Executive summary
Rodent infestation and subsequent allergen exposure can occur in a wide range of environments, including homes, schools, hospitals, stores, restaurants, and animal laboratory facilities.1 The amount of rodent allergen exposure in a particular environment depends on numerous factors, the most important of which is the presence of rodents. Other factors include reservoirs and ingress of allergens from other locations.
The health effects of rodent exposure start with sensitization, which leads to sensitivity (ie, allergy) and then to morbidity if exposure continues. Exposure to rodent allergens, particularly to levels above 1.6 μg/g of dust, is associated with an increased risk of developing rodent-specific IgE. For that reason, a recommendation is made to reduce rodent allergen exposure as much as is feasible.
It is important to recognize that exposure to a specific concentration of rodent allergen should not be interpreted to represent a rigid clinical threshold. Allergen levels associated with an increased risk of sensitization or disease may instead represent artifacts related to specific characteristics of a study population and to the distribution of allergen levels in their environments and to their prevalence of sensitization or disease. Reported thresholds may also represent purely statistical phenomena, which should not be assumed to be biologically relevant. Study thresholds also tend to ignore the underlying shape of the exposure-response relationship, presuming that there is a steady, low-level risk up until a specific allergen concentration at which the risk increases to a higher level and remains at that higher level. It is possible that a more linear or even sigmoidal type of relationship exists between exposure and morbidity, which could mean that any amount of exposure might potentially be harmful.
Once sensitization has occurred, continued exposure is associated with a risk of developing disease. If a sensitized person develops asthma or rhinitis, further exposure increases their risk of morbidity. With further exposure to rodent allergens, individuals who are sensitized will tend to develop symptoms, such as wheezing and rhinorrhea. To minimize this risk, exposure reduction is recommended for sensitized individuals. Individuals who are sensitized and who already have developed a respiratory disease are strongly advised to avoid further exposure. It is possible to develop tolerance to rodent allergens. Rodent-tolerant individuals may develop rodent-specific IgG4, which is believed to serve as a blocking antibody. Because this mechanism is still under investigation, no recommendation is made for the routine measurement of rodent-specific IgG4.
The clinical evaluation of rodent allergy begins by asking patients about their rodent exposure along with questions to determine whether there is a relationship between exposure to rodents and clinical symptoms. Animal laboratory workers can usually provide a reasonably accurate history of whether rodent exposure triggers their symptoms; however, homeowners are often incapable of providing an equally accurate history. That is because the extent of exposure to rodents is not always clear unless the home has an obvious infestation. Establishment of a relationship between rodent exposure and symptoms is further complicated because homes with increased concentrations of rodent allergens are likely to have other allergens of similar importance that also can serve as triggers of symptoms. This makes it difficult for a homeowner to report an exclusively rodent-related symptom history.
Patients with suspected exposure to rodents, including all urban dwellers and families living in rural areas, should be evaluated for the presence of specific IgE either with skin prick testing or specific IgE tests. The decision to perform this test should be guided by knowledge of where rodents are likely to be present and whether there is a history of rodent exposure in the patient’s home or workplace. This provides an estimate of the risk that rodent exposure is associated with the risk of developing symptoms. Tests for the presence of rodent sensitization have been studied most extensively in animal laboratory workers. The performance characteristics of skin prick test and measurement of specific IgE for mouse allergy are summarized in Table 1. Immunotherapy for rodent allergy has not been adequately studied, so no recommendations are provided regarding administration of rodent-specific immunotherapy.
The problem with obtaining high-level evidence to demonstrate the efficacy of rodent avoidance measures is that most interventions are nonspecific. Measures that remove rodent allergens also remove other allergens at the same time, making it difficult to prove that removal of the rodent allergen itself is responsible for any clinical benefit that is found. Environmental reduction of rodent allergens can most effectively be accomplished by complete removal of the animals. Unlike the situation with furry animals, homeowners generally are willing to comply with recommendations for rodent removal. Obviously, eradication of a rodent infestation is more challenging from a logistical standpoint than removal of a pet. Exposure assessment and reduction are best attained using integrated pest management (IPM).
IPM begins with identification and removal of facilitative factors (food, water, shelter) that allow populations of rodents to inhabit an environment, thus reducing its carrying capacity. It also includes blocking pathways of rodent ingress by sealing openings. The rodents themselves can be removed with use of bait traps, rodenticides, and, in some cases, introduction of rodent predators, such as cats. Measurement of rodent allergens in settled dust is of uncertain clinical utility because analytic tests are not standardized and clinically relevant exposure thresholds are not well defined. The most potentially helpful use of rodent dust analysis is to evaluate the effectiveness of an intervention by comparing samples from before and after the intervention.
Laboratory animal handlers require special consideration. Sensitization and sensitivity (ie, development of allergy symptoms with exposure to an allergen in a sensitized person) progress rapidly in predisposed individuals who work in such facilities, which is why monitoring of employees for sensitization is recommended. Specific techniques to limit animal allergen exposure in such facilities also are recommended.
Introduction
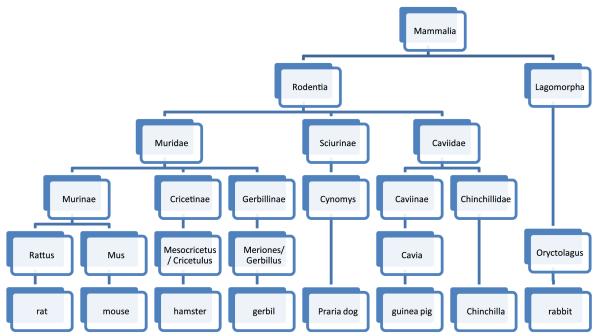
Rodents (Rodentia) are the largest order of mammals in the world, with estimates ranging from 1,500 to 2,000 different documented species. The order includes commonly known mammals, such as rats, mice, guinea pigs, hamsters, and gerbils. The order Rodentia also includes beavers, muskrats, porcupines, woodchucks, chipmunks, squirrels, prairie dogs, marmots, chinchillas, voles, lemmings, and many others, although significant human exposure to these rodents is uncommon. The presence of 2 sets of incisors (top and bottom), used for chewing, is the common characteristic of rodent species. Incidentally, rabbits, hares, and a few other species make up the Lagomorpha order and therefore are not classified as rodents. Figure 1 shows an abbreviated taxonomy of the order Rodentia.2
Figure 1.
Taxonomy of the order Rodentia. Adapted from Taxonomy of Common Rodent and Rodent-like pets.2
The common house mouse (Mus musculus) is a small, primarily nocturnal mammal. Although usually considered to be pests, mice also are popular pets. Mice are known to invade and establish residence in homes and other buildings, including schools, to obtain food and can at times be harmful, causing structural damage. Certain rodents, such as the deer mouse, can also spread diseases such as hantavirus through their feces and possibly also through their urine and saliva.3,4 Mice also are commonly used in research laboratories, where laboratory animal handlers come into contact with their allergens.
Mice tend to have litter sizes of 4 to 8 pups and can have 6 to 8 litters per year, depending on the availability of food. Their life span in the wild is less than 1 year because of predation, although under laboratory conditions they can live as long as 2 years.5 Mice have the ability to jump 12 inches up and down 8 feet to the floor. They can run up almost any vertical surface, including wood, brick, metal pipes, wire mesh, and cables, and can run along suspended electric wires and ropes. They can squeeze through a 1/4-inchdiameter hole, travel upside down, cling from 1/4-inch hardware mesh, swim well, and survive at 24 °F for many generations.6
Rats are long-tailed mammals also of the order Rodentia. The most important rats from a human perspective are the black or roof rat, Rattus rattus, and the brown or Norway rat, Rattus norvegicus. The Norway rat is found in every state of the United States, whereas the roof rat tends to be found in coastal states. Rats usually can be distinguished from mice by their larger size. Rats also can serve as vectors for certain pathogens, such as Lassa fever. They are popular as pets but also are commonly used in research laboratories, where laboratory animal handlers come into contact with their allergens.6
Rats tend to be nocturnal and search for food and water between dusk and dawn; however, they will come out in daylight if their habitat is overcrowded or food is lacking. They require daily water; prefer traveling along edges, pipes, and rafters; and can even travel along overhead utility lines. They prefer to not cross open spaces to avoid predation, and although they have poor visual acuity, they have acute senses of smell, taste, hearing, and touch using their whiskers.
Rats tend to have litter sizes of 5 to 12, and they can have up to 9 litters per year, depending on food supplies. Their life span generally is less than 1 year. Rats can pass through openings as small as 3/4 inch, theycan climb up vertical surfaces and even inside vertical pipes, and they can crawl horizontally on any type of pipe or conduit. They can jump vertically 24 inches and fall more than 50 feet and survive. They are able to swim under water for up to 30 seconds, tread water for up to 3 days, and swim up to ½ mile in open water. This permits them to enter buildings through drains and toilets.7
Although guinea pigs (Cavia porcellus), hamsters (Mesocritecus auratus), and gerbils (Gerbillus jerboa) also are rodents, they usually are kept as pets in a confined area and usually are not considered to be pests. Patients who keep such pets clearly can develop allergic symptoms after sensitization; however, other than in the case of laboratory animal workers, exposure to such rodents can usually be contained, and complete abatement, should it become necessary, is easily accomplished with removal of the offending animal.
Major rodent allergens
The currently identified major mouse and rat allergens are Mus m 1 and Rat n 1 respectively.
Mus m 1 (lipocalin/urinary protein)
Mouse allergen was originally identified as a component of mouse urine and initially was referred to as mouse urinary protein. The major allergen was subsequently isolated from mouse urine and now is designated as Mus m 1. Mus m 1 is also found on mouse hair follicles8 and also on the stratum corneum and along the skin surface.9 Mus m 1 is a pheromone-binding protein and is under regulation of sex hormones so that male mice produce substantially more Mus m 1 than females. One report found that female mice do not produce a significant amount of Mus m 1.10,11 Mus m 1 belongs to the lipocalin family of fatty acid–binding proteins. It consists of 180 amino acids with a molecular weight of 16 to 18 kDa. Most mouse-sensitive individuals have specific IgE to Mus m 1. Cross-reactivity to Can f 2, Fel d 4, and Rat n 1 has been described. Cross-reactivity with rat is substantial, with almost all mouse-sensitized people also being sensitized to rat allergens. Rat n 1 and Mus m 1 are highly homologous, sharing 80% of their amino acids. The cross-reactivity with cat and dog allergens is less and is certainly no greater than the cross-reactivity seen between other families of allergens.
