Abstract
Benzopyrano[2,3-b]pyridine is an important privileged medicinal scaffold. A three-component reaction of salicylaldehydes, thiols and 2 equiv of malononitrile that leads to the formation of a series of compounds incorporating 2,4-diamino-3-cyano-5-sulfanylbenzopyrano[2,3-b]pyridine framework is described. A proposed mechanism with the supporting experimental data is presented.
Keywords: Multicomponent reaction, Privileged medicinal scaffold, Drug-like heterocycle
The rapid assembly of molecular diversity utilizing multi-component reactions has received a great deal of attention, most notably for the construction of heterocyclic `drug-like' libraries.1 These methodologies are of particularly great utility when they lead to the formation of `privileged medicinal scaffolds,' defined as molecular frameworks serving as the basis for the generation of ligands for functionally and structurally discreet biological receptors.2 Such chemistry greatly facilitates the development of pharmaceutical agents for diverse applications.
Benzopyrano[2,3-b]pyridine scaffold is of a significant medicinal relevance. The examples of approved therapeutic agents incorporating this molecular framework include amlexanox and pranoprofen (Fig. 1).
Figure 1.
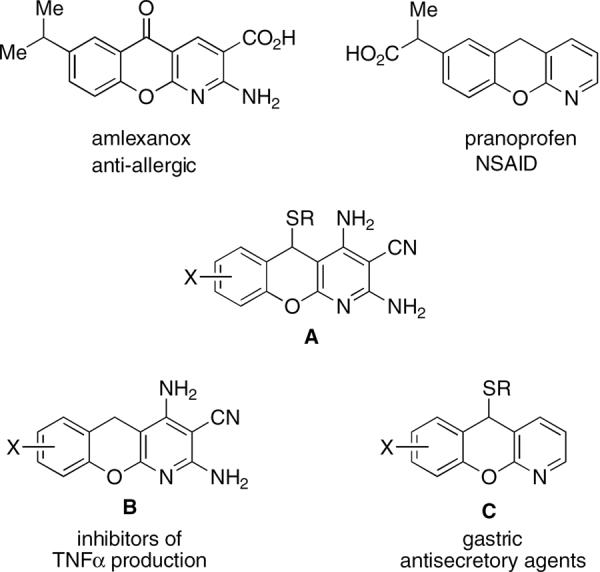
In addition, many of these compounds possess anti-pro-liferative,3 cancer chemopreventive,4 anti-bacterial (including anti-tubercular),5 anti-myopic,6 anti-histaminic,7 hypotensive,8 anti-rheumatic9 and anti-asthmatic activities.10 Many synthetic pathways to such medicinal libraries have been reported.11 However, the diverse pharmacological properties associated with benzopyranopyridines warrant the development of novel processes allowing the synthesis of previously inaccessible analogues for biological evaluation. In this letter, we describe a multi-component strategy for the rapid preparation of library A (Fig. 1). Examples of structurally relevant biologically active compounds are benzopyranopyridines B, found to inhibit mitogen-activated protein kinase-activated protein kinase 2 and attenuate the production of pro-inflammatory TNFα,12 and C, reported to inhibit histamine-stimulated gastric acid secretion in animals.13
We previously disclosed a one-step three-component synthesis of 3,5-dicyanopyridines D starting from various aldehydes, thiols and malononitrile (Fig. 2).14
Figure 2.
