Abstract
Background
Brugada syndrome is a potentially serious channelopathy that usually presents in adulthood and has only rarely been described in infancy. In the absence of metabolic or structural cardiac disease, rapid ventricular tachycardia (>200 bpm) and primary cardiac conduction disease are uncommon in infancy. We hypothesized that infants having rapid ventricular tachycardia and conduction abnormalities and not having structural or metabolic pathogeneses were likely to have mutations in depolarizing current channels.
Methods and Results
A retrospective review of all clinical materials from a single institution over a 9-year period from all infants <2 years old and having a discharge diagnosis of ventricular tachycardia or ventricular fibrillation was performed. Among 32 infants fulfilling inclusion criteria, 12 had a structurally normal heart, and 9 of them had either prolonged QRS duration or Brugada pattern while in sinus rhythm. Of those 5 infants not having a definitive pathogenesis, electrophysiological testing had been performed in 4, and genetic testing had been performed in all 5 of those infants. During electrophysiological testing, a prolonged HV interval was present in 2 of 4, inducible ventricular tachycardia was present in 1 of 4, and a type 1 Brugada pattern was induced by intravenous procainamide in 3 of 4. Genetic testing revealed disease-causing mutations in depolarizing sodium (SCN5A) or calcium (CaCNB2b) channels in all 5 infants.
Conclusions
Infants having rapid ventricular tachycardia and conduction abnormalities in the absence of structural or metabolic abnormalities are likely to have disease-causing mutations in cardiac depolarizing channels.
Keywords: Brugada syndrome, bundle-branch block, ion channel, ion channels, pediatrics, ventricular tachycardia
Brugada syndrome (BrS) is a potentially serious cardiac channelopathy first described as a new clinical entity in 1992.1 The diagnosis of BrS is based on electrocardiographic and clinical criteria. Although it is usually diagnosed in adults >30 years of age, it has been identified in children2 and, rarely, in infants.3 In the absence of a previously diagnosed family member, BrS typically presents as polymorphic ventricular tachycardia (VT) with syncope or sudden death, or it is suspected from incidental electrocardiographic findings. BrS has been associated with mutations in 11 different genes. More than 300 mutations in SCN5A (Nav1.5, BrS1) have been reported in 11% to 28% of BrS probands.4,5 Mutations in CACNA1C (Cav1.2, BrS3), CACNB2b (Cavβ2b, BrS4), and CACNA2D1 (Cavα2δ, BrS9) are found in approximately 13% of probands.6,7 Mutations in glycerol-3-phophate dehydrogenase 1-like enzyme gene (GPD1L, BrS2), SCN1B (β1-subunit of Na channel, BrS5), KCNE3 (MiRP2; BrS6), SCN3B (β3-subunit of Na channel, BrS7), KCNJ8 (BrS8), KCND3 (BrS10), and MOG1 (BrS11) are more rare.8–14 These genetic defects cause a loss of function of INa or ICa, or a gain of function of Ito or IK-ATP.
Ventricular tachycardia (VT) is an uncommon arrhythmia in people <18 years of age, with a published incidence of 1.1 per 100 000 patient-years.15 This figure drops to 0.6 per 100 000 patient-years after excluding patients having coexisting structural heart disease.15 Therefore, VT in children generally results in a comprehensive evaluation for potential etiologies.
Following an index case at Duke University Medical Center of rapid VT in an infant who was later demonstrated to have a structurally normal heart, intraventricular conduction delay while in sinus rhythm, and abnormal genetic testing compatible with BrS, it has been our practice to search for depolarizing channel gene mutations in all subsequent infants having a similar clinical phenotype. This report is based on a 9-year experience with all infants <2 years of age having rapid VT (>200 bpm). We then focused on the comprehensive evaluations of those 5 infants having, in addition, intraventricular conduction delay while in sinus rhythm and a structurally normal heart.
Methods
Patient Population
This retrospective descriptive study analyzed clinical information at Duke University Medical Center from all patients <2 years of age at presentation who had the diagnosis of ventricular tachycardia or ventricular fibrillation (VF) based on discharge diagnosis ICD-9 codes during the years 2001 to 2008. Historical information of interest included presence of congenital structural cardiac defects, prior cardiac surgery, presence of any form of cardiomyopathy, infectious disease evaluation, metabolic evaluation (including electrolyte abnormalities), and toxicological evaluation. Features of interest from the family history included sudden unexplained death (including sudden infant death syndrome [SIDS]), known BrS, recurrent syncope, recurrent seizures, and history of prior implanted pacemaker or implantable cardioverter-defibrillator (ICD). All electrocardiograms, electrophysiological testing results, clinical courses, and genetic testing results were analyzed.
Definitions
For the purposes of this study, Brugada-like syndrome was diagnosed under the following circumstances: 1) standard diagnostic criteria of BrS were met, including coved ST segments in 2 of 3 V1 through V3 leads (type 1 pattern),16 or conversion of saddleback ST segment pattern (type 2 pattern)16 to coved pattern in 2 of 3 V1 through V3 leads following intravenous procainamide infusion at 10 mg/kg, plus any one of the following: documented VF, documented self-terminating polymorphic VT, syncope, history of unexplained sudden death before 45 years of age in a first-degree family member, or history of coved ECG pattern in a first-degree family member (when available)17 or 2) presence of rapid VT, defined as rate >200 bpm, plus all of the following: (a) intraventricular conduction delay, defined as a QRS duration z score >2 for age and not having a classical right or left bundle branch block pattern; (b) structurally normal heart by echocardiography; and (c) negative evaluation for infectious, metabolic, and toxicological pathogeneses of VT. Serial echocardiograms were performed in patients having diminished left ventricular shortening fraction during or immediately following termination of VT. The possibility of Brugada-like syndrome was only considered if the left ventricular systolic function normalized.
This investigation was approved by the Duke University Medical Center Institutional Review Board.
Genetic Analysis
All exons and intron borders of sodium and calcium channel genes, including SCN5A, SCN1B, SCN2B, SCN3B, SCN4B, CaCNB2, CACNA2D1, and CACNA1C were amplified with intronic primers and sequenced in both directions to probe for mutations, with the use of an ABI PRISM 3100-Avant Automatic DNA sequencer (Applied Biosystem. Foster City, CA). The flanking primers used for PCR were published previously or designed with Oligo software (Molecular Biology Insights, Inc., Cascade, CO) and are available on request.
The degree to which variations uncovered are conserved among species was determined using VISTA browser (http://pipeline.lbl.gov/cgi-bin/gateway2).