Rat n 1 (lipocalin)
The major rat allergen is Rat n 1.12 Similar to Mus m 1, it is present in higher concentrations in male rat urine than female rat urine and may not be produced by females at all. Rat n 1 also belongs to the lipocalin family (PF00061) of fatty acid proteins. It consists of 181 amino acids usually as a tetramer. More than 90% of individuals sensitive to rats have specific IgE to Rat n 1. Cross-reactivity to Can f 2, Fel d 4, and Mus m 1 has been described.
Rat albumin
Rat serum albumin and its proteolytic fragments were able to induce IgE-mediated histamine release in 8 of 33 rat-allergic patients. Each fragment has at least 2 antigenic determinants for a total minimum of 4 sites on the intact rat serum albumin molecule.13
Exposure to rodent allergens
In 2 studies of inner-city homes, 100% of the homes in one had detectable mouse allergen in settled dust, with a median level of 14 μg/g, whereas the other reported detectable airborne Mus m 1 in more than 80% of bedrooms of children with asthma.14 Another study found that 75% to 80% of suburban Maryland homes had detectable mouse allergen,15 although levels were 100- to 1000-fold less than those found in inner-city Baltimore.16
Mus m 1 is one of the few allergens to span environments from inner-city to suburban homes and schools. It is widely distributed and commonly found even in homes that are not infested with mice.1 In a study evaluating the relationship between building-level characteristics and exposure, increased mouse allergen was associated with the presence of rodents, building size (low-rise or high-rise compared with a house or duplex), and with living in public housing.17 In a nationally representative US housing survey, 82% of homes had detectable Mus m 1. In this US survey, older homes, high-rise apartments, mobile homes, and low-income homes tended to have higher concentrations of Mus m 1. Concentration of Mus m 1 in inner-city homes are an order of magnitude greater than those found in non–inner-city homes and classrooms.18
In a subset of homes from the National Cooperative Inner-City Asthma Study (NCICAS), 95% of all homes had detectable Mus m 1 in at least one room. Mouse allergen levels correlated among rooms. Homes with evidence of mice, such as droppings or stains, in one or more rooms had higher levels of mouse allergen than those without such evidence.19 Of 994 allergic children from 7 US urban communities, mice or rats were reported in 40% of homes.20 Thirty-three percent of inner-city homes also had detectable rat allergen Rat n 1. The presence of rat allergen was associated with reported rat and mouse infestation, as well as evidence of mouse infestation on home inspection.21
Recent individual-city studies reported that 42% of homes in Boston have detectable mouse allergen.22 In addition, 89% of dustsamples from urban elementary schools in the northeastern United States had detectable levels of mouse allergen,23 with 68% having a Mus m 1 concentration greater than 0.5 μg/g. In contrast, mouse allergen was detectable in only 26% of bedrooms of the students who attended those schools. Higher mouse allergen levels were found in classrooms (6.45 μg/g) than in homes (0.44 μg/g).24 Mouse allergen was detected in all homes where there was sufficient dust in a study of homes in Toronto, Ontario, albeit at low concentrations. Self-reported presence of mice predicted measured indoor levels of mouse allergens.25
In another study of inner-city homes in Los Angeles, California, 51% of homes had detectable rodent allergen in kitchen dust. All families who reported the presence of rodents had detectable allergen in the home and had higher levels of allergen than those who did not have rodents. Unwashed dishes or food crumbs and lack of a working vacuum increased the risk of rodent sightings or detectable allergen.26 Other factors associated with mouse allergen levels in home include frequency of mouse sightings, use of traps or pesticides for mice, presence of holes in ceilings or walls, absence of a cat, and living in a building with fewer than 8 floors.27
Another study of multiple daycare centers in 2 North Carolina counties found 83% with detectable mouse allergen.28 A similar study of Head Start facilities in Arkansas revealed 100% with detectable Mus m 1, and the median allergen level was 0.18 μg/g of settled dust.29 The study had fewer centers with detectable cockroach allergen, but only a few centers were in urban locations.
Rat allergen was found to be less prevalent than mouse allergens in homes from one study from the NCICAS. Although Rat n 1 concentration ranged from 4.4 to 1,413 ng/g in bedrooms, 4.4 to 3,380 ng/g in living rooms, and 4.4 to 4,620 ng/g in kitchens, the median values for each of these room types was below the level of detection. The authors were careful to rule out cross-reactivity between Mus m 1 and Rat n 1 in their assay. There was no correlation between rat and mouse allergen concentrations in dust samples.21 The presence of rat allergen was associated with reported rat and mouse infestation. Evidence of mice or rats was found in 23.3% of studied homes.30 In another study of public housing residences in New York City, evidence of mice was found in 13% of the homes.31
It has been suggested that cross-reactivity between rodent allergens and dog and cat allergens could confound studies of rodent allergen exposure. The observation that cat allergen and mouse allergen levels in dust samples from homes are inversely correlated suggests that cross-reactivity is not an explanation for finding mouse allergen in many public places.14,27
Aerodynamic and environmental properties
The aerodynamic and environmental properties of Mus m 1 have been characterized and studied in laboratory, home, and school settings. It is carried on small and large particles and has the ability to migrate throughout a building.8 Rat allergens are carried on particles ranging from 1 to 20 μm in diameter, with most less than 7 μm,32 and can remain airborne for 60 minutes or longer after disturbance of the environment.
Studies have characterized mouse allergen in public areas of an animal facility and found that rooms connected to the animal facility, but not actually containing mice, had detectable allergen on particles ranging in size from 0.4 to 3.3 μm.33 In free-standing, independently ventilated areas, such as a cafeteria not connected to a mouse facility, Mus m 1 was predominantly found in particles smaller than 10 μm, suggesting that it is more of an airborne issue than often appreciated34 and possibly explaining why rodent allergens can be carried substantial distances in buildings.
Health effects
For rodent allergen exposure to cause adverse health effects, a chain of events is necessary. First, exposure to rodent allergens leads to the development of allergic IgE sensitization. Once that has occurred, further exposure leads to the development of asthma or rhinitis. Finally, once a patient has become sensitized and has developed respiratory disease, additional exposure causes morbidity in the form of exacerbation of respiratory symptoms.
1. Exposure to mouse allergen in homes should be minimized to reduce the risk of sensitization. (Rec, B evidence)
Mouse allergen exposure increases the risk of mouse sensitization in atopic children, for example, such sensitization, as determined by a skin prick or serum specific IgE tests, in a number of studies, ranges from 12% to 26%.35 Eighty-nine of 499 children (18%) had a positive mouse skin test result in a subset of children analyzed from the NCICAS. In addition, higher rates of mouse sensitization were found in children whose homes had mouse allergen levels above a median of 1.60 μg/g of settled dust.36 It is important to recognize that although this value is often referred to as a threshold for sensitization, it is actually the median value of Mus m 1 that was found in kitchens in this one study.
Subsequent research has confirmed that persons who are exposed to Mus m 1 concentrations above 1.6 μg/g are more likely to become sensitized than those who are exposed to lower amounts. What is not known is whether there is a linear relationship between exposure and sensitization risk or whether there is a threshold exposure above which the risk increases sharply. Because rodent allergen concentrations in settled dust correlates with the number of rodents in an environment, fewer sightings of rodents is a good indicator for lower exposure, although the absence of visible rodents is no guarantee for a complete lack of exposure. A more complete discussion of dust analysis for measurement of rodent allergen content is found later in this practice parameter.37
2. Exposure to rodent allergens should be minimized to reduce the risk that sensitized individuals will develop sensitivity in the form of respiratory symptoms. (Rec, C Evidence)
Little is known about the role of rodent allergen exposure and asthma development, although a prospective study of a Boston birth cohort suggested that early mouse exposure is associated with early wheeze in infancy.38 A recent analysis of data collected as part of the Boston Home Allergens and Asthma Study, which included 500 infants of atopic parents, found a significant association between parent report of mouse sightings and development of wheeze through 7 years of age; however, early exposure to mice at 2 to 3 months did not predict wheeze or asthma at 7 years.39 Although it is possible that mouse exposure may influence respiratory status as an irritant, the association between early exposure to mouse and the prevalence of atopy at 7 years argues that an early-life exposure influences the development of later disease through a progression from sensitization to disease. It is also possible that a separate, highly correlated exposure, such as dust mite, could be responsible for this observation.
In a cohort study, sensitization to mouse allergen by itself was found to be associated with more than twice the odds of asthma diagnosis among 853 women in Boston. Although it is likely that most of these women had substantial exposure to mouse allergens, the actual exposure was not measured in this study. The study also found that mouse allergy was more prevalent in women from economically disadvantaged areas and from ethnic minority groups.40
To further support these observations, children 2 to 3 years of age who had anti-cockroach and anti-mouse IgE were found to be at increased risk of wheeze and atopy, with higher amounts of specific IgE corresponding to an increased prevalence of wheeze, rhinitis, and atopic dermatitis.41 The amount of allergen-specific IgE also was associated with more severe asthma across a range of clinical and biologic markers, although exposure was not measured in this study.42
3. Mouse and rat allergen exposure should be minimized to reduce the risk of asthma morbidity in already sensitized individuals. (StrRec, B Evidence)
Exposure to mouse allergen may be an important cause of asthma morbidity. Of 127 children preschool children with asthma from inner-city Baltimore, mouse-sensitized children exposed to higher levels of Mus m 1 (>0.5 μg/g) had 50% more days of symptoms, 80% more days of β-agonist use, more unscheduled physician visits, emergency department visits, and hospitalizations than other children with asthma who were exposed to lower levels.43
Data from 831 households participating in the National Survey of Lead and Allergens in Housing found that 82% had detectable levels of Mus m 1 and concentrations exceeded 1.6 μg/g in 35% of the homes. This was associated with increased odds of having asthma symptoms.37
In a study of inner-city homes, exposure to rodent allergens was found to be an independent risk factor for asthma morbidity. A subanalysis of children with asthma enrolled in the Inner-City Asthma Study found that 22% tested positive to mouse. Most bedrooms (80%) had detectable mouse allergen. Sensitization and exposure were thus associated with increased asthma morbidity, including hospitalizations.44
Rat exposure and sensitization are also associated with increased asthma morbidity in inner-city children. Detectable rat allergen (Rat n 1) was found in 33% of inner-city homes, which correlated with reported rat and mouse infestation, as well as evidence of mouse infestation on home assessment. The numbers of hospitalizations and unscheduled medical visits were increased when sensitization and exposure to rat allergen were present.21
Tolerance to rodent allergens
4. Measurement of mouse-specific IgG4 may help to identify individuals with a reduced risk of mouse skin test sensitivity though the benefit of doing so is unclear. (NoRec, C Evidence)
Tolerance has been observed in some occupational laboratory workers who have been sensitized to rodent allergens. Although the exact mechanism behind this is not totally clear, the presence of specific IgG and IgG4 blocking antibodies has been proposed as a possible mechanism. This is based on the observation that among workers with detectable mouse IgE, higher specific IgG and IgG4 levels are associated with a decreased risk of developing mouse-related symptoms.45 It is not known whether this is also true for children or for the development of rodent-induced asthma.
