Abstract
The activity of the mammalian target of rapamycin (mTOR), an ubiquitously expressed serine/threonine kinase, is central to the regulation of translation initiation and, consequently protein synthesis required for long-term potentiation and new synaptic connections. Recent studies show that activation of the mTOR signaling pathway is required for the rapid antidepressant actions of glutamate N-methyl-D-aspartate (NMDA) receptor antagonists such as ketamine. Our prior work documented the first evidence of robust deficits in the mTOR signaling pathway in the prefrontal cortex (PFC) from subjects diagnosed with major depressive disorder (MDD). The goal of this study was to determine whether alterations in mTOR signaling can be observed in rats exposed to the chronic unpredictable stress (CUS) model of depression. In the present study, we examined the effect of CUS on the expression of phosphorylated mTOR and its downstream signaling components in the frontal cortex, hippocampus, amygdala, and dorsal raphe. We also examined the effect of CUS on the expression of kinases that phosphorylate mTOR such as extracellular signal-regulated kinase (ERK1/2) and protein kinase B/Akt (Akt1). In addition, we examined the effect of stress on the phosphorylation of GluR1 an α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor subunit. We found that eight-weeks of CUS exposure significantly decreased the phosphorylation levels of mTOR and its downstream signaling components in the amygdala. Reduced level of phospho-mTOR in the amygdala was accompanied by decreased phosphorylation of ERK-1/2, Akt-1, and GluR1. No significant changes were seen in the frontal cortex, hippocampus, or dorsal raphe. Our study demonstrates that long-term stress exposure results in brain region-specific abnormalities in signaling pathways previously linked to novel mechanisms for rapid antidepressant effects. These observations are in line with evidence showing that mTOR and its upstream and downstream signaling partners could be important targets for the development of novel antidepressants.
Keywords: mTOR signaling pathway, chronic unpredictable stress, rats, frontal cortex, hippocampus, amygdala, dorsal raphe
1. Introduction
Our previous postmortem studies showed robust deficits in the mammalian target of rapamycin (mTOR) signaling in the prefrontal cortex (PFC) of subjects diagnosed with major depressive disorder (MDD). Reduced protein expression of mTOR, and its two major downstream signaling effectors, the 70-kDa ribosomal protein S6 kinase (p70S6K) and eukaryotic translation initiation factor 4B (eIF-4B) has been previously demonstrated (Jernigan et al., 2011). These results indicate that deficits in mTOR-dependent protein synthesis may contribute to the molecular and cellular pathology detected in the PFC in MDD. Accordingly, deficits in prominent postsynaptic proteins including N-methyl-D-aspartate (NMDA) receptor subunits (NR2A and NR2B), metabotropic glutamate receptor subtype 5 (mGluR5) and postsynaptic density protein 95kDa (PSD-95) were previously demonstrated in the PFC from depressed subjects (Deschwanden et al., 2011; Feyissa et al., 2009). Based on these studies, it is tempting to hypothesize that an activation of mTOR function followed by enhanced mTOR-dependent protein synthesis may underlie antidepressant action. In fact, it has been recently demonstrated that a rapid increase in mTOR activity (measured by increased phosphorylation levels) and an induction of synaptogenesis in the rat PFC are responsible for the rapid and sustained antidepressant-like effects of ketamine (an NMDA receptor antagonist) and LY341495 (a metabotropic glutamate receptor subtype 2/3 antagonist) in animal screening procedures (Dwyer et al., 2012; Koike et al., 2011; Li et al., 2010; Li et al., 2011; reviewed by Hashimoto et al., 2011). Moreover, deficits in synaptic proteins induced by chronic unpredictable stress (CUS), animal model of depression, were normalized by rapid activation of the mTOR signaling pathway by ketamine (Li at al., 2011).
