Abstract
Background
Children reared in deprived environments, such as institutions for the care of orphaned or abandoned children, are at increased risk for attention and behavior regulation difficulties. This study examined the neurobehavioral correlates of executive attention in post-institutionalized (PI) children.
Methods
The performance and event-related potentials (ERPs) of 10- and 11-year-old internationally adopted PI children on two executive attention tasks, Go/No-go and Flanker, were compared to two groups: children internationally adopted early from foster care (PF) and non-adopted children (NA).
Results
Behavioral measures suggested problems with sustained attention, with PIs performing more poorly on Go trials and not on No-go trials of the Go/No-go and made more errors on both congruent and incongruent trials on the Flanker. ERPs suggested differences in inhibitory control and error monitoring, as PIs had smaller N2 amplitude on Go/No-go and smaller error-related negativity on Flanker.
Conclusions
This pattern of results raises questions regarding the nature of attention difficulties for PI children. The behavioral errors are not specific to executive attention and instead likely reflect difficulties in overall sustained attention. The ERP results are consistent with neural activity related to deficits in inhibitory control (N2) and error monitoring (error-related negativity). Questions emerge regarding the similarity of attention regulatory difficulties in PIs to those experienced by non-PI children with ADHD.
Keywords: Attention, event-related potentials, executive function, international adoption, institutional care
Regulatory abilities may be particularly sensitive to deprivation in early caregiving environments. Sensitive and responsive caregivers scaffold infant attention regulation and arousal (e.g., Carlson, Jacobvitz, & Sroufe, 1995). Infants lacking supportive and consistent caregivers, such as children reared in institutions (e.g., orphanages), are at increased risk of attention and behavior regulatory problems (e.g., Goldfarb, 1943; Kreppner, O’Connor, & Rutter, 2001; Tizard & Hodges, 1978), risk that increases with the length of time in institutional care (e.g., Gunnar & van Dulmen, 2007; Kumsta et al., 2010; Wiik et al., 2011).
To date, there is only emerging evidence regarding the specific nature of attention and regulatory problems in institutionalized and post-institutionalized children. For example, in two studies, as a group post institutionalized (PI) children performed more poorly than children adopted early from foster care and non-adopted children on tasks assessing visual attention and inhibition of immediate impulses (Bruce, Tarullo & Gunnar, 2009; Pollak et al., 2010). Additionally, toddlers adopted from Russian institutionswere more likely to score lower on parent-report measures of executive functioning than were children adopted earlier (Merz & McCall, 2010).
These results suggest that attentional difficulties noted across reports of post-institutionalized children (e.g., Ames, 1997; Maclean, 2003) may be related to difficulties with inhibitory control and selective attention. However, very little is known about the neurobiology specifically associated with these attention difficulties. Five published imaging studies implicate structural differences between PI and never-institutionalized children (Bauer, Hanson, Pierson, Davidson, & Pollak, 2009; Chugani et al., 2001; Eluvathingal et al., 2006; Mehta et al., 2009; Tottenham et al., 2010). However, because these studies did not include an adoption comparison group, such as children adopted from settings other than institutional care, the observed differences could have been due to factors beyond institutional care associated with being given up for adoption. A recent event-related potential study of attention did have an appropriate comparison group, examining institutionalized children placed in foster care versus those who remained in institutional “care as usual”. Although only the “care as usual” group showed behavioral deficits on the Go/No-go task, both groups showed smaller P300 amplitudes than the never-institutionalized children reared in their birth families (McDermott, Westerlund, Zeanah, Nelson, & Fox, 2012).
The goal of this study was to help fill in our understanding of the neurobiological correlates of attention problems that follow early institutional deprivation by focusing on executive attention, a multi-faceted construct involving inhibitory control, response monitoring, and conflict resolution (Rueda, Posner, & Rothbart, 2005). Specifically, we examined behavioral performance and event-related potentials (ERPs) during Go/No-go and Flanker tasks. ERPs represent summated electrical activity of the brain conducting to the scalp surface, where it is recorded by small sensors. Unlike fMRI, ERPs have excellent temporal resolution (Nelson & McCleery, 2008). Go/No-go assesses inhibitory control and is associated with activation of the ventrolateral prefrontal cortex and anterior cingulate cortex (e.g., Durston et al., 2002; Schulz et al., 2004). Flanker, a measure of selective attention and conflict monitoring, is associated with activation of the dorsolateral prefrontal cortex and anterior cingulate cortex (e.g., Botvinick, Nystrom, Fissell, Carter, & Cohen, 1999; Casey et al., 2000).
Our ERP analyses focused on five components: P2, N2, P300, error-related negativity (ERN), and Pe. P2 reflects a sensory-driven response to visual stimuli and the matching of visual perception to cognitive expectation (Fabiani, Gratton, & Coles, 2000). N2 is associated with cognitive control, including inhibitory control, stimulus discrimination and categorization (Nieuwenhuis, Yeung, Wildenberg, & Ridderinkhof, 2003). P300 reflects responses to a rare target among frequent non-targets and cognitive processes including inhibitory control and stimulus evaluation (Tekok-Kilic, Shucard, & Shucard, 2001). ERN reflects initial response monitoring immediately following an incorrect response (van Veen & Carter, 2002). Lastly, Pe represents further error processing and awareness (Overbeek, Nieuwenhuis, & Ridderinkhof, 2005).
We hypothesized that internationally adopted PI children would be more likely to demonstrate poorer inhibitory control and conflict monitoring than peers without extensive histories of institutionalization. Two comparison groups were included: non-adopted children raised in similarly-resourced families as those who adopt internationally (Loman, Wiik, Frenn, Pollak, & Gunnar, 2009) and children internationally adopted at earlier ages primarily from foster care to account for factors associated with international adoption (e.g., poverty, poor prenatal care; Johnson, 2000). Although this is the first study to explore ERPs associated with two types of attentional control tasks within the same sample of post-institutionalized children, previous ERP research (e.g., face processing, Moulson, Westerlund, Fox, Zeanah, & Nelson, 2009; inhibitory control, McDermott et al., 2012) suggests that post-institutionalized children may have smaller amplitudes and slower latencies for ERP waveforms.
