Abstract
Background
Evidence from prospective studies on intake of meat and fish and risk of squamous cell carcinoma (SCC) of the upper aero-digestive tract (UADT) is scarce. We prospectively investigated the association of meat and fish intake with risk of SCC of the UADT and the possible mechanism via heme iron in the large multi-center European Prospective Investigation into Cancer and Nutrition (EPIC) study.
Methods
Multivariable proportional hazards models were used to estimate relative risks of SCC of the UADT in relation to intake of total meat, as well as subtypes of meat, fish and heme iron among 348,738 individuals from 7 European countries.
Results
During an average follow-up of 11.8 years, a total of 682 incident cases of UADT SCC were accrued. Intake of processed meat was positively associated with risk of SCC of the UADT in the total cohort (highest versus lowest quintile: RR=1.41; 95% CI=1.03-1.94), however, in stratified analyses, this association was confined to the group of current smokers (highest versus lowest quintile: RR=1.89; 95% CI=1.22-2.93). Red meat, poultry, fish and heme iron were not consistently related to UADT SCC.
Conclusion
Higher intake of processed meat was positively associated with SCC of the UADT among smokers. Although this finding was stable in various sensitivity analyses, we cannot rule out residual confounding by smoking. Confirmation in future studies and identification of biological mechanisms is warranted.
Impact
Smokers may further increase their risk for SCC of the UADT if they additionally consume large amounts of processed meat.
Keywords: Meat intake, Heme iron, Upper aero-digestive tract cancer, Smoking, cohort study
INTRODUCTION
Cancers of the oral cavity, pharynx, larynx and esophagus, collectively referred to as cancers of the upper aero-digestive tract (UADT), account for 8% of all incident cancer cases worldwide (1). Despite improved methods of detection and advances in treatment, they continue to have a poor prognosis (2, 3). UADT cancers exist in two main histological subtypes, squamous cell carcinoma (SCC) and adenocarcinoma, which show distinct etiological and pathological characteristics. Although the incidence of adenocarcinomas of the esophagus has overtaken that of esophageal SCC in Western countries during the last decades, SCCs are still the dominant histological type for cancers of the mouth, pharynx and larynx, accounting for 90% of all cases worldwide (4).
Tobacco smoking and regular alcohol consumption are the main risk factors for SSC of the UADT (4-6). In terms of diet, the most consistent finding has been the protective effect of a high fruit and vegetable intake (4). Because meat may contain several carcinogenic compounds, including readily available heme iron, mutagens formed during high-temperature cooking and mutagens from nitrite-preserved meat, it has been the focus of many epidemiological studies. Specifically, high intake of red and processed meat is a well-established risk factor for colorectal cancer, its association to SCC of the UADT, however, is not yet clear (4). The few prospective studies on the relation of meat intake with SSC of the UADT have not resulted in a clear picture and, in many circumstances, lacked sufficient case numbers (7-13). Briefly, red meat was associated with higher risk of laryngeal and esophageal SCC in the NIH-AARP Diet and Health study (8, 13), while processed meat was not significantly related to risk of these cancer sites in that study. Although limited in size, a Norwegian study and a study among Hawaii Japanese men suggested a higher risk of SCC of the UADT for intake of bacon (7, 10). Among 33 UADT cancer cases of unspecified histology in the Iowa Women’s Health study, only combined risk estimates for processed meat and fish intake were reported and suggested a higher risk with higher frequency of intake (12).
In contrast to red and processed meat, cancer-protective effects have been ascribed to a high fish intake; the evidence for an association with SCC of the UADT, however, is sparse and was judged too limited to draw any conclusions (4).
The European Prospective Investigation into Cancer and Nutrition (EPIC) study includes individuals from 10 European countries with large differences in meat intake, a considerable number of UADT cancer cases and detailed data on smoking and alcohol consumption habits. Our aim was therefore to further elucidate the role of meat, its subtypes and fish intake in the development of SCC of the UADT and the potential mechanism via heme iron.
MATERIAL AND METHODS
Study population
EPIC is a large multi-center prospective cohort study designed primarily to investigate the relationship between diet, lifestyle and genetic factors and cancer incidence (14, 15). Briefly, between 1992 and 2000, a total of 521,448 participants were recruited in 23 administrative centers from 10 European countries: Denmark, Sweden, Norway, the United Kingdom, France, The Netherlands, Germany, Spain, Italy, and Greece. Participants provided written informed consent. Approval for this study was obtained from the ethical review boards of the International Agency for Research on Cancer and from all relevant local ethics committee in the participating countries.
We excluded participants if they reported prevalent cancer at baseline (n=23,785), if they had incomplete questionnaire data or missing dates of cancer diagnosis or follow-up (n=10,618), or if they were in the top or bottom 1% of the distribution of the ratio of energy intake vs. energy requirement (n=9,601). In addition, the cohorts of Norway (n=35,170) and Greece (n=26,032) were excluded due to very few cases of UADT SCC (9 in both centers) and the French cohort (n=67,386) because of incomplete case identification routines in this cohort for the cancer sites under study. The analytical cohort finally comprised 348,738 participants (131,453 men, 217,285 women).
Diet and lifestyle assessment
Habitual diet over the past 12 months was assessed at baseline by means of country-specific validated questionnaires (14, 16). In most countries, extensive quantitative food frequency questionnaires (FFQ) were used. In Denmark, Norway, Naples (Italy), and Umea (Sweden), semi-quantitative FFQs were administered. A combination of dietary methods (semi-quantitative FFQ and diet record) was adopted in Malmö (Sweden) and the UK. Diet history questionnaires were used in Spain. In addition to the dietary questionnaire, highly standardized, computer-based 24-hour dietary recall (24-HDR) measurements were obtained from representative sub-samples (5-12%) of each EPIC cohort (17). These 24-HDR data were used to correct for systematic differences between the dietary questionnaires and to minimize measurement error of the FFQ by calibration (18).
The exposure variables considered were daily intake of total meat, as well as its subgroups (red meat, processed meat, poultry), fish and heme iron from meat. Red meat included all fresh, minced and frozen beef, veal, pork, and lamb. Processed meats were mostly pork and beef that have undergone some form of preservation other than freezing, such as salting, smoking, marinating, air drying, or heating (e.g. ham, bacon, sausages, meat cuts, salami), and a small part of minced meat that has been bought as a ready-to-eat product (unknown recipe, e.g. hamburgers and meat balls). Lamb and poultry are rarely processed into these types of meats in Europe. Poultry included all fresh, frozen, minced chicken, and turkey. In some countries, rabbit (domestic) was also included, although 77% of the study population did not consume rabbit and among those who did, the contribution to total meat intake from rabbit was only 4%. Fish included whole fish, fish products, crustaceans, molluscs, and fish in crumbs. Food consumption data from the dietary questionnaires were used to calculate total dietary iron intake using country-specific food composition databases, which had been standardized across countries (19). Heme iron intake from meat was computed by applying type-specific proportions of heme iron to the total iron content of different types of meat and fish derived from published values: 65% for beef, 39% for pork, and 26% for chicken and fish, respectively (20). Since beef intake was estimated based on only 2 recipes in Umea (Sweden), this cohort was not included in the analysis on iron intake.
Lifestyle questionnaires included detailed questions on smoking habits at baseline and history of tobacco consumption, current alcohol consumption and lifetime history of alcoholic beverage consumption, occupation, medical history, and physical activity.
Body weight and height were measured in all centers, except for part of the Oxford cohort, for which self-reported anthropometric data were collected (21). Body-Mass-Index (BMI) was calculated by dividing weight in kilograms by height in meters squared (kg/m2).