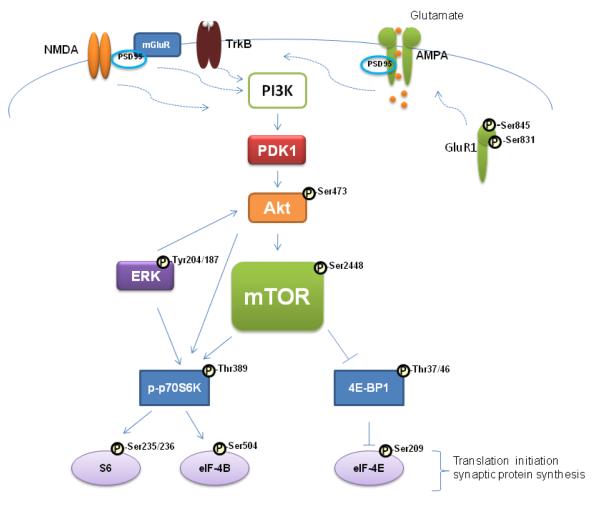
mTOR belongs to a family of serine/threonine kinases widely expressed in the brain. It regulates the initiation of protein translation, the rate limiting step in protein synthesis (Fig. 1). Activation of mTOR occurs via phosphorylation by Akt/protein kinase B (Akt). Akt can be activated by phosphoinositide – dependent kinase 1 (PDK1) and by the extracellular signal regulated protein kinase 1 (ERK1) (Hoeffer and Klann, 2010; Kim et al., 2010). Activated mTOR phosphorylates p70 ribosomal protein S6 kinase (p70S6K) followed by p70S6K-induced phosphorylation of ribosomal protein S6 and eukaryotic initiation factor 4B (eIF-4B) which promotes the initiation of protein translation. mTOR also phosphorylates and inactivates the eukaryotic initiation factor 4E-binding protein 1 (4E-BP1) reducing its affinity for the eukaryotic initiation factor 4E (eIF4E) thus releasing eIF4E to facilitate translation initiation. Thus, mTOR controls the efficiency of protein translation via its critical downstream targets.
Fig. 1.
Simplified diagram illustrating the mTOR signaling pathway. Neuronal receptors (NMDAR, mGluR5, TrkB) increase intracellular [Ca2+] and in turn activate PI3K, PDK1, Akt, and mTOR. Akt can be activated by PDK1 and by ERK1/2. Activated mTOR phosphorylates p70S6K followed by p70S6K-induced phosphorylation of S6 and eIF-4B, which promotes the initiation of protein translation. mTOR also phosphorylates and inactivates 4E-BP, reducing its affinity for eIF-4E and releasing eIF-4E to facilitate translation initiation. Abbreviations: Akt, Akt/protein kinase-B; AMPA, α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionic acid receptor; ERK, extra cellular signal-regulated kinase; eIF-4B, eukaryotic translation initiation factor 4B; eIF-4E, eukaryotic translation initiation factor 4E; GluR1, α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionic acid receptor subunit 1; mGluR, metabotropic glutamate receptor; mTOR, mammalian target of rapamycin; NMDA, N-methyl-D-aspartate receptor; PDK1, phosphoinositide – dependent kinase 1; PI3K, phosphoinositide-3 kinase; PSD-95, postsynapticdensity protein-95 kDa; p70S6K, 70-kDa ribosomal protein S6 kinase; S6, ribosomal protein S6 - a component of the 40S ribosomal subunit; TrkB, Tyrosine kinase-B receptor; 4E-BP, eukaryotic translation initiation factor 4E-binding protein.
In the present study, we sought to investigate whether the alterations in the phosphorylation of the mTOR signaling pathway components could be produced experimentally in rats exposed to CUS. The effect of CUS on the expression of kinases linked to the activation of mTOR such as phospho-ERK-1/2 and phospho-Akt1 was also examined. Additionally, the level of phospho-GluR1 (an indicator of synaptic activity), was examined. Brain areas investigated included the frontal cortex, hippocampus, amygdala, and dorsal raphe.
2. Methods
2.1. Animals
Adult male Wistar rats initially weighing 250-260 g were purchased from Harlan Sprague-Dawley Inc. (Indianapolis, IN). Animals were housed in groups of 2 per cage in a room maintained under standard conditions of light (12:12 h light-dark cycle), temperature (22 ± 3 °C) and humidity. Animals had ad libitum access to food and water. Control animals were housed in a separate room away from those in the CUS treatment group. Animals were allowed to habituate to their new environment for 7 days and thereafter to testing conditions for an additional 3 days. All procedures with the animal work were done in accordance to the guidelines established by the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23) and the Animal Care and Use Committee of the University of Mississippi Medical Center.