Methods
Participants
The participants included 82 children (M age=10.9 years, SD=0.6) divided into three groups: post-institutionalized children (PI; n=24, 13 female) internationally adopted at 12–78 months of age (M=24.9 months, SD=16.9) who spent at least 75% of their pre-adoptive lives in institutional care; post-foster care children (PF; n=31, 16 female) internationally adopted at 2–8 months of age (M=4.5 months, SD=1.6), predominantly from foster care, having spent two months or less in an institution; and non-adopted children (NA; n=27, 14 female) born and raised in their biological families in the United States (see Table 1 for participant demographics including region of origin for adopted groups).
Table 1.
Descriptive Statistics for Participant Characteristics
| PI n=24 M (SD) |
PF n=31 M (SD) |
NA n=27 M (SD) |
|
|---|---|---|---|
| Age (years) at session | 11.0 (0.6) | 10.8 (0.6) | 11.0 (0.7) |
| Age (months) at adoption | 24.9 (16.9) | 4.6 (1.5) | - |
| Time (months) in institution | 22.9 (14.6) | 1.8 (.45) | - |
| Region of origin (n) | |||
| Eastern Europe | 14 | 0 | - |
| Asia | 10 | 26 | - |
| Central & South America | 0 | 5 | - |
| WISC-III | |||
| Vocabulary | 9.5 (3.4) | 12.0 (3.0) | 13.3 (3.3) |
| Block Design | 8.5 (3.9) | 12.4 (3.3) | 12.5 (3.2) |
The children were recruited from registries of families interested in being contacted about research studies. The first contained contact information for internationally adopted children, the other contained information for children born in a metropolitan area. All participants were living in generally highly educated, highly resourced homes, consistent with families who adopt internationally (Hellerstedt et al., 2008; Loman et al., 2009). Across groups, the majority of parents completed 4-year college degrees or higher (68%), were living with a spouse/partner (95%) and reported family incomes greater than $75,000 (63%).
Participants were screened via parent report and excluded at the time of recruitment for neurological and congenital anomalies and medically diagnosed fetal alcohol spectrum disorders. Children taking stimulant medications were asked to participate on days when they had not taken their medication. In the laboratory, participants completed vision, fetal alcohol spectrum disorder (FASD), and estimated IQ screening following methods described by Loman and colleagues (2009). Eleven children were excluded based on the vision (n=7), FASD (n=1), and IQ (n=3) assessments. As expected (see Loman et al., 2009), PIs had lower estimated IQs than the other two groups; however, all participants in the final sample had estimated IQs above 75 and 95% had scores within the broad average range (>85) (Table 1). Participants and their parents completed informed assent and consent for participation at the laboratory session.
Measures
Go/No-go task
This computerized task required participants to press a button as quickly and accurately as possible for each letter presented (Go trials) except the letter X (No-go trials). Stimuli were presented for 600 ms with 1600 ms allowed for a response. Intertrial intervals were varied (200–400 ms). Following a short practice (8 Go trials, 6 No-go trials), two blocks of 164 trials were completed. Within each block, 20 Go trials were presented, followed by 144 trials consisting of 75% Go intermixed with 25% No-go trials.
Flanker task
This computerized task presented a row of five arrows and participants indicated on a button box the direction of the middle arrow (right or left), as quickly and accurately as possible. The middle arrow was flanked by arrows pointing in either the same direction (congruent, ≪≪<) or the opposite direction (incongruent, ≫<≫). Participants completed a 48 practice trials, with a 300 ms five asterisk (*****) fixation followed by stimulus presentation of 400 ms. The block consisted of equal numbers of left, right, congruent, incongruent trials of varying intertrial intervals (100–500 ms). The participant’s error rate at the 400 ms stimulus presentation determined the stimulus speed for experimental trials. If the error rate was below 25% or above 35%, participants completed the 250 or 550 ms stimulus presentation experiment, respectively. The experimental task consisted of four 100-trial blocks, counterbalanced across participants. Each trial included a 300 ms fixation and 250, 400, or 550 ms stimulus presentation of left or right congruent or incongruent arrows, with 1500 ms allowed for a response. Intertrial intervals varied (100–500 ms). 70% of trials were congruent and 30% were incongruent (Casey et al., 2000).
The two tasks, Go/No-go and Flanker, were administered in a counterbalanced order. Order of task delivery did not vary by group, χ2(2, N=72)=0.08, ns. Accuracy and reaction time were computed for both tasks.
Event-related potentials (ERPs)
ERPs were recorded using a 32-channel Electro-Cap (Electro-Cap International, Inc., Eaton, Ohio) from electrode sites Fz, Pz, FC1, FC2, FC5, FC6, F3, F4, F7, F8, C3, C4, CP1, CP2, CP5, CP6, P3, P4, PO3, PO4, PO7, PO8, O1, O2, T3, T4, T5, T6, referenced to Cz during collection. Bilateral mastoid activity and vertical EOG (eye blinks and movements) from above and below the left eye were recorded. Scalp electrodes were rereferenced to average mastoid sites post-collection. EEG and EOG channels were recorded using Grass Neurodata 12A5 amplifiers with gain of 50,000 for EEG and 5,000 for EOG, bandpass of 0.1–30 Hz. A 60-Hz notch filter was engaged. All data were acquired at 200 Hz.
Data were processed using the ERP32 data analysis software package (Version 3.82; New Boundary Technologies, Minneapolis, MN) and trials with excessive artifact (i.e., EOG >250 μV) were rejected. Following rereferencing to linked mastoids, EOG-related artifact was corrected (Gratton, Coles, & Donchin, 1983). Trials were baseline corrected (based on 100 ms prior to stimulus onset) and averaged within each condition for each participant. These averages were visually inspected for excessive mastoid, vertical EOG, and movement artifact that appeared to bias the waveform. A minimum of 10 usable ERP trials were required for analyses.