Results
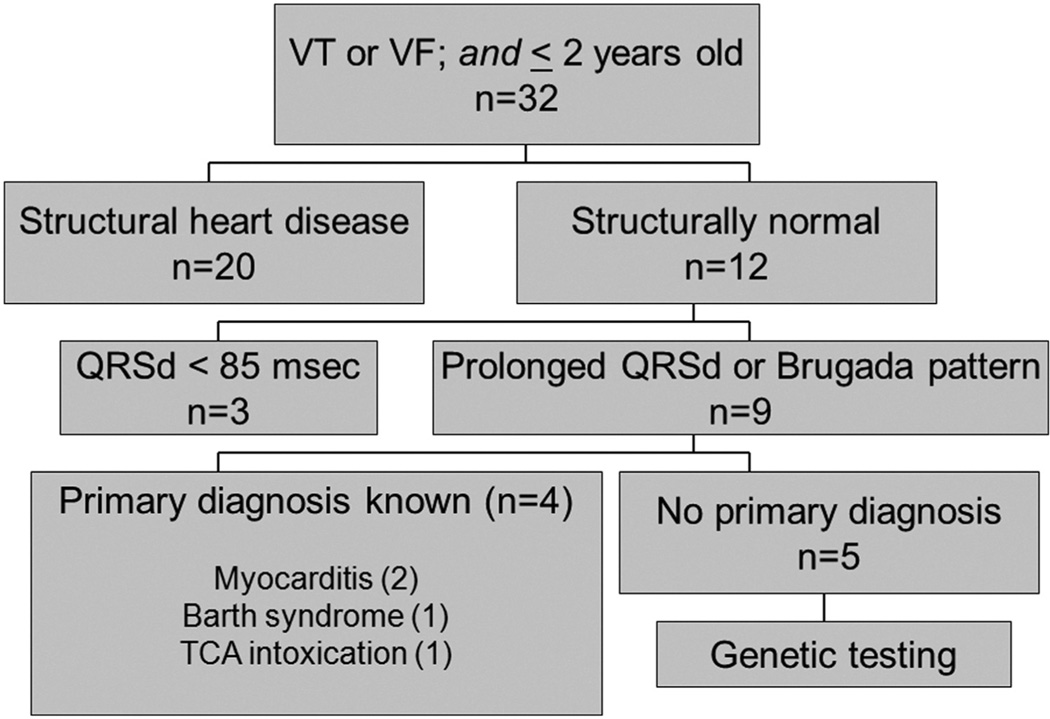
Between January 2001 and November 2008, 32 patients fulfilled the inclusion criteria of age <2 years and rapid VT or VF. Twenty had known structural heart disease, and 9 of the remaining 12 had intraventricular conduction delay or Brugada pattern (coved) (Figure 1). Of those 9, 2 had myocarditis (1 diagnosed by polymerase chain reaction from endomyocardial biopsy specimen and 1 from viral culture), 1 was diagnosed with Barth syndrome, and 1 had had a tricyclic antidepressant (TCA) intoxication. The clinicans caring for the infant having tricyclic antidepressant intoxication did not consider the possibility that she might have increased vulnerability to impaired depolarization due to an underlying channelopathy. Hence, no further work-up was instituted. Among the 3 patients who had VT or VF and a normal QRS duration while in sinus rhythm, final diagnoses were long QT syndrome, fascicular VT, and idiopathic VT (n = 1 each).
Figure 1.
Patients of interest based on age <2 years and an admission diagnosis of ventricular tachycardia or ventricular fibrillation.
The ages at presentation, sexes, presence of fever at presentation, arrhythmias and clinical signs at presentation, and other arrhythmias of the remaining 5 patients are presented in Table 1. As best as could be determined by history, none of these infants were related. None of these infants had fever at the time of initial VT or presumed arrhythmic event. However, patient 4 had received his 6-month immunizations earlier on the day of his presenting VT. Three infants presented with lethargy and pallor, and 1 had syncope while sitting in a flotation device in a swimming pool. The fifth patient, patient 1, had experienced a persistent fetal atrial tachycardia, variously described as chaotic atrial tachycardia and atrial flutter. Family histories were negative, except for patient 2, whose brother who died suddenly at 20 months of age. Patient 5 was adopted from Ecuador, and his family history was limited.
Table 1.
Patient Demographics and Presenting Arrhythmias
| Presenting | ||||||
|---|---|---|---|---|---|---|
| Patient | Age at Presentation (Months) |
Sex | Fever Present? |
Sign | Arrhythmia (Rate in bpm) |
Other Arrhythmias |
| 1 | 0 | F | No | (In utero) | AFl (2:1 AVC) | VF |
| 2 | 14 | F | No | Lethargy; respiratory distress | VT (280) | None |
| 3 | 10 | M | No | Lethargy; repiratory distress | VT (300) | None |
| 4 | 5 | M | No | Lethargy | VT (220–300) | None |
| 5 | 13 | M | No | Syncope | Unknown | None |
AFl indicates atrial flutter; AVC, atrioventricular conduction; bpm, beats per minute; F, female; M, male; VF, ventricular fibrillation; and VT, ventricular tachycardia.
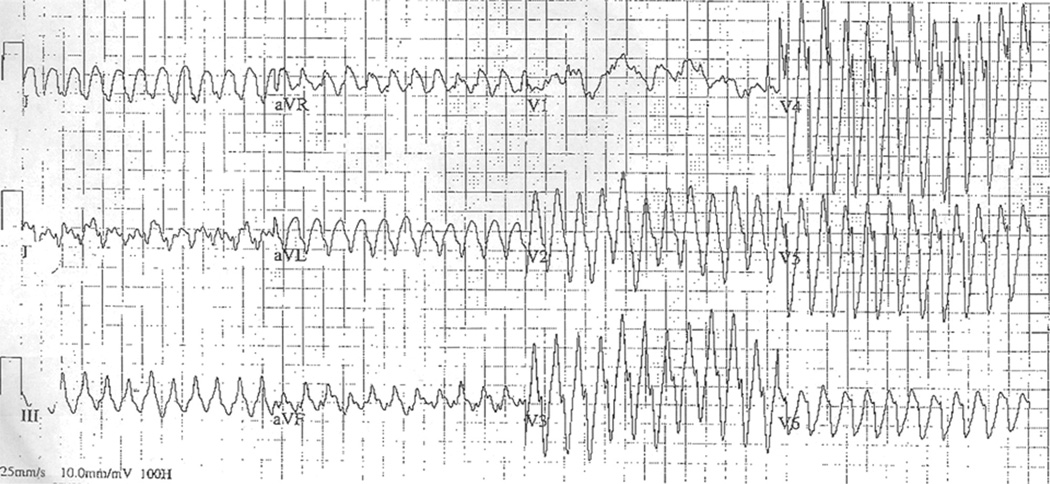
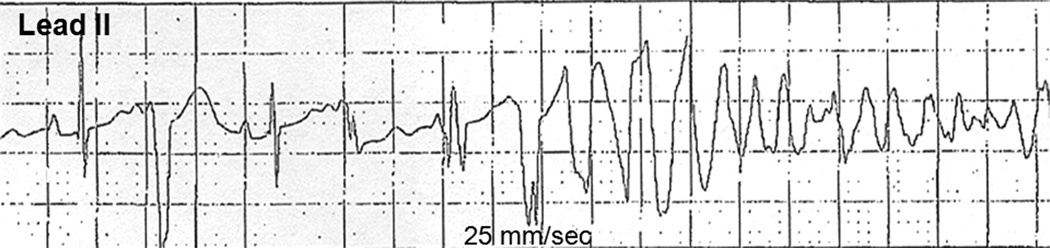
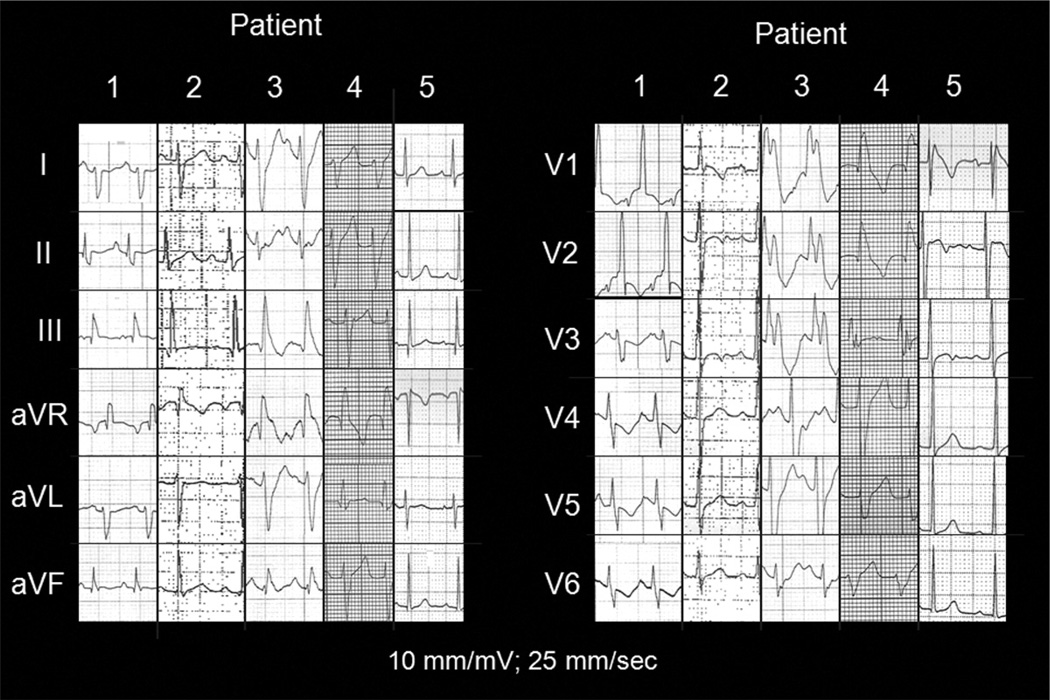
The VT from patient 2 (Figure 2) is representative of the presenting tachycardias from patients 2, 3, and 4. Patient 1’s initial atrial flutter was treated with a single dose of intravenous amiodarone 5 mg/kg over 30 minutes and within 60 minutes of delivery. This resulted in asystole requiring brief transcutaneous pacing. Ventricular fibrillation occurred multiple times over the next 8 days in the absence of further antiarrhythmic drugs (Figure 3). This infant was receiving various combinations of intravenous epinephrine, milrinone, and vasopressin for hypotension and reduced left ventricular function. A composite of the 12-lead ECGs during sinus rhythm from these 5 patients—and in the absence of antiarrhythmic drugs (except for any residual amiodarone effect in patient 1)—is shown in Figure 4.