2.2. CUS procedure
Rats were exposed to a variable sequence of mild and unpredictable stressors for 8 weeks. A total of 9 different stressors were used (2 stressors per day). The stressors included rotation on a shaker, placement in a 4°C ambient environment, lights off for 3 hours (10:00 AM– 1:00 PM), lights on overnight, restraint stress, swimming, food and water deprivation, crowded housing, and isolation housing (Banasr and Duman, 2008; Kim et al., 2003; Li et al., 2011; Orsetti et al., 2007; Sowa-Kućma et al., 2008). The control rats were not handled and left undisturbed (except for routine cage maintenance) in their home cage. Rats were sacrificed 48 h after the last stress exposure. The frontal cortex, hippocampus, amygdala, and dorsal raphe were immediately dissected and stored at -80°C until further use.
2.3. Immunoblotting and data analysis
Western blot experiments were performed as described in our previous studies (Feyissa at al., 2009, Feyissa et al., 2010) with phosphorylation-state-specific rabbit monoclonal antibodies against phospho-mTOR (Ser-2448), phosoho-eIF4B (Ser-504), phospho-eIF4E (Ser-209), phosohp-ERK1/2 (Tyr-204/187), phospho-Akt1 (Ser-473), phospho-GluR1 (Ser-831), phospho-GluR1 (Ser-845), (Epitomics Burlingame, CA, USA; 1:1000 or 1:500) and phosoho-p70S6K (Thr-389), phosoho-S6 (Ser-235/236), and phosoho-4E-BP1 (Thr-37/46) (1:500 or 1:1000; Cell Signaling Technology Danvers, MA, USA). After overnight incubation with primary antibodies, membranes were incubated with peroxidase labeled secondary antibodies for 1 h (1:3000; Amersham Biosciences, Piscataway, NY, USA). As a control for transfer and loading, actin was detected on each blot using mouse anti-actin primary antibody (Millipore, Temecula, CA, USA; 1:10,000). Immunoactive bands were analyzed using MCID Elite 7.0 (Imaging Research, St. Catherines, ON, Canada). The resulting data was analyzed statistically using a two-tailed unpaired t-test (GraphPad Prism 5, LaJolla, CA, USA). The final data are expressed as a ratio of the relative optical density (ROD) of protein of interest to the ROD of actin. A p-value <0.05 was considered significant.
3. Results
Amounts of phosphorylated forms of mTOR, p70S6K, S6, eIF-4B, eIF-4E, 4E-BP1, ERK1/2, Akt1, and GluR1 proteins were analyzed in the frontal cortex, hippocampus, amygdala and dorsal raphe from 11 stressed and 11 control rats.
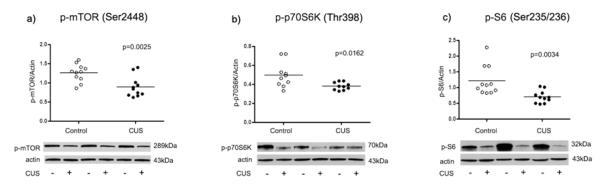
In the amygdala the amount of phospho-mTOR immunoreactivity from stressed rats (0.90 ± 0.08) was significantly decreased compared to controls (1.26 ± 0.07; t=3.45 df=20, p=0.0025, Fig. 2a). Equally reduced in the stressed rats were phospho-p70S6K immunoreactivity (0.38 ± 0.013) compared to control rats (0.50 ± 0.04; t=2.65 df=18, p=0.0162, Fig. 2b) and phospho-S6 (0.71 ± 0.06) compared to controls (1.21 ± 0.14, t=3.32 df=20, p= 0.0034, Fig. 2c).
Fig. 2.
Scatter plots of phosphorylated mTOR, p70S6K, and S6 protein levels normalized to actin. Significant reduction in phospho-mTOR, phospho-p70S6K, and phospho-S6 were observed in the amygdala from stressed rats (filled circles; n=11) as compared to controls (open circles; n=11). Normalized optical density values for the individual subjects and mean values (horizonatal lines) are presented.