Automatic detection identified peak amplitude (μV) and latency to peak (ms) for the stimulus-locked components of P2 (positive deflection between 150 and 275 ms for Go/No-go and 240–350 for Flanker) and N2 (negative deflection between 250–500 ms for Go/No-go and 360–500 for Flanker) at frontocentral scalp electrodes (Cz, FC1, FC2 and Fz, FC1, FC2, respectively). Average amplitude (μV within specified window) was used for P300 (positive deflection between 400–700 ms for Go/No-go and 650–950 for Flanker) at midline electrodes (Fz, Cz, Pz). Response-locked peak amplitude was recorded for ERN (negative deflection between 1–150 ms) and Pe (positive deflection between 100–500 ms) at frontocentral electrodes (Fz, Cz).
Data Analysis
Repeated measures analyses of variance were conducted to examine the behavioral and ERP measures. Group and sex were included as independent variables in each analysis. Unless otherwise noted, there were no significant sex effects or group by sex interactions. Greenhouse-Geisser correction was applied when the sphericity assumption was not met. Significant interactions were explored using simple effects analyses. Consistent with our hypotheses, the effect of institutionalization on each outcome was assessed using planned contrasts comparing PI to NA and PF to NA. For measures correlated with estimated IQ, results were recalculated controlling for IQ and reported with and without IQ as a covariate. Whenever a significant PI versus NA planned contrast or interaction involving group was found, the relation of duration of institutionalization and age at adoption with that outcome was assessed using Pearson’s correlations among internationally adopted children with histories of institutionalization. Only significant findings involving these preadoptive variables are reported.
Results
Behavioral Performance
Go/No-go
As expected, mean accuracy was higher for Go than for No-go trials across groups, F(1,70)=77.25, p<.001. Importantly, this trial type effect interacted with group, F(2,70)=4.44, p<.05. Contrary to hypotheses, PIs did not demonstrate increased false alarms on No-go trials, but instead made more omission errors (i.e., less accurate on Go trials) than NAs (Figure 1). PFs did not differ from NAs. The trial type by group interaction remained marginally significant with IQ covaried, F(2,67)=2.70, p=.075. Age at adoption and number of months in institutional care were negatively correlated with Go trial accuracy, r(26)=−0.48, p<.05, and r(26)=−0.42, p<.05, respectively.
Figure 1.
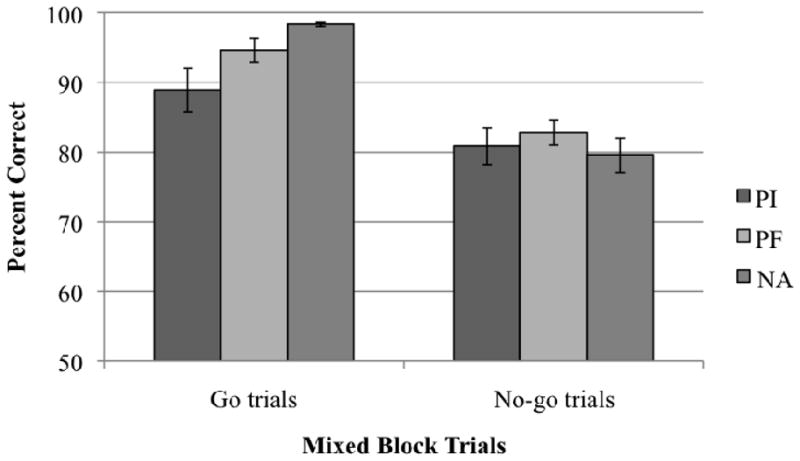
Accuracy for each group on Go and No-go trials of Go/No-go.
For Go trial reaction time, a group by sex interaction was observed, F(2,67)=3.52, p<.05. PI boys were significantly slower than NA boys, and girls did not differ across groups. Further, age at adoption and number of months in institutional care were positively correlated with Go trial reaction time, r(26)=−0.50, p<.01, and r(26)=−0.48, p<.05, respectively.
Flanker
Based on practice performance, PIs were more likely to need the slower stimulus presentation speed (500 ms) for the test trials than PFs or NAs, χ2(4, N=72)=10.85, p<.05. For Flanker accuracy, all groups were more accurate on congruent than incongruent trials, F(1,62)=108.69, p<.001. There was a main effect of sex, F(1,62)=6.16, p<.05: boys were less accurate than girls. Contrary to expectations regarding deficits only on incongruent trials, PIs had lower overall accuracy than NAs, F(1,65)=13.47, p<.001 (Figure 2). PFs and NAs did not differ, F(1,65)=.74, ns. The group effect remained marginally significant with IQ covaried, F(1,63)=3.04, p=.086. A trend was observed for age at adoption to be negatively correlated with overall accuracy, r(23)=-.402, p=.057,
Figure 2.
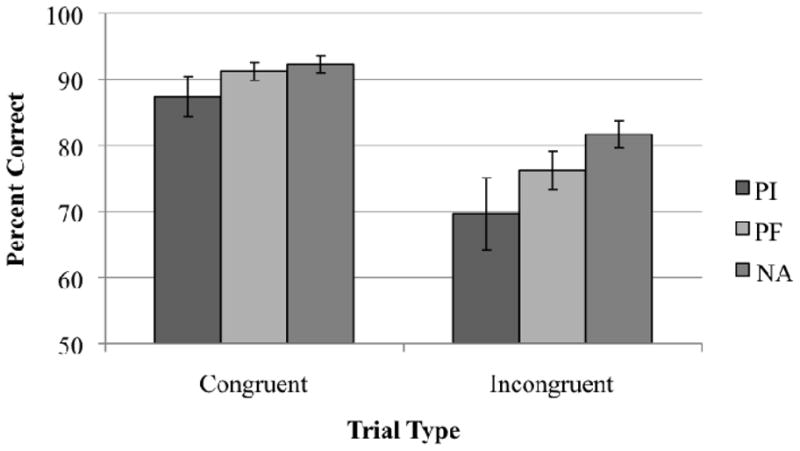
Accuracy for each group on Congruent and Incongruent trials of Flanker.