Follow-up and ascertainment of endpoints
The follow-up was based on population cancer registries (Denmark, The Netherlands, Spain, Sweden, the United Kingdom, and Italy) or a combination of methods including linkage with health insurance records, contacts with cancer and pathology registries, and active follow-up through study participants and their next-of-kin (Germany). Mortality data were also obtained from either the cancer or mortality registries at the regional or national level.
Each participant was followed for incidence of SCC of the UADT from study entry to cancer diagnosis, emigration, loss to follow-up, death, or end of follow-up, whichever came first. For centers covered by cancer registries, specific censoring dates were established depending on the dates in which the cancer registries were considered complete: December 2004 (Asturias), December 2006 (Florence, Varese, Ragusa, Granada, San Sebastian), December 2007 (Murcia, Navarra, Oxford, Bilthoven, Aarhus, Copenhagen), June 2008 (Cambridge), and December 2008 (Turin, Utrecht, Malmo, Umea). For those countries using individually based follow-up, the end of follow-up was considered to be the date of the last known contact, or date of diagnosis, or date of death, whichever came first. Cancer incidence data were collected following the rules of the second revision of the International Classification of Diseases for Oncology (ICD-O-2) and converted to ICD-10 for the analysis.
We included incident primary SCC of the oral cavity including the tongue (C01–C06), oropharynx (C09–C10) and hypopharynx (C13–C14), esophagus (C15) and larynx (C32) in our study. The majority of cancer diagnoses (90%) were based on a histological confirmation.
Statistical analysis
Associations between meat intake and risk of SCC of the UADT were analyzed by estimating relative risks (RR) as hazard ratios using Cox proportional hazards models. Age was taken as the underlying time variable with entry time t0 and exit time t1 defined as the participants’ age at recruitment and age at cancer diagnosis or censoring, respectively. In all models, the variables center and age at recruitment (1-year categories) were used as stratification variables to control for differences in questionnaire design, follow-up procedures, and other non-measured center effects, and allow for more flexibility with the assumption of proportionality of risks.
To control for the effect of energy intake, intake of meat and other dietary variables, except alcohol, was adjusted for energy intake by the multivariate nutrient density method (22). Individuals were classified into quintiles of energy-adjusted meat intake based on the distribution among the total cohort and RR were estimated for quintiles of intake with the first quintile as reference. To test for a linear trend across categories, the median value within quintiles was used as score variable. Meat intake was also analyzed as continuous variable with increments of 20g/1000 kcals for total meat, 10g/1000 kcals for red and processed meat, 5g/1000 kcals for poultry, 10g/1000kcals for fish, and 200μg/1000kcals for dietary heme iron, corresponding approximately to one standard deviation in intake of the respective intake variable. Except for poultry (12%), non-consumer status of meat intake only included a negligible number of participants and non-consumers were not investigated as a separate intake category. However, we included a variable indicating non-consumer status in all models.
In model 1, RRs were adjusted for non-consumer status (0/1), sex, energy intake from fat and non-fat sources, and education (none/primary, technical/professional, secondary school, university, not specified). Red meat, poultry and processed meat were mutually adjusted. Model 2 additionally included a comprehensive variable for smoking habits (lifelong non-smoking, former smoking with quitting ≥10 years, former smoking with quitting <10 years, current smoking with <15 cigarettes/day, current smoking with 15-24, current smoking with ≥25 cigarettes/day, current smoking other than cigarettes combined with smoking with unknown quantity, and missing). The final model (model 3) was further adjusted for alcohol consumption (g/d), drinking history (never, former, unknown), BMI (kg/m2), physical activity (inactive, moderately inactive, moderately active, and active), citrus and non-citrus fruits, and vegetables.
Departure from the proportional hazards assumption was evaluated for each exposure variable by Schoenfeld residuals. No violations were detected.
Sex-specific differences in the association of meat intake with UADT cancer were evaluated using interaction terms. P values for all tests of interaction were based on the likelihood ratio test for the comparison of a model with interaction term to a model without interaction term. Since there was no evidence of effect modification by sex (all P for interaction >0.05), we present the results for both sexes combined. Further, we investigated possible effect modification with smoking status (never, former, current) and drinking status (non-users versus users of alcohol) by conducting stratified analyses and evaluating interaction terms. To rule out reverse causation, sensitivity analyses were performed by excluding cases diagnosed during the first two, three and five years of follow-up. Analyses were performed using SAS (Statistical Analysis System, version 9.2; SAS Institute Inc, Cary, NC). For all analyses, 2-sided P values <0.05 were considered statistically significant.
A linear regression calibration approach was used to improve the comparability of dietary data across participating centers and to correct relative risk estimates for systematic over- or underestimation of dietary intake (18, 23, 24). Country- and sex-specific calibration models were applied to obtain individual predicted values of dietary exposures for all study participants. Specifically, the 24-HDR measurements were regressed on dietary questionnaires. For zero consumption values reported in the main dietary questionnaire a zero was directly imputed as the corrected value. Negative values occasionally arising after regression were set to zero as well. Age at recruitment, center, weight, and height were included as covariates in the calibration model, and data were weighted by the day of the week and season of the year on which the 24-HDR was collected. Predicted values were modeled as continuous variables in the risk models.
RESULTS
During an average follow-up of 11.8 ± 2.4 years (4,107,300 person-years), a total of 682 incident cases of SCC of the UADT have been accrued among 348,738 study participants (Table 1). According to cancer site, 325 (48%) cancers were located in the oral cavity and pharynx, 206 (30%) in the larynx and 151 (22%) in the esophagus. Mean age at recruitment was 51.1 years. The overall mean intake of total meat was 47.7 g/1000 kcals, with the highest intake observed in Spain (60.1 g/1000 kcals) and the lowest intake observed in the UK health conscious cohort (22.1 g/1000 kcals). Individuals consuming larger amounts of total meat intake were more likely to be men and to be current smokers, while they were less likely to have a university degree and to be physically active (Table 2). They further tended to have a higher BMI and reported higher intake of alcohol and fish but lower intake of fruits and vegetables than individuals with lower meat consumption.
Table 1. Number of incident cases during 12 years of follow-up across centers of the EPIC study and mean intake of meat and its subtypes.
| Country |
|
Meanintake (g/1000kcals) |
|||||
|---|---|---|---|---|---|---|---|
| Cohort size (n) |
Person- years |
Squamous cell carcinoma (n) |
Total meat | Red meat | Processed meat |
Poultry | |
| Italy | 44,541 | 515,923 | 45 | 46.1 (19.9) | 21.9 (13.2) | 10.7 (8.0) | 11.7 (8.7) |
| Spain | 40,002 | 493,380 | 88 | 60.1 (24.9 ) | 20.1 (15.4) | 17.0 (13.8) | 17.7 (14.3) |
| UK HC | 45,888 | 510,513 | 39 | 22.1 (12.2) | 9.1 (4.8) | 8.2 (4.8) | 5.1 (6.1) |
| UK GP | 29,510 | 354,000 | 58 | 43.0 (14.7) | 14.4 (8.8) | 14.3 (6.6) | 12.8 (6.4) |
| The Netherlands | 36,505 | 443,646 | 62 | 51.5 (24.2) | 30.4 (16.7) | 14.3 (11.5) | 6.3 (7.2) |
| Germany | 48,583 | 495,506 | 84 | 51.2 (24.7) | 15.0 (11.1) | 29.3 (17.3) | 6.3 (6.4) |
| Sweden | 48,693 | 669,709 | 94 | 45.0 (19.1) | 13.3 (10.5) | 20.0 (12.1) | 5.3 (6.4) |
| Denmark | 55,016 | 624,623 | 212 | 59.8 (20.6) | 34.8 (14.3) | 13.1 (8.6) | 10.1 (8.7) |
| Total | 348,738 | 4,107,300 | 682 | 47.7 (15.9) | 20.5 (16.1) | 15.5 (8.9) | 9.4 (10.2) |
All values are means (SD) as estimated from the dietary questionnaires.