Figure 2.
Twelve-lead ECG from patient 2 at the time of presentation illustrating rapid ventricular tachycardia.
Figure 3.
Lead II rhythm strip from patient 1 on postbirth day 9 while still critically ill and receiving intravenous epinephrine and milri-none infusions. Illustrated is sinus rhythm with a premature ventricular contraction, followed by an idioventricular couplet initiating polymorphic ventricular tachycardia that degenerated rapidly to ventricular fibrillation.
Figure 4.
Composite 12-lead ECGs during sinus rhythm from the 5 infants in our series. None were receiving antiarrhythmic drugs at the time. Only patient 5 displayed a type 1 Brugada pattern.
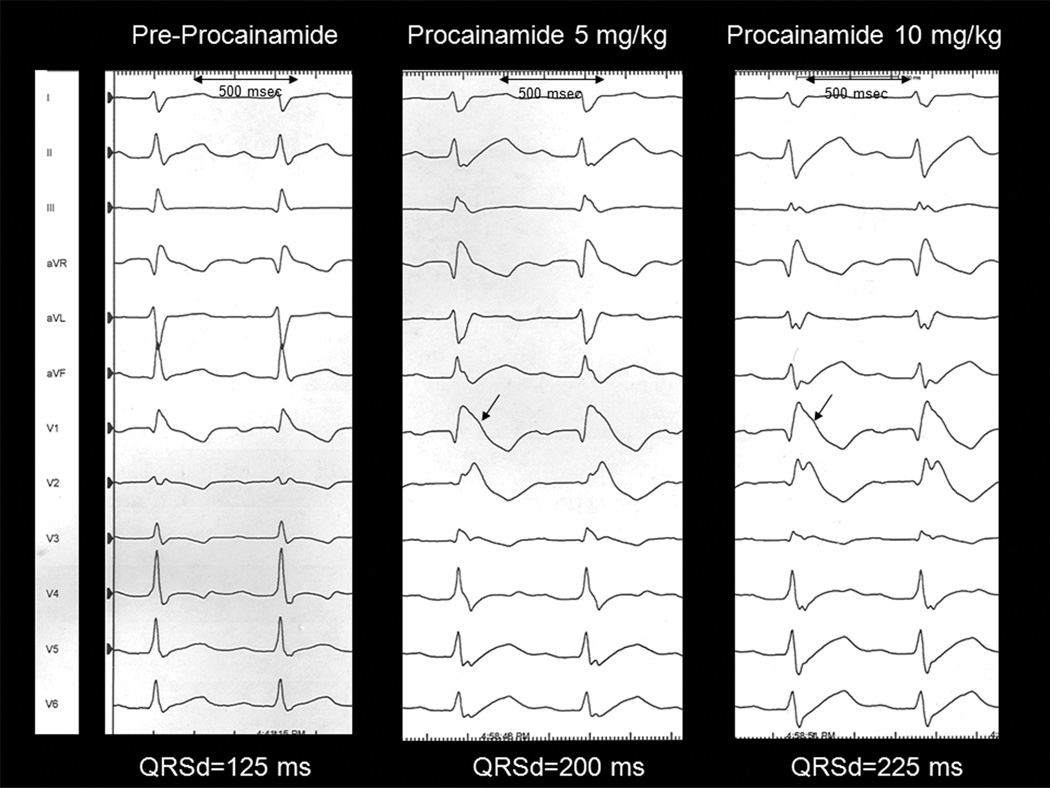
Prior to institution of chronic antiarrhythmic drug therapy, electrophysiological testing was performed on patients 2, 3, 4, and 5. This included interval measurements, programmed ventricular stimulation (up to 3 ventricular extrastimuli down to a minimal coupling interval of 180 ms, and from one pacing site), and intravenous infusion of procainamide (to a maximum of 15 mg/kg over 30 minutes). The results appear in Table 2. An example of a positive response to procainamide appears in Figure 5. Although the baseline QRS duration was prolonged, there was an abrupt additional QRS broadening with J-point elevation (arrows in Figure 5).
Table 2.
Selected Results From Electrophysiologic and Pharmacological Testing
| Patient | Sinus Rhythm | Inducible Ventricular Tachycardia |
Procainamide Response |
||
|---|---|---|---|---|---|
| QRS Duration (msec) | QRS Morphology | HV Interval (msec) | |||
| 1 | 92 | IVCD | NP | NP | NP |
| 2 | 102 | IVCD | 70 | No | Type 1 |
| 3 | 155 | IVCD/RBBB-like | 63 | Yes | Type 1 |
| 4 | 115 | IVCD/RBBB-like | 55 | No | Type 1 |
| 5 | 61 | Type 1 Brugada26 | NA | No | Negative |
IVCD indicates intraventricular conduction delay; NA, not available; and NP, not performed.
Figure 5.
Sequential 12-lead ECGs from patient 4 during intravenous procainamide administration. The arrows indicate J-point elevation characteristic of a type 1 Brugada pattern.