For reaction time, all groups were slower on incongruent compared to congruent trials, F(1,66)=114.03, p<.001. Groups did not differ in reaction time.
Go/No-go Event-Related Potentials
P2
For P2 amplitude, there was no main effect for lead or condition, and no group differences (see Table 2 for group means). However, a group by condition interaction was observed, F(2,50)=4.22, p<.05 in that PIs and PFs both had smaller amplitudes for Go (PI M=9.81 μV, SD=5.2; PF M=10.03 μV, SD=4.6) than for No-go (PI M=15.29 μV, SD=5.9; PF M=14.83 μV, SD=5.0) whereas the amplitudes did not differ for NAs (Go M=10.48 μV, SD=5.7; No-go M=11.97 μV, SD=5.5). For latency, P2 peaked later for No-go than for Go, F(1,53)=20.34, p<.001. PIs demonstrated later latencies than NAs F(1,53)=5.12, p<.05. PFs also had later latencies than NAs F(1,53)=11.49, p<.01.
Table 2.
Event-related Potential Components Amplitude (μV) and Latency (ms) for each Group
| PI M (SD) |
PF M (SD) |
NA M (SD) |
|
|---|---|---|---|
|
|
|||
| Go/No-go | |||
| P2 (Cz, FC1, FC2) | |||
| peak amplitude | 12.55 (5.2) | 12.43 (4.2) | 11.23 (5.2) |
| latencya,b | 219.63 (18.3) | 223.86 (16.8) | 206.77 (14.20) |
| N2 (Fz, FC1, FC2) | |||
| peak amplitudea | −4.85 (5.6) | −8.34 (6.3) | −8.88 (4.9) |
| latency | 342.60 (50.9) | 339.16 (23.4) | 331.59 (36.7) |
| P300 (Fz, Cz, Pz) | |||
| area | 4.89 (5.6) | 4.27 (4.9) | 4.22 (4.3) |
| ERN & Pe (Fz, Cz) | |||
| ERN peak amplitude | −6.67 (2.7) | −7.29 (3.9) | −7.96 (5.0) |
| Pe peak amplitude | 8.75 (8.5) | 10.30 (7.1) | 12.17 (5.4) |
| Flanker | |||
| P2 (Cz, FC1, FC2) | |||
| peak amplitude | 4.65 (4.7) | 8.59 (4.8) | 7.19 (4.1) |
| latencyb | 285.61 (13.3) | 292.41 (12.8) | 283.10 (15.6) |
| N2 (Fz, FC1, FC2) | |||
| peak amplitude | −8.62 (6.5) | −8.38 (4.5) | −8.69 (3.9) |
| latency | 402.75 (17.6) | 409.44 (21.0) | 405.89 (24.67) |
| P300 (Fz, Cz, Pz) | |||
| area | 4.87 (4.2) | 5.69 (4.6) | 5.92 (4.1) |
| ERN & Pe (Fz, Cz) | |||
| ERN peak amplitudea | −3.72 (5.4) | −6.15 (6.2) | −8.63 (8.0) |
| Pe peak amplitude | 8.25 (7.9) | 10.05 (7.5) | 9.44 (9.4) |
Bold indicates a group difference significant at p<.05.
Significant difference PI vs. NA
Significant difference PF vs NA
N2
The analysis for amplitude of N2 revealed a main effect of lead F(2,100)=9.89, p<.001 in that Fz (M=−8.76 μV, SD=6.3) was more negative than FC1 (M=−7.53 μV, SD=6.5) and FC2 (M=−6.70 μV, SD=5.5) and no main effect of condition. PIs showed a smaller N2 (less negative) than NAs, F(1,53)=4.21, p<.05 and PFs did not differ from NAs, F(1,53)=.10, ns. For N2 latency, there was no main effect for condition or group; however, there was a main effect of lead F(2,100)=4.73, p<.05: Fz M=332.51 ms, SD=38.3; FC1 M=342.52 ms, SD=37.1; FC2 M=336.91 ms, SD=38.9. There was also a condition by group by sex interaction, F(2,50)=4.80, p<.05; for females, PIs and PFs did not show the difference in latency by condition (Go later than No-go) demonstrated by NAs.
P300
As expected, there was a main effect for condition in that P300 amplitude was larger for No-go (M=6.76 μV, SD=5.4) than for Go (M=2.05 μV, SD=5.0), F(1,53)=62.92, p<.001 and for lead with amplitude largest at Pz (M=9.71 μV, SD=5.7), followed by Cz (M=4.79 μV, SD=6.0), and then Fz (M=−1.28 μV, SD=5.1), F(2,106)=124.78, p<.001. There was also a significant condition by lead interaction, F(1.60,84.95)=9.99, p<.01. At Fz, the difference between Go and No-go was smaller than it was at Cz and Pz. Groups did not differ.
ERN
Investigation into ERN amplitude revealed a main effect for trial accuracy, F(1,29)=21.74, p<.001, as amplitude was more negative for incorrect (M=−10.75 μV, SD=6.0) than correct trials (M=−3.59 μV, SD=4.5). There was no effect of lead and groups did not differ.
Pe
There was a main effect of trial accuracy for Pe amplitude, F(1,29)=16.57, p<.001, with greater amplitude to incorrect (M=13.61 μV, SD=10.0) than correct trials (M=7.30 μV, SD=5.9). There was also a main effect for lead, F(1,29)=14.88, p<.01; Pe amplitude was more positive at Cz (M=7.53 μV, SD=6.7) than Fz (M=3.30 μV, SD=5.1). Additionally, there was an interaction between trial accuracy and lead, F(1,29)=20.11, p<.001. Pe was more positive for incorrect trials at Cz (M=17.03, SD=11.9) than at Fz (M=9.85, SD=9.8) but did not differ correct trials (Cz (M=7.48, SD=6.6) Fz (M=7.12, SD=5.9). Groups did not differ.