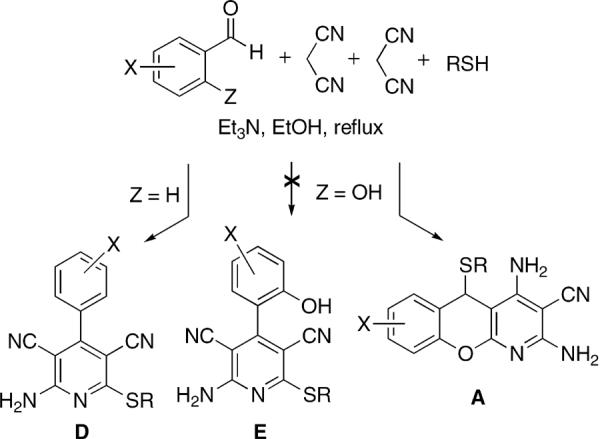
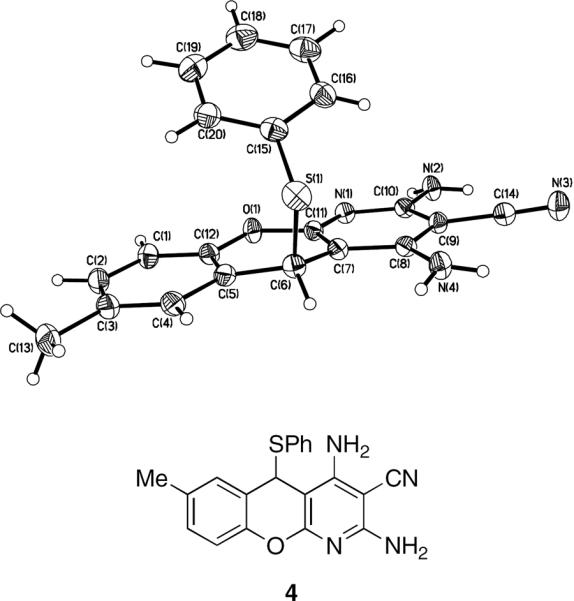
In an attempt to extend the method for the preparation of pyridines E we employed salicylic aldehydes and obtained a library of compounds whose elemental analysis data and the NMR spectra were inconsistent with the expected structures. The X-ray analysis showed these compounds to have a benzopyrano[2,3-b]pyridine framework A (Fig. 3).15
Figure 3.
The reaction works well for all salicylaldehyde and thiol combinations tested. The products precipitate from refluxing ethanolic solutions and are isolated by simple filtration. The yields of recrystallized benzopyranopyridines are given in Table 1.16,17
Table 1.
One-step synthesis of benzopyranopyridines
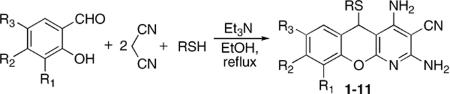
| Benzopyranopyridine | R1 | R2 | R3 | R | Yield (%) |
|---|---|---|---|---|---|
| 1 | H | H | H | Ph | 86 |
| 2 | H | H | H |
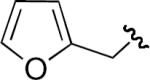
|
55 |
| 3 | H | H | H | PhCH2 | 51 |
| 4 | H | H | Me | Ph | 69 |
| 5 | H | H | Me | PhCH2 | 52 |
| 6 | H | H | Me |
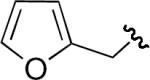
|
63 |
| 7 | H | H | Me | Mes | 56 |
| 8 | OMe | H | H | Ph | 57 |
| 9 | H | Et2N | H | Ph | 63 |
| 10 | H | OH | H | Ph | 63 |
| 11 | H | H | NO2 | 4-F–Ph | 73 |
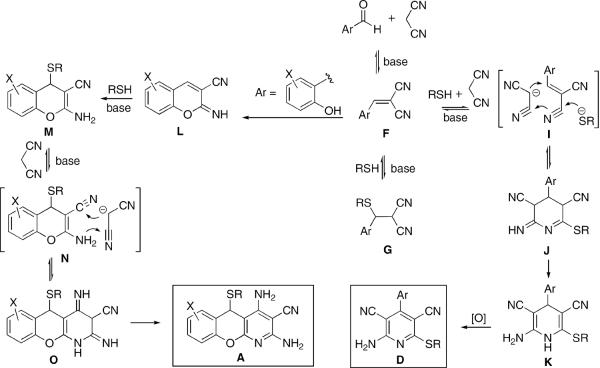
Our proposed mechanistic interpretation of the divergence in reaction paths for o,o-unsubstituted aldehydes, leading to formation of pyridines D, and salicylic aldehydes, resulting in benzopyranopyridines A, is shown in Figure 4.
Figure 4.
Base-catalyzed Michael addition of thiols to Knoevenagel adducts F, produced from o,o-unsubstituted aldehydes, to form G is reversible and non-productive. However, the formal assembly process I leads irreversibly to the formation of dihydropyridines K, thermodynamically stabilized by the push–pull interactions of the donor (positions 2 and 6) and acceptor (positions 3 and 5) substituents. Oxidative aromatization then affords pyridines D. In contrast, Knoevenagel intermediates F, produced from salicylaldehydes, undergo intramolecular cyclization to give powerful Michael acceptors L that react with strongly nucleophilic thiols to form thermodynamically stable chromenes M irreversibly. Lastly, the addition of another equivalent of malononitrile results in benzopyranopyridines A.