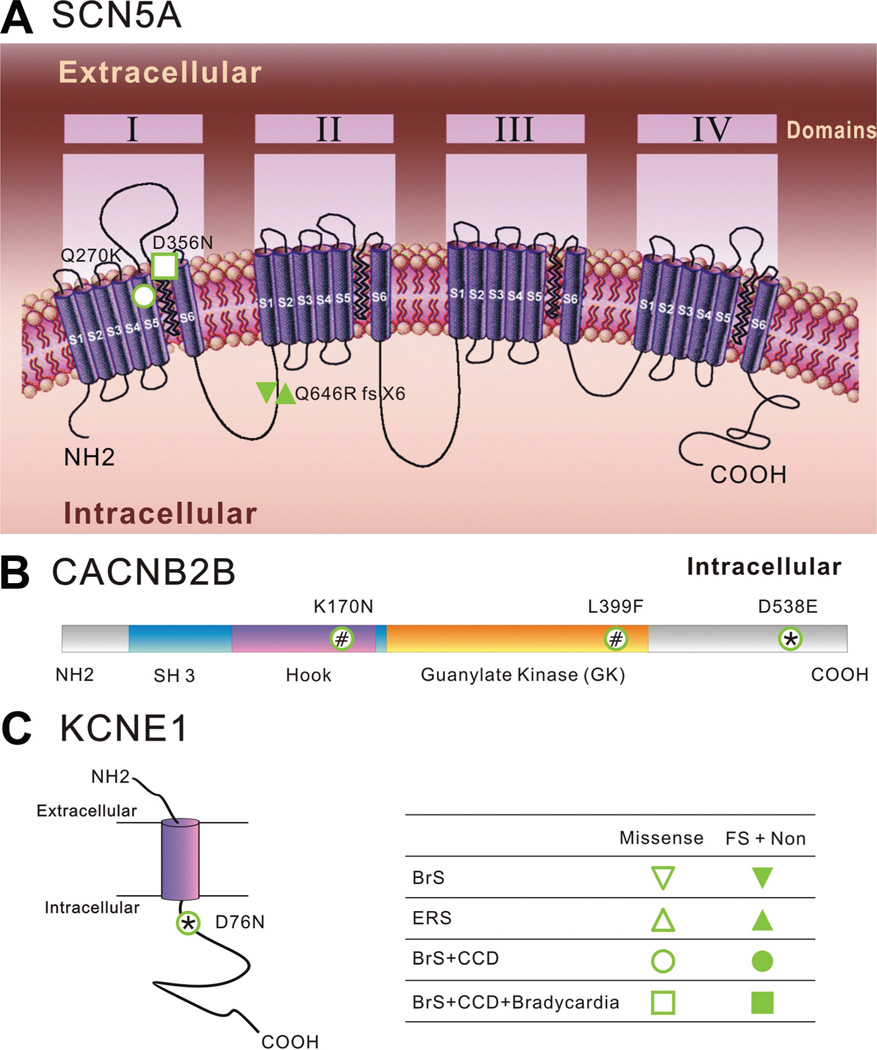
Each of these 5 infants had at least 1 disease-causing mutation in the genes coding for the sodium channel alpha subunit (SCN5A) or the calcium channel β-subunit (CaCNB2b). None of these mutations were found in >300 control alleles from ethnically matched healthy individuals. The mutations from 3 of the 5 patients have been previously described.5,18,19 Patient 3, who had mutations in both genes, also had the longest QRS duration while in sinus rhythm (Figure 4). In addition to a CaCNB2b mutation, patient 5 had an additional mutation in the KCNE1 gene, a known cause of type 5 long QT syndrome.19 The results from genetic testing are shown in Table 3 and Figure 6.
Table 3.
Genetic Screening Results
| Patient | Gene | Map Locus | Exon | Nucleotide Change | Amino Acid Change |
Mutation Type/ Degree Conserved |
Topological Location |
Additional Mutation |
|---|---|---|---|---|---|---|---|---|
| 1 | SCN5A | 3p21 | 7 | 808c>a | Q270K* | Missense/HC | DI-S5 | No |
| 2 | SCN5A | 3p21 | 9 | 1066 g>a | D356N† | Missense/HC | DI/S5–S6 | No |
| 3 | CACNB2 | 10p12 | 12/7b | 1195 c>t/510 g>t | L399F/K170N | Missense/HC | GK/HK | IVS10+2 t>a-SCN5A |
| 4 | SCN5A | 3p21 | 13 | 1936 del c | Q646R fs+X5 | Frame shift/stop codon | DI-DII | No |
| 5 | CACNB2 | 10p12 | 13 | 1614 c>a | D538E‡ | Missense/HC | C-terminal | KCNE1-D76N |
HC indicates highly conserved amount species; GK, guanylate kinase domain; and HK, hook region.
Q270K-SCN5A-previously reported as a Brugada syndrome mutation.5
Previously reported as mutation associated with Brugada syndrome and cardiac conduction disease.18
Previously reported as a Brugada syndrome mutation.19
Figure 6.
Topological location of identified mutations. A, Schematic diagram of α-subunits of the cardiac Na channel (Nav1.5, SCN5A). It consists of 4 domains (DI–DIV), each containing 6 transmembrane-spanning segments. Q646R(fx + X5) is a frame-shift mutation resulting in early truncation of the channel protein in the linker between domains I and II. It is associated with both Brugada syndrome (BrS) and ERS phenotypes. Q270K and D356N are 2 missense mutations located in the region of the pore. B, Diagrammatic representation of the topology of the calcium channel β2b subunit. The SH3–HOOK–GK motif interacts with the voltage-activated Ca2+ channel. K170N, L399F, and D538E are 3 missense mutations identified in infants displaying a BrS phenotype. C, Topology of the β-subunit, KCNE1, of the cardiac slowly activating delayed rectifier channel (IKs). The purple cylinder denotes the transmembrane segment of KCNE1. D76N is a missense mutation identified in patient 5, previously reported as being associated with LQT5 and JLNS2. ERS indicates early repolarization syndrome; FS, frameshift mutation; Non, nonsense mutation. * and #Compound mutations found in the same patient.
Table 4 includes therapies and clinical follow-up from these 5 patients. Patient 1’s episodes of spontaneous VF while in the intensive care unit completely abated following the first dose of enteral quinidine gluconate. Her family discontinued the quinidine approximately 3 months after hospital discharge. She has moved from the region, but we have learned that she remains well. Patient 2 developed extreme QRS prolongation during administration of quinidine, so it was discontinued. The choices of propranolol in patients 3 and 4 and of mexiletine in patient 3 were based on the apparent termination and control of VT by intravenous esmolol and lidocaine and by esmolol alone in these 2 patients, respectively.
Table 4.
Therapies and Follow-Up
| Patient | Anti-Arrhythmic Medications |
ICD? | Acute Ventricular Pacing Threshold |
Appropriate ICD Discharge at Follow-Up? |
Last Status (Months Since Diagnosis) |
|---|---|---|---|---|---|
| 1 | Quinidine | No | NA | NA | A&W (22) |
| 2 | Quinidine | Yes | 1.3 V | Yes | A&W (13) |
| 3 | Mexiletine; propranolol | Yes | 4.5 V | Yes | A&W (31) |
| 4 | Propranolol | Yes | 2.0 V | No | A&W (52) |
| 5 | None | No | NA | NA | A&W (128) |
ICD indicates implantable cardioverter-defibrillator; A&W, alive and well; and NA, not applicable.
The families of all patients were counseled prior to discharge and at follow-up outpatient appointments to aggressively treat fevers with ibuprofen and acetaminophen. They were also counseled to pretreat their children with ibuprofen or acetaminophen just prior to immunizations. At last follow-up, and using this strategy, all children were up-to-date with their immunizations, and none had associated fevers. The appropriate implantable cardioverter-defibrillators (ICD) discharge in patient 2 was identified during routine telemetry 5 months after the event and had occurred at 1:00 am. The family had no recollection of an illness or nocturnal awakening on that date. Patient 3’s ICD discharge occurred during fever related to otitis media.
In all 3 infants who had received ICDs with epicardial pace/sense leads and high-voltage conductors, the acute and chronic ventricular pacing thresholds were unexpectedly high. The shock vectors included an active can in the anterior abdominal wall and a transvenous high-voltage lead (single coil) attached to the posterior parietal pericardium. Because defibrillation was not accomplished at an energy 10 joules less than the device’s maximal output, patient 4 also required placement of an epicardial patch in an anterior subcutaneous location. In follow-up, 2 of these 3 patients (patients 2 and 3) had experienced 1 appropriate ICD discharge each, 1 during a febrile illness (patient 3). Patient 3 experienced fracture of his high-voltage coil 18 months after initial implantation, requiring the addition of an epicardial patch in a left lateral subcutaneous location and abandonment of the original coil. Fifteen months later, he suffered 3 inappropriate ICD discharges due to both atrial and ventricular pace/sense lead fractures, requiring replacement of both leads. At surgery, due to inability to defibrillate the heart during testing at maximal device output, the original high-voltage coil was replaced, finally enabling a 10-joule safety margin. The defibrillation thresholds of patients 2 and 4 have remained at least 10 joules less than their devices’ maximal outputs.