Flanker Event-Related Potentials
P2
For P2 amplitude, there were no effects of lead or condition and no group differences. For P2 latency, there were no lead or condition effects. Planned contrasts revealed that PIs did not differ from NAs, F(1,48)=.44, ns; instead, PFs had longer latencies than NAs, F(1,48)=5.42, p<.05.
N2
There were no condition effects or group differences for N2 amplitude. N2 amplitude varied by lead, F(2,88)=9.85, p<.001: Fz (M=−7.61 μV, SD=4.8) was smaller (i.e., less negative) than FC1 (M=−8.97 μV, SD=5.1) and FC2 (M=−8.90 μV, SD=5.0). For N2 latency, latencies were shorter for congruent (M=407.7 ms, SD=25.9) versus incongruent (M=411.9 ms, SD=23.1) trials, F(1,44)=4.24, p<.05. There was no effect of lead or group differences.
P300
P300 amplitude was larger for congruent (M=5.61 μV) than incongruent (M=4.64 μV), F(1,44)=5.86, p<.05 and the amplitude was largest at Pz (M=9.36 μV) followed by Cz (M=3.94 μV), then Fz (M=1.88 μV), F(1.45,63.88)=77.66, p<.001. Groups did not differ.
ERN
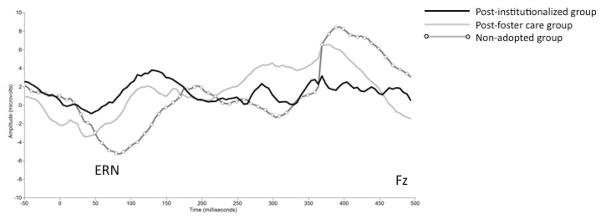
ERN was more negative at Fz (M=−7.64 μV, SD=8.3) than at Cz (M=−5.32 μV, SD=6.3). PIs had a smaller ERN amplitude (less negative) compared to NAs F(1,38)=4.75, p<.05, and PFs and NAs did not differ F(1,38)=1.11, ns (Figure 3).
Figure 3.
Grand-averaged group ERN waveforms at the front midline electrode (Fz) for Flanker. The x-axis represents latency in milliseconds and the y-axis represents amplitude in microvolts.
Pe
Analysis of Pe amplitude revealed a main effect of trial accuracy, F(1,38)=45.98, p<.001, with greater amplitude for incorrect (M=12.36 μV, SD=8.1) versus correct trials (M=3.62 μV, SD=7.8). There was an interaction between trial accuracy and lead, F(1,38)=10.55, p<.01. Pe was more positive for incorrect trials at Cz than at Fz; however, for correct trials, the leads did not differ. Planned contrasts revealed no group differences. However, there was a group by trial accuracy interaction, F(2,38)=3.38, p<.05, PIs had a smaller difference in Pe amplitude for incorrect versus correct trials than NAs.
Discussion
Overall, the Go/No-go and Flanker tasks elicited patterns of behavior and neural activation consistent with previous research. For Go/No-go, all groups were more accurate on Go than on No-go trials, indicating increased difficulty of the trials requiring inhibitory control. For Flanker, all children were more accurate and responded more quickly on congruent than incongruent trials, demonstrating the expected interference effect (Ridderinkhof, van der Molen, Band, & Bashore, 1997).
As expected, the amplitude of the target-sensitive P300 was larger for correct No-go and incongruent compared to Go and congruent trials. The amplitude of the sensory-driven P2 did not differ by trial type on either task. Unexpectedly, N2 was not larger on No-go or incongruent compared to Go or congruent trials. However, this pattern replicates previous developmental research among populations with atypical attention regulation (e.g., children with ADHD, Burden et al., 2010). Further, true cognitive and behavioral correlates of N2 are debated and some suggest that N2 should not be larger for conflict trials (Nieuwenhuis et al., 2003). Error monitoring also followed expected patterns of activation. ERN and Pe both averaged higher amplitudes (negative and positive, respectively) on incorrect compared to correct trials, although the trial type difference was not significant for Flanker-associated ERN. The finding of a more pronounced Pe than ERN during Flanker error trials has been previously reported (Bruce et al., 2009; Davies, Segalowitz, & Gavin, 2004).
The literature to date examining children with early histories of institutionalization suggests that PI children may have difficulties with executive attention (e.g. Maclean, 2003; Kreppner et al., 2001; Wiik et al., 2011). This is one of the first studies to explicitly assess behavioral performance and ERPs related to inhibitory control and response monitoring. As expected, PI children made more errors on the two tasks, required slower stimulus presentation on Flanker, and PI males had slower reaction times during Go/No-go. The specific pattern of errors, however, raises questions regarding the nature of attention difficulties for PI children. The results suggest that PI children may not have difficulties specific to response inhibition or selective attention, and instead their difficulties may relate to overall sustained attention. PI children demonstrated more omission errors on Go/No-go than NA children, but there was no group difference in commission errors. Omission errors on continuous performance tasks reflect deficits in sustained attention whereas false alarm or commission errors reflect response inhibition (e.g., Willcutt et al., 2005). McDermott and colleagues (2012) observed a similar pattern of increased omission errors among institutionalized children in Romania randomly assigned to care as usual (i.e., primarily institutional care), but not among those assigned to foster care.
Further, while the PI children demonstrated the expected pattern of more errors on incongruent Flanker trials, they also made more errors on congruent trials, resulting in poorer accuracy overall. Their deficits were not more apparent on incongruent trials. While it could be that PIs demonstrated typical age-related difficulties with selective attention due to their similar incongruent trial error rate to NAs, it is difficult to draw this conclusion given their low accuracy for congruent trials. Instead, the low accuracy rate on congruent trials suggests PI children may have had difficulty sustaining attention to the task. This pattern ties in with previous literature suggesting PI children have difficulties monitoring their actions (e.g., Merz & McCall, 2010).