A high level of exposure to mouse allergens does not necessarily prevent sensitization because the presence of detectable mousespecific IgG4 is associated with an increased risk of skin test sensitivity to mouse. The highest IgG4 levels, however, appear to be associated with an attenuated risk of mouse skin test sensitivity, suggesting that induction of high levels of IgG4 through natural exposure may protect against the development of allergic sensitization or may simply be a marker for tolerance.46 In fact, in one study IgG4 antibodies were present before IgE antibodies developed and IgG4 levels were stable over time. Despite these findings, the authors concluded that the modified TH2 response had no protective effect on development of sensitization or on allergic symptoms.47
In a study of new employees at a mouse facility, exposure and sensitization were monitored prospectively along with measurements of mouse-specific IgG. By 24 months, 23% developed sensitization and 8% had specific IgG. The frequency of sensitization increased with increased exposure. Once exposure exceeded 1.2 ng/M3, sensitization rates decreased, suggesting that extremely high exposures are associated with development of tolerance.48
Clinical evaluation
5. Patients with possible rodent allergy should be asked whether they have seen rodents in their home. (StrRec, B Evidence)
Direct measurement of rodent allergens in homes is not generally available for clinical use. Fortunately, a patient report of rodent infestation has been shown to have a high positive predictive value for high levels of mouse allergen in the home.25 In one study, when patients reported the presence of mice, 90% of those homes had Mus m 1 levels greater than 0.5mcg/g of settled dust. However, a negative report was not as reliable at ruling out exposure to rodent allergens.49 Neighborhood-level characteristics, and more specifically the presence of housing code violations, also correlate with the concentration of indoor rodent allergens in homes.50
6. Patients with atopy and likely rodent exposure, such as patients with persistent asthma living in inner-city areas, should be evaluated for sensitization to rodent allergens by skin prick testing, rodent-specific IgE testing, or both if indicated. (StrRec, B Evidence)
Diagnostic allergy tests, such as skin tests and specific IgE tests, can help to determine whether symptoms are allergic in origin. The decision to perform diagnostic testing must rely on clinical judgment to select patients who would benefit most from determining their allergic status while minimizing unnecessary testing. All patients with persistent asthma living in inner-city areas should be tested for rodent sensitization. Patients with a low probability of allergic sensitization should not be tested for specific IgE due to the increased likelihood of a false positive test result.51 The use of diagnostic tests to identify the presence of sensitization in clinical practice has been described in detail in “Allergy Diagnostic Testing: An Updated Practice Parameter.”52
Mouse and rat epithelia extracts are commercially available for skin testing. However, such extracts are not available for rodent urine. Mus m 1 has been assayed in several commercially available mouse extracts by means of enzyme-linked immunosorbent assay using immunosorbent purified sheep anti-Mus m 1. Mus m 1 concentrations appear to differ substantially by manufacturer and extract type (Greer glycerinated 1:20 wt/vol extract 8270 ng/mL, Greer Laboratories, Lenoir, North Carolina; Allermed glycerinated 1:10 wt/vol extract 3185 ng/mL, Allermed Laboratories, San Diego, California; and ALK-Abello glycerinated 1:20 wt/vol extract 546 ng/mL, ALK-Abello, Round Rock, Texas).53
Specific IgE tests are available for mouse and rat epithelium, serum proteins, and urine proteins though specific IgE to individual recombinant components are not yet available commercially. There have been a number of recent advances in diagnostic approaches to patients with suspected mouse allergy.16,54 Recombinant Mus m 1 has been demonstrated to be a suitable alternative to traditional Mus m 1 for specific IgE antibody testing.55,56
In a study of diagnostic tests for mouse allergy in laboratory animal workers, nasal allergen challenge responses to mouse epithelia extract were used as a gold standard. Mouse urine–specific IgE measured by ImmunoCap was compared with skin prick tests with mouse epithelia. The mouse extract that was used for all skin testing and the nasal challenge procedures consisted of aqueous mouse epithelia extract (1:10 wt/vol) obtained from Greer Laboratories. The skin test corresponded better with allergic symptoms and with responses to the nasal allergen challenge than did the specific IgE test. Intracutaneous tests did not correspond well with clinical allergic responses to mouse allergen in the nasal allergen challenge.53
In this widely cited study, the specific IgE and skin tests were performed with qualitatively and quantitatively different extracts.
It is possible that the skin test correlated better with the nasal challenge because that is the extract that was used for the challenges. When the authors looked at both mouse urine–specific IgE and mouse epithelial–specific IgE, the mouse epithelial–specific IgE was no more sensitive than mouse urine–specific IgE and in fact was slightly less sensitive than mouse urine–specific IgE. Thus, the results of this study do not appear to depend on the use of mouse urine IgE rather than mouse epithelial IgE. This study is applicable to laboratory animal workers who reported mouse-associated symptoms. This is a different population than a community-based one that cannot report symptoms associated with mouse exposure because they do not have direct contact with mice to the degree that laboratory workers do. The performance characteristics of skin and specific IgE tests from this study are given in Table 1.
Another study in a community population with asthma found similar results in that 67% of patients who either had a positive skin or specific-IgE test result had a positive nasal challenge response. Skin prick test or the combination of skin test plus measurement of specific-IgE performed best for diagnosing mouse allergy in that population.57
Immunotherapy for rodents
7. Immunotherapy with rodent extracts has not been adequately studied to determine whether it is effective. (NoRec, D Evidence)
Evidence for treatment of rodent-allergic patients with allergen immunotherapy is sparse. A case report of a rat-allergic laboratory worker who received immunotherapy with rat epithelium for 18 months described a decrease in rat-specific IgE and reduced symptoms with exposure to rats.58 In a study of 23 patients, a significant increase in blocking antibody titers as determined by serum inhibition of allergen-induced histamine release was demonstrated after immunotherapy with extracts to a variety of laboratory animals; however, the clinical effect of this was not determined. The investigators demonstrated a lack of cross-reactivity in the IgG response to the major animal allergens.59
Exposure assessment and reduction
Assessment for rodents is a qualitative process that requires a careful walk-through of the property and systematically collecting qualitative evidence for the presence of rodents. The goal of rodent exposure reduction is to eliminate or minimize the source of rodent allergens from the environment and to remove or abate their reservoirs. In the furry animal practice parameter, removal of sources was impractical because most families are reluctant to give up a beloved pet. The situation is different for rodents because most building occupants usually prefer that they be completely removed, with the infrequent exception of a pet rodent. Specific interventions include thorough cleaning, education on allergen removal, filling of holes to prevent ingress, application of rodenticides, traps, and, in some situations, professional extermination.
Facilitative factors
Assessment
8. An assessment for facilitative factors of rodent exposure should focus on identifying food, water, routes of ingress, and the presence of rodent habitats. (Rec, C Evidence)
When performing an assessment for factors that lead to an infestation by rodents, it helps to guide the inspection with a schematic of the building. The goal is to find areas that might provide shelter, food, water, or access to the building. Because rodents require food and water, the presence of these factors is an invitation for rodents to enter an environment and usually indicates where they will be found. Rats can gain access to buildings through virtually any opening, including toilets. Entry holes can be as small as 1/4 inch for mice and 3/4 inch for rats. Such openings can be found in walls, in pipe entries, in sewer outlets, and under doors. Rodent nests are composed of material scavenged from the local. A typical nest can range from 5 inches for mice to 12 inches for rats. Rodents can also find shelter in piles of trash and miscellaneous debris.60
Mitigation
9. Habitat modification should be performed to remove means of rodent ingress, food, water, and shelter. (StrRec, C Evidence)
Elimination of causal factors of rodent infestations consists largely of habitat modification, which is an integral component of IPM. If the habitat is incapable of supporting a given population of rodents, they will go elsewhere or die. Rodents require a number of ingredients to survive, including a means of ingress, food, water, and shelter. Removal of any or all of these factors reduces the carrying capacity of the environment, causing the population of rodents to decline.61
If rodents are unable to gain entry to a home, there will not be a rodent infestation. The challenge is that rodents are able to pass through holes that are smaller than their body size. This means that all openings, regardless of how small, need to be sealed or covered with metal mesh to prevent ingress. Debris and clutter located adjacent to or near a building can mask evidence of rodent ingress and provide shelter. In some cases, it may be necessary to place rodent barriers made of metal in walls to prevent rodents from gnawing their way into the building.
Like all animals, rodents need a source of food and water. Foods commonly eaten by rodents include pet food, grains, and bird seed that may be near feeders. To deny them a food source, cereal, grain, and pet food should be stored in thick plastic containers and seeds should be kept in rodent-proof containers. Food should be placed in covered containers with a properly sealed lid. Garbage should be taken out regularly and should not be allowed to accumulate where rodents can get to it.
Contaminant sources
Assessment
10. Evidence for the presence of rodents should be identified to determine the likely extent of an infestation. (Rec, C Evidence)
The US Department of Housing and Urban Development’s “Guidance on Integrated Pest Management” recommends establishing a zero threshold for when implementation of rodent pest management should be implemented. This means that if even one rodent is observed, action should be taken to eliminate the pest.62
The most obvious evidence for the presence of rodents is seeing live rodents or signs of their presence. Because rodents tend to be nocturnal, a rodent that is seen during the daytime suggests either that the infestation is extremely heavy or that the rodent has recently arrived and has not yet found shelter. Reports of mice as pests in the previous 12 months in a home assessment questionnaire was found to predict increased concentrations of mouse allergen exposure in settled dust.25 This was also seen in a Baltimore inner-city study in which mouse infestations predicted detectable mouse allergen.14 Reports of rodents also were associated with increased mouse allergen exposure in both of the 2 large US studies.18,63 Examples of visual evidence include living and dead rodents; mouse droppings; urine staining of floors, carpets, and ceilings; tracks in dust; and rub marks on surfaces, including on floors and baseboards, inside cabinets, along countertops, and in most areas near food and water sources. A clear sign of rodent activity is the presence of droppings, particularly if they are still moist, which indicates recent deposition. Damage to a building, its furnishings, and food storage containers commonly manifests as gnaw marks and holes, wood chips and wood dust from gnawing, and small holes in containers, allowing access. Grease or rub marks on rafters, pipes, walls, and other areas indicate pathways of frequent rodent travel. They are caused by oil and dirt rubbing off the rodent’s fur. Footprints and tail tracks or whip marks also can be found along frequently traveled routes and near entry holes.64
Shelter near a building, such as debris, shrubbery, and other places where rodents can hide, allows rodents to migrate between outside and inside. To reduce their willingness to enter a building, a nonvegetative border is recommended around the perimeter that creates a space across which they are unwilling to travel because of fear of predators.