Discussion
Our main finding from this observational investigation is that loss-of-function mutations in depolarizing channels may present as rapid monomorphic VT, VF, or syncope in infants having an intraventricular conduction delay or coved ST segment pattern while in sinus rhythm. When these 2 criteria alone were satisfied, at least 5 of 9 patients (56%) had loss-of-function mutations in L-type calcium or sodium channels. Genetic testing was not performed for the remaining 4 patients because alternate diagnoses had been confirmed. Of those 4, 1 infant who had TCA intoxication might have increased vulnerability to sodium channel-blocking agents. Going forward, we would recommend investigation of any such infant. The a priori strategy of the electrophysiology team to only perform genetic testing based on the aforementioned criteria mitigated a more complete investigation among infants having rapid VT or VF, a structurally normal heart, and normal QRS duration and morphology while in sinus rhythm. Later, 1 of those 3 children was discovered to have a disease causing loss-of-function mutation in the KCNH2 gene. If none of these 32 infants had 2 unrelated cardiac conditions, the presence of rapid VT or VF and intraventricular conduction delay or coved ST segment pattern in sinus rhythm had a predictive accuracy of 100% for a depolarizing channel gene mutation. The simple diagnosis of rapid VT or VF in an infant not having an alternate confirmed diagnosis and irrespective of QRS and ST segment morphology was at least 71% sensitive (5 of 7) for a mutation in a depolarizing channel.
The combination of VF, rapid VT, ventricular flutter, syncope, or positive family history of ventricular arrhythmias with right bundle-branch block pattern, Brugada pattern, or positive procainamide/ajmaline test has been described in 20 infants <3 years of age within 15 families in 12 separate reports.2,3,20–29 SCN5A gene mutations were found in 7 families.2,3,21,25,26,28,29 Some of these infants fell into the SIDS age range, officially defined as 1 month to 1 year of age. The remaining infants in these reports, similar to 2 of the patients in our series, likely represent an arbitrary age extension of SIDS or near-SIDS. Based on the genetic autopsy or family history studies, Ackerman et al has estimated that 5% to 10% of SIDS is related to mutations in the α-subunit of the SCN5A gene or in the channel-interacting proteins, caveolin3, and GPD1-L.30 We believe that the patients in our series fall well within this disease spectrum.
The electrocardiographic Brugada pattern is not specific for BrS in adults. In infants, the fatty acid oxidation disorder, medium chain acyl-CoA dehydrogenase deficiency,31 and rapid infusion of the anesthetic agent propofol32 have been reported to create the electrocardiographic Brugada pattern, and both have been implicated in ventricular tachyarrhythmias. An even longer list of etiologies of non-specific QRS duration lengthening, especially myocardial ischemia, hyperkalemia, and sodium channel-blocking drugs, mandates a thorough metabolic and toxicological evaluation prior to genetic testing for a channelopathy.
The typical patterns in the right precordial leads associated with BrS are thought to be caused by an exaggerated phase 1 or notch in the action potential of right ventricular epicardium. Phase 1 of the action potential is due to activation of the transient outward current (Ito), giving rise to a notch that is much greater in right ventricular epicardium versus endocardium, thus creating a transmural gradient across the ventricular wall, which inscribes the normal J wave. Reduced depolarizing currents can exaggerate this transmural gradient and accentuate the J wave, thus creating the familiar Brugada pattern. Depending on lead positioning, the accentuated J wave may appear as an ST segment elevation or an accentuated r′ in the right precordial leads or in aVr (especially in the presence of a loss-of-function mutation in the SCN5A channel).33 In our 5 cases, patients 3, 4, and 5 displayed an ST-segment elevation or prominent r′ in V1 and/or V2, whereas patients 1 and 2 displayed a prominent r′ in aVr. However, a classical BrS pattern was only present in patient 5.
Progressive conduction system disease is another clinical phenotype resulting from certain SCN5A gene mutations. Patients 1, 2, 3, and 4 in our series had findings compatible with this diagnosis: diffusely prolonged QRS complexes while in sinus rhythm, additional QRS duration prolongation from quinidine (patient 2), high ventricular pacing thresholds (patients 2, 3, and 4), and prolonged HV intervals (patients 2 and 3). Favoring the diagnosis of BrS was the J-point elevation during procainamide infusion in patients 2, 3, and 4. Also, patient 1’s QRS duration normalized during quinidine effect. Moreover, 3 of the 4 missense mutations uncovered in our patients (patients 1, 2 and 5) have previously been associated with BrS in adults (Table 3). The nonsense mutation in SCN5A in patient 4 as well as the combined sodium and calcium channel gene mutations in patient 3 lead to a loss of function in the respective currents (Barajas and Antzelevitch, unpublished data), consistent with the substrate known to underlie the development of a BrS phenotype.
Normal developmental changes in channel density and function could contribute to the absence of a classical Brugada pattern in 4 of our patients. There is relatively less Ito density in immature rodents,34 rabbits,34 and dogs35 compared with mature animals. Similar information is not available in humans, but, if present, could explain why type 1 or type 2 patterns were not observed in our patients. This possibility does not explain the diffuse QRS prolongation observed in our infants and in infants from other reports.2,20,21 An intriguing explanation is related to the depolarization disorder hypothesis of the BrS pattern and pathophysiology.36 This hypothesis is supported by the finding of areas of prolonged, low-amplitude electrograms in the right ventricular outflow tract of some affected adults. Local catheter ablation has even been shown to eliminate the BrS phenotype and reduce arrhythmia burden.37 Given that the infant human heart has a greater right ventricle-to-left ventricle mass ratio compared with the mature heart, it could be argued that the mass of conduction slowing could be proportionately greater and therefore more generally effect the QRS duration. Alternately, the mere fact that these patients developed VT at such young ages may indicate such a severe global reduction in depolarizing currents that gross conduction delay exists even at sinus rates. These observations notwithstanding, it is noteworthy that patient 5, the only patient displaying a classical BrS phenotype, had a calcium channel mutation that does not lead to a slowing of conduction. We propose the term “Brugada-like” to describe what may be a heterogeneous electrophysiological milieu in this small group of infants.
Pharmacological therapy for BrS has been largely limited to quinidine because of its Ito current blocking effect. This drug had a clearly ameliorative effect on patient 1 but not on patient 2. The apparent termination of VT during administration of esmolol (β-adrenergic blocking drug) in patients 3 and 4 requires explanation because β-adrenergic stimulation improves function in voltage-gated sodium and L-type calcium depolarizing channels. Hence, β-adrenergic blocking agents would be expected to further reduce conduction velocity, favoring a reentrant mechanism.27 However, if the tachycardia wavelength was sufficiently lengthened by esmolol, due to disproportionate prolongation of refractoriness, tachycardia could be expected to terminate. Adding to the complexity of the interactions between defective depolarizing channels and sympathetic stimulation is the role of use-dependence. Simple slowing of the sinus rate by β-adrenergic blocking drugs may ameliorate rate-dependent conduction delay. In a recent case report by Chockalingam et al of a toddler with life-threatening VT and an SCN5A loss-of-function mutation, this was the postulated favorable effect of high-dose metoprolol.38
The apparent acute efficacy of intravenous lidocaine in patient 3 and the subsequent use of mexiletine in that patient require explanation, as these drugs are also not conventional therapies for BrS. Lidocaine is thought not to influence the Brugada phenotype in patients harboring loss-of-function depolarizing channel mutations. However, expression studies from specific mutations have shown either exacerbation39 or suppression40 of the Brugada phenotype. Because our knowledge of channel kinetics-drug interactions for each encountered mutation is insufficiently complete, the inclusion of lidocaine as a therapy option in these critically ill infants seems reasonable.