When group differences were present on the electrophysiological measures, PIs had smaller amplitudes and later latencies than NAs. Group differences noted in P2, a stimulus identification component, augment our behavioral findings suggesting PIs may struggle with sustaining attention to the tasks. PIs’ smaller ERN amplitudes similarly map onto the behavioral results, indicating decreased error monitoring, and suggesting that perhaps they are not attending well enough to realize an error was made. Similarly, the trend toward PIs having a smaller difference in Pe amplitude for incorrect versus correct trials may indicate reduced awareness of behavioral errors (Overbeek et al., 2005). While the behavioral and ERP results in the current study suggest deficits in sustained attention, additional study is needed.
Interestingly, despite not demonstrating more false alarms behaviorally on the Go/No-go task, PIs did demonstrate differential brain activity (smaller amplitude) associated with response inhibition-related N2. This is not the first study involving children with early adversity to find that behavioral and ERP results did not reveal convergent findings. McDermott and colleagues (2012) identified a similar pattern among post-institutionalized children living in foster care; specifically, they did not show deficits in Go/No-go behavioral performance, but did demonstrate reduced amplitude of P300 during No-go trials. Further, among a sample of domestic foster care children, Bruce and colleagues (2009) found group differences for ERP components associated with feedback, but no group differences in behavioral performance. Similar results have also been reported in studies involving children with ADHD (e.g., Karayanidis et al., 2000). This pattern may represent processing deficits that behavioral measures are not sensitive enough to detect. This explanation would suggest that PIs might have an underlying deficit related to inhibitory control. It could also be that PIs struggled with stimulus discrimination, perhaps due to poor sustained attention, explaining the decreased N2 during Go/No-go (e.g., Nieuwenhuis et al., 2003).
Together with reports that PI children demonstrate difficulties with inattention, impulsivity, and hyperactivity (Kumsta et al., 2010; Maclean, 2003), these results suggest that PI children demonstrate attention regulation similar to children with ADHD. Like the current behavioral results, children with ADHD demonstrate increased omission errors on continuous performance tasks (e.g., Sartory, Heine, Müller, & Elvermann-Hallner, 2002). Children with ADHD also generally make more commission errors. Similar to our results, there is evidence supporting decreased N2 in ADHD samples (Kenemans et al., 2005); however, there is also evidence for decreased P300 among children with ADHD (e.g., Spronk, Jonkman, & Kemner, 2008). Further, on Flanker, children with ADHD had increased error rates and decreased ERN amplitude (van Meel, Heslenfeld, Oosterlaan, & Sergeant, 2007). Overall, PIs may demonstrate deficits similar to non-institutionalized children with ADHD. Future studies will benefit from directly comparing executive attention task performance and ERPs from PIs to those from non-institutionalized, non-adopted children with ADHD.
The reported findings are most notably limited by the small sample size. However, electrophysiological studies often have smaller sample sizes, which was coupled here with recruitment within unique populations of PI and PF children. Nevertheless, even with this sample size, previously reported task-related ERP patterns were replicated. Second, a potential limitation in the type of design/sampling parameters in this study is the role that general cognitive functioning (i.e., IQ) may or may not play a role in our outcomes. IQ might affect attention regulation; at the same time, IQ testing is highly susceptible to attention. Within the current sample, covarying IQ only changed our results in that they became trend-level effects. Third, an inherent limitation of the current design is the inability to link outcomes to specific aspects of institutional deprivation because direct observation is not possible across the multiple institutions. However, certain characteristics are common within institutional environments, dictated by few caregivers caring for many infants (i.e., assembly-line care, little one-to-one interaction, multiple caregivers; Zeanah, Smyke, & Settles, 2006). A strength of our wider sampling parameters is that these findings generalize across a broader range of internationally adopted children and are not limited to one institution or one country of origin. Indeed, not all children adopted from institutions are equally affected by their experiences and the relation between early institutional care and difficulties with inattention may be moderated by genes (e.g., DAT1 Stevens et al., 2009). Finally, a primary strength of this study was the PF group. PFs did not differ from the NAs on most of the outcomes with group differences, suggesting that the PI effects were related to institutionalization. This argument was strengthened in several cases by evidence that duration of institutional care was a significant correlate.
Conclusion
Overall, the present findings suggest that attention regulatory problems consistently reported for PI children may reflect overall difficulties sustaining attention, rather than inhibitory control or selective attention. Corresponding with clinical reports of behavior problems (e.g., Gunnar & van Dulmen, 2007; Maclean, 2003), these sustained attention difficulties may be specifically related to early institutionalization rather than other care situations (e.g., international foster care). Lacking consistent and responsive caregiving likely contributes to these attention patterns, which may in fact be adaptive within the context of institutional care. This is further supported by evidence that longer time in institutional care is related to hallmark findings of poor sustained attention (i.e., increased omission errors). Future studies will benefit from functional magnetic resonance imaging investigations of attention regulatory difficulties within PI children to better understand the specific neurobehavioral correlates. Further, the current findings support previous research (e.g., Carlson et al., 1995) and suggest that high-quality caregiver-child interactions support the development of attention regulation. Moreover, they highlight the need for future research to examine which specific aspects of early experiences support the development of attention systems. Lastly, comparisons with other populations demonstrating similar attention difficulties (e.g., children with ADHD) will aid in understanding the nature of these deficits and ultimately contribute to the successful implementation of interventions for PI children.
Key Points.
Previous research indicates that post-institutionalized (PI) children are at increased risk for attention and behavior regulation difficulties.
This study is among the first to examine both behavioral and neurobiological correlates of attention processes in PI children.
Behavioral performance on Go/No-go and Flanker tasks indicated that internationally adopted PI children had more problems with sustained attention.
Event-related potentials suggested that PI children might have deficits in processes underlying inhibitory control and error monitoring.
The findings raise questions regarding the similarity of attention regulatory difficulties in PI children to those of children with ADHD.