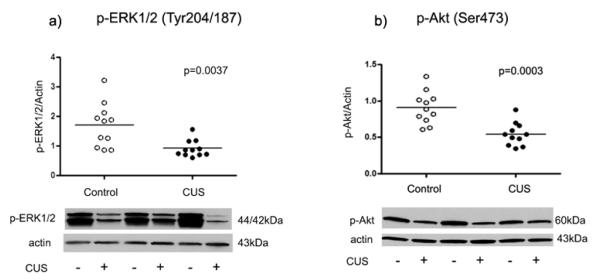
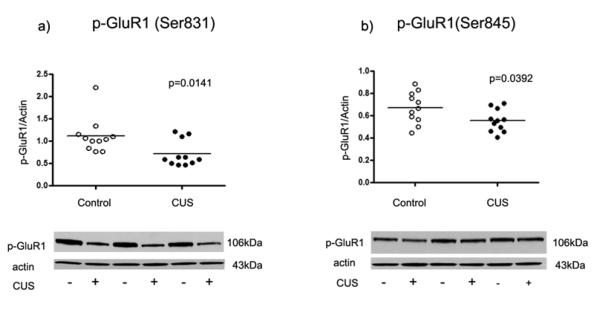
Moreover, the amount of phosoho-ERK1/2 immunoreactivity in amygdala of stressed rats (0.91 ± 0.09) was significantly decreased compared to control rats (1.70 ± 0.22, t=3.28 df=20, p= 0.0037, Fig. 3a). There was a robust reduction in the level of phospho-Akt immunoreactivity in stressed rats (0.54 ± 0.05) compared to controls (0.91 ± 0.07, t=4.40 df=20, p=0.0003, Fig. 3b). The amount of phospho-GluR1 (Ser-831) from stressed rats (0.72 ± 0.09) was significantly decreased compared to controls (1.12 ± 0.12, t=2.69 df=20, p=0.0141, Fig. 4a). There was also a significant reduction in the amount of GluR1 phosphorylated at Ser-845 in stressed rats (0.56±0.03) as compared to controls (0.67±0.04, t=2.21 df=20, p=0.0392, Fig. 4b).
Fig. 3.
Scatter plots of phosphorylated ERK1/2 and Akt1 protein levels normalized to actin. Significant reduction in phospho-ERK1/2 and phospho-Akt1 were observed in the amygdala from stressed rats (filled circles; n=11) as compared to controls (open circles; n=11). Normalized optical density values for the individual subjects and mean values (horizonatal lines) are presented.
Fig. 4.
Scatter plot of phosphorylated GluR1 (Ser-831 and Ser-845) protein levels normalized to actin. Significant reductions in phospho-GluR1 (Ser-831 and Ser-845) were observed in the amygdala from stressed rats (filled circles; n=11) as compared to controls (open circles; n=11). Normalized optical density values for the individual subjects and mean values (horizonatal lines) are presented.
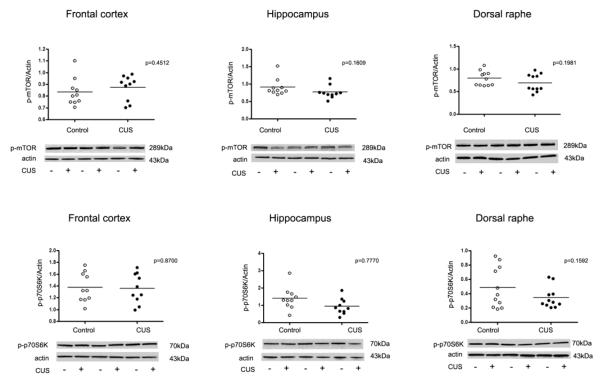
Interestingly, no significant changes in phosphorylation level of the mTOR signaling pathway components and associated kinases were detected in the frontal cortex, hippocampus or dorsal raphe from rats exposed to eight-week CUS paradigm (Fig. 5).
Fig. 5.