Rodents constantly urinate as they travel from place to place; thus, rodent infestation is often associated with a strong urine odor. Fresh and dry urine fluoresces with UV light as do other biological materials. Some individuals trained to mitigate rodent infestation can smell the difference between mouse and rat urine when they inspect contaminated buildings. The presence of sounds, such as climbing, running, squeaks, gnawing, and chewing, also is evidence that living rodents are present in a home.64
Source reduction
A variety of techniques have been studied for rodent removal from urban homes. These interventions include initial removal of the rodents in addition to habitat modification to prevent recurrence of the infestation.
11. Cats and other rodent predators could be considered as a possible method for reducing rodent populations, although they generally do not completely eliminate an infestation and individuals may become sensitized to a cat. (Opt, C Evidence)
Cats can lead to a reduction in a mouse population, but they generally do not eliminate it completely. They also can serve as a deterrent to new mouse immigration. In one study, both the number of cats and higher cat allergen levels were associated with lower airborne and settled dust mouse allergen levels in homes of inner-city children with asthma.65 Cats also can kill rats, particularly when they are young; however, they tend not to be a significant rat deterrent. The use of cats to reduce rodent exposure could potentially cause harm because 84% of the patients who were sensitized to mice in one study also were sensitized to cats.65 Given the known cross-reactivity between some cat allergens and rodent allergens, this intervention might cause more harm than the initial exposure. Owls and snakes also are rat predators; however, most homeowners are reluctant to have such predators roaming freely in their homes.
12. Rodent traps are an effective way to remove rodents from an infested building. (Rec, C Evidence).
Rodent traps fall into 3 general categories: snap traps, live traps, and glue boards. Each type of trap is better suited to certain situations than others.
Snap traps are triggered by the mouse or rat running over the trap. They should be placed where rodents often go, such as near food sources. They also should be baited properly and inspected frequently. A map should be used to record the location of each trap and the date it was set to prevent unpleasant smells due to decomposing rodents left in forgotten traps. Rats may learn to avoid traps, so it may be necessary to remove them for a week and then to set new traps in different locations.
Live traps consist of baited cages with a one-way door. They are available for both rats and mice and are useful when rodent populations are high because multiple catch traps can capture more rodents than snap traps. If the occupant prefers to release captured rodents rather than kill them, they should be released in natural areas at least 1500 m from their home habitat.
Glue boards consist of boards that are covered with a sticky material that can catch rodents. An advantage of glue boards is that they also can catch and retain rodent hair and droppings. Glue board traps should be inspected daily to reduce unnecessary suffering by the trapped animal.
13. Rodenticides should be used if other interventions are ineffective. They should be applied according to the manufacturer’s instructions that are part of the label. Many pesticides should only be applied by a licensed professional exterminator. (Rec, C Evidence)
Rodenticides are indicated if nonchemical methods alone prove insufficient to eliminate the infestation. Rodenticides should be used in accordance with their Environmental Protection Agency–approved label directions. Most rodenticides are anticoagulants, such as warfarin, chlorophacinone, and pindone. These last for a few days in a dead rodent body and thus pose a hazard to nontarget animals that might eat a dead rodent.66 Rats and mice can become resistant to some anticoagulants if they are overused. Such resistance should be suspected if the bait is eaten regularly but the same or a greater number of rodents, holes, and droppings are seen.67
Reservoirs
The main reservoirs of rodent allergens include carpeting, areas with rodent feces and urine, and areas where rodents are present. The allergens become incorporated into dust, which can remain allergenic for long periods.
Assessment
14. Measurement of rodent allergens in house dust may be considered; however, it has an unclear clinical benefit given the wide range of observed values and uncertain clinically relevant exposure thresholds. (Opt, D Evidence)
Measurement of rodent allergens in samples of dust to determine a patient’s exposure is optional at this time. This section consists of a series of suggestions that rely heavily on expert opinion with limited evidence to support them. Although analysis of settled dust for allergen content has been performed in research settings, it has not been widely used in clinical practice, which is the reason for this conservative stance. Dust analysis as is currently available has a number of limitations, including unclear indications for performing such an analysis, unstandardized methods for collecting appropriate samples, lack of accredited laboratories for performing the analysis, and uncertain clinically relevant exposure thresholds. In addition, many third-party payers do not reimburse for the cost of dust analysis, and there are no procedure codes available for such analysis.
Given the earlier recommendations to minimize exposure to rodent allergens to reduce the likelihood of sensitization, development of disease, and morbidity, it would seem reasonable that measurement of rodent allergens in settled dust could give an indication of what the exposure actually is. A problem with this approach is that people are exposed to many different environments. As a result, knowledge about exposure in one environment does not necessarily indicate that there is a similar amount of exposure in others and it does not provide information about overall exposure or how long the exposure has been present. Because we do not understand the shape of the exposure-response curve with respect to rodent allergen and asthma symptoms, it is difficult to determine what concentration of allergen is associated with a clinical effect. Added to this uncertainty about the shape of the dose-response curve is the substantial interindividual heterogeneity in degree of clinical sensitivity. Therefore, it is problematic to draw conclusions about the clinical implications of a given exposure to rodent allergens in an individual patient’s home.
One possible use for dust analysis would be to determine the success (or failure) of an environmental intervention. Although the absence of further rodent sightings is most likely to correlate with reduced allergen exposure, a sensitized patient who remains highly symptomatic after an intervention may want to determine whether the intervention was successful so that other causes of the continued illness may be sought. This would ideally require that a sample be obtained before and after the intervention so that a comparison can be made. Unfortunately, once the intervention has taken place, it is impossible to obtain a preintervention sample. For that reason, it might be wise for a homeowner to collect a dust sample before an extensive intervention and store it for possible future analysis should it become necessary. Because of the heterogeneity across individuals in clinical sensitivity to aeroallergens and our limited understanding of the dose-response relationship for mouse allergen exposure and its clinical effects, allergen measurements before and after an intervention must be interpreted with caution.
It is not currently possible to confidently predict the magnitude of reduction that would be required to achieve a clinical benefit. For example, if the dose-response relationship is linear, any decrease in allergen exposure would be expected to be associated with an incremental improvement in asthma. On the other hand, if there is a threshold effect, improvement in asthma would only be expected if the allergen concentration is reduced below that threshold. Finally, it might not be clear why someone has persistent symptoms. Dust analysis could be used to determine what types of exposures are present in an environment, which could help to direct a subsequent intervention.
Methods to collect dust
If a homeowner is going to collect dust for rodent allergen analysis, it is important that it be done with an uncontaminated vacuum. A variety of locations should be sampled because some may be more contaminated than others. If a preintervention and postintervention dust analysis is planned, dust collection should be from the same location and for the same duration both times. Patients should be informed that reimbursement for dust collection and rodent allergen analysis is unlikely to be reimbursed by their health plan, although it may be possible to obtain prior authorization to do so in some cases. Detailed protocols for collecting dust samples for rodent allergen analysis have been described.68,69 To determine the extent of rodent contamination in settled dust, dust samples can be collected from floor surfaces with adapters fitted to a vacuum cleaner nozzle or by using a filter cassette attached to a vacuum pump.70 Few studies of comparisons of collection efficiency have indicated that collection efficiency for rodent allergen collection from settled dust varies.68,71,72 Dust sampling ideally should be performed from a premeasured area in a methodical fashion. Sampling from hard floors typically requires collection from a larger surface area than from carpets because there usually is less dust on hard floors.
Analysis of dust samples
Currently, no laboratories have accreditation for measurement of rodent allergens. Most laboratories that perform such analysis use monoclonal antibody–based assays with reagents obtained from Indoor Biotechnologies (Charlottesville, Virginia). Other reagents also have been used, including polyclonal antibody–based assays (Greer Laboratories) and assays developed in individual research laboratories. Accreditation of medical laboratories generally is performed using CAP surveys, which are administered by the College for American Pathologists. This procedure involves measurement of samples by different laboratories to determine the interlaboratory and intralaboratory variation in results. It would be helpful for such a survey to be developed for the measurement of indoor allergens.
Interpretation of results
It is not clear how the results of dust rodent allergen analysis are to be interpreted given the wide range of exposures and lack of thresholds. When evaluating the efficacy of an intervention, it is likely that at least a 75% to 90% reduction in allergen exposure or even more might be necessary to be clinically useful.
Abatement
15. Rodent allergens in homes can be reduced using integrated pest management techniques. (StrRec, A Evidence)
IPM is a combination of control tactics that focuses on mitigating or reducing causal factors, eliminating sources of contamination, and removing or abating reservoirs of rodent allergens.73 Although most interventional studies of IPM have focused on cockroach allergen reduction, IPM also has been used to reduce rodent allergen exposure.