Fever is a well-described accompaniment of ventricular arrhythmias in children having BrS.2,26 None of these 5 infants had fever during initial VT, VF, or syncope occurrences based on parental report and hospital records, although patient 4’s VT may have occurred related to undetected fever, as he had just received immunizations. The presenting ages of these patients and subsequent durations of follow-up encompassed the peak ages of initial childhood exposure to infectious agents that typically result in febrile illnesses, and minor febrile illnesses were, in fact, known to have occurred in these youngsters. That 3 of these 5 patients have had a symptom-free course 22–128 months after their initial events may be related to the normal developmental changes in autonomic innervation of the heart. In several mammalian species, progressive postnatal sympathetic cardiac innervation, in particular β-adrenergic innervation, is linked to increased density of L-type calcium and sodium channels.41 These changes would be expected to shift the balance of current during the early phases of the epicardial action potential in the inward direction, thus preventing the development of the arrhythmogenic substrate underlying BrS. Despite this rationale, other authors have recommended that affected children be hospitalized for telemetry during any febrile illness and even immunizations.38 Based on the present limited experience, we agree with that approach for children not having an ICD and for those whose family is unreliable in administration of antipyretic therapy.
ICD implantation in infants is known to be fraught with a higher than expected incidence of complications, including inappropriate shocks, lead fractures, and need for early reoperation.42 Patient 3 highlights these issues. However, in our small series of 5 children, life-saving device discharges were documented in 2, including 1 late at night. Until reliable pharmacotherapy can be established in this difficult and probably heterogeneous patient group, device therapy seems reasonable to the extent that it can be physically accomplished.
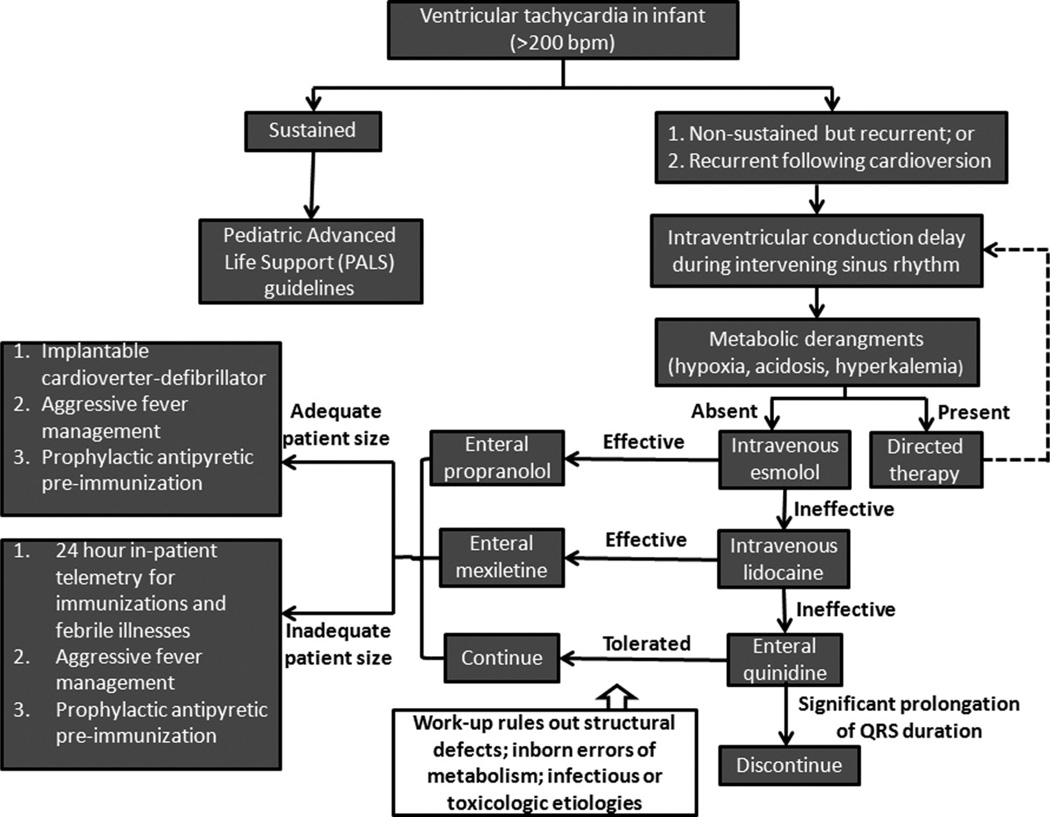
A summary schema of therapy for infants having rapid ventricular tachycardia and in whom intraventricular conduction delay is present while in sinus rhythm is shown in Figure 7.
Figure 7.
A proposed treatment schema for infants presenting with rapid ventricular tachycardia and having intraventricular conduction delay while in sinus rhythm, based on the presumption that they have Brugada-like syndrome.
Conclusions
Infants having rapid ventricular arrhythmias and intraventricular conduction delay while in sinus rhythm may have a loss-of-function depolarizing channel mutation, especially in the absence of other structural or functional heart disease. We consider their clinical phenotype to be Brugada-like, because there are differences from classical adult BrS and from many reports of childhood BrS. Although their intermediate-term follow-up is mostly favorable, additional life-threatening arrhythmias may occur.
CLINICAL PERSPECTIVE.
In clinical practice, the occurrence of rapid ventricular tachycardia in an infant is sufficiently uncommon that a comprehensive diagnostic evaluation usually ensues. This includes cardiac structural and functional assessment and metabolic, infectious disease, and toxicological analysis. In the absence of a clear etiology among these categorical entities (and perhaps even in the presence of drug-induced ventricular tachycardia), and, especially if the infant’s ECG shows ventricular conduction delay while in sinus rhythm, a cardiac depolarizing channel defect should be considered. This diagnosis is supported by characteristic prolongation of the QRS duration during intravenous infusion of procainamide or by demonstration of a disease-causing gene mutation coding for a subunit of the cardiac L-type calcium channel (CACNB2) or the cardiac sodium channel (SCN5A). Making the diagnosis of what may be considered Brugada-like syndrome is of clinical importance for 2 reasons: reliable pharmacotherapy to prevent potentially fatal ventricular tachycardia recurrences has not yet been established; and infants may be especially vulnerable to such recurrences during febrile illnesses and following immunizations, both common events during infancy. Although medical therapy may be individualized based on response to drugs attempted during the initial event, antiarrhythmic drug use should be considered adjunctive therapy. Effective management strategies should include aggressive antipyretic therapy during febrile illnesses, prophylactic antipyretic drugs with immunizations, and perhaps inpatient telemetry associated with these events. Implantable cardioverter-defibrillator implantation may be life-saving once the infant is of sufficient size, acknowledging the high complication rate of device use in infancy.
Acknowledgments
Sources of Funding
This work was supported in part by NHLBI grant HL47678 and by the Masons of New York State and Florida.
Footnotes
Disclosures
Charles Antzelevitch received an NIH grant of >10 k.
