Abstract
Epidemiological, cross-sectional, and prospective studies suggest that insomnia, chronic pain, and depression frequently co-occur and are mutually interacting conditions. However, the mechanisms underlying these comorbid disorders have yet to be elucidated. Overlapping mechanisms in the central nervous system suggest a common neurobiological substrate(s) may underlie the development and interplay of these disorders. We propose that the mesolimbic dopamine system is an underappreciated and attractive venue for the examination of neurobiological processes involved in the interactions, development, exacerbation, and maintenance of this symptom complex. In the present article, studies from multiple disciplines are reviewed to highlight the role of altered dopaminergic function in the promotion of arousal, pain sensitivity, and mood disturbance. We argue that studies aiming to elucidate common factors accounting for the comorbidity of insomnia, chronic pain, and depression should evaluate functioning within the mesolimbic dopaminergic system and its effect on common processes known to be dysregulated in all three disorders.
Keywords: Insomnia, Chronic Pain, Depression, Dopamine, Stress, Affect, Opioids
Insomnia is a prevalent sleep disorder, with a least 6% of the population meeting stringent diagnostic criteria, and as many as 48% presenting with symptoms (1). Psychiatric and medical comorbidities commonly present with insomnia, necessitating a transdisciplinary approach to research and treatment conceptualization. Chronic pain is one of the most common comorbidities, and has been linked to insomnia at the population level through epidemiological surveys (2). Finer-grained prospective and experimental investigations have convincingly characterized sleep and pain symptoms as reciprocally interacting (3–5). Current research efforts have focused on identifying common pathways through which neurobiological and psychosocial mechanisms of chronic pain and insomnia covary to enhance risk for symptom amplification and functional decline. Similarly, separate inquiries at multiple levels of analysis have implicated depression as a prominent comorbidity of both insomnia (for a review, see: 6) and chronic pain (for a review, see: 7).
Taken together, epidemiological, cross-sectional, and prospective studies converge on the general notion that insomnia, chronic pain, and depression are mutually interacting, each increasing the risk for the emergence and/or exacerbation of the other. However, few studies have systematically conceptualized and investigated all three disorders and their symptoms as a cluster. The relative dearth of studies, together with differences in methodologies across them, has resulted in a lack of clarity regarding which common factors account for the clustering of these disorders. The inconsistencies present across studies may be due to the fact that most have employed symptom-level measurements, which introduce heterogeneity into the classification of the disease phenotype (e.g., insomnia). Smith et al. (8) reported that 88% of patients with one of a variety of chronic pain disorders (N = 51) self-reported at least one insomnia complaint, and that their overall sleep quality index was statistically indistinguishable from that of primary insomniacs. Despite these descriptive findings, pain severity did not predict severity of sleep disturbance, a result that runs counter to traditional conceptualizations of secondary insomnia. Similar findings elsewhere have led researchers to consider insomnia and its treatment from a multidimensional perspective that integrates the evaluation of sleep quality with temporally adjacent changes in physical (e.g., pain) and psychological (e.g., cognitive-behavioral) concomitants (8–10).
The two most prominent studies to have investigated the population prevalence of insomnia in chronic pain disorders have offered dramatically different estimates, but methodological differences make it difficult to resolve the discrepancies. Taylor et al (11) defined insomnia as the presence in the past month of problems either with sleep latency or mid-sleep awakenings and found population insomnia prevalence estimates of 39.8% in fibromyalgia, 27.3% in arthrosis, 25.6% in rheumatoid arthritis, 17.6% in tension-type headache, and 16.8% in migraine, among a sample constituted of 29% African Americans in Tennessee. In contrast, Sivertsen et al (12) defined insomnia based on a self-report affirming the presence of insomnia for at least 6 months, described by a combination of sleep-onset latency, wake after sleep onset, and daytime complaints obtained through a daily sleep diary. Using these criteria, the authors observed population insomnia prevalence estimates of 48.6% among white Norwegians with chronic pain, broadly defined. In light of these discrepancies, it is evident that population comorbidity estimates between diagnoses are limited by a lack of standardization in measurement.
To ameliorate problems due to methodological inconsistencies and improve our understanding of how and when symptoms cluster in insomnia, chronic pain, and depression, an approach that addresses putative mechanisms is needed. As all three conditions are principally regulated by central nervous system mechanisms, it is intuitive to turn to the brain for answers about which common pathways explain variance in the observed comorbidities and risk estimates.
Although there is likely a large degree of equifinality, we hypothesize that the mesolimbic dopamine (DA) system is an attractive starting point in the effort to unravel common mechanisms, given its direct role in the regulation of the hallmark symptoms of insomnia, chronic pain, and depression. We acknowledge the likely role that other neurotransmitter systems, such as serotonin and norepinephrine, play in providing a broad central nervous system substrate linking sleep, pain, and mood. Readers are referred to prior reviews linking these systems and symptom clusters (13–15). Relative to other neurotransmitter systems, the mesolimbic DA system, however, has received less attention in the literatures on insomnia, chronic pain, and depression. To our knowledge, no study has attempted to explain the influence of the mesolimbic DA system on the interrelations of these three disorders. The goal of this review, then, is to draw together otherwise disparate lines of evidence and advance a potentially unifying, though not exhaustive, mechanistic model of the comorbidity of insomnia, chronic pain, and depression. A comprehensive pharmacologic review of DA and closely linked neuropetide systems is outside the scope of this paper, and has been conducted elsewhere (16;17). Rather, here we provide an overview of the evidence in support of the common mechanisms through which mesolimbic DA activity may promote the comorbidity of insomnia, chronic pain, and depression. We start by first briefly reviewing evidence for the clustering of symptoms of the triad of insomnia, chronic pain, and depression. Subsequently, we review the evidence in support of DA as a common substrate of each disorder and propose a model through which DAergic dysfunction may increase vulnerability for the development and/or maintenance of these comorbidities.
Summary of Studies Simultaneously Investigating Insomnia, Pain, and Depression
Although relatively fewer studies have examined the triad of insomnia, chronic pain, and depression—or their symptoms—together in one model, there is growing support for the hypothesis that the three disorders are linked through common mechanisms. Wilson et al. (18) examined comorbidities in a sample of chronic musculoskeletal pain patients and found that those diagnosed with depression were significantly more likely to have insomnia. Further, patients with both comorbidities reported greater affective distress and pain severity than either those with comorbid insomnia alone or those without comorbid depression or insomnia. The causal ordering of these relationships is not clear. A prospective mediation study found that negative mood mediates the relationship between sleep and pain (19), but a cross-sectional study demonstrated that sleep quality mediates the relationship between pain and symptoms of depression (20). Still other studies have pointed to a direct relationship between sleep and pain over and above the effect of depression symptoms. For example, Chung and Tso (21) found that three months of pharmacotherapy for depression did not attenuate the effects of either self-reported insomnia symptoms or objective polysomnography parameters on pain symptoms observed during an acute depressive episode. A number of other studies have found that significant associations between insomnia severity and musculoskeletal or headache pain remain after controlling for depression symptoms in chronic pain samples (18;22–24). These latter studies, however, were limited in that formal mediational analyses were not conducted.
Two additional prospective studies have sought to establish temporal and directional parameters for these relations, but the results are still mixed. Nicassio & Wallston (25) reported that pain and sleep interact to prospectively predict depression symptoms at 2-year follow-up for rheumatoid arthritis patients. Specifically, individuals with higher pain and worse sleep at time 1 had more depressive symptoms at time 2 than individuals with either problem in isolation (25). A recent study of fibromyalgia patients evaluated sleep disturbance, pain, depression symptoms, and physical function at baseline and one-year follow-up (26). Through path analysis, the authors demonstrated that baseline sleep predicted follow-up pain, baseline pain predicted follow-up physical function, and baseline physical function predicted follow-up depression symptoms, while no baseline variable predicted follow-up sleep. This analysis highlights the unique findings that emerge when the relationships between sleep, pain, and depression are examined prospectively, but is limited in its explanatory power due to the extensive temporal separation of its two assessment points.
To combat this potential confound and evaluate the temporal dynamics of sleep, pain, and depression symptoms, microlongitudinal designs, incorporating repeated assessments in close to real-time, have been employed. A recent daily diary study found that poor nighttime sleep quality predicted increases in next-day pain severity to a greater extent among chronic pain patients with higher versus lower levels of depression symptoms (19). In an ecological momentary assessment study, rheumatoid arthritis and fibromyalgia patients who reported poor sleep quality perceived greater negative affect and lower positive affect—both risk factors for depression (27)-- when pain was elevated relative to when pain was closer to the mean (28). Although there were no diagnostic differences found in the experience of negative and positive affect, fibromyalgia patients reported greater pain and worse sleep quality than rheumatoid arthritis patients (28).
Despite these promising results from cross-sectional and prospective studies demonstrating complex interactions between sleep disturbance, pain, and symptoms of depression, the directionality of effects is mixed, and it is not clear which mechanisms account for them. Although more prospective studies measuring all three symptoms complexes are needed to clarify the temporal complexities, the evidence suggests that each has the potential to moderate and/or mediate the expression of the other. The robust, multidirectional linkages between these symptoms observed in extant longitudinal data raises the distinct possibility that they are regulated by a common neurobiologic substrate or set of substrates. A systematic, multilevel research agenda seeking to identify these putative substrates for the insomnia, chronic pain, and depression triad has the potential to improve our understanding, treatment and prevention of these disorders. Below we assemble evidence implicating altered mesolimbic DA processing as a putative mechanism underlying insomnia, chronic pain, and depression.