UK HC = United Kingdom Health Conscious; UK GP = United Kingdom General Population.
Table 2. Baseline characteristics of the cohort according to study-wide quintiles of total meat intake in the EPIC study (n=348,738).
|
Quintiles of total meat intake (g/1000 kcals)
|
|||||
|---|---|---|---|---|---|
| Characteristics | Q1 | Q2 | Q3 | Q4 | Q5 |
| Total meat, g/1000 kcals (median, range) | 12.1 (0-27) | 35.0 (27-41) | 47.2 (41-53) | 59.8 (53-68) | 79.8 (68-332) |
| Men (%) | 27.1 | 34.1 | 37.6 | 42.0 | 57.7 |
| Age, y (SD) | 47.0 (13.0) | 51.8 (10.3) | 52.2 (9.8) | 52.3 (9.3) | 52.2 (8.9) |
| Mean BMI, kg/m2 (SD) | 24.1 (3.8) | 25.5 (4.0) | 25.9 (4.1) | 26.3 (4.2) | 27.1 (4.3) |
| Energy from fat, kcal/d (mean, SD) | 668 (263) | 727 (264) | 741 (260) | 749 (262) | 745 (274) |
| Energy from nonfat, kcal/d (mean, SD) | 1346 (408) | 1417 (426) | 1409 (414) | 1388 (406) | 1324 (406) |
| Citrus fruits, g/1000 kcals (median, IQR) | 17.1 (32.8) | 16.8 (31.5) | 15.0 (29) | 13.3 (27.4) | 10.9 (27.1) |
| Non-citrus fruits, g/1000 kcals (median, IQR) | 89.5 (92.5) | 74.4 (78.3) | 69.0 (74) | 63.6 (70.8) | 57.8 (72.4) |
| Vegetables, g/1000 kcals (median, IQR) | 103.4 (61.8) | 70.4 (63.5) | 71.0 (61.2) | 73.1 (60.2) | 77.5 (66.3) |
| Fish, g/1000kcals (mean, SD) | 4.4 (12.9) | 8.4 (12.3) | 9.5 (12.7) | 10.4 (13.4) | 11.1 (14.8) |
| Alcohol, non-consumer (%) | 9,3 | 12,6 | 11,7 | 11,7 | 13,8 |
| Alcohol, g/d (median, IQR) * | 5.8 (11.5) | 6.9 (14.9) | 8.7 (17.9) | 10.1 (20.0) | 10.6 (20.4) |
| Lifelong non-smokers (%) † | 51,8 | 43,9 | 42,6 | 40,6 | 38,1 |
| Former smokers (%) † | 27.8 | 27,1 | 27,3 | 27,8 | 27,8 |
| Current smokers (%) † | 19,7 | 28,1 | 29,4 | 30,9 | 33,4 |
| University degree (%) | 36.8 | 22.5 | 20,2 | 18,9 | 17,2 |
| Physically active (%) | 22,0 | 20,3 | 20,8 | 21,1 | 20,5 |
All continuous variables are expressed either as mean (SD) or median and (IQR).
Only among alcohol consumers at baseline.
Percentages do not add up to 100% because information on smoking status was missing for 2,508 individuals (0.7%).
Table 3 shows the relative risks and corresponding 95% confidence intervals (95% CI) of UADT cancer by quintiles of intake of total meat, its subtypes, fish and heme iron. In the fully adjusted model (model 3), higher intake of total meat was associated with higher risk of UADT SCC (highest compared with the lowest quintile: RR=1.37; 95% CI=1.00-1.88, P for trend = 0.01). On a continuous scale, a 20g/1000 kcals higher intake of total meat was related to a 9% (95% CI=1.02-1.17) higher risk for SCC of the UADT. Red meat was not associated with UADT cancer risk. For poultry, significant inverse relations were observed from third to fifth quintile, while the trend test was not significant and there was no association on a continuous scale. With respect to processed meat, individuals in the highest quintile of intake had a 41% higher risk for UADT SCC (95% CI=1.03-1.94, P for trend = 0.01) compared to individuals in the first quintile. Per 10g/1000kcals of processed meat, UADT cancer risk increased by 13% (95% CI=1.06-1.20). According to subtype of processed meat, a positive association was found for ham and meatballs (RR=1.11 (1.02-1.20) and 1.21 (0.99-1.47) per 5g/1000kcals, respectively), while bacon and hamburger were not related to UADT cancer risk (RR=1.00 (0.98-1.02) and 0.77 (0.50-1.20) per 5g/1000kcals, respectively, data not shown). Fish intake was not related to risk of UADT SCC. Also, we observed no clear association for heme iron intake in categorical analyses, in continuous analyses, risk increased by 8% per 200 μg/1000kcals with bordering on significance.
Table 3. Relative risks and 95% CIs of UADT cancer according to quintiles of total meat, its subtypes, fish and heme iron intake in th EPIC study (n=348,738).
|
Quintiles of meat, fish and heme iron intake *
|
Continuous intake | |||||||
|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | Ptrend | Observed * | Predicted * | |
|
Total meat (g/1000 kcals) |
12.1 (0-27) | 35.0 (27-41) | 47.2 (41-53) | 59.8 (53-68) | 79.8 (68-332) | |||
| Total no. of cases | 67 | 102 | 140 | 172 | 201 | per 20g/1000 kcals | ||
| Model 1 † | Ref. | 1.10 (0.79-1.52) | 1.32 (0.97-1.81) | 1.47 (1.08-2.01) | 1.62 (1.19-2.21) | 0.0002 | 1.15 (1.08-1.23) | 1.21 (1.06-1.38) |
| Model 2 ‡ | Ref. | 1.05 (0.76-1.46) | 1.26 (0.92-1.72) | 1.33 (0.97-1.81) | 1.38 (1.01-1.89) | 0.01 | 1.09 (1.02-1.17) | 1.12 (0.98-1.28) |
| Model 3 ¶ | Ref. | 1.04 (0.75-1.44) | 1.24 (0.91-1.70) | 1.32 (0.97-1.80) | 1.37 (1.00-1.88) | 0.01 | 1.09 (1.02-1.17) | 1.16 (1.02-1.33) |
| Red meat (g/1000 kcals) | 1.9 (0-6) | 9.8 (6-13) | 17.5 (13-22) | 27.1 (22-33) | 42.4 (33-252) | |||
| Total no. of cases | 79 | 93 | 128 | 179 | 203 | per 10g/1000 kcals | ||
| Model 1 † | Ref. | 0.88 (0.63-1.23) | 1.02 (0.74-1.41) | 1.22 (0.88-1.68) | 1.21 (0.87.-1.69) | 0.05 | 1.07 (1.02-1.13) | 1.06 (1.95-1.18) |
| Model 2 ‡ | Ref. | 0.86 (0.62-1.20) | 0.95 (0.69-1.32) | 1.08 (0.78-1.49) | 1.00 (0.72-1.40) | 0.56 | 1.02 (0.97-1.08) | 0.97 (0.86-1.08) |
| Model 3 ¶ | Ref. | 0.86 (0.62-1.20) | 0.95 (0.68-1.31) | 1.07 (0.77-1.48) | 0.98 (0.70-1.37) | 0.69 | 1.02 (0.97-1.08) | 1.00 (0.90-1.12) |
| Poultry (g/1000 kcals) | 0 (0-2) | 3.5 (2-5) | 6.6 (5-8) | 10.9 (8-15) | 22.3 (15-324) | |||
| Total no. of cases | 129 | 165 | 129 | 144 | 115 | per 5g/1000 kcals | ||
| Model 1 † | Ref. | 0.75 (0.57-0.99) | 0.55 (0.41-0.74) | 0.58 (0.43-0.77) | 0.50 (0.37-0.68) | 0.0002 | 0.90 (0.86-0.95) | 0.77 (0.69-0.88) |
| Model 2 ‡ | Ref. | 0.77 (0.58-1.02) | 0.61 (0.45-0.82) | 0.66 (0.49-0.89) | 0.62 (0.45-0.85) | 0.03 | 0.96 (0.91-1.00) | 1.01 (0.90-1.13) |
| Model 3 ¶ | Ref. | 0.79 (0.60-1.05) | 0.64 (0.48-0.86) | 0.72 (0.54-0.97) | 0.70 (0.51-0.96) | 0.17 | 0.96 (0.91-1.01) | 1.00 (0.89-1.12) |
|
Processed meat (g/1000 kcals) |