References
- 1.Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome: a multicenter report. J Am Coll Cardiol. 1992;20:1391–1396. doi: 10.1016/0735-1097(92)90253-j. [DOI] [PubMed] [Google Scholar]
- 2.Probst V, Denjoy I, Meregalli PG, Amirault JC, Sacher F, Mansourati J, Babuty D, Villain E, Victor J, Schott JJ, Lupoglazoff JM, Mabo B, Veltmann C, Jesel L, Chevalier P, Clur SA, Haïssaguerre M, Wolpert C, Le Marec H, Wilde AA. Clinical aspects and prognosis of Brugada syndrome in children. Circulation. 2007;115:2042–2048. doi: 10.1161/CIRCULATIONAHA.106.664219. [DOI] [PubMed] [Google Scholar]
- 3.Priori SG, Napolitano C, Giordano U, Collisani G, Memmi M. Brugada syndrome and sudden cardiac death in children. Lancet. 2000;355:808–809. doi: 10.1016/S0140-6736(99)05277-0. [DOI] [PubMed] [Google Scholar]
- 4.Chen Q, Kirsch GE, Zhang D, Brugada R, Brugada J, Brugada P, Potenza D, Moya A, Borggrefe M, Breithardt G, Ortiz-Lopez R, Wang Z, Antzelevitch C, O’Brien RE, Schulze-Bahr E, Keating MT, Towbin JA, Wang Q. Genetic basis and molecular mechanism for idiopathic ventricular fibrillation. Nature. 1998;392:293–296. doi: 10.1038/32675. [DOI] [PubMed] [Google Scholar]
- 5.Kapplinger JD, Tester DJ, Alders M, Benito B, Berthet M, Brugada J, Brugada P, Fressart V, Guerchicoff A, Harris-Kerr C, Kamakura S, Kyndt F, Koopmann TT, Miyamoto Y, Pfeiffer R, Pollevick GD, Probst V, Zumhagen S, Vatta M, Towbin JA, Shimizu W, Schulze-Bahr E, Anztelevitch C, Salisbury BA, Gueicheney P, Wilde AA, Brugada R, Schott JJ, Ackerman MA. An international compendium of mutations in the SCN5A-encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing. Heart Rhythm. 2010;7:33–46. doi: 10.1016/j.hrthm.2009.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antzelevitch C, Pollevick GD, Cordeiro JM, Casis O, Sanguinetti MC, Aizawa Y, Guerchicoff A, Pfeiffer R, Oliva A, Wollnik B, Gelber P, Bonaros EP, Jr, Burashnikov E, Wu Y, Sargent JD, Schickel S, Oberheiden R, Bhatia A, Hsu LF, Haïssaguerre M, Schimpf R, Borggrefe M, Wolpert C. Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT intervals, and sudden cardiac death. Circulation. 2007;115:442–449. doi: 10.1161/CIRCULATIONAHA.106.668392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burashnikov E, Pfeiffer R, Barajas-Martinez H, Helpón E, Hu D, Desai M, Borggrefe M, Haïssaguerre M, Kanter R, Pollevick GD, Laiño R, Marieb M, Nademanee K, Nam GB, Robles R, Schimpf R, Stapleton DD, Viskin S, Winters S, Wolpert C, Zimmern S, Veltmann C, Antzelevitch C. Mutations in the cardiac L-type calcium channel associated with inherited J-wave syndromes and sudden cardiac death. Heart Rhythm. 2010;7:1872–1882. doi: 10.1016/j.hrthm.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.London B, Michalec M, Mehdi H, Zhu X, Kerchner L, Sanyal S, Viswanathan PC, Pfahnl AE, Shang LL, Madhusudanan M, Baty CJ, Lagana S, Aleong R, Gutmann R, Ackerman MJ, McNamara DM, Weiss R, Dudley SC., Jr Mutation in glycerol-3-phosphate dehydrogenase 1 like gene (GPD1-L) decreases cardiac Na+ current and causes inherited arrhythmias. Circulation. 2007;116:2260–2268. doi: 10.1161/CIRCULATIONAHA.107.703330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watanabe H, Koopmann TT, Le Scouarnec S, Yang T, Ingram CR, Schott JJ, Demolombe S, Probst V, Anselme F, Escande D, Eiesfeld AC, Pfeufer A, Kääb S, Wichmann HE, Hasdemir C, Aizawa Y, Wilde AA, Roden DM, Bezzina CR. Sodium channel beta1 subunit mutations associated with Brugada syndrome and cardiac conduction disease in humans. J Clin Invest. 2008;118:2260–2268. doi: 10.1172/JCI33891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delpón E, Cordeiro JM, Núñez L, Thomsen PE, Guerchicoff A, Pollevick GD, Wu Y, Kanters JK, Larsen CT, Hofman-Bang J, Burashnikov E, Christiansen M, Antzelevitch C. Functional effects of KCNE3 mutation and its role in the development of Brugada syndrome. Circ Arrhythm Electrophysiol. 2008;1:209–218. doi: 10.1161/CIRCEP.107.748103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu D, Barajas-Martinez H, Burashnikov E, Springer M, Wu Y, Varro A, Pfeiffer R, Koopmann TT, Cordeiro JM, Guerchicoff A, Pollevick GD, Antzelevitch C. A mutation in the beta 3 subunit of the cardiac sodium channel associated with Brugada ECG phenotype. Circ Cardiovasc Genet. 2009;2:270–278. doi: 10.1161/CIRCGENETICS.108.829192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medeiros-Domingo A, Tan BH, Crotti L, Tester DJ, Eckhardt L, Cuoretti A, Kroboth SL, Song C, Zhou Q, Kopp D, Schwartz PJ, Makielski JC, Ackerman MJ. Gain-of-function mutation S422L in the KCNJ8-encoded cardiac K(ATP) channel Kir6.1 as a pathogenic substrate for J-wave syndromes. Heart Rhythm. 2010;7:1466–1471. doi: 10.1016/j.hrthm.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giudicessi JR, Ye D, Tester DJ, Crotti L, Mugione A, Nesterenko VV, Alberson RM, Antzelevitch C, Schwartz PJ, Ackerman MJ. Transient outward current (I(to)) gain-of-function mutations in the KCND3-encoded Kv4.3 potassium channel and Brugada syndrome. Heart Rhythm. 2011;8:1024–1032. doi: 10.1016/j.hrthm.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kattygnarath D, Maugenre S, Neyroud N, Balse E, Ichai C, Denjoy I, Dilanian G, Martins RP, Fressart V, Berthet M, Schott JJ, Leenhardt A, Probst V, Le Marec H, Hainque B, Coulombe A, Hatem SN, Guicheney P. MOG1: a new susceptibility gene for Brugada syndrome. Circ Cardiovasc Genet. 2011;4:261–268. doi: 10.1161/CIRCGENETICS.110.959130. [DOI] [PubMed] [Google Scholar]
- 15.Roggen A, Pavlovic M, Pfammatter JP. Frequency of spontaneous ventricular tachycardia in a pediatric population. Am J Cardiol. 2008;101:852–854. doi: 10.1016/j.amjcard.2007.10.047. [DOI] [PubMed] [Google Scholar]
- 16.Wilde AA, Antzelevitch C, Borggrefe M, Brugada J, Brugada R, Brugada P, Corrado D, Hauer RN, Kass RS, Nademanee K, Priori SG, Towbin JA. Proposed diagnostic criteria for the Brugada syndrome. Eur Heart J. 2002;23:1648–1654. doi: 10.1053/euhj.2002.3382. [DOI] [PubMed] [Google Scholar]
- 17.Benito B, Brugada R, Brugada J, Brugada P. Brugada syndrome. Prog Cardiovasc Dis. 2008;51:1–22. doi: 10.1016/j.pcad.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Makiyama T, Akao M, Tsuji K, Doi T, Ohno S, Takenaka K, Kobori A, Ninomya T, Yoshida H, Takano M, Nakita N, Yanagisawa F, Higashi Y, Takeyama Y, Kita T, Horie M. High risk for bradycardiac complications in patients with Brugada syndrome caused by SCN5A gene mutations. J Am Coll Cardiol. 2005;46:2100–2106. doi: 10.1016/j.jacc.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 19.Barajas-Martinez H, Hu D, Burashnikov E, Pfeiffer R, Kanter R, Antzelevitch C. A novel mutation (D538E) in CACNB2b associated with infant Brugada syndrome. Circulation. 2010;122:A15855. [Google Scholar]