The Role of DA in Sleep Disorders
Mesolimbic DA and Arousal
DA is a neurotransmitter from the catecholamine family that is considered to be a key neurobiological substrate of reward, and has traditionally been the target of pharmaceutical therapies for movement disorders, psychotic disorders, and to a lesser extent depression. Evidence also indicates, however, that DA is integral to the promotion and maintenance of arousal states (for reviews, see: 29;30).
Mesolimbic DA neurons originate in the ventral tegmental area and the substantia nigra pars compacta, and project to both subcortical and cortical structures relevant to the sleep/wake cycle, including the nucleus accumbens, striatum, prefrontal cortex, and anterior cingulate cortex (31;32). DA receptors are abundant in key structures of the ascending reticular activating system, including the dorsal and median raphe nuclei and the ventral periaqueductal gray, which are critical in modulating sleep (16;33). Depletion of DA in humans via a competitive inhibitor of a key DA enzymatic precursor provokes sleepiness, which correlates with prolactin increase and increased glucose metabolism in the periaqueductal gray and left insula (34). Experimentally/pharmacologically-induced arousal states, too, are associated with altered DA neurotransmission.
DA neurotransmission has been proposed as a primary mechanism for the arousal-promoting effects of exogenous stimulants (i.e., amphetamine, cocaine, and methylphenidate), which are known to be related to sleep disturbance and insomnia, during both acute and abstinence phases (35–37). An elegant series of studies by Wisor et al. (38) revealed that both methamphetamine and modafinil, an analeptic drug designed to treat narcolepsy and daytime sleepiness, increased extracellular DA by blocking the DA transporter (DAT) receptor, which regulates DA reuptake. DAergic tone and wakefulness were increased in narcoleptic dogs following treatment with amphetamine and modafinil, while neither drug had a wake-promoting effect in DAT-knockout mice, supporting the notion that the DAT receptor mediates amphetamine and modafinil-induced wakefulness (38). More recent mouse studies have implicated D1 and D2 receptors in the wake-promoting effect of modafinil (39;40). PET imaging has confirmed these effects in humans, with differences in radiotracer binding observed in the striatum and nucleus accumbens (41). Thus, exogenously administered stimulants appear to promote wakefulness directly through action in the mesolimbic DAergic system. Indeed, DAergic agents are often utilized to treat daytime sleepiness and disorders associated with failing to maintain adequate daytime arousal. These basic science findings, however, also provide a general basis for DA neurotransmission involvement in sleep abnormalities associated with excessive central nervous system arousal, with functional alterations observed in brain regions associated with the mesolimbic DAergic system.
DA signaling alterations have long been understood to be integral to the pathophysiology of a variety of sleep disorders, and understanding the role of DA in these disorders may inform the pursuit of a shared pathophysiological basis of insomnia, chronic pain, and depression. One such disorder is narcolepsy, a sleep/arousal disorder characterized by REM sleep abnormalities and excessive daytime sleepiness. The hypothalamic peptide orexin (also known as hypocretin), which binds to orexin 1 and orexin 2 receptors on ventral tegmental DAergic neurons (42;43), increases nucleus accumbens DA release (43), and is strongly linked to arousal and the maintenance of wakefulness (44). Convincing evidence suggests orexin is deficient in narcolepsy (45).
An additional disorder for which DA abnormalities have been observed is restless legs syndrome (RLS), a sleep-related movement disorder characterized by a chronic urge to move the limbs during wakefulness with a pronounced circadian pattern in symptom expression that often interferes with sleep onset. Allen (46) reviewed the evidence for DA in the pathophysiology of RLS and tentatively concluded that nigrostriatal DA dysfunction may contribute to RLS symptoms, while low-dose DA agonists generally reduce symptoms. A more recent study of postmortem RLS brain tissue supported those earlier findings by demonstrating reduced D2 receptors in the putamen of RLS patients (47). Clinically, RLS is associated with insomnia (48), as well as objective markers of sleep dysfunction and arousal, including increased sleep latency, decreased sleep efficiency, and excessive sleep instability (49–51). A recent pharmacological trial revealed superior efficacy of the preferential D3 agonist pramipexole, relative to the preferential D2 agonist bromocriptine, in treating insomnia symptoms in RLS (52). These findings highlight the often underappreciated feature of arousal in RLS and provide a further basis from which to hypothesize that DA abnormalities, mediated by activity at multiple receptor subtypes, may contribute to sleep-interfering states of arousal and, by extension, insomnia.
Genetic findings implicating the potential role of DA Neurtransmission in Arousal
Genetic studies offer another tool to evaluate the role of DA in arousal-related sleep problems. A well-studied single nucleotide polymorphism (SNP; val158met) on the catechol-O-methyltransferase gene (COMT) leads to differences in DA metabolism by effecting alterations to the thermostability of the COMT enzyme. Individual differences in the val158met genotype have been associated with differences in alpha wave oscillations in REM and N-REM sleep. Specifically, the met/met genotype, which is associated with decreased DA metabolism, and therefore abnormally elevated levels of tonic DA, is associated with higher peak amplitude of the alpha wave form during wakefulness, and greater 11–13 Hz activity in both REM and N-REM sleep relative to the val/val genotype, which is associated with greater DA metabolism (53). Although the sample size was small (N = 22), and the finding has not yet been replicated, to our knowledge, it nonetheless provides preliminary evidence that genetic differences in a key DA gene known to be implicated in both chronic pain and depression (reviewed below) are associated with heightened states of arousal during sleep.
Other DA-relevant genes have also been implicated in sleep disturbance. A variable number tandem repeat (VNTR) polymorphism on the D4 receptor gene (DRD4) was associated with insomnia-like symptoms in people with Alzheimer’s disease (54), as well as smokers following cessation (55). At present, however, the literature on DA genes and sleep is inconclusive and prone to inconsistencies across studies. For example, a recent study of a VNTR polymorphism on the DA transporter (DAT1) gene that had previously been associated with cocaine abuse and DAT1 transcriptional differences found that the polymorphism was not associated with variability in sleep measured through polysomnography (56). Thus, more research is needed to clarify early genetic findings as genomic and phenotypic methods are refined. If genetic variation associated with functional alterations in DA neurotransmission does, indeed, contribute to a general arousal-based phenotype, we would expect to observe downstream differences in DA processing among people with clinical sleep disorders relative to good sleepers. Ultimately, translational studies linking genetic variants to clinical sleep disorders via intermediate phenotypes descriptive of genetic function (e.g., transcription factors), objective clinical markers (e.g., polysomnagraphy), and subjective psychosocial variables (e.g., affect regulation) will need to be conducted.
Is DA Involved in Primary Insomnia?
Following the observation that DA is associated with arousal in animal models, human neuroimaging models, genetic models, and sleep-related movement disorders, it is intriguing to question whether DA may also contribute to arousal-related sleep dysfunction in other sleep disorders with a less well-established pathophysiological basis, such as insomnia. Decades of research on insomnia frame it as a disorder of 24 hour hyperarousal (57;58). Although the precise neurobiology of insomnia remains poorly elucidated, there is growing evidence for a neurobiological basis for the hallmark hyperarousal symptoms (59;60). DA neurotransmission, however, has rarely been targeted as a possible mechanism for chronic insomnia despite its rather robust association with arousal. Some notable exceptions highlight the promise of targeting DA neurotransmission in insomnia. Vgontzas et al. (61) reported positive correlations between polysomnographic indicators of sleep disturbance (i.e., sleep latency; wake after sleep onset) and urinary levels of the DA precursor dihydroxyphenylalanine (DOPA) and the DA metabolites dihydroxyphenylacetic acid (DOPAC) and dihydroxyphenylglycol (DHPG) in individuals with primary insomnia. In regression analyses, the DA-related variables were stronger predictors of sleep disturbance indices than plasma cortisol. The authors interpreted the results as evidence that sleep-related arousal in chronic insomnia activates a sympathetic stress response mediated by catecholamines. More recently, pilot neuroimaging studies of primary insomnia provide indirect, exploratory support for the possibility of a DA-insomnia link. Prior work by our group measured regional cerebral blood flow via SPECT imaging during NREM sleep shortly after the emergence of the first sleep spindling or K complex (62). This study found the basal ganglia, a major DAergic structure implicated in movement disorders, to demonstrate the most robust perfusion abnormality relative to good sleeper controls. Other work using FDG PET imaging to compare differences between the pre-sleep state and NREM sleep in primary insomnia and controls found several brain regions to be hypermetabolic in primary insomnia during NREM (63). Consistent with the hyperarousal hypothesis of insomnia, these authors found primary insomniacs to demonstrate greater activations in the ascending reticular activating system, mesial temporal cortex, thalamus, hypothalamus, cingulate gyrus, and insula.
There is clearly a need to conduct studies that directly investigate the role of DA as a putative pathophysiological mechanism in insomnia. Some of the above referenced studies directly implicating DA-related genetic variation in insomnia symptoms related to Alzheimer’s disease (54) and nicotine withdrawal (55), raise the possibility that DA might play a role in insomnia disorders more generally, including primary insomnia. Interestingly, several experiments have found that sleep deprivation increases endogenous DA tone, as evidenced by reduced [11C]raclopride binding to D2/D3 receptors in the striatum and thalamus of healthy human subjects (64;65). A recent study in mice similarly showed increases in endogenous DA binding to D3 receptors following sleep deprivation (66). These findings are particularly intriguing to consider with respect to a potential role of DA in the maintenance of chronic insomnia. Although hypervigilence is not typically associated with acute sleep deprivation in healthy subjects, this may not be the case for patients with insomnia who often exhibit mild, chronic partial sleep deprivation. Acute sleep deprivation in healthy subjects is most commonly associated with excessive daytime sleepiness, presumably because sleep-related homeostatic processes override wake promoting arousal mechanisms that might be influenced by enhanced DAergic tone. Indeed, Volkow et al. (64;65) interpreted their findings to indicate that enhanced endogenous DA tone during sleep deprivation may have resulted as a compensatory opponent process combating the primary drive to sleep, and suggested that the suprachiasmatic nucleus, with direct innervations of the striatum and mesencephalon, may regulate this homeostatic process. With respect to chronic primary insomnia, Stepanski and colleagues (67) demonstrated that patients exhibit a diminished homeostatic response to sleep deprivation. This finding suggests the possibility that patients with insomnia may be more vulnerable to sleep-interfering states of arousal occurring in the context of mild chronic sleep deprivation.