One study in Boston homes used a combination of filling holes with copper mesh, vacuuming, cleaning, and the baited traps with low-toxicity pesticides. This IPM intervention delivered by a licensed pest management professional resulted in a reduction of more than 75% in Mus m 1 levels in kitchens and bedrooms.74 More recently, researchers from the Inner City Asthma Study examined the efficacy of an IPM intervention that was facilitated and supported by research staff but primarily performed by study participants. This facilitated IPM strategy had minimal success because Mus m 1 levels were only reduced by approximately 27%.44,75 However, the subset of children whose homes had at least a 50% reduction in Mus m 1 had fewer missed school days, reduced sleep disruption, and reduced caretaker burden, suggesting that an intervention that can achieve at least a 50% reduction in allergen levels may also have positive effects on measures of asthma health. On the other hand, the symptom and health care use outcomes were not statistically significant, so the outcomes were mixed.44
Laboratory animal handlers
16. Monitoring for rodent sensitization should be considered at least for the first 3 years of employment in an laboratory animal facility. (Rec, C Evidence)
Approximately one-third of laboratory animal workers develop occupational allergy to animal allergens, and a third of these have symptomatic asthma. Sensitization generally occurs with the first 3 years of employment.76 Risk factors include atopic background and the intensity of exposure.77 In one study, skin prick test results were positive to laboratory animals in 27.6% of workers with direct animal exposure and 17.0% had allergic symptoms, with 6.2% of them also having asthmatic symptoms.78
A cohort of employees at the Jackson Laboratory, including animal caretakers, scientists, administrative and support personnel, materials and supplies handlers, and laboratory technicians, was evaluated for exposure and sensitization to rodents. Breathing zone Mus m 1 was detected in 82% of participants, with a mean exposure of 1.02 ng/M3. Mouse handlers had greater exposure to mouse allergen than nonhandlers did; however, 66% of nonhandlers still were exposed to detectable mouse allergen.79
17. Extra avoidance precautions should be taken for individuals with an increased risk of animal sensitization, including those with an atopic background and with high-intensity exposure. (Rec, B Evidence)
18. Extra avoidance precautions should be taken when contaminated bedding and high numbers of conscious animals are handled. (Rec, B Evidence)
Allergen levels have been studied extensively in laboratory settings, and the level of exposure has been found to be primarily dependent on activity, with the highest exposures occurring among cage changers, room cleaners, and animal feeders.80 Levels are also increased with higher animal density and decreased relative humidity.81-83 Allergens in animal facilities primarily occur as particles less than 10 μm in diameter. The concentration of airborne animal allergen is proportionate to the number of animals in the room. Allergen levels are twice as high on Mondays as on other days because of the tasks performed on Mondays. Filter tops on animal cages can substantially reduce airborne allergen levels. The highest personal exposure occurs when contaminated bedding and high numbers of conscious animals are handled.84 In one study, high exposure to mouse urinary protein was associated with handling mice, indirect contact with mice, and washing floors.34 Positive skin prick, specific IgE, and inhalation test reactions have been demonstrated mouse to urine proteins.85
19. Allergen exposure in laboratory facilities should be reduced by engineering controls, staff training, and appropriate personal protection. (Rec, B Evidence)
The most important approaches to reduced exposure are engineering and administrative controls. Because rodent exposure cannot be completely avoided, workers who are sensitized can be protected from rodent allergens with personal protective devices86 and laminar flow caging, frequent wet washing of animals, and careful maintenance of ventilation systems. However, not all these measures are possible in all situations.77 Increased humidity also is associated with reduced airborne allergen concentrations.87 Engineering controls, including well-maintained ventilation systems and training of staff and students involved in animal experiments, are essential to reduce the risk of sensitization88,89; however, it is not clear whether the amount of obtainable exposure reduction is sufficient to prevent sensitization in workers.
Care is needed to prevent transport of the allergen to other parts of the laboratory building29 and to homes. In one study, rat and mouse allergen concentrations were higher in the home mattresses of laboratory animal workers than in controls. This was reduced when the workers wore hair caps, suggesting that hair is a significant source of allergen transfer.90 In addition, children of laboratory workers have been found to have an increased risk of sensitization to the animals with which their parents work, suggesting that this transfer of allergens from work to home is of clinical importance.91
Pet shop workers are also at some risk for occupational allergens. In a study of workers from 24 pet shops, 33% reported respiratory symptoms at work, mostly against rodents, birds, insects, and hay, and 29% were sensitized to work-related allergens, mainly rodents.92
Table 1.
Performance Characteristics of Skin and Specific IgE Tests (ImmunoCAP) for Mouse Sensitivity
| Mouse | Skin | Specific IgE |
|---|---|---|
| Sensitivity, % | 0.67 | 0.47 |
| Specificity, % | 0.94 | 0.91 |
| PPV, % | 0.83 | 0.70 |
| NPV, % | 0.86 | 0.79 |
| LR+ | 11.20 | 5.20 |
| LR− | 0.35 | 0.58 |
Abbreviations: LR+, likelihood ratio for a positive test result; LR−, likelihood ratio for a negative test result; NPV, negative predictive value; PPV, positive predictive value.
Acknowledgments
This parameter was developed by the Joint Task Force on Practice Parameters, representing the American Academy of Allergy, Asthma and Immunology, the American College of Allergy, Asthma and Immunology, and the Joint Council of Allergy, Asthma and Immunology.
The Joint Task Force has made a concerted effort to acknowledge all contributors to this parameter. If any contributors have been excluded inadvertently, the task force will ensure that appropriate recognition of such contributions is made subsequently.
Appendix
Classification of recommendations and evidence
Recommendation rating scale
| Statement | Definition | Implication |
|---|---|---|
| Strong recommendation (StrRec) | A strong recommendation means the benefits of the recommended approach clearly exceed the harms (or that the harms clearly exceed the benefits in the case of a strong negative recommendation) and that the quality of the supporting evidence is excellent (grade A or B*). In some clearly identified circumstances, strong recommendations may be made based on lesser evidence when high-quality evidence is impossible to obtain and the anticipated benefits strongly outweigh the harms. |
Clinicians should follow a strong recommendation unless a clear and compelling rationale for an alternative approach is present. |
| Recommendation (Rec) | A recommendation means the benefits exceed the harms (or that the harms clearly exceed the benefits in the case of a negative recommendation) but the quality of evidence is not as strong (grade B or C).* In some clearly identified circumstances, recommendations may be made based on lesser evidence when high-quality evidence is impossible to obtain and the anticipated benefits outweigh the harms. |
Clinicians should also generally follow a recommendation but should remain alert to new information and sensitive to patient preferences. |
| Option (Opt) | An option means that, either well-done studies (grade A, B, or C)* show little clear advantage to one approach vs another or that the quality of evidence that exists is suspect (grade D).* |
Clinicians should be flexible in their decision making regarding appropriate practice, although they may set bounds on alternatives; patient preference should have a substantial influencing role. |
| No recommendation (No Rec) | No recommendation means there is both a lack of pertinent evidence (grade D)* and an unclear balance between benefits and harms. |
Clinicians should feel little constraint in their decision making and be alert to new published evidence that clarifies the balance of benefit vs harm; patient preference should have a substantial influencing role. |
Category of evidence
-
Ia
Evidence from meta-analysis of randomized controlled trials
-
Ib
Evidence from at least 1 randomized controlled trial
-
IIa
Evidence from at least 1 controlled study without randomization
-
IIb
Evidence from at least 1 other type of quasiexperimental study
-
III
Evidence from nonexperimental descriptive studies, such as comparative studies
-
IV
Evidence from expert committee reports or opinions or clinical experience of respected authorities or both
Strength of evidence
-
A
Directly based on meta-analysis of randomized controlled trials; well-designed randomized controlled trials or diagnostic studies performed on a population similar to the guideline’s target population
-
B
Directly based on randomized controlled trials or diagnostic studies with minor limitations; overwhelmingly consistent evidence from observational studies
-
C
Directly based on observational studies (case-control and cohort design)
-
D
Directly based on expert opinion, case reports, and nonsystematic reviews.
-
LB
Laboratory based on reasoning from first principles (bench research or animal studies)
-
NR
Not rated
How this practice parameter was developed
The Joint Task Force on Practice Parameters
The Joint Task Force (JTF) on Practice Parameters is a 13-member task force consisting of 6 representatives assigned by the American Academy of Allergy, Asthma & Immunology, 6 by the American College of Allergy, Asthma & Immunology, and 1 by the Joint Council of Allergy and Immunology. This task force oversees the development of practice parameters; selects the workgroup chair(s); and reviews drafts of the parameters for accuracy, practicality, clarity, and broad utility of the recommendations for clinical practice.
The Environment Practice Parameter Work Group
The Environment Practice Parameter Work Group was asked by the JTF to develop practice parameters that address environmental assessment and remediation. This rodent practice parameter is one segment of the overall series of environment practice parameters. James Sublet, MD, and Kevin Kennedy, MPH, MD, cochairs, with Jay Portnoy, MD, liaison from the JTF, invited work group members considered to be experts in environmental assessment and contaminant reduction to participate in this practice parameter. Work group members have been evaluated for financial conflicts of interest (COIs) by the JTF, and their COIs have been listed in this document and are posted on the JTF website at http://www. allergyparameters.org. Where a potential COI is present, the potentially conflicted work group member was excluded from discussing relevant issues.
The charge to the workgroup was to use a systematic literature review, in conjunction with consensus expert opinion and work group–identified supplementary documents, to develop practice parameters that provide a comprehensive approach to identify and manage environmental exposures and their health effects based on the current state of the science.
Protocol for finding evidence
A search of the medical literature was performed for a variety of terms that were considered to be relevant to this practice parameter. In particular, search terms included a combination of allergy or asthma and one of the following: mouse, rat, or rodent. Literature searches were performed on PubMed and the Cochrane Database of Systematic Reviews, much of which can be found in PubMed. This document includes references from 1970 through June 1, 2012, which is when the work group finished responding to reviewers’ comments. All reference types were included in the results. References identified as being relevant were searched for additional references, and these also were searched for citable references. In addition, members of the work group were asked for references they were aware of that were missed by this initial search. Although the ideal type of reference would consist of a randomized, doubleblind, placebo-controlled study, the topic of this practice parameter is represented by very few such studies. Consequently, it was necessary to use observational studies, basic laboratory reports, and regulatory requirements to develop a document that addresses most of the issues included in this practice parameter.
Glossary
Terms related to evaluation of exposures
Contaminant is any physical, chemical, biological, or radioactive substance that can have an adverse effect on air, water, or soil or on any interior or exterior surface and that has the potential to cause harm to a building’s occupants. Contaminants can be allergens, irritants, or other types of substances, including biologically active ones. Rodents generally produce contaminants in the form of rodent allergens.
Facilitating factors are conditions that facilitate production of allergens by rodents. Examples include moisture, food, appropriate temperature, and shelter, which are conditions essential for rodent survival and growth.
Pathways are transport mechanisms by which contaminants can travel from sources and reservoirs to occupants. Contaminants can travel via air or water or on fomites or vectors.
Reservoirs are contained spaces or microenvironments in which contaminants can accumulate for subsequent release into the environment. Examples include carpeting, bedding, and contaminated building materials.
Source of contaminant is a mechanism for the production of contaminants. Rodents are the source of contaminant in this practice parameter because they produce rodent allergens.
Terms Related to Interventions
Abatement is a diminution in amount, degree, or intensity. Abatement of rodents can be attained by removing, treating, or isolating reservoirs of rodent allergens and could include air filtration, vacuuming or removal of carpeting, use of denaturing chemicals, and removal of contaminated building materials.
Exposure reduction is interventions that reduce occupant exposure to rodent allergens, usually by placing barriers in the pathways from reservoirs and sources to occupants or by eliminating the allergens altogether. The goal is to keep contaminant exposure below a threshold where adverse health effects can occur.
Mitigation is the process of removing facilitative factors, either completely or partially. Once the facilitative factors are reduced, production of contaminants will no longer be facilitated, leading to reduced exposure. Mitigation should occur early in the exposure reduction process so that production of the contamination does not continue. Once mitigation is done, restoration and remediation can commence.
Remediation encompasses mitigation, restoration, and other restorative processes, leading to removal of contaminants and conditions that facilitate contamination.
Source control is the process of reducing or eliminating sources of allergens, which, in this case, derive from rodents. If rodents are removed, exposure will decrease over time as the previously released contaminant is eliminated from the environment.