1.5 (0-5) | 7.2 (5-10) | 12.4 (10-15) | 19.2 (15-24) | 32.8 (24-196) | |||
| Total no. of cases | 73 | 117 | 149 | 152 | 191 | per 10g/1000 kcals | ||
| Model 1 † | Ref. | 1.08 (0.79-1.47) | 1.24 (0.92-1.69) | 1.29 (0.94-1.75) | 1.81 (1.32-2.49) | <.0001 | 1.19 (1.12-1.26) | 1.19 (1.13-1.27) |
| Model 2 ‡ | Ref. | 1.04 (0.77-1.42) | 1.15 (0.85-1.56) | 1.14 (0.84-1.56) | 1.51 (1.10-2.08) | 0.002 | 1.13 (1.07-1.20) | 1.22 (1.07-1.38) |
| Model 3 ¶ | Ref. | 1.01 (0.74-1.38) | 1.11 (0.81-1.50) | 1.09 (0.80-1.48) | 1.41 (1.03-1.94) | 0.01 | 1.13 (1.06-1.20) | 1.22 (1.07-1.40) |
| Fish (g/1000 kcals) | ||||||||
| Total no. of cases | 0.3 (0-1.9) | 4.0 (1.9-6.4) | 8.9 (6.4-11.5) | 14.7 (11.5-19.1) | 27.5 (19.0-267) | per 10g/1000 kcals | ||
| Model 1 † | Ref. | 1.17 (0.85-1.62) | 0.98 (0.70-1.39) | 1.10 (0.78-1.56) | 0.83 (0.57-1.20) | 0.05 | 0.93 (0.86-1.00) | 0.93 (0.79-1.10) |
| Model 2 ‡ | Ref. | 1.16 (0.84-1.59) | 1.01 (0.72-1.43) | 1.16 (0.82-1.64) | 0.87 (0.60-1.25) | 0.10 | 0.94 (0.87-1.02) | 0.95 (0.81-1.12) |
| Model 3 ¶ | Ref. | 1.15 (0.84-1.58) | 1.02 (0.72-1.44) | 1.19 (0.84-1.69) | 0.94 (0.65-1.36) | 0.32 | 0.96 (0.89-1.04) | 1.02 (0.87-1.21) |
|
Haem iron (pg/1000 kcals) |
53 (0-116) | 160 (116-202) | 245 (202-293) | 351 (293-426) | 542 (426-460) | |||
| Total no. of cases | 84 | 116 | 123 | 144 | 193 | per 10g/1000 kcals | ||
| Model 1 † | Ref. | 1.05(0.79-1.41) | 1.05 (0.78-1.41) | 1.05 (0.77-1.41) | 1.31 (0.97-1.76) | 0.03 | 1.10 (1.03-1.18) | 1.02 (0.98-1.07) |
| Model 2 ‡ | Ref. | 1.01 (0.75-1.35) | 1.00 (0.74-1.35) | 0.96 (0.71-1.29) | 1.18 (0.88-1.59) | 0.16 | 1.07 (1.00-1.15) | 1.02 (0.98-1.07) |
| Model 3 ¶ | Ref. | 1.04 (0.78-1.39) | 1.03 (0.76-1.38) | 1.00 (0.74-1.35) | 1.23 (0.91-1.65) | 0.10 | 1.08 (1.00-1.16) | 1.08 (1.00-1.16) |
Quintiles of meat/heme iron intake were calculated based on nutrient density energy adjusted meat/heme iron intake. Intakes are medians and ranges as estimated from the dietary questionnaire.
Observed = intake of meat/iron was estimated from the dietary questionnaire; Predicted = Intake of meat/iron was calibrated using data of the 24-hour diet recall of the calibration study participants.
Model 1 is derived from Cox regression stratified by age at recruitment and center, adjusted for non-consumer status (0/1), sex, energy intake from fat and non-fat sources, and education (none/primary, technical/professional, secondary school, university, not specified). Red meat, poultry and processed meat were mutually adjusted.
Model 2: Model 1 + smoking (lifelong non-smoking, former smoking with quitting ≥10 years, former smoking with quitting <10 years, current smoking with <15 cigarettes/day, current smoking with 15-24, current smoking with ≥25 cigarettes/day, current smoking other than cigarettes combined with smoking with unknown quantity, and missing).
Model 3: Model 2 + alcohol consumption (g/d), drinking history (never, former, unknown), BMI (kg/m2), physical activity (inactive, moderately inactive, moderately active, and active), citrus and non-citrus fruits, and vegetables.
We also addressed the role of anatomic location by fitting separate models on a continuous scale for cancers of the oral cavity/pharynx, esophagus and larynx (Table 4). The positive association of processed meat observed with combined UADT cancer was also present for all three cancer sites, though not significant for laryngeal cancer. Further, a significant inverse relation was found for poultry with esophageal cancer (RR=0.86 (0.76-0.98) per 5g/1000kcals).
Table 4. Relative risks and 95% CIs for quintiles of intake of meat, its subtypes, fish and heme iron according to anatomic location of squamous cell carcinoma of the UADT in EPIC.
| Relative risk (95% CI) ** | |||||
|---|---|---|---|---|---|
|
| |||||
| Food item | Increment | Model * |
Oral cavity/ Pharynx (325 cases) |
Esophagus (151 cases) |
Larynx (206 cases) |
| Total meat | Per 20g/1000kcals | Observed | 1.13 (1.02-1.24) | 1.24 (1.09-1.41) | 0.91 (0.79-1.04) |
| Calibrated | 1.27 (1.06-1.53) | 1.22 (0.97-1.52) | 0.88 (0.66-1.67) | ||
| Red meat | Per 10g/1000kcals | Observed | 1.04 (0.96-1.12) | 1.09 (0.99-1.20) | 0.92 (0.82-1.02) |
| Calibrated | 1.07 (0.92-1.25) | 1.03 (0.86-1.23) | 0.84 (0.67-1.06) | ||
| Poultry | Per 5g/1000kcals | Observed | 1.01 (0.95-1.08) | 0.86 (0.76-0.98) | 0.92 (0.83-1.01) |
| Calibrated | 1.12 (0.98-1.27) | 0.86 (0.66-1.13) | 0.87 (0.69-1.10) | ||
| Processed meat | Per 10g/1000kcals | Observed | 1.09 (1.00-1.19) | 1.31 (1.18-1.46) | 1.03 (0.91-1.16) |
| Calibrated | 1.17 (0.97-1.42) | 1.42 (1.13-1.78) | 1.09 (0.84-1.41) | ||
| Fish | Per 10g/1000kcals | Observed | 0.99 (0.88-1.10) | 0.92 (0.77-1.09) | 0.97 (0.85-1.12) |
| Calibrated | 1.00 (0.78-1.28) | 0.96 (0.66-1.40) | 1.10 (0.83-1.46) | ||
| Heme iron | Per 200ug/1000kcals | Observed | 1.10 (0.99-1.22) | 1.06 (0.92-1.22) | 1.06 (0.92-1.21) |
| Calibrated | 1.05 (0.98-1.12) | 0.99 (0.90-1.10) | 1.09 (0.99-1.21) | ||
Relative risks are derived from multivariate Cox regression stratified by age at recruitment and center, and adjusted for non-consumer status (0/1), sex, energy intake from fat and non-fat sources, education (none/primary, technical/professional, secondary school, university, not specified), smoking (lifelong non-smoking, former smoking with quitting ≥10 years, former smoking with quitting <10 years, current smoking with <15 cigarettes/day, current smoking with 15-24, current smoking with ≥25 cigarettes/day, current smoking other than cigarettes combined with smoking with unknown quantity, and missing), alcohol consumption (g/d), drinking history (never, former, unknown), BMI (kg/m2), physical activity (inactive, moderately inactive, moderately active, and active), citrus and non-citrus fruits, and vegetables. Red meat, poultry and processed meat were mutually adjusted.