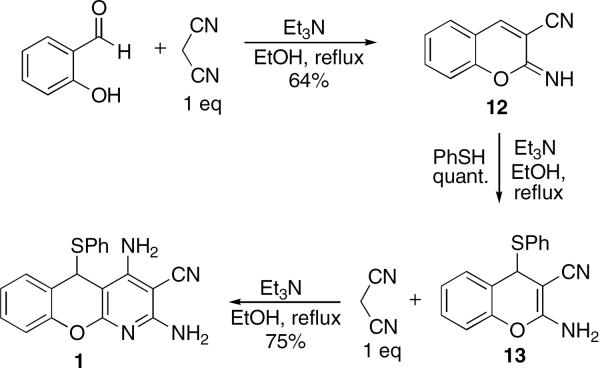
In support of the proposed mechanism we obtained experimental evidence for each of the key steps in Figure 4. Thus, o-hydroxybenzaldehyde reacts with 1 equiv of malononitrile to form iminochromene 12 in a 64% isolated yield after recrystallization (Fig. 5). This compound undergoes an addition with thiophenol to afford phenylsulfanylchromene 13 in a quantitative yield. Finally, when 13 is treated under the same reaction conditions with another equivalent of malononitrile, benzopyranopyridine 1 forms in a good isolated yield.
Figure 5.
Further exploration of this chemistry and biological testing of the synthesized benzopyranopyridines are in progress and will be reported in due course.
Acknowledgements
A.K. thanks the US National Institutes of Health (CA-99957 and RR-16480) for financial support of this work. A.A.Y. and M.Yu.A. are grateful to NSF/DMR (Grant 0420863) for the acquisition of X-ray single crystal diffractometer and to the Distributed Nanomaterials Characterization Network in the framework of New Mexico NSF EPSCoR Nanoscience initiative.
References and notes
- 1.(a) Gerencsér J, Dormán G, Darvas F. QSAR Comb. Sci. 2006:439–448. [Google Scholar]; (b) Ramón DJ, Yus M. Angew. Chem., Int. Ed. 2005;44:1602–1634. doi: 10.1002/anie.200460548. [DOI] [PubMed] [Google Scholar]; (c) Orru RVA, de Greef M. Synthesis. 2003:1471–1499. [Google Scholar]; (d) Hulme C, Gore V. Curr. Med. Chem. 2003;10:51–80. doi: 10.2174/0929867033368600. [DOI] [PubMed] [Google Scholar]; (e) Ugi I, Heck S. Comb. Chem. High Throughput Screen. 2001;4:1–34. doi: 10.2174/1386207013331291. [DOI] [PubMed] [Google Scholar]; (f) Weber L, Illgen K, Almstetter M. Synlett. 1999:366–374. [Google Scholar]; For recent reviews, see:
- 2.(a) Evans BE, Rittle KE, Bock MG, DiPardo RM, Freidinger RM, Whitter WL, Lundell GF, Veber DF, Anderson PS, Chang RSL, Lotti VJ, Cerino DJ, Chen TB, Kling PJ, Kunkel KA, Springer JP, Hirshfield JJ. Med. Chem. 1988;31:2235–2246. doi: 10.1021/jm00120a002. [DOI] [PubMed] [Google Scholar]; (b) Patchett AA, Nargund RP. Ann. Rep. Med. Chem. 2000;35:289–298. [Google Scholar]; The term `privileged scaffolds or structures' was originally introduced by Merck researchers in their work on benzodiazepines:
- 3.Kolokythas G, Pouli N, Marakos P, Pratsinis H, Kletsas D. Eur. J. Med. Chem. 2006;41:71–79. doi: 10.1016/j.ejmech.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Azuine MA, Tokuda H, Takayasu J, Enjyo F, Mukainaka T, Konoshima T, Nishino H, Kapadia GJ. Pharmacol. Res. 2004;49:161–169. doi: 10.1016/j.phrs.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 5.(a) Srivastava SK, Tripathi RP, Ramachandran RJ. Biol. Chem. 2005;280:30273–30281. doi: 10.1074/jbc.M503780200. [DOI] [PubMed] [Google Scholar]; (b) Brötz-Oesterhelt H, Knezevic I, Bartel S, Lampe T, Warnecke-Eberz U, Ziegelbauer K, Häbich D, Labischinski HJ. Biol. Chem. 2003;278:39435–39442. doi: 10.1074/jbc.M306479200. [DOI] [PubMed] [Google Scholar]
