole 8-h APF forelegs shown in Fig. 2 . Male (A) and female (B). The left panels show 22C10 staining, while the right panels are a merge of 22C10 (magenta), poxn-GAL4 driving UAS-mCD8::GFP (green), and DNA stained with DAPI (blue). Tarsal segments boundaries are indicated with light blue lines in left panels. Cells marked with 22C10 were classified based on both colocalization of poxn-GAL4 and morphology of the cells or cell clusters: GSO lineage cells (magenta arrows); non-GSO cells that lack poxn-GAL4 in T1 and T3 (dark blue arrows); non-GSO cells marked by poxn-GAL4 but lacking GSO morphology in T2 and T4 (light blue arrows). In panel (A), the row of 22C10-positive cells (bracket) in T1 are likely the sex comb SOPs. Scale bars, 50 µm.
(TIF)
Gustatory SOPs and daughter cells at 8 h APF. Male foreleg disc at 8 h APF with poxn-GAL4 driving UAS-mcd8::GFP (green), stained with 22C10 (red) and DAPI (blue). (A) Whole foreleg disc. Cells marked with the barbed arrowhead or arrowhead are enlarged in (B) and (C), respectively. (B) A large single cell in T3 is likely to be a pre- mitotic SOP. (C) Pair of large cells in T2 in which the lower cell has metaphase chromosomes (arrow). Note that poxn-GAL4 is expressed strongly in all cells of the T2 epithelium. Projections of confocal slices shown. Scale bars, (A) 50 µm and (B,C) 10 µm.
(TIF)
DSX is present in the daughters of a recently divided SOP. Shown is DAPI staining of the ase-lacZ–expressing, anti-DSXDBD-stained cells from Fig. 4B. Two masses of DNA can be distinguished (green arrows), indicating separate nuclei. This pair of tightly associated cells expressing ase-lacZ is assumed to be the immediate daughters of a recently divided SOP.
(TIF)
DSXM is not present in the male foreleg disc epithelium at 32 h 3I or preceding time points. (A–C) Male foreleg discs from the indicated time points of third instar larval development were stained for AC (left panels) and DSXM (middle panels). Right panels show merged images of DSXM (magenta), AC (green) and DAPI-stained DNA (blue). From 24–32 h 3I, DSXM is not detected in the foreleg disc, while AC is present in single cells and cell clusters mostly in T5 at the center of the discs. The number of AC-positive cells increases over time. All images are projections of only those confocal sections that encompass the majority of AC signal from a given disc as no DSXM signal was detected. Scale bars, 50 µm.
(TIF)
Some gustatory SOPs are dividing at 6 h APF. (A and B) T4–T2 region of male foreleg disc with poxn-GAL4 driving UAS-mCD8::GFP (green in middle and right panels) stained with DAPI (white in left panel, blue in middle and right panels) and 22C10 (red in right panel). (A) Several 22C10-positive cells expressing poxn-GAL4 have mitotic figures indicating cell division (arrows). (B) Enlargement of the dividing cell in the T3 region of (A) (arrow). Projection of only a few confocal sections shown. Scale bars (A) 10 µm and (B) 5 µm.
(TIF)