As we have discussed, insomnia is rarely a disorder that occurs in isolation, and often presents comorbid with chronic pain and/or depression. Understanding a more clearly defined role of DA dysfunction in those disorders may shed light on how similar abnormalities may precipitate or result from insomnia, and may help clarify the well-recognized but poorly elucidated comorbidity of insomnia, chronic pain, and depression. Indeed, many of the mesolimbic DAergic system structures found to be activated in insomnia and other conditions of disturbed sleep have also been associated with pain processing and mood, suggesting that DA may impact both the development and descending control of common symptoms of chronic pain, and depression. We turn here to the evidence supporting DA’s role in chronic pain.
The Role of DA in Chronic Pain
The notion that DA is involved in pain regulation follows from the observation that endogenous DA in the mesolimbic DA system may produce analgesia. Specifically, lesions to the ventral tegmental area lead to increased sensitivity to pain, while stimulation to the same area produces analgesia (68). The process by which DA is thought to promote chronic pain states, however, is complex and first requires an understanding of the homeostatic checks and balances of DA neurotransmission in steady state. Under normal conditions, tonic DA is released into the extracellular fluid in a phase-independent fashion by glutamate, and builds to a steady state level. Phasic DA, in contrast, is characterized by rapid burst-firing of activated DA neurons, and is released in quantities sufficient to saturate the D2 receptors in the synaptic cleft in response to elevations in pain (69). To maintain homeostatic DA levels at steady state, increases in tonic DA concentrations result in compensatory inhibition of phasic DAergic firing by modulating presynaptic glutamatergic inputs (69). Presynaptic activation of prefrontal cortical D2 receptors by tonic DA has been shown to inhibit DA neuron firing in the nucleus accumbens, whereas presynaptic activation of hippocampal (ventral subliculum) D1 receptors by phasic DA has the opposite effect (70). The opposing processes of presynaptic D1 and D2 receptor regulation of afferent DA neuronal inputs in the nucleus accumbens requires a delicate balance of tonic and phasic DA firing to maintain homeostasis. An excess of tonic versus phasic DA, then, would be expected to inhibit mesolimbic DA neuronal activity. Clinically, an excess of tonic DA may be maladaptive for individuals with chronic pain in that it should both promote arousal and inhibit DA analgesia in response to pain flares. Through this lens, we speculate that the high comorbidity between chronic pain and insomnia may be partially accounted for by a fundamental dysfunction in tonic versus phasic DAergic neurotransmission.
Patients with facial pain, as well as those with fibromyalgia, an idiopathic widespread musculoskeletal pain disorder thought to be maintained primarily by central nervous system abnormalities (71;72), have reduced DA metabolite concentrations in the cerebrospinal fluid (73–75). On the surface, these findings would appear to contradict the hypothesis that DA abnormalities underlie the comorbidity of insomnia and chronic pain in light of the wealth of data pointing to abnormally elevated levels of DA in states of arousal. However, the seemingly paradoxical findings can be clarified through our understanding of the homeostatic regulation of tonic versus phasic pain. If the phasic DA response to pain is chronically inhibited by elevated tonic levels, then we would expect less DA metabolite in the cerebral spinal fluid, which should primarily consist of postsynaptic phasic DA cleared from the synapse through reuptake. Providing further support for the tonic/phasic hypothesis, one study showed that in response to injection of hypertonic saline, fibromyalgia patients experience more pain and release less phasic DA than control subjects (76). Moreover, healthy controls release DA in the striatum in response to sustained pain, whereas fibromyalgia patients do not (76;77).
COMT, which directly regulates tonic, but not phasic DA metabolism, has been proposed as a pain-modulating gene, and allelic differences have been reported in fibromyalgia (78–80), osteoarthritis (81), migraine (82), and tension-type headache (83), such that the low DA metabolism genotype (met/met) tends to be more frequent in cases than controls. Further, three COMT haplotypes have been associated with high, low, and average pain sensitivity. Possession of the average or high pain sensitivity haplotypes increases the odds of developing temporomandibular joint disorder by 2.5 times (84). Recent gene × environment studies indicate the effect of COMT on pain may be moderated by cognitive-affective processes that are relevant to DAergic processing. Carriers of a low activity COMT diplotype who tended to catastrophize about their pain reported greater pain following knee replacement surgery than carriers of the high activity diplotype and non-catastrophizing carriers of the low activity diplotype (85). In a separate within-person microlongitudinal study, fibromyalgia patients who were homozygous for the low activity met158 allele on the val158met SNP reported greater pain on days in which their catastrophizing was elevated than patients homozygous for the high activity val158 allele (86). Some evidence indicates that the changes to DA metabolism conferred by COMT may be limited to tonic DA in the prefrontal cortex (87), which is in line with the genotype-specific effects observed for pain-modulating effects of catastrophizing, a negative affectively laden cognition that may be regulated by prefrontal cortical circuits (88;89). To the extent that dysfunction in prefrontal cortical DA neurotransmission affects striatal, cingulate, and insular DA burst firing (89–92), chronic pain may be perpetuated among individuals with poor tonic DA regulation (76).
The Role of DA in Depression
Although serotonin and norepinephrine signaling in the hippocampus and frontal cortex has been the target of a large proportion of the research on depression and its treatment, a growing body of research has turned the attention toward DA neurotransmission in the mesolimbic DA system (93–95). Meta-analysis has confirmed that depression is a significant comorbidity of cocaine addiction, which is associated with mesolimbic DA abnormalities (96). The dysregulation of emotions characteristic of depression involves not only an increase in negative emotions, such as hopelessness or anger, but also a relative depletion of positive affective resources, such as the ability to experience reward. A hallmark of depression is a breakdown of the motivational drive to cope with life’s challenges, leading to behavioral withdrawal and inhibition (97;98). Mesolimbic DA-mediated anhedonia, or the inability to experience reward from natural reinforcers, may underlie this motivational-affective disturbance (99).
Although neuroimaging studies have yielded equivocal results with regard to DA binding differences in depressed patients relative to controls (94), psychological stress, a general but fundamental symptom of depression, has been convincingly linked with DA. Stress has been shown to acutely activate DA neurons in the ventral tegmental area and promotes increases in tonic DA in the nucleus accumbens (100;101). In the short term, stress-induced DAergic neurotransmission may be adaptive, in as much as activation within the nucleus accumbens may drive motivation to cope with stress and enhance the experience of natural reward in the event of coping success and/or stress relief (102). Affect, however, is especially dysregulated during periods of chronic stress (103), suggesting that sustained mesolimbic DA activation is maladaptive (104). Studies of chronic cocaine use support these findings. For example, by repeatedly potentiating phasic DA release, chronic cocaine use is associated with reduced D2 receptor availability in the striatum and nucleus accumbens of detoxified cocaine addicts (105). Over time, D2 receptor function may change as a result of chronic phasic activation, thereby creating a state in which natural rewards lose their ability to stimulate phasic DA activity at the D2 receptor (106;107) and affect becomes dysregulated (108). Diminished mesolimbic phasic DA function, a process also engendered by inhibitory tonic DA activity, may contribute to the elevated risk for depression to develop during and/or subsequent to active substance abuse (109).
Early major life events, too, may prime the mesolimbic DAergic system for later life dysregulation (110). For example, in animal models, stress associated with maternal deprivation leads to increased tonic DA in the nucleus accumbens and amygdala during adulthood (111), as well as an increased sensitivity to cocaine (112). In humans, exposure to negative early life events is a major risk factor for substance use disorders (113) and depression (114). These findings are underscored by the recent findings that early childhood trauma predicts disordered sleep (115;116) and chronic pain (117) in adulthood. Relating these findings to DA neurotransmission in humans, it has been shown that in response to an experimental psychosocial stressor, adults who reported poor maternal care evidenced greater DA release in the ventral striatum than those who reported healthy maternal rearing (118).
One mechanism through which stress-induced mesolimbic DA activation can be appreciated is the transcription of cAMP response element binding (CREB) protein (for a review, see: 119). Stress stimulates CREB in the nucleus accumbens (120), where its activity is mediated by DA through both D1 (121) and D2 (122) receptors. Overexpression of CREB has been found to diminish the rewarding value of cocaine (123), suggesting a role in promoting anhedonia. Interestingly, behavioral responses to aversive stimuli are also muted when CREB is elevated (120), and depressive behavior has been observed during a forced-swim test in animal models (124). Taken together, the evidence suggests that CREB elevations contribute to emotional dysregulation and may be considered a putative risk factor for depression. As DA neurons may be chronically activated in response to chronic stress, the CREB findings provide further support for the notion that altered mesolimbic DAergic neurotransmission contributes to the development and maintenance of depression.