- 6.Toshiro S, Noriko W. Eur. Pat. Appl. 1995 EP 647445 A1 19950412. [Google Scholar]
- 7.Ito Y, Kato H, Yasuda S, Kato N, Iwasaki N, Nishino H, Takeshita M. Jpn. Kokai Tokkyo Koho. 1994 JP 06107664 A2 19940419. [Google Scholar]
- 8.Goto K, Yaoka O, Oe T. PCT Int. Appl. 1984 WO 8401711 A1 19840510. [Google Scholar]
- 9.Maruyama Y, Goto K, Terasawa M. Ger. Offen. 1981 DE 3010751 19810806. [Google Scholar]
- 10.Ukawa K, Ishiguro T, Kuriki H, Nohara A. Chem. Pharm. Bull. 1985;33:4432–4437. doi: 10.1248/cpb.33.4432. [DOI] [PubMed] [Google Scholar]
- 11.(a) Abdel-Rahman AH, Hammouda MAA, El-Desoky SI. Heteroat. Chem. 2005;16:20–27. [Google Scholar]; (b) Langer P, Appel B. Tetrahedron Lett. 2003;44:5133–5135. [Google Scholar]; (c) Daia DE, Gabbutt CD, Heron BM, Hepworth JD, Hursthouse MB, Abdul Malik KM. Tetrahedron Lett. 2003;44:1461–1464. [Google Scholar]; (d) Fujiwara H, Kitagawa K. Heterocycles. 2000;53:409–418. [Google Scholar]; (e) O'Callaghan CN, McMurry TBH, O'Brien JE, Draper SMJ. Chem. Res. (S) 1997:312–313. [Google Scholar]; (f) O'Callaghan CN, McMurry TBH, O'Brien J. Chem. Soc., Perkin Trans. 1. 1995:417–420. [Google Scholar]; For recent synthetic work, see:
- 12.Anderson DR, Hegde S, Reinhard E, Gomez L, Vernier WF, Lee L, Liu S, Sambandam A, Snider PA, Masih L. Bioorg. Med. Chem. Lett. 2005;15:1587–1590. doi: 10.1016/j.bmcl.2005.01.067. [DOI] [PubMed] [Google Scholar]
- 13.Bristol JA, Gold EH, Gross I, Lovey RG, Long JFJ. Med. Chem. 1981;24:1010–1013. doi: 10.1021/jm00140a020. [DOI] [PubMed] [Google Scholar]
- 14.Evdokimov NM, Magedov IV, Kireev AS, Kornienko A. Org. Lett. 2006;8:899–902. doi: 10.1021/ol052994+. [DOI] [PubMed] [Google Scholar]
- 15.Crystallographic data for compound 4: C20H16N4OS, Mr = 360.43, triclinic space group , a = 7.3717(6), b = 9.5840(7), c = 12.8924(10)Å, α = 103.820(2), β = 92.975(2), γ = 99.441(2)°, V = 868.55(12)Å3, Z = 2, T = 120(2) K, F(000) = 376, Dcalcd = 1.378 g cm−3, ηmax = 29.99°, 9671 reflections measured and 4882 unique (Rint = 0.0190) reflections, full matrix least-squares refinement on F2, R1 (obs) = 0.0494, and wR2 (all data) = 0.1037. Supplementary data in the form of CIFs have been deposited with the Cambridge Crystallographic Data Centre (CCDC 622796). Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK [fax: +44 1223 336033 or e-mail: deposit@ccdc.cam.ac.uk]
- 16.General procedure for benzopyranopyridine synthesis: To a mixture of a selected salicylaldehyde (1.5 mmol), malononitrile (3 mmol) and a desired thiol (1.5 mmol) in 7 mL of anhydrous ethanol was added Et3N (0.1 mmol) dropwise at room temperature. The resulting mixture was refluxed for 3–3.5 h and then allowed to cool to room temperature. The formed precipitate was isolated by filtration. The product was dissolved in DMF (3 mL), and the remaining undissolved material was removed by filtration. To the filtrate was added water (4 mL), which resulted in the crystallization of the product. The formed crystals were isolated by filtration to yield a corresponding pure benzopyranopyridine (1–11).