Acknowledgments
This research was supported by grants from the National Institute of Mental Health (NIMH) to S Pollak (RO1 MH068858), CA Nelson (1RO1 MH091363), and Megan Gunnar (R01 MH068857). Additional support was provided by predoctoral fellowships to M Loman and A Johnson from the Center for Cognitive Sciences at the University of Minnesota through NIH T32HD007151. The authors thank the children and families who participated, Kristin Frenn, Sandi Wewerka, and the University of Minnesota Center for Neurobehavioral Development.
Abbreviations
- PI
post-institutionalized
- PF
post foster care
- NA
non-adopted
- ERP
event-related potentials
- ERN
error-related negativity
Footnotes
Conflict of interest statement: No conflicts declared
This paper was presented in part at the Society for Research in Child Development Biennial meeting, Montreal, March 31, 2011.
References
- Ames E. Final Report to the National Welfare Grants Program: Human Resources Development Canada. Burnaby, British Columbia: Simon Fraser University; 1997. The Development of Romanian Oprhanage Children Adopted to Canada. [Google Scholar]
- Bauer PM, Hanson JL, Pierson RK, Davidson RJ, Pollak SD. Cerebellar volume and cognitive functioning in children who experienced early deprivation. Biological Psychiatry. 2009;66:1100–1106. doi: 10.1016/j.biopsych.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Bruce J, Tarullo AR, Gunnar MR. Disinhibited social behavior among internationally adopted children. Development and Psychopathology. 2009;21(01):157–171. doi: 10.1017/S0954579409000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden MJ, Jacobson JL, Westerlund A, Lundahl LH, Morrison A, Dodge NC, Jacobson SW. An event-related potential study of response inhibition in ADHD with and without prenatal alcohol exposure. Alcoholism: Clincial and Experimental Research. 2010;34:617–627. doi: 10.1111/j.1530-0277.2009.01130.x. [DOI] [PubMed] [Google Scholar]
- Carlson E, Jacobvitz D, Sroufe LA. A developmental investigation of inattentiveness and hyperactivity. Child Development. 1995;66:37–54. doi: 10.1111/j.1467-8624.1995.tb00854.x. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Thomas KM, Welsh TF, Badgaiyan RD, Eccard CH, Jennings JR, Crone EA. Dissociation of response conflict, attentional, selection, and expectancy with functional magnetic resonance imaging. Proceedings of the National Academy of Sciences. 2000;97(15):8728–8733. doi: 10.1073/pnas.97.15.8728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani HT, Behen ME, Muzik O, Juhasz C, Nagy F, Chugani DC. Local Brain Functional Activity Following Early Deprivation: A Study of Postinstitutionalized Romanian Orphans. NeuroImage. 2001;14:1290–1301. doi: 10.1006/nimg.2001.0917. [DOI] [PubMed] [Google Scholar]
- Davies PL, Segalowitz SJ, Gavin WJ. Development of response-monitoring ERPS in 7- to 25-year-olds. Developmental Neuropsychology. 2004;25:355–376. doi: 10.1207/s15326942dn2503_6. [DOI] [PubMed] [Google Scholar]
- Durston S, Thomas KM, Yang Y, Ulug AM, Zimmerman RD, Casey BJ. A neural basis for the development of inhibitory control. Developmental Science. 2002;5(4):F9–F16. [Google Scholar]
- Eluvathingal TJ, Chugani HT, Behen ME, Juhász C, Muzik O, Maqbool M, Makki M. Abnormal brain connectivity in children after early severe socioemotional deprivation: A diffusion tensor imaging study. Pediatrics. 2006;117:2093–2100. doi: 10.1542/peds.2005-1727. [DOI] [PubMed] [Google Scholar]
- Fabiani M, Gratton G, Coles MG. Event-related brain potentials: Methods, theory, and applications. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. 2. Cambridge, UK: Cambridge University Press; 2000. pp. 53–84. [Google Scholar]
- Goldfarb W. The effects of early institutional care on adolescent personality. Journal of Experimental Education. 1943;12:106–129. [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, van Dulmen M. Behavior problems in post-institutionalized internationally-adopted children. Development & Psychopathology. 2007;19:129–148. doi: 10.1017/S0954579407070071. [DOI] [PubMed] [Google Scholar]
- Hellerstedt WL, Madsen NJ, Gunnar MR, Grotevant HD, Lee RM, Johnson DE. The International Adoption Project: Population-based Surveillance of Minnesota Parents Who Adopted Children Internationally. Maternal Child Health Journal. 2008;12:162–171. doi: 10.1007/s10995-007-0237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DE. The impact of orphanage rearing on growth and development. In: Nelson CA, editor. The Effects of Adversity on Neurobehavioral Development: Minnesota Symposia on Child Psychology. Vol. 31. Mahwah, NJ: Lawrence Erlbaum; 2000. pp. 113–162. [Google Scholar]
- Karayanidis F, Robaey P, Bourassa M, De Koning D, Geoffroy G, Pelletier G. ERP differences in visual attention processing between attention-deficit hyperactivity disorder and control boys in the absence of performance differences. Psychophysiology. 2000;37:319–333. [PubMed] [Google Scholar]
- Kenemans JL, Bekker EM, Lijffijt M, Overtoom CC, Jonkman LM, Verbaten MN. Attention deficit and impulsivity: Selecting, shifting, and stopping. International Journal of Psychophysiology. 2005;58(1):59–70. doi: 10.1016/j.ijpsycho.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Kreppner JA, O’Connor TG, Rutter M. Can inattention/overactivity be an institutional deprivation syndrome? Journal of Abnormal Child Psychology. 2001;29(6):513–528. doi: 10.1023/a:1012229209190. [DOI] [PubMed] [Google Scholar]
- Kumsta R, Kreppner J, Rutter M, Beckett C, Castle J, Stevens S, et al. III. Deprivation-specific psychological patterns. Monographs of the Society for Research in Child. 2010;75:48–78. doi: 10.1111/j.1540-5834.2010.00550.x. [DOI] [PubMed] [Google Scholar]