Summary and Future Directions
The evidence implicates DA as a neurobiological factor associated with symptoms of insomnia, chronic pain, and depression. However, the exact nature of these associations has yet to be elucidated, and the putative role for DA in insomnia rests primarily on documented associations of DA with arousal and other arousal-related disorders, and therefore requires rigorous empirical evaluation in insomnia itself. It is likely that the DAergic influence on the comorbidity triad is dependent on receptor subtype, receptor pharmacokinetics, homeostatic regulation of DA release, functional connectivity between brain regions, activity of functionally related neurotransmitters, metabolic and catabolic factors, genetic differences, environmental differences, and stimulus type, intensity, and duration. Further, although the majority of studies reviewed above point to elevated tonic DA levels as a potential risk factor in the manifestation of sleep, pain, and mood problems, other studies have found reduced DA concentrations in clinical populations (73;74;125). As such, this review runs the risk of oversimplifying the function of DA as a substrate linking the symptom triad. For example, DA has been proposed as a key substrate in chronic migraine given the abundance of D2 receptors found in the trigeminovascular system, and the increased aura presentation resulting from administration of D2 agonists (for a review, see: 126). Despite these findings, DA hypersensitivity in migraine has not been consistently linked to migraine-related pain, suggesting that the role for DA in chronic pain may be nuanced and vary as a function of clinical presentation.
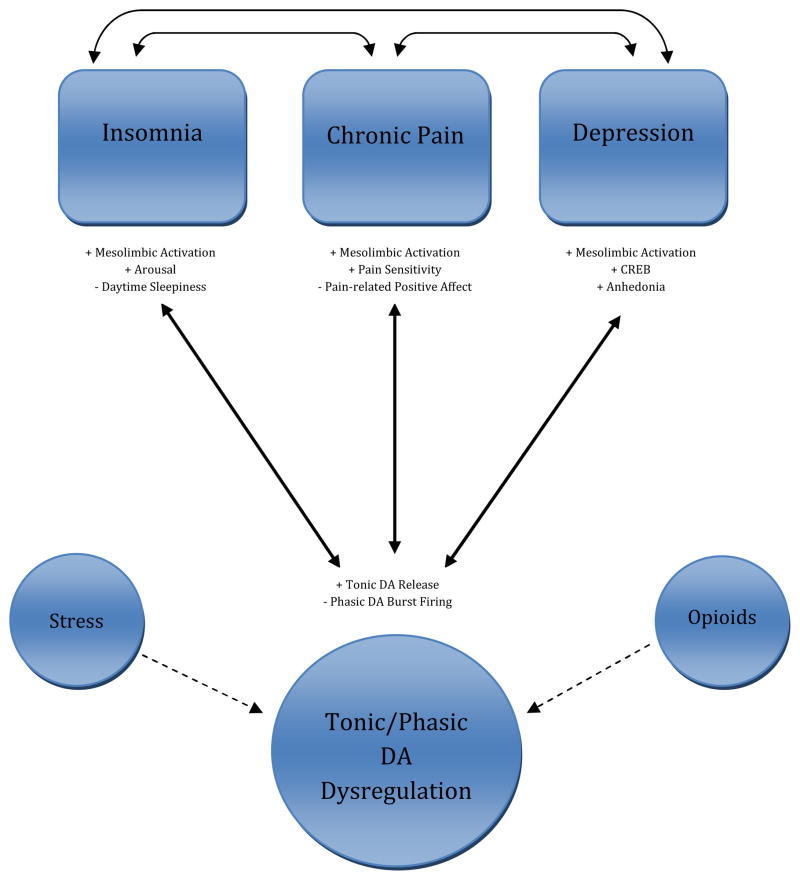
Nonetheless, it is reasonable to glean from the evidence presented in this review that a common DAergic abnormality or set of abnormalities may underlie insomnia, chronic pain, and depression. As reflected in Figure 1, we propose that DA abnormalities may perpetuate the experience of insomnia, chronic pain, and depression, and that persistent exacerbations of the symptoms of these disorders may feed back to promote further DA dysregulation, as represented by the bidirectional arrows. The current literature does not provide guidance regarding the temporal dynamics of DA’s relationship with these symptoms, so our model refrains from making causal predictions. Therefore, we are limited to making the more general prediction that tonic DAergic functioning will be dysregulated among individuals who have comorbid insomnia, chronic pain, and depression. A critical path to test with this model is the extent to which psychological stress moderates the relations of DA and the comorbidity triad. Stress has been shown to have a bidirectional relation with insomnia (61;127), chronic pain (128;129), and depression (130), whereby stress can both contribute to symptom exacerbation and be generated in response to symptoms themselves. As described above, stress is thought to result in increased tonic DA release, providing a rationale for including stress as a moderator of the paths from DA dysfunction to illness in Figure 1.
Figure 1.
Vulnerability model of tonic/phasic DA dysregulation. Solid arrows represent putative bidirectional pathways through which abnormalities in the homeostatic regulation of tonic and phasic DA contribute to the comorbid triad of insomnia, chronic pain, and depression. Dashed arrows represent putative moderators of DA function in this model.
Another intriguing future research direction is to prospectively assess DAergic function among patients with recently acquired chronic pain. As many of these patients will subsequently develop insomnia and/or depression, the evaluation of DAergic function before and after the development of comorbidities would provide information regarding the potential presence or absence of a comorbidity “dose” effect, whereby DAergic function would be increasingly compromised as sleep and mood become increasingly disturbed. Additionally, it would be important to know whether dysfunctional DA processing can be reversed with symptom reduction following treatment. By studying the separate and combined effects of DA neurotransmission on insomnia, chronic pain, and depression, we can test whether, in the presence of DAergic dysfunction, improvement in one domain (e.g., sleep efficiency) is sufficient to bring about changes in DA function, or whether altered DA processing persists until symptoms in all domains are remitted. Alternatively, the effects of selective DA receptor agonists on the symptom cluster may be informative. Several of the atypical antipsychotic medications, which demonstrate strong D2 receptor antagonism have demonstrated efficacy for depression (131) and several preliminary studies suggest low doses of these medications may be promising in treating refractory insomnia (e.g., 132;133). Unfortunately, most of these medications such as quietapine have broader effects on serotonergic, adrenergic and histaminergic receptors, making it difficult to conclude that improvement in sleep or depression results from specific DAergic mechanisms.
Given the complexity of the mesolimbic DAergic system, it is likely that other interacting neurotransmitter systems may contribute to, and perhaps augment, the experience of these comorbidities. Although discussing in depth the interactive role of other potential neurotransmitter systems is beyond the scope of this review, we highlight the endogenous opioid system as a particularly attractive and relatively novel target given its especially strong linkages to the mesolimbic DAergic systems (for a review, see: 134). Endogenous opioids have been shown to functionally interact with DA (135–139), and are directly implicated in pain processing (140) and depression symptoms (141) in regions with heavy DAergic innervation. Further, recent data suggests opioids are related to arousal, providing additional motivation to consider the opioid-DA interaction in the comorbidity triad. For example, a handful of studies indicate that sleep deprivation dysregulates endogenous opioid systems and diminishes the analgesic efficacy of μ-opioid receptor agonists (142;143). Animal studies have found that sleep deprivation alters μ and δ opioid receptor function in mesolimbic DA circuits(144), diminishes basal endogenous opioid levels in the brain(145) and down regulates central opioid receptors (146). Both opioids and DA are elevated in the limbic system in response to sleep deprivation, an effect that can be reversed with administration of selective μ and δ-opioid and DA D1 receptor antagonists (147;148). Work by our group in human subjects (149) found that experimental sleep disruption impairs pain inhibitory capacity in healthy females using a diffuse noxious inhibitory control (DNIC) test, a procedure that has been shown in prior studies to be mediated by endogenous opioid peptides (150). Thus, as future research efforts target DAergic functioning in the context of the comorbidity of insomnia, chronic pain, and depression, designs that test the complementary and interacting influence of opioids will be essential. An excellent example of such a design is the influential PET neuroimaging study by Zubieta and colleagues (139), which showed that healthy COMT met158 carriers (i.e., higher tonic DA) have greater pain, reduced μ-opioid system activation, and increased μ-opioid receptor concentrations in the nucleus accumbens and other limbic regions in response to a sustained pain challenge, suggesting that genetic alterations in DA processing affect the efficacy of the opioid system in producing endogenous analgesia. These same investigators have demonstrated a role for the endogenous opioid system during experimental manipulations of sadness (141). A next step will be to extend such a design to account for differences in affect regulation and objective sleep parameters.
A final research direction we will mention here is the examination of positive affect-DA relations in the context of insomnia, chronic pain, and depression. As we have discussed above, DA is directly implicated in the processing of natural reward, and disruptions in DA regulation of reward perception promote affective instability and poor pain outcomes. It remains to be seen if these findings help explain variance in the triad of insomnia, chronic pain, and depression, but preliminary research with fibromyalgia patients, who tend to have elevated rates of all three symptom clusters, suggests this may be a promising direction. Fibromyalgia patients evidence a global deficit in positive affect, as well as a diminished ability to sustain positive affect during pain flares, relative to other chronic pain populations (151;152). A recent candidate gene study revealed COMT genotype-driven differences in the dynamics of positive affect and pain among fibromyalgia patients (153). Specifically, patients homozygous for the met158 allele had greater difficulty sustaining positive affect during daily elevations in pain compared to those homozygous for the high COMT activity val158 allele (i.e., lower tonic DA). Further, fibromyalgia patients who carried the asp40 allele of the asn40asp polymorphism on the μ-opioid receptor gene (OPRM1), which results in a 7–10 fold reduction in μ-opioid receptor protein in cultured cells (154;155), reported higher positive affect compared to those with the genotype conferring higher levels of μ-opioid receptor protein (153). Thus, the relative balance of DA and μ-opioid receptor activity may be an important component of the etiology of both pain processing and affect regulation in fibromyalgia. As this patient population commonly experiences dysregulated sleep (156–158), it is reasonable to predict that DA and opioid-driven positive affective decrements may explain the manifestation of insomnia and depression together with ongoing dysregulation of pain processing. This possibility is underscored by recent research highlighting the positive emotional benefits of restorative sleep (159).