- 17.Selected characterization data: 4: 69%; mp 275–276 °C (DMF); 1H NMR (DMSO-d6) δ 2.24 (s, 3H), 5.69 (s, 1H), 6.32 (br s, 2H), 6.72 (d, J = 8.2 Hz, 1H), 6.78 (br s, 2H), 6.83 (dd, J = 1.3, 7.3 Hz, 2H), 6.91 (d, J = 1.5 Hz, 1H), 7.01 (dd, J = 1.5, 8.2 Hz, 1H), 7.13 (t, J = 7.3 Hz, 2H), 7.28 (dd, J = 1.2, 7.3 Hz, 1H); 13C NMR (DMSO-d6) δ 160.2, 160.0, 156.9, 149.2, 136.6, 132.9, 131.2, 129.5, 129.4, 129.2, 129.0, 128.6, 121.8, 116.9, 116.0, 86.6, 70.6, 43.3, 20.7; IR (KBr) 3480, 3410, 3374, 3144, 2926, 2204, 1658, 1616, 1500, 1478, 1398, 1332, 1262, 1226, 1164, 1076, 1024, 900, 820, 780, 740, 692, 644, 532 cm−1. Anal. Calcd for C20H16N4OS (360.442): C, 66.64; H, 4.48; N, 15.55; S, 8.89. Found: C, 66.51; H, 4.52; N, 15.68; S, 8.83. Compound 6: 63%; mp 240–242 °C (DMF); 1H NMR (DMSO-d6) δ 2.31 (s, 3H), 3.50 (d, J = 14.0 Hz, 1H), 3.57 (d, J = 14.0 Hz, 1H), 5.43 (s, 1H), 6.02 (dd, J = 0.9, 3.0 Hz, 1H), 6.26 (d, J = 1.8 Hz, 1H), 6.39 (br s, 2H), 6.65 (br s, 2H), 6.98 (d, J = 8.8 Hz, 1H), 7.09 (d, J = 1.8 Hz, 1H), 7.11 (dd, J = 1.8, 8.8 Hz, 1H), 7.41 (dd, J = 0.9, 1.8 Hz, 1H); 13C NMR (DMSO-d6) δ 160.7, 160.3, 157.1, 151.2, 149.4, 142.7, 133.8, 129.7, 129.0, 116.9, 116.5, 111.0, 107.8, 87.1, 71.0, 25.9, 20.9; IR (KBr) 3458, 3350, 3240, 3160, 2918, 2200, 1628, 1606, 1575, 1498, 1472, 1398, 1332, 1262, 1226, 1155, 1008, 780, 745, 538 cm−1. Anal. Calcd for C19H16N4O2S (364.431): C, 62.62; H, 4.43; N, 15.38; S, 8.80. Found: C, 62.45; H, 4.31; N, 15.52; S, 8.91. Compound 8: 57%; mp 254–255 °C (DMF); 1H NMR (DMSO-d6) δ 3.72 (s, 3H), 5.74 (s, 1H), 6.30 (br s, 2H), 6.74 (dd, J = 1.4, 7.9 Hz, 1H), 6.80 (br s, 2H), 6.84 (dd, J = 1.2, 7.3 Hz, 2H), 6.90 (dd, J = 1.4, 7.9 Hz, 1H), 7.01 (t, J = 7.9 Hz, 1H), 7.12 (t, J = 7.3 Hz, 2H), 7.28 (dt, J = 1.2, 7.3 Hz, 1H); 13C NMR (DMSO-d6) δ 160.3, 160.1, 156.9, 147.5, 141.3, 136.4, 131.3, 129.4, 128.8, 123.9, 123.0, 120.5, 117.1, 111.9, 86.7, 70.9, 56.5, 43.4; IR (KBr) 3452, 3340, 3272, 2866, 2208, 1656, 1630, 1606, 1590, 1486, 1408, 1350, 1270, 1220, 1172, 1116, 1096, 794, 778, 764, 748, 710 cm−1. Anal. Calcd for C20H16N4O2S (376.442): C, 63.81; H, 4.29; N, 14.89; S, 8.52. Found: C, 63.95; H 4.08; N, 14.81; S, 8.63.