- 20.Iturralde-Torres P, Nava-Townsend S, Gómez-Flores J, Medeiros-Domingo A, Colín-Lizalde L, Hermosillo AG, Vicoria D, Márquez MF. Association of congenital, diffuse electrical disease in children with normal heart: sick sinus syndrome, intraventricular conduction block, and monomorphic ventricular tachycardia. J Cardiovasc Electrophysiol. 2008;19:550–555. doi: 10.1111/j.1540-8167.2007.01006.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee YS, Baek JS, Kim SY, Seo SW, Kwon BS, Kim GB, Bae EJ, Park SS, Noh CI. Childhood Brugada syndrome in two Korean families. Korean Circ J. 2010;40:143–147. doi: 10.4070/kcj.2010.40.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moltedo JM, Abello M. Ventricular flutter in a child with Brugada syndrome. Resuscitation. 2010;81:643–644. doi: 10.1016/j.resuscitation.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 23.Plunkett A, Hulse JA, Mishra B, Gill J. Variable presentation of Brugada syndrome: lessons from three generations with syncope. BMJ. 2003;326:1078–1079. doi: 10.1136/bmj.326.7398.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sastry BK, Narasimhan C, Soma Raju B. Brugada syndrome with monomorphic ventricular tachycardia in a one-year-old child. Indian Heart J. 2001;53:203–205. [PubMed] [Google Scholar]
- 25.Skinner JR, Chung SK, Montgomery D, McCulley CH, Crawford J, French J, Rees MI. Near-miss SIDS due to Brugada syndrome. Arch Dis Child. 2005;90:528–529. doi: 10.1136/adc.2004.058115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skinner JR, Chung SK, Nel CA, Shelling AN, Crawford JR, McKenzie N, Pinnock R, French JK, Rees MI. Brugada syndrome masquerading as febrile seizures. Pediatrics. 2007;119:e1206–e1211. doi: 10.1542/peds.2006-2628. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki H, Torigoe K, Numata O, Yazaki S. Infant case with a malignant form of Brugada syndrome. J Cardiovasc Electrophysiol. 2000;11:1277–1280. doi: 10.1046/j.1540-8167.2000.01277.x. [DOI] [PubMed] [Google Scholar]
- 28.Todd SJ, Campbell MJ, Roden DM, Kannankeril PJ. Novel Brugada SCN5A mutation causing sudden death in children. Heart Rhythm. 2005;2:540–543. doi: 10.1016/j.hrthm.2005.01.029. [DOI] [PubMed] [Google Scholar]
- 29.Vatta M, Dumaine R, Varghese G, Richard TA, Shimizu W, Aihara N, Nademanee K, Brugada R, Brugada J, Veerakul G, Li H, Bowles NE, Brugada P, Antzelevitch C, Towbin JA. Genetic and biophysical basis of sudden unexplained nocturnal death syndrome (SUNDS), a disease allelic to Brugada syndrome. Hum Mol Genet. 2002;11:337–345. doi: 10.1093/hmg/11.3.337. [DOI] [PubMed] [Google Scholar]
- 30.Ackerman MJ, Siu BL, Sturner WQ, Tester DJ, Valdivia CR, Makielski JC, Towbin JA. Postmortem molecular analysis of SCN5A defects in sudden infant death syndrome. JAMA. 2001;286:2264–2269. doi: 10.1001/jama.286.18.2264. [DOI] [PubMed] [Google Scholar]
- 31.Sanatani S, Mahkseed N, Vallance H, Brugada R. The Brugada ECG pattern in a neonate. J Cardiovasc Electrophysiol. 2005;16:342–344. doi: 10.1046/j.1540-8167.2005.40607.x. [DOI] [PubMed] [Google Scholar]
- 32.Robinson JD, Melman Y, Walsh EP. Cardiac conduction disturbances and ventricular tachycardia after prolonged propofol infusion in an infant. Pacing Clin Electrophysiol. 2008;31:1070–1073. doi: 10.1111/j.1540-8159.2008.01138.x. [DOI] [PubMed] [Google Scholar]
- 33.Lizotte E, Junttila MJ, Dube MP, Hong K, Benito B, DE Zutter M, Henkens S, Sarkozy A, Huikuri HV, Towbin J, Vatta M, Brugada P, Brugada J, Brugada R. Genetic modulation of Brugada syndrome by a common polymorphism. J Cardiovasc Electrophysiol. 2009;20:1137–1341. doi: 10.1111/j.1540-8167.2009.01508.x. [DOI] [PubMed] [Google Scholar]
- 34.Bassani RA. Transient outward potassium current and Ca2+ homeostasis in the heart: beyond the action potential. Braz J Med Biol Res. 2006;39:393–403. doi: 10.1590/s0100-879x2006000300010. [DOI] [PubMed] [Google Scholar]
- 35.Plotnikov AN, Sosunov EA, Patberg KW, Anykhovsky EP, Gainullin RZ, Shlapakova IN, Krishnamurthy G, Danilo P, Jr, Rosen MR. Cardiac memory evolves with age in association with development of the transient outward current. Circulation. 2004;110:489–495. doi: 10.1161/01.CIR.0000137823.64947.52. [DOI] [PubMed] [Google Scholar]
- 36.Postema PG, van Dessel PF, de Bakker JM, Dekker LR, Linnenbank AC, Hoogendijk MG, Coronel R, Tijssen JG, Wilde AA, Tan HL. Slow and discontinuous conduction conspire in Brugada syndrome: a right ventricular mapping and stimulation study. Circ Arrhythm Electrophysiol. 2008;1:379–386. doi: 10.1161/CIRCEP.108.790543. [DOI] [PubMed] [Google Scholar]
- 37.Nademanee K, Veerakul G, Chandanamattha P, Chaothawee L, Ariyachaipanich A, Jirasirirojanakorn K, Likittanasombat K, Bhuripanyo K, Ngarmulko T. Prevention of ventricular fibrillation episodes in Brugada syndrome by catheter ablation over the anterior right ventricular outflow tract epicardium. Circulation. 2011;123:1270–1279. doi: 10.1161/CIRCULATIONAHA.110.972612. [DOI] [PubMed] [Google Scholar]
- 38.Chockalingam P, Rammeloo LA, Postema PG, Hruda J, Clur SA, Blom NA, Wilde AA. Fever-induced life-threatening arrhythmias in children harboring an SCN5A mutation. Pediatrics. 2011;127:e239–e244. doi: 10.1542/peds.2010-1688. [DOI] [PubMed] [Google Scholar]
- 39.Barajas-Martinez HM, Hu D, Cordeiro JM, Wu Y, Kovacs RJ, Meltser H, Kui H, Elena B, Brugada R, Anztelevitch C, Dumaine R. Lidocaine-induced Brugada syndrome phenotype linked to a novel double mutation in the cardiac sodium channel. Circ Res. 2008;103:396–404. doi: 10.1161/CIRCRESAHA.108.172619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Itoh H, Tsuji K, Sakaguchi T, Nagaoka I, Oka Y, Nakazawa Y, Yao T, Jo H, Ashihara T, Ito M, Horie M, Imoto K. A paradoxical effect of lidocaine for the N406S mutation of SCN5A associated with Brugada syndrome. Int J Cardiol. 2007;121:239–248. doi: 10.1016/j.ijcard.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 41.Qu J, Robinson RB. Cardiac ion channel expression and regulation: the role of innervation. J Mol Cell Cardiol. 2004;37:439–448. doi: 10.1016/j.yjmcc.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 42.Stephenson EA, Batra AS, Knilans TK, Gow RM, Gradaus R, Balaji S, Dubin AM, Rhee EK, Ro PS, Thørgersen AM, Cecchin F, Triedman JK, Walsh EP, Berul CI. A multicenter experience with novel implantable cardioverter defibrillator configurations in the pediatric and congenital heart disease population. J Cardiovasc Electrophysiol. 2006;17:41–46. doi: 10.1111/j.1540-8167.2005.00271.x. [DOI] [PubMed] [Google Scholar]