As more data continue to be gathered in support of the observation that insomnia, chronic pain, and depression are highly comorbid disorders, the search for common mechanisms will intensify. The mesolimbic DA system has been implicated separately in each disorder, and is related to processes, such as arousal, that are present in all three disorders. Thus, there is evidence to suggest that a dysfunction in mesolimbic DAergic functioning may underlie the comorbidity of insomnia, chronic pain, and depression. As research methods are refined and more mechanistic data are reported, there may be hope that pharmacological and psychosocial treatments will more efficiently and effectively alleviate the symptoms of this comorbidity triad.
Box 1. Practice Points.
Patients being treated for primary insomnia, chronic pain, or depression should be thoroughly evaluated for the presence of other disorders in the triad. The symptoms of each disorder appear to be functionally related, although no single pathway has been definitively established, and individual differences in the time course and expression of symptoms are likely. Clinicians should consider the possibility that specifically targeting each symptom may have beneficial effects on the other members of the triad. Some evidence for this is already beginning to emerge, e.g. (160;161).
Patients with centrally-mediated chronic pain, such as fibromyalgia, may be more likely to experience comorbid insomnia and depression. Therefore, preventative counseling to address behavioral issues pertinent to these disorders, such as sleep hygiene, may prove beneficial. Such preventative approaches, however, are only beginning to be tested.
Evaluation of the presence of life stressors and the individual’s coping skills may provide insight into the onset and/or maintenance of insomnia, chronic pain, and depression.
Evaluation of substance abuse may aid in treatment conceptualization and planning. Current and/or past stimulant abusers may be especially vulnerable to the development of comorbid insomnia, chronic pain, and depression because their dopaminergic circuits may be altered as a consequence of repeated activation associated with drug intake.
Box 2. Research Agenda.
Future research efforts should evaluate
the role of the mesolimbic dopaminergic system in the development and maintenance of insomnia, chronic pain, and depression with prospective studies that incorporate current methods of assessment of dopaminergic function, including neuroimaging and genetics.
the role of psychological stress as a mediator in models seeking to establish unidirectional or bidirectional paths between dopamine and comorbidity of insomnia, chronic pain, and depression.
the complementary and interacting influence of opioids on dopaminergic processes in the comorbidity of insomnia, chronic pain, and depression.
whether people with comorbid insomnia, chronic pain, and depression are especially vulnerable to deficits in positive affect, whether those positive affective deficits influence and interact with symptoms of each disorder, and whether those dysfunctional positive affective processes are attributable to altered mesolimbic dopaminergic functioning.
Acknowledgments
The authors would like to acknowledge funding support from NINDS/NINR grant T32 NS070201 (PHF) and R01 AR05487, R01 AR059410, R01 DE019731 (MTS).
Abbreviation
- DA
Dopamine
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 2.Ohayon MM. Relationship between chronic painful physical condition and insomnia. J Psychiatr Res. 2005;39:151–9. doi: 10.1016/j.jpsychires.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Kelly GA, Blake C, Power CK, O’Keefe D, Fullen BM. The association between chronic low back pain and sleep: a systematic review. Clin J Pain. 2011;27:169–81. doi: 10.1097/AJP.0b013e3181f3bdd5. [DOI] [PubMed] [Google Scholar]
- 4.Menefee LA, Cohen MJ, Anderson WR, Doghramji K, Frank ED, Lee H. Sleep disturbance and nonmalignant chronic pain: a comprehensive review of the literature. Pain Med. 2000;1:156–72. doi: 10.1046/j.1526-4637.2000.00022.x. [DOI] [PubMed] [Google Scholar]
- 5.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004;8:119–32. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 6.Staner L. Comorbidity of insomnia and depression. Sleep Med Rev. 2010;14:35–46. doi: 10.1016/j.smrv.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433–45. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 8.Smith MT, Perlis ML, Smith MS, Giles DE, Carmody TP. Sleep quality and presleep arousal in chronic pain. J Behav Med. 2000;23:1–13. doi: 10.1023/a:1005444719169. [DOI] [PubMed] [Google Scholar]
- 9.Lichstein KL. Secondary insomnia: a myth dismissed. Sleep Med Rev. 2006;10:3–5. doi: 10.1016/j.smrv.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Stepanski EJ, Rybarczyk B. Emerging research on the treatment and etiology of secondary or comorbid insomnia. Sleep Med Rev. 2006;10:7–18. doi: 10.1016/j.smrv.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Riedel BW, Bush AJ. Comorbidity of chronic insomnia with medical problems. Sleep. 2007;30:213–8. doi: 10.1093/sleep/30.2.213. [DOI] [PubMed] [Google Scholar]
- 12.Sivertsen B, Krokstad S, Overland S, Mykletun A. The epidemiology of insomnia: associations with physical and mental health. The HUNT-2 study. J Psychosom Res. 2009;67:109–16. doi: 10.1016/j.jpsychores.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Argoff C. Mechanisms of pain transmission and pharmacologic management. Curr Med Res Opin. 2011;27:2019–31. doi: 10.1185/03007995.2011.614934. [DOI] [PubMed] [Google Scholar]
- 14.Carver CS, Johnson SL, Joormann J. Serotonergic function, two-mode models of selfregulation, and vulnerability to depression: what depression has in common with impulsive aggression. Psychol Bull. 2008;134:912–43. doi: 10.1037/a0013740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ursin R. Serotonin and sleep. Sleep Med Rev. 2002;6:55–69. doi: 10.1053/smrv.2001.0174. [DOI] [PubMed] [Google Scholar]
- 16.Bannon MJ, Roth RH. Pharmacology of mesocortical dopamine neurons. Pharm Rev. 1983;35:68. [PubMed] [Google Scholar]
- 17.Seeman P, Van Tol HH. Dopamine receptor pharmacology. Trends on Pharmacological Science. 1994;15:264–70. doi: 10.1016/0165-6147(94)90323-9. [DOI] [PubMed] [Google Scholar]
- 18.Wilson KG, Eriksson MY, D’Eon JL, Mikail SF, Emery PC. Major depression and insomnia in chronic pain. Clin J Pain. 2002;18:77–83. doi: 10.1097/00002508-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 19.O’Brien EM, Waxenberg LB, Atchison JW, Gremillion HA, Staud RM, McCrae CS, et al. Negative mood mediates the effect of poor sleep on pain among chronic pain patients. Clin J Pain. 2010;26:310–9. doi: 10.1097/AJP.0b013e3181c328e9. [DOI] [PubMed] [Google Scholar]
- 20.Miro E, Martinez MP, Sanchez AI, Prados G, Medina A. When is pain related to emotional distress and daily functioning in fibromyalgia syndrome? The mediating roles of self-efficacy and sleep quality. Br J Health Psychol. 2011;16:799–814. doi: 10.1111/j.2044-8287.2011.02016.x. [DOI] [PubMed] [Google Scholar]
- 21.Chung KF, Tso KC. Relationship between insomnia and pain in major depressive disorder: A sleep diary and actigraphy study. Sleep Med. 2010;11:752–8. doi: 10.1016/j.sleep.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Boardman HF, Thomas E, Millson DS, Croft PR. Psychological, sleep, lifestyle, and comorbid associations with headache. Headache. 2005;45:657–69. doi: 10.1111/j.1526-4610.2005.05133.x. [DOI] [PubMed] [Google Scholar]
- 23.Smith MT, Klick B, Kozachik S, Edwards RE, Holavanahalli R, Wiechman S, et al. Sleep onset insomnia symptoms during hospitalization for major burn injury predict chronic pain. Pain. 2008;138:497–506. doi: 10.1016/j.pain.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vgontzas A, Cui L, Merikangas KR. Are sleep difficulties associated with migraine attributable to anxiety and depression? Headache. 2008;48:1451–9. doi: 10.1111/j.1526-4610.2008.01175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicassio PM, Wallston KA. Longitudinal relationships among pain, sleep problems, and depression in rheumatoid arthritis. J Abnorm Psychol. 1992;101:514–20. doi: 10.1037//0021-843x.101.3.514. [DOI] [PubMed] [Google Scholar]
- 26.Bigatti SM, Hernandez AM, Cronan TA, Rand KL. Sleep disturbances in fibromyalgia syndrome: relationship to pain and depression. Arthritis Rheum. 2008;59:961–7. doi: 10.1002/art.23828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cicchetti D, Ackerman BP, Izard CE. Emotions and emotion regulation in develoopmental psychopathology. Dev Psychopathol. 1995;7:1–10. [Google Scholar]
- 28.Hamilton NA, Catley D, Karlson C. Sleep and the affective response to stress and pain. Health Psychol. 2007;26:288–95. doi: 10.1037/0278-6133.26.3.288. [DOI] [PubMed] [Google Scholar]
- 29*.Monti JM, Monti D. The involvement of dopamine in the modulation of sleep and waking. Sleep Med Rev. 2007;11:113–33. doi: 10.1016/j.smrv.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Monti JM, Jantos h. The roles of dopamine and serotonin, and of their receptors, in regulating sleep and waking. Prog Brain Res. 2008;172:625–46. doi: 10.1016/S0079-6123(08)00929-1. [DOI] [PubMed] [Google Scholar]