Observed = intake of meat/iron was estimated from the dietary questionnaire; Predicted = Intake of meat/iron was calibrated using data of the 24-hour diet recall of the calibration study participants.
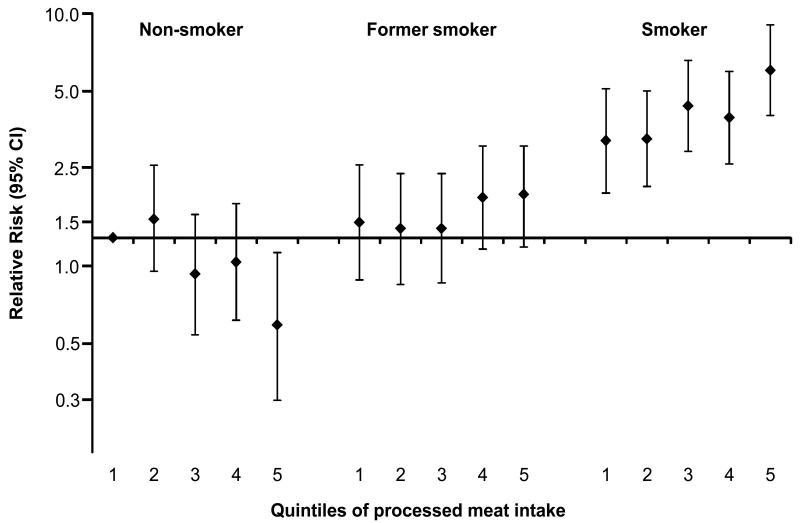
There was evidence of effect modification by smoking status with processed meat (Table 5). While intake of processed meat was not related to SCC of the UADT among lifelong non-smokers and former smokers, it was positively associated with these cancers among smokers at recruitment (P for interaction <0.0001). Specifically, per 10g/1000 kcals processed meat intake, UADT cancer risk increased by 18% among smokers. This observation was consistent across countries (P for heterogeneity = 0.62). A similar pattern was observed when a single reference category was chosen and combined effects were determined for quintiles of processed meat intake in combination with categories of smoking status in relation to UADT SCC (Figure 1). Smokers in the highest quintile of processed meat intake had a 5.5-fold higher risk than non-smokers in the lowest intake category of intake. When we further divided the subgroup of smokers into mild and heavy smokers based on sex-specific medians of number of cigarettes per day (15 cigarettes for men and 12 for women), the higher risk for UADT cancer was present both in mild and heavy smokers, though it was more pronounced among heavy smokers in comparison to the combined reference category of lowest quintile of processed meat intake among lifelong non-smokers (RR=3.73 (2.22-6.72) and 8.32 (5.12-13.5) for mild and heavy smokers, respectively). In additional analyses among smokers, relative risks were further adjusted for intensity of smoking (number of cigarettes) and smoking duration in order to control for heterogeneous smoking habits; however, relative risks were hardly affected (RR (95% CI) per increase of 10g/1000kcals: 1.16 (1.08-1.25)).
Table 5. Relative risks and 95% CIs of UADT cancer according to quintiles of total meat, its subtypes, fish and heme iron intake in the EPIC study stratified by smoking status.
| Lifelong non-smokers | Former smokers | Current smokers | ||||
|---|---|---|---|---|---|---|
| Cases (N) |
RR (95% CI) | Cases (N) |
RR (95% CI) | Cases (N) |
RR (95% CI) | |
| Total meat (g/1000 kcals) | ||||||
| Q1 (Reference) | 21 | 1.00 | 14 | 1.00 | 31 | 1.00 |
| Q2 | 21 | 0.97 (0.51- 1.86) |
23 | 1.25 (0.63- 2.49) |
57 | 1.09 (0.67- 1.73) |
| Q3 | 25 | 1.13 (0.59- 2.15) |
26 | 1.27 (0.64- 2.54) |
87 | 1.40 (0.90- 2.17) |
| Q4 | 14 | 0.64 (0.30- 1.34) |
35 | 1.54 (0.78- 3.02) |
122 | 1.65 (1.07- 2.54) |
| Q5 | 19 | 0.88 (0.43- 1.81) |
50 | 1.97 (1.02- 3.19) |
131 | 1.60 (1.03- 2.47) |
| Ptrend | 0.50 | 0.02 | 0.01 | |||
| P for interaction with smoking = 0.01 ¶ | ||||||
| Per 20g/1000kcals, observed | 0.98 (0.81- 1.18) |
1.20 (1.04- 1.38) |
1.12 (1.03- 1.22) |
|||
| Per 20g/1000kcals, predicted | 0.86 (0.58- 1.27) |
1.28 (0.97- 1.69) |
1.30 (1.10- 1.54) |
|||
| Red meat (g/1000 kcals) | ||||||
| Q1 (Reference) | 17 | 1.00 | 14 | 1.00 | 47 | 1.00 |
| Q2 | 21 | 1.59 (0.75- 3.39) |
18 | 1.00 (0.47- 2.15) |
53 | 0.74 (0.48- 1.14) |
| Q3 | 19 | 1.28 (0.58- 2.83) |
31 | 1.45 (0.70- 2.97) |
75 | 0.77 (0.51- 1.19) |
| Q4 | 22 | 1.37 (0.61- 3.07) |
43 | 1.90 (0.93- 3.87) |
113 | 0.89 (0.59- 1.36) |
| Q5 | 21 | 1.24 (0.53- 2.92) |
42 | 1.70 (0.81- 3.57) |
140 | 0.89 (0.58- 1.37) |
| Ptrend | 0.99 | 0.07 | 0.68 | |||
| P for interaction with smoking = 0.10 ¶ | ||||||
| Per 10g/1000kcals, observed | 1.03 (0.89- 1.20) |
1.12 (1.00- 1.25) |
1.03 (0.96- 1.10) |
|||
| Per 10g/1000kcals, predicted | 0.90 (0.63- 1.29) |
1.16 (0.92- 1.45) |
1.04 (0.91- 1.20) |
|||
| Poultry (g/1000 kcals) | ||||||
| Q1 (Reference) | 26 | 1.00 | 24 | 1.00 | 78 | 1.00 |
| Q2 | 16 | 0.56 (0.26- 1.21) |
29 | 0.80 (0.40- 1.59) |
119 | 0.85 (0.61- 1.20) |
| Q3 | 16 | 0.47 (0.22- 1.04) |
27 | 0.70 (0.35- 1.43) |
86 | 0.67 (0.47- 0.97) |
| Q4 | 19 | 0.56 (0.25- 1.21) |
37 | 0.99 (0.50- 1.98) |
86 | 0.67 (0.46- 0.96) |
| Q5 | 23 | 0.61 (0.28- 1.32) |
31 | 0.87 (0.42- 1.80) |
59 | 0.64 (0.42- 0.96) |
| Ptrend | 0.81 | 0.76 | 0.04 | |||
| P for interaction with smoking = 0.13 ¶ | ||||||
| Per 5g/1000kcals, observed | 0.97 (0.86- 1.09) |
1.03 (0.94- 1.12) |
0.91 (0.84- 0.98) |
|||
| Per 5g/1000kcals, predicted | 0.88 (0.64- 1.20) |
1.11 (0.91- 1.36) |
0.92 (0.78- 1.10) |
|||
|
Processed meat (g/1000 kcals) |
||||||
| Q1 (Reference) | 20 | 1.00 | 20 | 1.00 | 32 | 1.00 |
| Q2 | 32 | 1.64 (0.86- 3.12) |
25 | 0.76 (0.41- 1.43) |
59 | 0.98 (0.63- 1.54) |
| Q3 | 18 | 0.96 (0.46- 2.00) |
27 | 0.74 (0.40- 1.39) |
102 | 1.34 (0.87- 2.05) |
| Q4 | 20 | 1.10 (0.53- 2.29) |
37 | 1.01 (0.55- 1.86) |
94 | 1.16 (0.75- 1.80) |
| Q5 | 10 | 0.65 (0.26- 1.60) |
39 | 1.00 (0.52- 1.90) |
141 | 1.89 (1.22- 2.93) |
| Ptrend | 0.12 | 0.50 | <.0001 | |||
| P for interaction with smoking < 0.0001 ¶ | ||||||
| Per 10g/1000kcals, observed | 0.87 (0.69- 1.08) |
1.11 (0.97- 1.26) |
1.18 (1.10- 1.27) |
|||
| Per 10g/1000kcals, predicted | 0.84 (0.54- 1.30) |
1.16 (0.87- 1.56) |
1.33 (1.15- 1.55) |
|||
| Fish (g/1000 kcals) | ||||||
| Q1 (Reference) | 15 | 1.00 | 21 | 1.00 | 62 | 1.00 |
| Q2 | 17 | 1.74 (0.63-4.84) | 29 | 1.28 (0.63-2.60) | 91 | 1.07 (0.73-1.58) |
| Q3 | 18 | 1.57 (0.53-4.69) | 35 | 1.15 (0.54-2.44) | 90 | 0.91 (0.60-1.38) |
| Q4 | 30 | 2.31 (0.79-6.76) | 38 | 1.20 (0.55-2.58) | 104 | 1.07 (0.70-1.64) |
| Q5 | 20 | 1.43 (0.46-4.40) | 25 | 0.81 (0.35-1.85) | 91 | 0.90 (0.57-1.42) |
| Ptrend | 0.84 | 0.20 | 0.52 | |||
| P for interaction with smoking = 0.95 ¶ | ||||||
| Per 10g/1000kcals, observed | 0.96 (0.79-1.16) | 0.85 (0.71-1.02) | 1.01 (0.91-1.11) | |||