Terms related to health effects of exposure
Sensitization is the presence of specific IgE antibodies to a particular allergen.
Sensitivity is the development of symptoms on exposure to the substance to which a person is sensitized (ie: the person has an “allergy” to the exposure)
Preface
“Environmental Assessment and Exposure Reduction of Rodents: A Practice Parameter” is a practice parameter that addresses health problems associated with exposure to these animals. The previous practice parameter on furry animals focused on voluntary exposure to intentionally introduced animals into the environment, whereas this practice parameter focuses on involuntary exposure caused by rodent infestation.
Rodent infestation of a home or a building requires the help of professionals trained to identify the problems and take remedial action to eliminate the rodents and their residual debris. Environmental assessment professionals are members of a relatively new field in which individuals with expertise in evaluating homes and other nonoccupational buildings are trained to identify the potential for exposure to indoor contaminants that are known to have potential adverse effects on health, with an emphasis on allergens, irritants, and safety hazards. The environmental health expert ideally should work closely with physicians and other health care professionals to determine what exposures are likely to be causing health problems in building occupants. In that respect, the 2 fields are complementary, requiring cooperation and collaboration. The goal with respect to rodents is to identify evidence for their presence and to recommend methods for elimination and long-term management when necessary.
Reduction of exposure to involuntary pests, such as rodents, requires a deeper understanding of how contaminants, such as allergens, are generated, where they are deposited, and how they are transported from sources and reservoirs to occupants. It is only by addressing all of these issues, in addition to their underlying causal factors, that exposure to contaminants, such as rodent allergens, can be reduced sufficiently to have a beneficial effect on health.
An exception to involuntary rodent exposure is in laboratory animal handlers. Animal facilities have known exposures to allergens that can be controlled with appropriate interventions. In addition, workers can be monitored for sensitization, sensitivity, and morbidity caused by their exposure so that appropriate interventions, such as filters, masks, and reassignment to less exposed environments, can be provided.
Future practice parameters on specific exposures are planned for fungi, dust mites, cockroaches and other insects, and irritants. The reader should note that this practice parameter is the first one developed by the Joint Task Force to include an indication of strength of recommendation in addition to the strength of evidence supporting the recommendation. The summary statements have been designed to recommend (or not recommend) that a particular action be taken.
List of summary statements
Exposure to mouse allergen in homes should be minimized to reduce the risk of sensitization. (Rec, B Evidence)
Exposure to rodent allergens should be minimized to reduce the risk that sensitized individuals will develop sensitivity in the form of respiratory symptoms. (Rec, C Evidence)
Mouse and rat allergen exposure should be minimized to reduce the risk of asthma morbidity in already sensitized individuals. (StrRec, B Evidence)
Measurement of mouse-specific IgG4 may help to identify individuals with a reduced risk of mouse skin test sensitivity though the benefit of doing so is unclear. (NoRec, C Evidence)
Patients with possible rodent allergy should be asked whether they have seen rodents in their home. (StrRec, B Evidence)
Patients with atopy and likely rodent exposure, such as patients with persistent asthma living in inner-city areas, should be evaluated for sensitization to rodent allergens by skin prick testing, rodent-specific IgE testing, or both if indicated. (StrRec, B Evidence)
Immunotherapy with rodent extracts has not been adequately studied to determine whether it is effective. (NoRec, D Evidence)
An assessment for facilitative factors of rodent exposure should focus on identifying food, water, routes of ingress, and the presence of rodent habitats. (Rec, C Evidence)
Habitat modification should be performed to remove means of rodent ingress, food, water, and shelter. (StrRec, C Evidence)
Evidence for the presence of rodents should be identified to determine the likely extent of an infestation. (Rec, C Evidence)
Cats and other rodent predators could be considered as a possible method for reducing rodent populations, although they generally do not completely eliminate an infestation and individuals may become sensitized to a cat. (Opt, C Evidence)
Rodent traps are an effective way to remove rodents from an infested building. (Rec, C Evidence).
Rodenticides should be used if other interventions are ineffective. They should be applied according to the manufacturer’s instructions that are part of the label. Many pesticides should only be applied by a licensed professional exterminator. (Rec, C Evidence)
Measurement of rodent allergens in house dust may be considered; however, it has an unclear clinical benefit given the wide range of observed values and uncertain clinically relevant exposure thresholds. (Opt, D Evidence)
Rodent allergens in homes can be reduced using integrated pest management techniques (StrRec, A Evidence)
Monitoring for rodent sensitization should be considered at least for the first 3 years of employment in an laboratory animal facility. (Rec, C Evidence)
Extra avoidance precautions should be taken for individuals with an increased risk of animal sensitization, including those with an atopic background and with high-intensity exposure. (Rec, B Evidence)
Extra avoidance precautions should be taken when contaminated bedding and high numbers of conscious animals are handled. (Rec, B Evidence)
Allergen exposure in laboratory facilities should be reduced by engineering controls, staff training, and appropriate personal protection. (Rec, B Evidence)
Footnotes
Disclaimer: The American Academy of Allergy, Asthma and Immunology (AAAAI) and the American College of Allergy, Asthma and Immunology (ACAAI) have jointly accepted responsibility for establishing “Environmental Assessment and Remediation: A Practice Parameter.” This is a complete and comprehensive document at the current time. The medical environment is a changing environment, and not all recommendations will be appropriate for all patients. Because this document incorporated the efforts of many participants, no single individual, including those who served on the Joint Task Force, is authorized to provide an official AAAAI or ACAAI interpretation of these practice parameters. Any request for information about or an interpretation of these practice parameters by the AAAAI or ACAAI should be directed to the executive offices of the AAAAI, the ACAAI, and the Joint Council of Allergy, Asthma and Immunology. These parameters are not designed for use by pharmaceutical companies in drug promotion.
Disclosures: The following is a summary of interests disclosed on Work Group members’ Conflict of Interest Disclosure Statements (not including information concerning family member interests). Completed Conflict of Interest Disclosure Statements are available upon request. Dr Sublett is the owner of AllergyZone. Dr Portnoy is a consultant for ThermoFisher (Phadia). Dr Barnes is a consultant for and has received research funding from Clorox Corporation. Mr Grimes is the owner of Healthy Habitats LLC. Dr Matsui is speaker for Indoor BioTechnologies. Dr Seltzer is the president of James M. Seltzer, Assoc. The other Work Group members have no conflicts to disclose. The Joint Task Force recognizes that experts in a field are likely to have interests that could come into conflict with development of a completely unbiased and objective practice parameter. To take advantage of that expertise, a process has been developed to prevent potential conflicts from influencing the final document in a negative way. At the workgroup level, members who have a potential conflict of interest either do not participate in discussions concerning topics related to the potential conflict or, if they do write a section on that topic, the workgroup completely rewrites it without their involvement to remove potential bias. In addition, the entire document is reviewed by the Joint Taskforce and any apparent bias is removed at that level. Finally, the practice parameter is sent for review both by invited reviewers and by anyone with an interest in the topic by posting the document on the web sites of the ACAAI and the AAAAI. These reviewers make comments which often change the document and would further delete any bias on the part of any one individual involved in the drafting of the document.
References
- [1].Cohn RD, Arbes SJ, Jr, Jaramillo R, Reid LH, Zeldin DC. National prevalence and exposure risk for cockroach allergen in U.S. households. Environ Health Per-spect. 2006;114:522–526. doi: 10.1289/ehp.8561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. [Assessed February 26, 2012];Taxonomy of common rodent and rodent-like pets. 2012 [updated Feb 26, 2012]. http://www.ratbehavior.org/pet_rodent_classification.htm.
- [3].Quarles W. Deer mice spread new hantavirus. Common Sense Pest Control Q. 1995;11:13–15. [Google Scholar]
- [4].Centers for Disease Control and Prevention Hantavirus infection-Southwestern United States: interim recommendations for risk reduction. MMWR Morb Mortal Wkly Rep. 1993;42(No. R-11):1–20. [Google Scholar]
- [5].Frantz S, Davis D. Bionomics and integrated pest management of commensal rodents. In: Ecology and Management of Food-Industry Pests. Association of Of ficial Analytical Chemists; Arlington, VA: 1991. pp. 243–313. [Google Scholar]
- [6].Pinto L, Module III. Urban Integrated Pest Management: A Guide for Commercial Applicators. Dual & Associates Inc; Arlington, VA: 1992. vertebrates; pp. 1–7. [Google Scholar]
- [7].Caslick J, Decker D. Rat and mouse control. Cornell Cooperative Extension Bull. 1980:163. [Google Scholar]
- [8].Phipatanakul W. Rodent allergens. Curr Allergy Asthma Rep. 2002;2:412–416. doi: 10.1007/s11882-002-0075-1. [DOI] [PubMed] [Google Scholar]
- [9].Price JA, Longbottom JL. Allergy to mice, II: further characterization of two major mouse allergens (AG 1 and AG 3) and immunohistochemical investigations of their sources. Clin Exp Allergy. 1990;20:71–77. doi: 10.1111/j.1365-2222.1990.tb02778.x. [DOI] [PubMed] [Google Scholar]
- [10].Mus m1. The Allergome Project [Accessed August 29, 2012];2010 [updated 2010]. http://www.allergome.org/script/dettaglio.php?id_molecule=478.
- [11].Ferrari E, Lodi T, Sorbi RT, Tirindelli R, Cavaggioni A, Spisni A. Expression of a lipocalin in Pichia pastoris: secretion, purification and binding activity of a recombinant mouse major urinary protein. FEBS Lett. 1997;401:73–77. doi: 10.1016/s0014-5793(96)01436-6. [DOI] [PubMed] [Google Scholar]
- [12].Rat n 1. The Allergome Project [Accessed August 29, 2012];2010 [updated 2010]. http://www.allergome.org/script/dettaglio.php?id_molecule=611.