- 31.Lena I, Parrot S, Deschaux O, Muffat-Joly S, Sauvinet V, Renaud B, et al. Variations in extracellular levels of dopamine, noradrenaline, glutamate, and aspartate across the sleep--wake cycle in the medial prefrontal cortex and nucleus accumbens of freely moving rats. J Neurosci Res. 2005;81:891–9. doi: 10.1002/jnr.20602. [DOI] [PubMed] [Google Scholar]
- 32.Van Der Kooy D, Hattori T. Single subthalamic nucleus neurons project to both the globus pallidus and substantia nigra in rat. J Comp Neurol. 1980;192:751–68. doi: 10.1002/cne.901920409. [DOI] [PubMed] [Google Scholar]
- 33.Lu J, Jhou TC, Saper CB. Identification of wake-active dopaminergic neurons in the ventral periaqueductal gray matter. J Neurosci. 2006;26:193–202. doi: 10.1523/JNEUROSCI.2244-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meyers N, Fromm S, Luckenbaugh DA, Drevets WC, Hasler G. Neural correlates of sleepiness induced by catecholamine depletion. Psychiatry Res. 2011;194:73–8. doi: 10.1016/j.pscychresns.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brower KJ, Maddahian E, Blow FC, Beresford TP. A comparison of self-reported symptoms and DSM-III_R criteria for cocaine withdrawal. Am J Drug Alcohol Abuse. 1988;14:347–56. doi: 10.3109/00952998809001556. [DOI] [PubMed] [Google Scholar]
- 36.Morgan PT, Pace-Schott EF, Sahul ZH, Coric V, Stickgold R, Malison RT. Sleep, sleepdependent procedural learning and vigilance in chronic cocaine users: Evidence for occult insomnia. Drug Alcohol Depend. 2006;82:238–49. doi: 10.1016/j.drugalcdep.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 37.Valladares EM, Irwin MR. Polysomnographic sleep dysregulation in cocaine dependence. Sci World J. 2007;7:213–6. doi: 10.1100/tsw.2007.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38*.Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM. Dopaminergic role in stimulantinduced wakefulness. J Neurosci. 2001;21:1787–94. doi: 10.1523/JNEUROSCI.21-05-01787.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qu WM, Huang ZL, Xu XH, Matsumoto N, Urade Y. Dopaminergic D1 and D2 receptors are essential for the arousal effect of modafinil. J Neurosci. 2008;28:8462–9. doi: 10.1523/JNEUROSCI.1819-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qu WM, Xu XH, Yan MM, Wang YQ, Urade Y, Huang ZL. Essential role of dopamine D2 receptor in the maintenance of wakefulness, but not in homeostatic regulation of sleep, in mice. J Neurosci. 2010;30:4382–9. doi: 10.1523/JNEUROSCI.4936-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41***.Volkow ND, Fowler JS, Logan J, Alexoff D, Zhu W, Telang F, et al. Effects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implications. JAMA. 2009;301:1148–54. doi: 10.1001/jama.2009.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 43.Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake M, et al. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26:398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–4. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baumann CR, Bassetti CL. Hypocretins (orexins) and sleep-wake disorders. Lancet Neurol. 2005;4:673–82. doi: 10.1016/S1474-4422(05)70196-4. [DOI] [PubMed] [Google Scholar]
- 46.Allen R. Dopamine and iron in the pathophysiology of restless legs syndrome (RLS) Sleep Med. 2004;5:385–91. doi: 10.1016/j.sleep.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 47.Connor JR, Wang XS, Allen RP, Beard JL, Wiesinger JA, Felt BT, et al. Altered dopaminergic profile in the putamen and substantia nigra in restless leg syndrome. Brain. 2009;132:2403–12. doi: 10.1093/brain/awp125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 49.Ferri R, Manconi M, Arico D, Sagrada C, Zucconi M, Bruni O, et al. Acute dopamine-agonist treatment in restless legs syndrome: effects on sleep architecture and NREM sleep instability. Sleep. 2010;33:793–800. doi: 10.1093/sleep/33.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Montplaisir J, Boucher S, Poirier G, Lavigne G, Lapierre O, Lesperance P. Clinical, polysomnographic, and genetic characteristics of restless legs syndrome: a study of 133 patients diagnosed with new standard criteria. Mov Disord. 2011;12:61–5. doi: 10.1002/mds.870120111. [DOI] [PubMed] [Google Scholar]
- 51.Saletu B, Gruber G, Saletu M, Brandstatter N, Hauer C, Prause W, et al. Sleep laboratory studies in restless legs syndrome patients as compared with normals and acute effects of ropinirole. 1. Findings on objective and subjective sleep and awakening quality. Neuropsychobiology. 2000;41:181–9. doi: 10.1159/000026658. [DOI] [PubMed] [Google Scholar]
- 52.Manconi M, Ferri R, Zucconi M, Clemens S, Giarolli L, Bottasini V, et al. Preferential D2 or preferential D3 dopamine agonists in restless legs syndrome. Neurology. 2011;77:110–7. doi: 10.1212/WNL.0b013e3182242d91. [DOI] [PubMed] [Google Scholar]
- 53.Bodenmann S, Rusterholz T, Durr R, Stoll C, Bachmann V, Geissler E, et al. The functional Val158Met polymorphism of COMT predicts interindividual differences in brain alpha oscillations in young men. J Neurosci. 2009;29:10855–62. doi: 10.1523/JNEUROSCI.1427-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Proitsi P, Lupton MK, Reeves SJ, Hamilton G, Archer N, Martin BM, Iyegbe C, Hollingworth P, Lawlor B, Gill M, Brayne C, Rubinsztein DC, Owen MJ, Williams J, Lovestone S, Powell JF. Association of serotonin and dopamine gene pathways with behavioral subphenotypes in dementia. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.06.011. In press. [DOI] [PubMed] [Google Scholar]
- 55.Vandenbergh DJ, O’Connor RJ, Grant MD, Jefferson Al, Vogler GP, Strasser AA, et al. Dopamine receptor genes (DRD2, DRD3 and DRD4) and gene-gene interactions associated with smoking-related behaviors. Addiction Biol. 2007;12:106–16. doi: 10.1111/j.1369-1600.2007.00054.x. [DOI] [PubMed] [Google Scholar]
- 56.Guindalini C, Martins RC, Andersen ML, Tufik S. Influence of genotype on dopamine transporter availability in human striatum and sleep architecture. Psychiatry Res. 2010;179:238–40. doi: 10.1016/j.psychres.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 57.Bonnet MH, Arand DL. Hyperarousal and insomnia. Sleep Med Rev. 1997;1:97–108. doi: 10.1016/s1087-0792(97)90012-5. [DOI] [PubMed] [Google Scholar]
- 58.Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14:9–15. doi: 10.1016/j.smrv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 59.Perlis ML, Giles DE, Mendelson WB, Bootzin RR, Wyatt JK. Psychophysiological insomnia: the behavioral model and a neurocognitive perspective. J Sleep Res. 1997;6:179–88. doi: 10.1046/j.1365-2869.1997.00045.x. [DOI] [PubMed] [Google Scholar]
- 60.Riemann D, Spiegelhalder K, Feige B, Voderholzer U, Berger M, Perlis M, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14:19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 61.Vgontzas AN, Tsigos C, Bixler EO, Stratakis CA, Zachman K, Kales A, et al. Chronic insomnia and activity of the stress system: a preliminary study. J Psychosom Res. 1998;45:21–31. doi: 10.1016/s0022-3999(97)00302-4. [DOI] [PubMed] [Google Scholar]
- 62.Smith MT, Perlis ML, Chengazi VU, Pennington J, Soeffing J, Ryan JM, et al. Neuroimaging of NREM sleep in primary insomnia: a Tc-99-HMPAO single photon emission computed tomography study. Sleep. 2002;25:325–35. [PubMed] [Google Scholar]
- 63.Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161:2126–8. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]
- 64.Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Wong C, et al. Sleep deprivation decreases binding of [11C]raclopride to dopamine D2/D3 receptors in the human brain. J Neurosci. 2008;28:8454–61. doi: 10.1523/JNEUROSCI.1443-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Volkow ND, Tomasi D, Wang GJ, Telang F, Fowler JS, Wang RN, et al. Hyperstimulation of striatal D2 receptors with sleep deprivation: Implications for cognitive impairment. Neuroimage. 2009;45:1232–40. doi: 10.1016/j.neuroimage.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lim MM, Xu J, Holtzman DM, Mach RH. Sleep deprivation differentially affects dopamine receptor subtypes in mouse striatum. Neuroreport. 2011;22:489–93. doi: 10.1097/WNR.0b013e32834846a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stepanski E, Zorick F, Roehrs T, Roth T. Effects of sleep deprivation on daytime sleepiness in primary insomnia. Sleep. 2000;23:215–9. [PubMed] [Google Scholar]
- 68.Sotres-Bayon F, Torres-Lopez E, Lopez-Avila A, del AR, Pellicer F. Lesion and electrical stimulation of the ventral tegmental area modify persistent nociceptive behavior in the rat. Brain Res. 2001;898:342–9. doi: 10.1016/s0006-8993(01)02213-2. [DOI] [PubMed] [Google Scholar]
- 69.Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- 70*.Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–7. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 71.Lee YC, Nassikas NJ, Clauw DJ. The role of the central nervous system in the generation and maintenance of chronic pain in rheumatoid arthritis, osteoarthritis and fibromyalgia. Arthritis Res Ther. 2011;13:211. doi: 10.1186/ar3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meeus M, Nijs J. Central sensitization: a biopsychosocial explanation for chronic widespread pain in patients with fibromyalgia and chronic fatigue syndrome. Clin Rheumatol. 2007;26:465–73. doi: 10.1007/s10067-006-0433-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bouckoms AJ, Sweet WH, Poletti C, Lavori P, Carr D, Matson W, et al. Monoamines in the brain cerebrospinal fluid of facial pain patients. Anesth Prog. 1992;39:201–8. [PMC free article] [PubMed] [Google Scholar]