| Per 10g/1000kcals, predicted | 0.97 (0.59-1.64) | 0.77 (0.52-1.13) | 1.12 (0.91-1.37) | |||
| Haem iron (μg/1000 kcals) | ||||||
| Q1 (Reference) | 21 | 1.00 | 14 | 1.00 | 47 | 1.00 |
| Q2 | 23 | 1.14 (0.61-2.14) | 26 | 1.50 (0.77-2.94) | 65 | 0.95 (0.64-1.40) |
| Q3 | 12 | 0.62 (0.29-1.33) | 36 | 2.03 (1.05-3.91) | 74 | 0.92 (0.62-1.37) |
| Q4 | 17 | 0.83 (0.40-1.73) | 28 | 1.58 (0.78-3.19) | 98 | 0.99 (0.67-1.46) |
| Q5 | 24 | 1.17 (0.57-2.39) | 42 | 2.14 (1.08-4.24) | 127 | 1.19 (0.81-1.75) |
| Ptrend | 0.68 | 0.06 | 0.13 | |||
| P for interaction with smoking = 0.32 ¶ | ||||||
| Per 200μg/1000kcals, observed | 1.06 (0.86-1.32) | 1.16 (0.99-1.36) | 1.10 (1.01-1.21) | |||
| Per 200μg/1000kcals, predicted | 1.03 (0.92-1.17) | 1.02 (0.91-1.14) | 1.05 (0.99-1.12) | |||
Relative risks are derived from multivariate Cox regression stratified by age at recruitment and center, and adjusted for non-consumer status (0/1), sex, energy intake from fat and non-fat sources, education (none/primary, technical/professional, secondary school, university, not specified), alcohol consumption (g/d), drinking history (never, former, unknown), BMI (kg/m2), physical activity (inactive, moderately inactive, moderately active, and active), citrus and non-citrus fruits, and vegetables. Red meat, poultry and processed meat were mutually adjusted.
Observed = intake of meat/iron was estimated from the dietary questionnaire; Predicted = Intake of meat/iron was calibrated using data of the 24-hour diet recall of the calibration study participants.
Figure 1. Cross-classification of smoking and processed meat intake.
Multivariable-adjusted relative risks and 95% CI for the joint effect of processed meat intake and smoking status on the association of upper aero-digestive tract cancer in EPIC. RRs were adjusted for sex, energy from fat and non-fat sources, education, alcohol intake, BMI, physical activity, intake of citrus and non-citrus fruits, and vegetables, red meat, and poultry (non-smoking participants in the lowest category of processed meat intake constitute the reference group). Note that the RRs (Y axis) are plotted on a logarithmic scale.
We found no evidence of different associations between meat, meat subtypes, fish and heme iron with risk of UADT cancer by status of alcohol use at baseline. Although P for interaction was significant (P=0.04) for processed meat, stratified analyses showed higher risks in both strata of alcohol consumption (RR=1.11 (0.95-1.30) and 1.15 (1.07-1.23) among non-users and users, respectively).
After exclusion of UADT cancer cases occurring during the first two, three and five years of follow-up, the associations between meat and UADT cancer hardly changed in the total cohort and in stratified analyses (data not shown). When we restricted the analysis to cancer cases for which diagnosis was based on histology (90%), results did not change (data not shown).
DISCUSSION
In this large prospective study based on data from almost 350,000 European men and women, higher intake of total meat was associated with higher risk of SCC of the UADT which was mainly driven by the effect of processed meat. Red meat, poultry, fish and heme iron were not consistently related to UADT SCC. Importantly, the higher risk with higher processed meat intake was only observed among smokers at recruitment.
To our knowledge, seven prospective studies (7-13) have investigated associations between consumption of total meat or its subtypes and (single) SCC of the UADT. Among them, five were conducted in Western populations (7, 8, 10, 12, 13) and two in Asian individuals for whom only risk estimates for total meat were reported (9, 11). Consistent with our observation of an increased UADT SCC risk with higher processed meat intake, a Norwegian study based on 71 UADT cancer cases, of which 61 were SCC, and a study among Hawaii Japanese men including 92 UADT SCC cases suggested a higher risk of these cancers for higher consumption of bacon (7, 10).
Interestingly, processed meat was positive, though non-significantly, associated with esophageal SCC in the large NIH-AARP Diet and Health study (13). Instead, an elevated risk of laryngeal cancer and esophageal SCC with higher red meat was reported (8, 13) which is not supported by the findings of our study. Case-control studies on the evaluation of meat intake and UADT cancer reported positive, though non-significant associations for red and processed meat (25), significant positive associations for red meat but not for processed meat (26) or positive associations for both red and processed meat (27, 28). An explanation for the divergent observations between the NIH-AARP study and our study may relate to different groupings of meat items and different intake ranges. In the NIH-AARP study, all types of beef, pork and lamb were considered red meat, including those types that had undergone some form of preparation and were defined as processed meat in our study. As a consequence from this grouping, red meat intake was higher in NIH-AARP than in our study. In contrast, intake of processed meat was remarkably lower in NIH-AARP than in the present study (median of 23.2g/1000kcals and 32.8g/1000kcals in the highest quintile in NIH-AARP and in our study, respectively). Thus, it may be possible that the contrast between first and fifth quintile of processed meat consumption was high enough for us to reveal a significant association with processed meat while in the NIH-AARP study it may have been too small. Overall, the findings of these two large prospective studies clearly emphasize the importance of identifying the agents responsible for an association between meat and UADT cancer.