- [13].Wahn U, Peters T, Jr, Siraganian RP. Studies on the allergenic significance and structure of rat serum albumin. J Immunol. 1980;125:2544–2549. [PubMed] [Google Scholar]
- [14].Matsui EC, Simons E, Rand C, et al. Airborne mouse allergen in the homes of inner-city children with asthma. J Allergy Clin Immunol. 2005;115:358–363. doi: 10.1016/j.jaci.2004.11.007. [DOI] [PubMed] [Google Scholar]
- [15].Matsui EC, Wood RA, Rand C, Kanchanaraksa S, Swartz L, Eggleston PA. Mouse allergen exposure and mouse skin test sensitivity in suburban, middle-class children with asthma. J Allergy Clin Immunol. 2004;113:910–915. doi: 10.1016/j.jaci.2004.02.034. [DOI] [PubMed] [Google Scholar]
- [16].Matsui EC, Eggleston PA, Breysse PN, Rand CS, Diette GB. Mouse allergen specific antibody responses in inner-city children with asthma. J Allergy Clin Immunol. 2007;119:910–915. doi: 10.1016/j.jaci.2006.12.663. [DOI] [PubMed] [Google Scholar]
- [17].Rosenfeld L, Chew GL, Rudd R, et al. Are building-level characteristics associated with indoor allergens in the household? J Urban Health. 2011;88:14–29. doi: 10.1007/s11524-010-9527-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cohn RD, Arbes SJ, Jr, Yin M, Jaramillo R, Zeldin DC. National prevalence and exposure risk for mouse allergen in US households. J Allergy Clin Immunol. 2004;113:1167–1171. doi: 10.1016/j.jaci.2003.12.592. [DOI] [PubMed] [Google Scholar]
- [19].Phipatanakul W, Eggleston PA, Wright EC, Wood RA. Mouse allergen, I: the prevalence of mouse allergen in inner-city homes: The National Cooperative Inner-City Asthma Study. J Allergy Clin Immunol. 2000;106:1070–1074. doi: 10.1067/mai.2000.110796. [DOI] [PubMed] [Google Scholar]
- [20].Crain EF, Walter M, O’Connor GT, et al. Home and allergic characteristics of children with asthma in seven U.S. urban communities and design of an environmental intervention: the Inner-City Asthma Study. Environ Health Perspect. 2002;110:939–945. doi: 10.1289/ehp.02110939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Perry T, Matsui E, Merriman B, Duong T, Eggleston P. The prevalence of rat allergen in inner-city homes and its relationship to sensitization and asthma morbidity. J Allergy Clin Immunol. 2003;112:346–352. doi: 10.1067/mai.2003.1640. [DOI] [PubMed] [Google Scholar]
- [22].Phipatanakul W, Gold DR, Muilenberg M, Sredl DL, Weiss ST, Celedon JC. Predictors of indoor exposure to mouse allergen in urban and suburban homes in Boston. Allergy. 2005;60:697–701. doi: 10.1111/j.1398-9995.2005.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chew GL, Correa JC, Perzanowski MS. Mouse and cockroach allergens in the dust and air in northeastern United States inner-city public high schools. Indoor Air. 2005;15:228–234. doi: 10.1111/j.1600-0668.2005.00363.x. [DOI] [PubMed] [Google Scholar]
- [24].Sheehan WJ, Rangsithienchai PA, Muilenberg ML, et al. Mouse allergens in urban elementary schools and homes of children with asthma. Ann Allergy Asthma Immunol. 2009;102:125–130. doi: 10.1016/S1081-1206(10)60242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Loo CK, Foty RG, Wheeler AJ, et al. Do questions reflecting indoor air pollutant exposure from a questionnaire predict direct measure of exposure in owneroccupied houses? Int J Environ Res Public Health. 2010;7:3270–3297. doi: 10.3390/ijerph7083270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Berg J, McConnell R, Milam J, et al. Rodent allergen in Los Angeles inner city homes of children with asthma. J Urban Health. 2008;85:52–61. doi: 10.1007/s11524-007-9232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chew GL, Perzanowski MS, Miller RL, et al. Distribution and determinants of mouse allergen exposure in low-income New York City apartments. Environ Health Perspect. 2003;111:1348–1351. doi: 10.1289/ehp.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Arbes SJ, Sever M, Mehta J, Collette N, Thomas B, Zeldin DC. Exposure to indoor allergens in day-care facilities: results from 2 North Carolina counties. J Allergy Clin Immunol. 2005;116:133–139. doi: 10.1016/j.jaci.2005.04.022. [DOI] [PubMed] [Google Scholar]
- [29].Perry TT, Vargas PA, Bufford J, et al. Classroom aeroallergen exposure in Arkansas head start centers. Ann Allergy Asthma Immunol. 2008;100:358–363. doi: 10.1016/S1081-1206(10)60599-6. [DOI] [PubMed] [Google Scholar]
- [30].Gruchalla RS, Pongracic J, Plaut M, et al. Inner City Asthma Study: relationships among sensitivity, allergen exposure, and asthma morbidity. J Allergy Clin Immunol. 2005;115:478–485. doi: 10.1016/j.jaci.2004.12.006. [DOI] [PubMed] [Google Scholar]
- [31].Chew GL, Carlton EJ, Kass D, et al. Determinants of cockroach and mouse exposure and associations with asthma in families and elderly individuals living in New York City public housing. Ann Allergy Asthma Immunol. 2006;97:502–513. doi: 10.1016/S1081-1206(10)60942-8. [DOI] [PubMed] [Google Scholar]
- [32].Price JA, Longbottom JL. ELISA method for measurement of airborne levels of major laboratory animal allergens. Clin Allergy. 1988;18:95–107. doi: 10.1111/j.1365-2222.1988.tb02848.x. [DOI] [PubMed] [Google Scholar]
- [33].Ohman JL, Jr, Hagberg K, MacDonald MR, Jones RR, Jr, Paigen BJ, Kacergis JB. Distribution of airborne mouse allergen in a major mouse breeding facility. J Allergy Clin Immunol. 1994;94:810–817. doi: 10.1016/0091-6749(94)90147-3. [DOI] [PubMed] [Google Scholar]
- [34].Gordon S, Kiernan LA, Nieuwenhuijsen MJ, Cook AD, Tee RD, Newman Taylor AJ. Measurement of exposure to mouse urinary proteins in an epidemiological study. Occup Environ Med. 1997;54:135–140. doi: 10.1136/oem.54.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gergen PJ, Arbes SJ, Jr, Calatroni A, Mitchell HE, Zeldin DC. Total IgE levels and asthma prevalence in the US population: results from the National Health and Nutrition Examination Survey 2005-2006. J Allergy Clin Immunol. 2009;124:447–453. doi: 10.1016/j.jaci.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Phipatanakul W, Eggleston PA, Wright EC, Wood RA. Mouse allergen, II: the relationship of mouse allergen exposure to mouse sensitization and asthma morbidity in inner-city children with asthma. J Allergy Clin Immunol. 2000;106:1075–1080. doi: 10.1067/mai.2000.110795. [DOI] [PubMed] [Google Scholar]
- [37].Salo PM, Jaramillo R, Cohn RD, London SJ, Zeldin DC. Exposure to mouse allergen in U.S. homes associated with asthma symptoms. Environ Health Perspect. 2009;117:387–391. doi: 10.1289/ehp.11847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Phipatanakul W, Celedon JC, Sredl DL, Weiss ST, Gold DR. Mouse exposure and wheeze in the first year of life. Ann Allergy Asthma Immunol. 2005;94:593–599. doi: 10.1016/S1081-1206(10)61139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Phipatanakul W, Celedon JC, Hoffman EB, Abdulkerim H, Ryan LM, Gold DR. Mouse allergen exposure, wheeze and atopy in the first seven years of life. Allergy. 2008;63:1512–1518. doi: 10.1111/j.1398-9995.2008.01679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Phipatanakul W, Litonjua AA, Platts-Mills TA, et al. Sensitization to mouse allergen and asthma and asthma morbidity among women in Boston. J Allergy Clin Immunol. 2007;120:954–956. doi: 10.1016/j.jaci.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Donohue KM, Al-alem U, Perzanowski MS, et al. Anti-cockroach and anti-mouse IgE are associated with early wheeze and atopy in an inner-city birth cohort. J Allergy Clin Immunol. 2008;122:914–920. doi: 10.1016/j.jaci.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Matsui EC, Sampson HA, Bahnson HT, et al. Allergen-specific IgE as a biomarker of exposure plus sensitization in inner-city adolescents with asthma. Allergy. 2010;65:1414–1422. doi: 10.1111/j.1398-9995.2010.02412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Matsui EC, Eggleston PA, Buckley TJ, et al. Household mouse allergen exposure and asthma morbidity in inner-city preschool children. Ann Allergy Asthma Immunol. 2006;97:514–520. doi: 10.1016/S1081-1206(10)60943-X. [DOI] [PubMed] [Google Scholar]
- [44].Pongracic JA, Visness CM, Gruchalla RS, Evans R, III, Mitchell HE. Effect of mouse allergen and rodent environmental intervention on asthma in innercity children. Ann Allergy Asthma Immunol. 2008;101:35–41. doi: 10.1016/S1081-1206(10)60832-0. [DOI] [PubMed] [Google Scholar]
- [45].Matsui EC, Diette GB, Krop EJ, et al. Mouse allergen-specific immunoglobulin G and immunoglobulin G4 and allergic symptoms in immunoglobulin E-sensitized laboratory animal workers. Clin Exp Allergy. 2005;35:1347–1353. doi: 10.1111/j.1365-2222.2005.02331.x. [DOI] [PubMed] [Google Scholar]
- [46].Matsui EC, Diette GB, Krop EJ, Aalberse RC, Smith AL, Eggleston PA. Mouse allergen-specific immunoglobulin G4 and risk of mouse skin test sensitivity. Clin Exp Allergy. 2006;36:1097–1103. doi: 10.1111/j.1365-2222.2006.02534.x. [DOI] [PubMed] [Google Scholar]
- [47].Krop EJ, Doekes G, Heederik DJ, Aalberse RC, van der Zee JS. IgG4 antibodies against rodents in laboratory animal workers do not protect against allergic sensitization. Allergy. 2011;66:517–522. doi: 10.1111/j.1398-9995.2010.02508.x. [DOI] [PubMed] [Google Scholar]
- [48].Peng RD, Paigen B, Eggleston PA, et al. Both the variability and level of mouse allergen exposure influence the phenotype of the immune response in workers at a mouse facility. J Allergy Clin Immunol. 2011;128:390–396. e7. doi: 10.1016/j.jaci.2011.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Curtin-Brosnan J, Matsui EC, Breysse P, et al. Parent report of pests and pets and indoor allergen levels in inner-city homes. Ann Allergy Asthma Immunol. 2008;101:517–523. doi: 10.1016/S1081-1206(10)60291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Rosenfeld L, Rudd R, Chew GL, Emmons K, Acevedo-Garcia D. Are neighborhood-level characteristics associated with indoor allergens in the household? J Asthma. 2010;47:66–75. doi: 10.3109/02770900903362676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Gendo K, Larson EB. Evidence-based diagnostic strategies for evaluating suspected allergic rhinitis. Ann Intern Med. 2004;140:278–289. doi: 10.7326/0003-4819-140-4-200402170-00010. [DOI] [PubMed] [Google Scholar]
- [52].Bernstein IL, Li JT, Bernstein DI, et al. Allergy diagnostic testing: an updated practice parameter. Ann Allergy Asthma Immunol. 2008;100(3 suppl 3):S1–S148. doi: 10.1016/s1081-1206(10)60305-5. [DOI] [PubMed] [Google Scholar]
- [53].Sharma HP, Wood RA, Bravo AR, Matsui EC. A comparison of skin prick tests, intradermal skin tests, and specific IgE in the diagnosis of mouse allergy. J Allergy Clin Immunol. 2008;121:933–939. doi: 10.1016/j.jaci.2008.01.023. [DOI] [PubMed] [Google Scholar]
- [54].Platts-Mills TA, Satinover SM, Naccara L, et al. Prevalence and titer of IgE antibodies to mouse allergens. J Allergy Clin Immunol. 2007;120:1058–1064. doi: 10.1016/j.jaci.2007.06.032. [DOI] [PubMed] [Google Scholar]
- [55].Matsui EC, Krop EJ, Diette GB, Aalberse RC, Smith AL, Eggleston PA. Mouse allergen exposure and immunologic responses: IgE-mediated mouse sensitization and mouse specific IgG and IgG4 levels. Ann Allergy Asthma Immunol. 2004;93:171–178. doi: 10.1016/S1081-1206(10)61471-8. [DOI] [PubMed] [Google Scholar]
- [56].Krop EJ, Matsui EC, Sharrow SD, et al. Recombinant major urinary proteins of the mouse in specific IgE and IgG testing. Int Arch Allergy Immunol. 2007;144:296–304. doi: 10.1159/000106318. [DOI] [PubMed] [Google Scholar]
- [57].Chong LK, Ong MJ, Curtin-Brosnan J, Matsui EC. Skin test sensitivity to mouse predicts allergic symptoms to nasal challenge in urban adults. Allergy Asthma Proc. 2010;31:472–476. doi: 10.2500/aap.2010.31.3372. [DOI] [PubMed] [Google Scholar]
- [58].Hansen I, Hormann K, Klimek L. Specific immunotherapy in inhalative allergy to rat epithelium [in German] Laryngorhinootologie. 2004;83:512–515. doi: 10.1055/s-2004-814505. [DOI] [PubMed] [Google Scholar]
- [59].Wahn U, Siraganian RP. Efficacy and specificity of immunotherapy with laboratory animal allergen extracts. J Allergy Clin Immunol. 1980;65:413–421. doi: 10.1016/0091-6749(80)90233-x. [DOI] [PubMed] [Google Scholar]
- [60].Harmon J. Classy vermin: controlling rodents in schools. Pest Control Technol. 1995;23:38, 40, 49, 52, 54, 112. [Google Scholar]
- [61].Timm R, Bodman G. Prevention and Control of Wildlife Damage. University of Nebraska; Lincoln: 1983. Rodent-proof construction. [Google Scholar]
- [62].HUD’s Guidance on Integrated Pest Management [Accessed August 29, 2012];2008 http://www.nchh.org/Portals/0/HUD%20Guidance%20on%20IPM.pdf.