- Loman MM, Wiik KL, Frenn KA, Pollak SD, Gunnar MR. Postinstitutionalized children’s development: growth, cognitive, and language outcomes. J Dev Behav Pediatr. 2009;30(5):426–434. doi: 10.1097/DBP.0b013e3181b1fd08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclean K. The impact of institutionalization on child development. Development and Psychopathology. 2003;15:853–884. doi: 10.1017/s0954579403000415. [DOI] [PubMed] [Google Scholar]
- McDermott JM, Westerlund A, Zeanah CH, Nelson CA, Fox NA. Early adversity and neural correlates of executive function: Implications for academic adjustment. Developmental Cognitive Neuroscience. 2012;2S:S59–S66. doi: 10.1016/j.dcn.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Golembo NI, Nosarti C, Colvert E, Mota A, Williams SC, Sonuga-Barke EJ. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: The English and Romanian Adoptees Study Pilot. Journal of Child Psychology and Psychiatry. 2009;50:943–951. doi: 10.1111/j.1469-7610.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- Merz EC, McCall RB. Behavior problems in children adopted from psychosocially depriving institutions. Journal of Abnormal Child Psychology. 2010;38:459–470. doi: 10.1007/s10802-009-9383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulson MC, Westerlund A, Fox NA, Zeanah CH, Nelson CA. The effects of early experience on face recognition: An event-related potential study of institutionalized children in Romania. Child Development. 2009;80:1039–1056. doi: 10.1111/j.1467-8624.2009.01315.x. [DOI] [PubMed] [Google Scholar]
- Nelson CA, McCleery JP. Use of Event-Related Potentials in the Study of Typical and Atypical Development. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47:1252–1261. doi: 10.1097/CHI.0b013e318185a6d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, van den Wildenberg W, Ridderinkhof KR. Electrophysiological correlates of anterior cingulate function in a go/no-go task: effects of response conflict and trial type frequency. Cognitive, Affective, and Behavioral Neuroscience. 2003;3(1):17–26. doi: 10.3758/cabn.3.1.17. [DOI] [PubMed] [Google Scholar]
- Overbeek TJ, Nieuwenhuis S, Ridderinkhof KR. Dissociable components of error processing: On the functional significance of the Pe vis-à-vis the ERN/Ne. Journal of Psychophysiology. 2005;19:319–329. [Google Scholar]
- Pollak SD, Nelson CA, Schlaak MF, Roeber BJ, Wewerka SS, Wiik KL, Gunnar MR. Neurodevelopmental Effects of Early Deprivation in Post-Institutionalized Children. Child Development. 2010;81(1):224–236. doi: 10.1111/j.1467-8624.2009.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, van der Molen MW, Band GP, Bashore TR. Sources of interference from irrelevant information: a developmental study. Journal of Experimental Child Psychology. 1997;65(3):315–341. doi: 10.1006/jecp.1997.2367. [DOI] [PubMed] [Google Scholar]
- Rueda MR, Posner MI, Rothbart MK. The development of executive attention: contributions to the emergence of self-regulation. Developmental Neuropsychology. 2005;28(2):573–594. doi: 10.1207/s15326942dn2802_2. [DOI] [PubMed] [Google Scholar]
- Sartory G, Heine A, Müller BW, Elvermann-Hallner A. Event- and Motor-Related Potentials During the Continuous Performance Task in Attention-Deficit/Hyperactivity Disorder. Journal of Psychophysiology. 2002;16(2):97–106. [Google Scholar]
- Schulz KP, Fan J, Tang CY, Newcorn JH, Buchsbaum MS, Cheung AM, Halperin JM. Response inhibition in adolescents diagnosed with attention deficit hyperactivity disorder during childhood: An event-related fMRI study. American Journal of Psychiatry. 2004;161:1650–1657. doi: 10.1176/appi.ajp.161.9.1650. [DOI] [PubMed] [Google Scholar]
- Spronk M, Jonkman LM, Kemner C. Response inhibition and attention processing in 5- to 7-year-old children with and without symptoms of ADHD: An ERP study. Clinical Neurophysiology. 2008;119:2738–2752. doi: 10.1016/j.clinph.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Stevens S, Kumsta R, Kreppner J, Brookes K, Rutter M, Sonuga-Barke EJ. Dopamine transporter gene polymorphism moderates the effects of severe deprivation on ADHD symptoms: Developmental continuities in gene-environment interplay. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2009;150B:753–761. doi: 10.1002/ajmg.b.31010. [DOI] [PubMed] [Google Scholar]
- Tekok-Kilic A, Shucard JL, Shucard DW. Stimulus modality and Go/NoGo effects on P3 during parallel visual and auditory continuous performance tasks. Psychophysiology. 2001;38:578–589. doi: 10.1017/s0048577201991279. [DOI] [PubMed] [Google Scholar]
- Tizard B, Hodges J. The effect of early institutional rearing on the development of eight-year-old children. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1978;19:99–118. doi: 10.1111/j.1469-7610.1978.tb00453.x. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, Casey BJ. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Developmental Science. 2010;13:46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meel CS, Heslenfeld DJ, Oosterlaan J, Sergeant JA. Adaptive control deficits in attention-deficit/hyperactivity disorder (ADHD): The role of error processing. Psychiatry Research. 2007;151(3):211–220. doi: 10.1016/j.psychres.2006.05.011. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiology and Behavior. 2002;77(4–5):477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- Wiik KL, Loman MM, Van Ryzin MJ, Armstrong JM, Essex MJ, Pollak SD, Gunnar MR. Behavioral and emotional symptoms of post-institutionalized children in middle childhood. Journal of Child Psychology and Psychiatry. 2011;52(1):56–63. doi: 10.1111/j.1469-7610.2010.02294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the Executive Function Theory of Attention-Deficit/Hyperactivity Disorder: A Meta-Analytic Review. Biological Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Zeanah CH, Smyke AT, Settles L. Children in orphanages. In: McCartney K, Phillips DA, editors. Handbook of Early Childhood Development. Malden, MA: Blackwell Publishing; 2006. pp. 224–254. [Google Scholar]