- 74.Legangneux E, Mora JJ, Spreux-Varoquaux O, Thorin I, Herrou M, Alvado G, et al. Cerebrospinal fluid biogenic amine metabolites, plasma-rich platelet serotonin and [3H]imipramine reuptake in the primary fibromyalgia syndrome. Rheumatology (Oxford) 2001;40:290–6. doi: 10.1093/rheumatology/40.3.290. [DOI] [PubMed] [Google Scholar]
- 75.Russell IJ, Vaeroy H, Javors M, Nyberg F. Cerebrospinal fluid biogenic amine metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritis. Arthritis Rheum. 1992;35:550–6. doi: 10.1002/art.1780350509. [DOI] [PubMed] [Google Scholar]
- 76*.Wood PB, Schweinhardt P, Jaeger E, Dagher A, Hakyemez H, Rabiner EA, et al. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci. 2007;25:3576–82. doi: 10.1111/j.1460-9568.2007.05623.x. [DOI] [PubMed] [Google Scholar]
- 77.Scott DJ, Heitzeg MM, Koeppe RA, Stohler CS, Zubieta JK. Variations in the human pain stress experience mediated by ventral and dorsal basal ganglia dopamine activity. J Neurosci. 2006;26:10789–95. doi: 10.1523/JNEUROSCI.2577-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gursoy S, Erdal E, Herken H, Madenci E, Alasehirli B, Erdal N. Significance of catechol-Omethyltransferase gene polymorphism in fibromyalgia syndrome. Rheumatol Int. 2003;23:104–7. doi: 10.1007/s00296-002-0260-5. [DOI] [PubMed] [Google Scholar]
- 79.Limer KL, Nicholl BI, Thomson W, McBeth J. Exploring the genetic susceptibility of chronic widespread pain: the tender points in genetic association studies. Rheumatology (Oxford) 2008;47:572–7. doi: 10.1093/rheumatology/ken027. [DOI] [PubMed] [Google Scholar]
- 80.Vargas-Alarcon G, Fragoso JM, Cruz-Robles D, Vargas A, Vargas A, Lao-Villadoniga JI, et al. Catechol-O-methyltransferase gene haplotypes in Mexican and Spanish patients with fibromyalgia. Arthritis Res Ther. 2007;9:R110. doi: 10.1186/ar2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van Meurs JB, Uitterlinden AG, Stolk L, Kerkhof HJ, Hofman A, Pols HA, et al. A functional polymorphism in the catechol-O-methyltransferase gene is associated with osteoarthritisrelated pain. Arthritis Rheum. 2009;60:628–9. doi: 10.1002/art.24175. [DOI] [PubMed] [Google Scholar]
- 82.Emin EM, Herken H, Yilmaz M, Bayazit YA. Significance of the catechol-Omethyltransferase gene polymorphism in migraine. Brain Res Mol Brain Res. 2001;94:193–6. doi: 10.1016/s0169-328x(01)00219-4. [DOI] [PubMed] [Google Scholar]
- 83.Hagen K, Pettersen E, Stovner LJ, Skorpen F, Zwart JA. The association between headache and Val158Met polymorphism in the catechol-O-methyltransferase gene: the HUNT Study. J Headache Pain. 2006;7:70–4. doi: 10.1007/s10194-006-0281-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84*.Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, et al. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 2005;14:135–43. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- 85.George SZ, Wallace MR, Wright TW, Moser MW, Greenfield WH, Sack BK, et al. Evidence for a biopsychosocial influence on shoulder pain: Pain catastrophizing and catechol-Omethyltransferase (COMT) diplotype predict clinical pain ratings. Pain. 2008;136:53–61. doi: 10.1016/j.pain.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Finan PH, Zautra AJ, Davis MC, Lemery-Chalfant K, Covault J, Tennen H. COMT moderates the relation of daily maladaptive coping and pain in fibromyalgia. Pain. 2011;152:300–7. doi: 10.1016/j.pain.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001;98:6917–22. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gracely RH, Geisser ME, Giesecke T, Grant MA, Petzke F, Williams DA, et al. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain. 2004;127:835–43. doi: 10.1093/brain/awh098. [DOI] [PubMed] [Google Scholar]
- 89.Seminowicz DA, Davis KD. Cortical responses to pain in healthy individuals depends on pain catastrophizing. Pain. 2006;120:297–306. doi: 10.1016/j.pain.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 90.Akil M, Kolachana BS, Rothmond DA, Hyde TM, Weinberger DR, Kleinman JE. Catechol-Omethyltransferase genotype and dopamine regulation in the human brain. J Neurosci. 2003;23:2008–13. doi: 10.1523/JNEUROSCI.23-06-02008.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000;20:3864–73. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Haber SN, Fudge JL. The primate substantia nigra and VTA: integrative circuitry and function. Crit Rev Neurobiol. 1997;11:323–42. doi: 10.1615/critrevneurobiol.v11.i4.40. [DOI] [PubMed] [Google Scholar]
- 93***.Cabib S, Puglisi-Allegra S. Stress, depression and the mesolimbic dopamine system. Psychopharmacology (Berl) 1996;128:331–42. doi: 10.1007/s002130050142. [DOI] [PubMed] [Google Scholar]
- 94.Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64:327–37. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- 95.Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–9. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 96.Conner KR, Pinquart M, Holbrook AP. Meta-analysis of depression and substance use and impairment among cocaine users. Drug Alcohol Depend. 2008;98:13–23. doi: 10.1016/j.drugalcdep.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. J Pers Soc Psychol. 1994;67:319–33. [Google Scholar]
- 98.Kasch KL, Rottenberg J, Arnow BA, Gotlib IH. Behavioral activation and inhibition systems and the severity and course of depression. J Abnorm Psychol. 2002;111:589–97. doi: 10.1037//0021-843x.111.4.589. [DOI] [PubMed] [Google Scholar]
- 99.Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35:537–55. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem. 1989;52:1655–8. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- 101.Horvitz JC. Dopamine gating of glutamatergic sensorimotor and incentive motivational input signals to the striatum. Behav Brain Res. 2002;137:65–74. doi: 10.1016/s0166-4328(02)00285-1. [DOI] [PubMed] [Google Scholar]
- 102.Cabib S, Puglisi-Allegra S. The mesoaccumbens dopamine in coping with stress. Neurosci Biobehav Rev. 2012;36:79–89. doi: 10.1016/j.neubiorev.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 103.Zautra AJ, Affleck GG, Tennen H, Reich JW, Davis MC. Dynamic approaches to emotions and stress in everyday life: Bolger and Zuckerman reloaded with positive as well as negative affects. J Pers. 2005;73:1511–38. doi: 10.1111/j.0022-3506.2005.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Andersen SL, Teicher MH. Desperately driven and no brakes: developmental stress exposure and subsequent risk for substance abuse. Neurosci Biobehav Rev. 2009;33:516–24. doi: 10.1016/j.neubiorev.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch Neurol. 2007;64:1575–9. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- 106.Spanagel R, Weiss F. The dopamine hypothesis of reward: past and current status. Trends Neurosci. 1999;22:521–7. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- 107.Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry. 2004;9:557–69. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- 108.Koob GF, Le MM. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–8. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- 109.Rounsaville BJ, Anton SF, Carroll K, Budde D, Prusoff BA, Gawin F. Psychiatric diagnoses of treatment-seeking cocaine abusers. Arch Gen Psychiatry. 1991;48:43–51. doi: 10.1001/archpsyc.1991.01810250045005. [DOI] [PubMed] [Google Scholar]
- 110.Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003;27:33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- 111.Andersen SL, Lyss PJ, Dumont NL, Teicher MH. Enduring neurochemical effects of early maternal separation on limbic structures. Ann N Y Acad Sci. 1999;877:756–9. doi: 10.1111/j.1749-6632.1999.tb09317.x. [DOI] [PubMed] [Google Scholar]
- 112.Kosten TA, Zhang XY, Kehoe P. Chronic neonatal isolation stress enhances cocaineinduced increases in ventral striatal dopamine levels in rat pups. Brain Res Dev Brain Res. 2003;141:109–16. doi: 10.1016/s0165-3806(03)00003-8. [DOI] [PubMed] [Google Scholar]
- 113.Hawkins JD, Catalano RF, Miller JY. Risk and protective factors for alcohol and other drug problems in adolescence and early adulthood: implications for substance abuse prevention. Psychol Bull. 1992;112:64–105. doi: 10.1037/0033-2909.112.1.64. [DOI] [PubMed] [Google Scholar]