Various biological mechanisms have been hypothesized to explain associations between intake of meat and cancer at various sites. First, both red and processed meat may be a source of several known mutagens, including heterocyclic amines (HCA) and polycyclic aromatic hydrocarbons (PAH) (29-31). The lack of association between red meat and UADT cancer in the present analysis, however, does not provide support for the hypothesis that those carcinogenic substances may play a major role in the etiology of neoplasms of the UADT. Second, meat, particularly red meat, is a source of readily available heme iron, which can act as a pro-oxidant and catalyze lipid peroxidation and DNA damage in the tissues (32), and may also induce endogenous formation of N-nitroso compounds (NOCs) (33). Nevertheless, we did not observe a clear association between heme iron and UADT cancer risk. Third, processed meat is an important source of nitrites and exogenous N-nitroso compounds (NOCs) (34), that have been found to be carcinogenic to multiple organs in 39 different animal species and may be specifically involved in the etiology of SCC of the esophagus (35).
The results of the present study indicate that carcinogenic compounds specifically present in processed but not in red meat, such as NOCs, might affect risk of SCC of the UADT. In this respect, the effect modification by smoking is interesting and suggests that eating processed meat and smoking cigarettes might exert a synergistic effect. Hence, it is tempting to speculate that a metabolic interplay of carcinogenic substances present in tobacco smoke and processed meat might be responsible for the observed associations. Tobacco smoke contains as many as 60 carcinogens (36) and the UADT is directly exposed to these inhaled substances. Much is known about the mechanisms by which carcinogens present in tobacco smoke can act as both initiators and promoters of cancer at various sites (6). Thus, one might hypothesize that the tobacco-initiated cells may be more susceptible to the deleterious effects of NOCs in processed meat. In addition, tobacco smoke has been shown to induce several phase I and phase II enzymes in human tissues (6) and one might speculate that tobacco smoke induces enzymes that are responsible for the metabolic activation of carcinogens present in processed meat. Finally, chronic exposure to tobacco smoke may cause irritation and inflammation of epithelial cells lining the UADT and renewing epithelial cells might be more susceptible to the detrimental effects of carcinogenic compounds in processed meat. However, the exact underlying biological mechanism is currently unknown and future research is needed to confirm the present finding of an effect modification by smoking. Because smoking is such a strong risk factor for UADT cancer, residual confounding is always an issue. Although we evaluated the stability of this result in various sensitivity analyses, we cannot rule out residual confounding by smoking.
Among the strengths of the present study are its prospective design, the large number of incident cases of UADT SCC allowing for stratified analyses, the distinctly diverging dietary habits due to inclusion of participants from several European countries and the detailed assessment of important confounders. Some limitations of our study should be acknowledged as well. First, the results could have been affected by measurement error, a common limitation in epidemiologic studies; however, the wide range of meat intake reduced potential effects of measurement error and we additionally corrected risk estimates in the calibrated models. Second, information on dietary habits was assessed only at recruitment and may therefore not perfectly represent long-term intake. Third, we were not able to evaluate associations of NOCs from processed meat with UADT cancer risk, which may explain the association observed for processed meat. Fourth, we did not perform direct measures of heme iron, but used specific factors for each type of meat obtained from published data. Nevertheless, in a recent study on colorectal adenocarcinoma it was shown that heme iron intake estimated using published data and heme iron estimated from own analysis was highly correlated and individuals were classified in the same quartile (37). Finally, since low consumers of meat tended to be healthier, this study might be subject to residual confounding by unknown factors if healthier behaviors of low-meat consumers have other underlying factors than those assessed here.
In conclusion, our study suggests that higher intake of processed meat may be associated with risk of SCC of the UADT among smokers. Confirmation in future studies and identification of biological mechanism is warranted.
Acknowledgments
Financial support:
This work was supported by the “Europe Against Cancer” Programme of the European Commission (SANCO); German Cancer Aid; German Cancer Research Center; German Federal Ministry of Education and Research; Danish Cancer Society; Health Research Fund of the Spanish Ministry of Health, Grant Number: Network RCESP C03/09); Spanish Regional Governments of Andalucia, Asturias, Basque Country, Murcia and Navarra; ISCIII, Red de Centros RETIC(RD06/0020); Grant Number: C03/09; Cancer Research UK; Medical Research Council, UK; Stroke Association, UK; British Heart Foundation; Department of Health, UK; Food Standards Agency, UK; Wellcome Trust, UK; Italian Association for Research on Cancer (AIRC); Compagnia di San Paolo; Dutch Ministry of Public Health, Welfare and Sports; National Cancer Registry and the Regional Cancer Registries Amsterdam, East and Maastricht of the Netherlands; World Cancer Research Fund (WCRF); Nordforsk (Centre of Excellence Programme HELGA); Swedish Cancer Society; Swedish Scientific Council; Regional Government of Skåne and Västerbotten, Sweden; Hellenic Health Foundation and the J.F. Costopoulos Foundation.
Footnotes
Conflict of interest:
No financial or personal conflict of interest was declared.
REFERENCES
- 1.Ferlay J, Shin H, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v1.2, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10. 2010 [cited 2011 09/12]; Available from: http://globocan.iarc.fr.
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Zigon G, Berrino F, Gatta G, Sanchez MJ, van Dijk B, Van Eycken E, et al. Prognoses for head and neck cancers in Europe diagnosed in 1995-1999: a population-based study. Ann Oncol. 2011;22:165–74. doi: 10.1093/annonc/mdq306. [DOI] [PubMed] [Google Scholar]
- 4.World Cancer Research Fund, American Institute for Cancer Research . Food, Nutrition, Physical Actitivity, and the Prevention of Cancer: A Global Perspective. AICR; Washington DC: 2007. [Google Scholar]
- 5.IARC Consumption of Alcoholic Beverages and Ethyl Carbamate (Urethane) 2007 [cited January 2010]; Available from: http://monographs.iarc.fr/ENG/Meetings/96-alcohol.pdf.