- [63].Wilson J, Dixon SL, Breysse P, et al. Housing and allergens: a pooled analysis of nine US studies. Environ Res. 2010;110:189–198. doi: 10.1016/j.envres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- [64].Meehan A. Rats and Mice: Their Biology and Control. Rentokil Ltd; East Grinstead, England: 1984. [Google Scholar]
- [65].Curtin-Brosnan J, Saams J, Breysse P, Diette G, Bradley H, Matsui E. Relationship between cat and mouse allergen levels in the homes of inner city children with asthma. J Allergy Clin Immunol. 2009;123:64. Abstract 233. [Google Scholar]
- [66].Dodds W, Frantz S, Story K. The Professional’s Guide to Managing Poisoning by Anticoagulant Rodenticides. Chempa Products Division, Lipha Chemicals Inc; New York, NY: 1986. [Google Scholar]
- [67].Frantz S, Padula C. Recent Developments in Anticoagulant Rodenticide Resistance Studies: Surveillance and Application in the United States. University of California Press; Davis: 1990. pp. 80–88. [Google Scholar]
- [68].Hung L, Miller J, Dillon H. Field Guide for the Determination of Biological Contaminants in Environmental Samples. 2nd ed American Industrial Hygiene Association; Fairfax, VA: 2005. [Google Scholar]
- [69].HUD Office of Healthy Homes and Lead Hazard Control. Vacuum Dust Sample Collection Protocol for Allergens. US Dept of Housing and Urban Development; Washington, DC: 2008. [Google Scholar]
- [70].Lewis RD, Breysse PN, Lees PS, Diener-West M, Hamilton RG, Eggleston P. Factors affecting the retention of dust mite allergen on carpet. Am Ind Hyg Assoc J. 1998;59:606–613. doi: 10.1080/15428119891010776. [DOI] [PubMed] [Google Scholar]
- [71].Sercombe JK, Liu-Brennan D, Garcia ML, Tovey ER. Evaluation of home allergen sampling devices. Allergy. 2005;60:515–520. doi: 10.1111/j.1398-9995.2004.00657.x. [DOI] [PubMed] [Google Scholar]
- [72].Dreborg S, Einarsson R, Lau S, Munir AK, Wahn U. Dust sampling for determination of allergen content. Allergy. 1995;50:188–189. doi: 10.1111/j.1398-9995.1995.tb05079.x. [DOI] [PubMed] [Google Scholar]
- [73].Kass D, McKelvey W, Carlton E, et al. Effectiveness of an integrated pest management intervention in controlling cockroaches, mice, and allergens in New York City public housing. Environ Health Perspect. 2009;117:1219–1225. doi: 10.1289/ehp.0800149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Phipatanakul W, Cronin B, Wood RA, et al. Effect of environmental intervention on mouse allergen levels in homes of inner-city Boston children with asthma. Ann Allergy Asthma Immunol. 2004;92:420–425. doi: 10.1016/S1081-1206(10)61777-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Krieger J, Jacobs DE, Ashley PJ, et al. Housing interventions and control of asthma-related indoor biologic agents: a review of the evidence. J Public Health Manag Pract. 2010;16(5 suppl):S11–S20. doi: 10.1097/PHH.0b013e3181ddcbd9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Gautrin D, Ghezzo H, Infante-Rivard C, Malo JL. Natural history of sensitization, symptoms and occupational diseases in apprentices exposed to laboratory animals. Eur Respir J. 2001;17:904–908. doi: 10.1183/09031936.01.17509040. [DOI] [PubMed] [Google Scholar]
- [77].Bush RK, Wood RA, Eggleston PA. Laboratory animal allergy. J Allergy Clin Immunol. 1998;102:99–112. doi: 10.1016/s0091-6749(98)70060-0. [DOI] [PubMed] [Google Scholar]
- [78].Son T, Bae J, Rhee C, Seong W. Prevalence of laboratory animal allergy in laboratory workers. Lab Anim Res. 2010;26:165–171. [Google Scholar]
- [79].Curtin-Brosnan J, Paigen B, Hagberg KA, et al. Occupational mouse allergen exposure among non-mouse handlers. J Occup Environ Hyg. 2010;7:726–734. doi: 10.1080/15459624.2010.530906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Eggleston PA, Ansari AA, Adkinson NF, Jr, Wood RA. Environmental challenge studies in laboratory animal allergy: effect of different airborne allergen concentrations. Am J Respir Crit Care Med. 1995;151(3 pt 1):640–646. doi: 10.1164/ajrccm.151.3.7881650. [DOI] [PubMed] [Google Scholar]
- [81].Gordon S, Fisher SW, Raymond RH. Elimination of mouse allergens in the working environment: assessment of individually ventilated cage systems and ventilated cabinets in the containment of mouse allergens. J Allergy Clin Immunol. 2001;108:288–294. doi: 10.1067/mai.2001.117258. [DOI] [PubMed] [Google Scholar]
- [82].Gordon S, Tee RD, Newman Taylor AJ. Analysis of the allergenic composition of rat dust. Clin Exp Allergy. 1996;26:533–541. [PubMed] [Google Scholar]
- [83].Gordon S, Tee RD, Stuart MC, Newman Taylor AJ. Analysis of allergens in rat fur and saliva. Allergy. 2001;56:563–567. doi: 10.1034/j.1398-9995.2001.056006563.x. [DOI] [PubMed] [Google Scholar]
- [84].Hollander A, Heederik D, Doekes G, Kromhout H. Determinants of airborne rat and mouse urinary allergen exposure. Scand J Work Environ Health. 1998;24:228–235. doi: 10.5271/sjweh.303. [DOI] [PubMed] [Google Scholar]
- [85].Taylor AN, Longbottom JL, Pepys J. Respiratory allergy to urine proteins of rats and mice. Lancet. 1977;2:847–849. doi: 10.1016/s0140-6736(77)90783-8. [DOI] [PubMed] [Google Scholar]
- [86].Sakaguchi M, Inouye S, Miyazawa H, Kamimura H, Kimura M, Yamazaki S. Evaluation of dust respirators for elimination of mouse aeroallergens. Lab Anim Sci. 1989;39:63–66. [PubMed] [Google Scholar]
- [87].Jones RB, Kacergis JB, MacDonald MR, et al. The effect of relative humidity on mouse allergen levels in an environmentally controlled mouse room. Am Ind Hyg Assoc J. 1995;56:398–401. doi: 10.1080/15428119591017033. [DOI] [PubMed] [Google Scholar]
- [88].Harrison DJ. Controlling exposure to laboratory animal allergens. Inst Lab Anim Resources J. 2001;42:17–36. doi: 10.1093/ilar.42.1.17. [DOI] [PubMed] [Google Scholar]
- [89].Fontes B. Institutional responsibilities in contamination control in research animals and occupational health and safety for animal handlers. Inst Lab Anim Resources J. 2008;49:326–337. doi: 10.1093/ilar.49.3.326. [DOI] [PubMed] [Google Scholar]
- [90].Krop EJ, Doekes G, Stone MJ, Aalberse RC, van der Zee JS. Spreading of occupational allergens: laboratory animal allergens on hair-covering caps and in mattress dust of laboratory animal workers. Occup Environ Med. 2007;64:267–272. doi: 10.1136/oem.2006.028845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Krakowiak A, Szulc B, Gorski P. Allergy to laboratory animals in children of parents occupationally exposed to mice, rats and hamsters. Eur Respir J. 1999;14:352–356. doi: 10.1034/j.1399-3003.1999.14b19.x. [DOI] [PubMed] [Google Scholar]
- [92].Renstrom A, Olsson M, Hedren M, Johansson SG, van Hage M. Pet shop workers: exposure, sensitization, and work-related symptoms. Allergy. 2011;66:1081–1087. doi: 10.1111/j.1398-9995.2011.02591.x. [DOI] [PubMed] [Google Scholar]