- 114.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14:245–58. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 115.Greenfield EA, Lee C, Friedman EL, Springer KW. Childhood abuse as a risk factor for sleep problems in adulthood: evidence from a U.S. national study. Ann Behav Med. 2011;42:245–56. doi: 10.1007/s12160-011-9285-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Koskenvuo K, Hublin C, Partinen M, Paunio T, Koskenvuo M. Childhood adversities and quality of sleep in adulthood: A population-based study of 26,000 Finns. Sleep Med. 2010;11:17–22. doi: 10.1016/j.sleep.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 117.Raphael KG, Widom CS. Post-traumatic stress disorder moderates the relation between documented childhood victimization and pain 30 years later. Pain. 2011;152:163–9. doi: 10.1016/j.pain.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J Neurosci. 2004;24:2825–31. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–45. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 120.Barrot M, Olivier JD, Perrotti LI, DiLeone RJ, Berton O, Eisch AJ, et al. CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci U S A. 2002;99:11435–40. doi: 10.1073/pnas.172091899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dudman JT, Eaton ME, Rajadhyaksha A, Macias W, Taher M, Barczak A, et al. Dopamine D1 receptors mediate CREB phosphorylation via phosphorylation of the NMDA receptor at Ser897-NR1. J Neurochem. 2003;87:922–34. doi: 10.1046/j.1471-4159.2003.02067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yan Z, Feng J, Fienberg AA, Greengard P. D(2) dopamine receptors induce mitogenactivated protein kinase and cAMP response element-binding protein phosphorylation in neurons. Proc Natl Acad Sci U S A. 1999;96:11607–12. doi: 10.1073/pnas.96.20.11607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Carlezon WA, Jr, Thome J, Olson VG, Lane-Ladd SB, Brodkin ES, Hiroi N, et al. Regulation of cocaine reward by CREB. Science. 1998;282:2272–5. doi: 10.1126/science.282.5397.2272. [DOI] [PubMed] [Google Scholar]
- 124.Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci. 2001;21:7397–403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hagelberg N, Forssell H, Rinne JO, Scheinin H, Taiminen T, Aalto S, et al. Striatal dopamine D1 and D2 receptors in burning mouth syndrome. Pain. 2003;101:149–54. doi: 10.1016/s0304-3959(02)00323-8. [DOI] [PubMed] [Google Scholar]
- 126.Akerman S, Goadsby PJ. Dopamine and migraine: biology and clinical implications. Cephalalgia. 2007;27:1308–14. doi: 10.1111/j.1468-2982.2007.01478.x. [DOI] [PubMed] [Google Scholar]
- 127.Morin CM, Rodrigue S, Ivers H. Role of stress, arousal, and coping skills in primary insomnia. Psychosom Med. 2003;65:259–67. doi: 10.1097/01.psy.0000030391.09558.a3. [DOI] [PubMed] [Google Scholar]
- 128.Davis MC, Zautra AJ, Reich JW. Vulnerability to stress among women in chronic pain from fibromyalgia and osteoarthritis. Ann Behav Med. 2001;23:215–26. doi: 10.1207/S15324796ABM2303_9. [DOI] [PubMed] [Google Scholar]
- 129.Davis MC, Zautra AJ, Smith BW. Chronic pain, stress, and the dynamics of affective differentiation. J Pers. 2004;72:1133–59. doi: 10.1111/j.1467-6494.2004.00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hammen C. Stress and depression. Annu Rev Clin Psychol. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- 131.Thase ME. What role do atypical antipsychotic drugs have in treatment-resistant depression? J Clin Psychiatry. 2002;63:95–103. doi: 10.4088/jcp.v63n0202. [DOI] [PubMed] [Google Scholar]
- 132.Tassniyom K, Paholpak S, Tassniyom S, Kiewyoo J. Quetiapine for primary insomnia: a double blind, randomized controlled trial. J Med Assoc Thai. 2010;93:729–34. [PubMed] [Google Scholar]
- 133.Wine JN, Sanda C, Caballero J. Effects of quetiapine on sleep in nonpsychiatric and psychiatric conditions. Ann Pharmacother. 2009;43:707–13. doi: 10.1345/aph.1L320. [DOI] [PubMed] [Google Scholar]
- 134.Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–38. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 135.Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, et al. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–32. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- 136.Spanagel R, Herz A, Shippenberg TS. The effects of opioid peptides on dopamine release in the nucleus accumbens: an in vivo microdialysis study. J Neurochem. 1990;55:1734–40. doi: 10.1111/j.1471-4159.1990.tb04963.x. [DOI] [PubMed] [Google Scholar]
- 137.Steiner H, Gerfen CR. Role of dynorphin and enkephalin in the regulation of striatal output pathways and behavior. Exp Brain Res. 1998;123:60–76. doi: 10.1007/s002210050545. [DOI] [PubMed] [Google Scholar]
- 138.Unterwald EM, Horne-King J, Kreek MJ. Chronic cocaine alters brain mu opioid receptors. Brain Res. 1992;584:314–8. doi: 10.1016/0006-8993(92)90912-s. [DOI] [PubMed] [Google Scholar]
- 139*.Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, et al. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240–3. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]
- 140.Schmidt BL, Tambeli CH, Barletta J, Luo L, Green P, Levine JD, et al. Altered nucleus accumbens circuitry mediates pain-induced antinociception in morphine-tolerant rats. J Neurosci. 2002;22:6773–80. doi: 10.1523/JNEUROSCI.22-15-06773.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kennedy SE, Koeppe RA, Young EA, Zubieta JK. Dysregulation of endogenous opioid emotion regulation circuitry in major depression in women. Arch Gen Psychiatry. 2006;63:1199–208. doi: 10.1001/archpsyc.63.11.1199. [DOI] [PubMed] [Google Scholar]
- 142.Ukponmwan OE, Rupreht J, Dzoljic MR. REM sleep deprivation decreases the antinociceptive property of enkephalinase-inhibition, morphine and cold-water-swim. Gen Pharmacol. 1984;15:255–8. doi: 10.1016/0306-3623(84)90170-8. [DOI] [PubMed] [Google Scholar]
- 143.Nascimento DC, Andersen ML, Hipolide DC, Nobrega JN, Tufik S. Pain hypersensitivity induced by paradoxical sleep deprivation is not due to altered binding to brain microopioid receptors. Behav Brain Res. 2007;178:216–20. doi: 10.1016/j.bbr.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 144.Fadda P, Martellotta MC, De Montis MG, Gessa GL, Fratta W. Dopamine D1 and opioid receptor binding changes in the limbic system of sleep deprived rats. Neurochem Int. 1992;20 (Suppl):153S–6S. doi: 10.1016/0197-0186(92)90229-k. [DOI] [PubMed] [Google Scholar]
- 145.Przewlocka B, Mogilnicka E, Lason W, van Luijtelaar EL, Coenen AM. Deprivation of REM sleep in the rat and the opioid peptides beta-endorphin and dynorphin. Neurosci Lett. 1986;70:138–42. doi: 10.1016/0304-3940(86)90452-0. [DOI] [PubMed] [Google Scholar]
- 146.Fadda P, Tortorella A, Fratta W. Sleep deprivation decreases mu and delta opioid receptor binding in the rat limbic system. Neurosci Lett. 1991;129:315–7. doi: 10.1016/0304-3940(91)90489-g. [DOI] [PubMed] [Google Scholar]
- 147.Fadda P, Martellotta MC, Gessa GL, Fratta W. Dopamine and opioids interactions in sleep deprivation. Prog Neuropsychopharmacol Biol Psychiatry. 1993;17:269–78. doi: 10.1016/0278-5846(93)90047-v. [DOI] [PubMed] [Google Scholar]
- 148.Fratta W, Collu M, Martellotta MC, Pichiri M, Muntoni F, Gessa GL. Stress-induced insomnia: opioid-dopamine interactions. Eur J Pharmacol. 1987;142:437–40. doi: 10.1016/0014-2999(87)90084-7. [DOI] [PubMed] [Google Scholar]
- 149*.Smith MT, Edwards RR, McCann UD, Haythornthwaite JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30:494–505. doi: 10.1093/sleep/30.4.494. [DOI] [PubMed] [Google Scholar]
- 150.Gear RW, Aley KO, Levine JD. Pain-induced analgesia mediated by mesolimbic reward circuits. J Neurosci. 1999;19:7175–81. doi: 10.1523/JNEUROSCI.19-16-07175.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Finan PH, Zautra AJ, Davis MC. Daily affect relations in fibromyalgia patients reveal positive affective disturbance. Psychosom Med. 2009;71:474–82. doi: 10.1097/PSY.0b013e31819e0a8b. [DOI] [PubMed] [Google Scholar]
- 152.Zautra AJ, Fasman R, Reich JW, Harakas P, Johnson LM, Olmsted ME, et al. Fibromyalgia: evidence for deficits in positive affect regulation. Psychosom Med. 2005;67:147–55. doi: 10.1097/01.psy.0000146328.52009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153*.Finan PH, Zautra AJ, Davis MC, Lemery-Chalfant K, Covault J, Tennen H. Genetic influences on the dynamics of pain and affect in fibromyalgia. Health Psychol. 2010;29:134–42. doi: 10.1037/a0018647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Beyer A, Koch T, Schroder H, Schulz S, Hollt V. Effect of the A118G polymorphism on binding affinity, potency and agonist-mediated endocytosis, desensitization, and resensitization of the human mu-opioid receptor. J Neurochem. 2004;89:553–60. doi: 10.1111/j.1471-4159.2004.02340.x. [DOI] [PubMed] [Google Scholar]
- 155.Zhang Y, Wang D, Johnson AD, Papp AC, Sadee W. Allelic expression imbalance of human mu opioid receptor (OPRM1) caused by variant A118G. J Biol Chem. 2005;280:32618–24. doi: 10.1074/jbc.M504942200. [DOI] [PubMed] [Google Scholar]
- 156.Branco J, Atalaia A, Paiva T. Sleep cycles and alpha-delta sleep in fibromyalgia syndrome. J Rheumatol. 1994;21:1113–7. [PubMed] [Google Scholar]
- 157.Harding SM. Sleep in fibromyalgia patients: subjective and objective findings. Am J Med Sci. 1998;315:367–76. doi: 10.1097/00000441-199806000-00005. [DOI] [PubMed] [Google Scholar]
- 158.Moldofsky HK. Disordered sleep in fibromyalgia and related myofascial facial pain conditions. Dent Clin North Am. 2001;45:701–13. [PubMed] [Google Scholar]
- 159.Vandekerckhove M, Cluydts R. The emotional brain and sleep: an intimate relationship. Sleep Med Rev. 2010;14:219–26. doi: 10.1016/j.smrv.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 160.Manber R, Edinger JD, Gress JL, San Pedro-Salcedo MG, Kuo TF, Kalista T. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31:489–95. doi: 10.1093/sleep/31.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Vitiello M, Rybakowski J, von Korff M, Stepanski E. Cognitive behavioral therapy for insomnia improves sleep and decreases pain in older adults with co-morbid insomnia and osteoarthritis. J Clin Sleep Med. 2009;5:355–62. [PMC free article] [PubMed] [Google Scholar]