- 6.Tobacco Smoke and Involuntary Smoking. Vol. 83. Lyon; 2004. (IARC Monographs on the Evaluation of Carcinogenic Risk to Humans). [PMC free article] [PubMed] [Google Scholar]
- 7.Chyou PH, Nomura AM, Stemmermann GN. Diet, alcohol, smoking and cancer of the upper aerodigestive tract: a prospective study among Hawaii Japanese men. Int J Cancer. 1995;60:616–21. doi: 10.1002/ijc.2910600508. [DOI] [PubMed] [Google Scholar]
- 8.Cross AJ, Leitzmann MF, Gail MH, Hollenbeck AR, Schatzkin A, Sinha R. A prospective study of red and processed meat intake in relation to cancer risk. PLoS Med. 2007;4:e325. doi: 10.1371/journal.pmed.0040325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan Y, Yuan JM, Wang R, Gao YT, Yu MC. Alcohol, tobacco, and diet in relation to esophageal cancer: the Shanghai Cohort Study. Nutr Cancer. 2008;60:354–63. doi: 10.1080/01635580701883011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kjaerheim K, Gaard M, Andersen A. The role of alcohol, tobacco, and dietary factors in upper aerogastric tract cancers: a prospective study of 10,900 Norwegian men. Cancer Causes Control. 1998;9:99–108. doi: 10.1023/a:1008809706062. [DOI] [PubMed] [Google Scholar]
- 11.Tran GD, Sun XD, Abnet CC, Fan JH, Dawsey SM, Dong ZW, et al. Prospective study of risk factors for esophageal and gastric cancers in the Linxian general population trial cohort in China. Int J Cancer. 2005;113:456–63. doi: 10.1002/ijc.20616. [DOI] [PubMed] [Google Scholar]
- 12.Zheng W, Sellers TA, Doyle TJ, Kushi LH, Potter JD, Folsom AR. Retinol, antioxidant vitamins, and cancers of the upper digestive tract in a prospective cohort study of postmenopausal women. Am J Epidemiol. 1995;142:955–60. doi: 10.1093/oxfordjournals.aje.a117743. [DOI] [PubMed] [Google Scholar]
- 13.Cross AJ, Freedman ND, Ren J, Ward MH, Hollenbeck AR, Schatzkin A, et al. Meat consumption and risk of esophageal and gastric cancer in a large prospective study. Am J Gastroenterol. 2011;106:432–42. doi: 10.1038/ajg.2010.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5:1113–24. doi: 10.1079/PHN2002394. [DOI] [PubMed] [Google Scholar]
- 15.Riboli E, Kaaks R. The EPIC Project: rationale and study design. European Prospective Investigation into Cancer and Nutrition. Int J Epidemiol. 1997;26(Suppl 1):S6–14. doi: 10.1093/ije/26.suppl_1.s6. [DOI] [PubMed] [Google Scholar]
- 16.Kaaks R, Slimani N, Riboli E. Pilot phase studies on the accuracy of dietary intake measurements in the EPIC project: overall evaluation of results. European Prospective Investigation into Cancer and Nutrition 10.1093/ije/26.suppl_1.S26. Int J Epidemiol. 1997;26:S26–36. doi: 10.1093/ije/26.suppl_1.s26. [DOI] [PubMed] [Google Scholar]
- 17.Slimani N, Ferrari P, Ocke M, Welch A, Boeing H, Liere M, et al. Standardization of the 24-hour diet recall calibration method used in the european prospective investigation into cancer and nutrition (EPIC): general concepts and preliminary results. Eur J Clin Nutr. 2000;54:900–17. doi: 10.1038/sj.ejcn.1601107. [DOI] [PubMed] [Google Scholar]
- 18.Slimani N, Kaaks R, Ferrari P, Casagrande C, Clavel-Chapelon F, Lotze G, et al. European Prospective Investigation into Cancer and Nutrition (EPIC) calibration study: rationale, design and population characteristics. Public Health Nutr. 2002;5:1125–45. doi: 10.1079/PHN2002395. [DOI] [PubMed] [Google Scholar]
- 19.Slimani N, Deharveng G, Unwin I, Southgate DA, Vignat J, Skeie G, et al. The EPIC nutrient database project (ENDB): a first attempt to standardize nutrient databases across the 10 European countries participating in the EPIC study. Eur J Clin Nutr. 2007;61:1037–56. doi: 10.1038/sj.ejcn.1602679. [DOI] [PubMed] [Google Scholar]
- 20.Balder HF, Vogel J, Jansen MC, Weijenberg MP, van den Brandt PA, Westenbrink S, et al. Heme and chlorophyll intake and risk of colorectal cancer in the Netherlands cohort study. Cancer Epidemiol Biomarkers Prev. 2006;15:717–25. doi: 10.1158/1055-9965.EPI-05-0772. [DOI] [PubMed] [Google Scholar]
- 21.Haftenberger M, Lahmann PH, Panico S, Gonzalez CA, Seidell JC, Boeing H, et al. Overweight, obesity and fat distribution in 50- to 64-year-old participants in the European Prospective Investigation into Cancer and Nutrition (EPIC) Public Health Nutr. 2002;5:1147–62. doi: 10.1079/PHN2002396. [DOI] [PubMed] [Google Scholar]
- 22.Willett WC. Nutritional Epidemiology. Second ed Oxford University Press; New York: 1998. [Google Scholar]
- 23.Ferrari P, Day NE, Boshuizen HC, Roddam A, Hoffmann K, Thiébaut A, et al. The evaluation of the diet/disease relation in the EPIC study: considerations for the calibration and the disease models. Int J Epidemiol. 2008 doi: 10.1093/ije/dym242. doi:10.1093/ije/dym242. [DOI] [PubMed] [Google Scholar]
- 24.Kaaks R, Riboli E, van Staveren W. Calibration of dietary intake measurements in prospective cohort studies. Am J Epidemiol. 1995;142:548–56. doi: 10.1093/oxfordjournals.aje.a117673. [DOI] [PubMed] [Google Scholar]
- 25.Silvera SAN, Mayne ST, Risch H, Gammon MD, Vaughan TL, Chow W-H, et al. Food group intake and risk of subtypes of esophageal and gastric cancer. International Journal of Cancer. 2008;123:852–60. doi: 10.1002/ijc.23544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bosetti C, La Vecchia C, Talamini R, Simonato L, Zambon P, Negri E, et al. Food groups and risk of squamous cell esophageal cancer in northern Italy. Int J Cancer. 2000;87:289–94. [PubMed] [Google Scholar]
- 27.De Stefani E, Deneo-Pellegrini H, Ronco AL, Boffetta P, Brennan P, Munoz N, et al. Food groups and risk of squamous cell carcinoma of the oesophagus: a case-control study in Uruguay. Br J Cancer. 2003;89:1209–14. doi: 10.1038/sj.bjc.6601239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levi F, Pasche C, Lucchini F, Bosetti C, Franceschi S, Monnier P, et al. Food groups and oesophageal cancer risk in Vaud, Switzerland. Eur J Cancer Prev. 2000;9:257–63. doi: 10.1097/00008469-200008000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Phillips DH. Polycyclic aromatic hydrocarbons in the diet. Mutat Res. 1999;443:139–47. doi: 10.1016/s1383-5742(99)00016-2. [DOI] [PubMed] [Google Scholar]
- 30.Sinha R. An epidemiologic approach to studying heterocyclic amines. Mutat Res. 2002;506-507:197–204. doi: 10.1016/s0027-5107(02)00166-5. [DOI] [PubMed] [Google Scholar]
- 31.Skog K, Steineck G, Augustsson K, Jagerstad M. Effect of cooking temperature on the formation of heterocyclic amines in fried meat products and pan residues. Carcinogenesis. 1995;16:861–7. doi: 10.1093/carcin/16.4.861. [DOI] [PubMed] [Google Scholar]
- 32.Andrews NC. Disorders of iron metabolism. N Engl J Med. 1999;341:1986–95. doi: 10.1056/NEJM199912233412607. [DOI] [PubMed] [Google Scholar]
- 33.Cross AJ, Pollock JR, Bingham SA. Haem, not protein or inorganic iron, is responsible for endogenous intestinal N-nitrosation arising from red meat. Cancer Res. 2003;63:2358–60. [PubMed] [Google Scholar]
- 34.Tricker AR. N-nitroso compounds and man: sources of exposure, endogenous formation and occurrence in body fluids. Eur J Cancer Prev. 1997;6:226–68. [PubMed] [Google Scholar]
- 35.Mirvish SS. Role of N-nitroso compounds (NOC) and N-nitrosation in etiology of gastric, esophageal, nasopharyngeal and bladder cancer and contribution to cancer of known exposures to NOC. Cancer Lett. 1995;93:17–48. doi: 10.1016/0304-3835(95)03786-V. [DOI] [PubMed] [Google Scholar]
- 36.Taioli E. Gene-environment interaction in tobacco-related cancers. Carcinogenesis. 2008;29:1467–74. doi: 10.1093/carcin/bgn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferrucci LM, Sinha R, Graubard BI, Mayne ST, Ma X, Schatzkin A, et al. Dietary meat intake in relation to colorectal adenoma in asymptomatic women. Am J Gastroenterol. 2009;104:1231–40. doi: 10.1038/ajg.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]