Scatter plots of phosphorylated mTOR and p70S6K protein levels normalized to actin. No significant changes in phospho-mTOR and phospho-p70S6K were observed in the frontal cortex, hippocampus and dorsal raphe (filled circles; n=10) as compared to controls (open circles; n=10). Normalized optical density values for the individual subjects and mean values (horizontal lines) are presented.
2. Discussion
The results obtained from this study demonstrate that an eight-week exposure to stress induces a significant reduction in activated/phosphorylated components of the mTOR signaling pathway in the amygdala. The components of the pathway that were reduced as a result of CUS exposure included phospho-mTOR and its downstream effectors (phospho-p70S6K and phospho-S6). It is known that mTOR phosphorylates p70S6K followed by p70S6K-induced phosphorylation of eIF4B and S6 (Kim et al., 2010) (Fig. 1). Interestingly, in our study we found a reduction in the levels of phospho-p70S6K and phospho-S6, but not phospho-eIF4B indicating that the mTOR/p70S6K /S6 signaling cascade in the amygdala is negatively influenced by chronic stress. Interestingly, no significant changes in phosphorylation of mTOR or p70S6K were detected in the PFC from rats exposed to a three-week CUS procedure (Li et al., 2011). Taken together, these results indicate that the effect of chronic stress on mTOR phosphorylation could be brain region-specific and/or dependent on the length of exposure to stress (e.g. three-week vs. eight-week CUS).
Reduced phosphorylation of the upstream regulators of mTOR, Akt1 and ERK1/2, was detected in the amygdala from rats exposed to CUS. Previously, Krishnan et al. demonstrated reduced levels of phosphorylated Akt1 in the ventral tegmental area (VTA) from mice exposed to chronic social defeat. Blockade of the Akt1 (with PI3K inhibitor – LY294002) in the VTA increased susceptibility to depressive-like behavior (Krishnan et al., 2008). These findings highlight a crucial role for Akt1 in stress-induced pathology. Interestingly, decreased activity of Akt1 (measured by kinase assays) was detected in the PFC from depressed subjects in, both, suicide and non suicide groups (Karege et al., 2007). A significant decrease in the catalytic activity and immunoreactivity levels of phospho-Ser473-Akt and phospho-Thr308-Akt were also reported in the PFC and hippocampus of suicide victims (Dwivedi et al., 2010). These findings indicate a strong association between dysfunction in Akt activity and depressive-like pathophysiology. Extracellular signal-regulated kinase (ERK1/2) plays a crucial role in synaptic and structural plasticity. Several studies have directly or indirectly shown that ERK1/2 plays a role in depressive behavior. A recent study demonstrated that chronic stress decreased ERK in the hippocampus and PFC, and that this effect was antagonized by fluoxetine treatment (Qi et al., 2008, confirmed by First et al., 2011). Another study has shown that chronic corticosterone exposure selectively reduced phosphorylated ERK1/2 in the dentate gyrus, amygdala and striatum, but not in the PFC of treated mice (Gourley et al., 2008). While Dwivedi et al. (2001 and 2006) have reported decreased level of ERK in the prefrontal cortex and hippocampus of suicide subjects. On the contrary, another group has shown that there was no statistically significant difference in total ERK1/2 protein levels in the postmortem frontal cortex of individuals with schizophrenia, major depressive disorder and bipolar disorder compared to control patients (Yuan et al., 2010). However, it can be argued that changes in total protein may not be crucial, since activation of ERK depends on phosphorylation (Charest et al. 1993). Our study showed markedly reduced phosho-ERK1/2 in the amygdala of stressed rats compared to controls. Taken together, these studies confirm the potential of targeting both Akt and ERK as an innovative strategy for the discovery of novel antidepressants.
We have documented reduced expression of the GluR1 subunit of the AMPA receptor phosphorylated at Ser-831 and Ser-845 in the amygdala. These data indicate that long-term exposure to stress could have a detrimental effect on synaptic activity. Phosphorylation of GluR1 potentiates receptor conductance, and is essential for long term potentiation (LTP) (Derkach et al., 1999; Esteban, 2003). Reduced levels of GluR1 phosphorylated at Ser-831 and Ser-845 may indicate reduced AMPA receptor currents (Derkach at al., 2007, Lee at al., 2010). It is important to note that phosphorylation of GluR1 at both Ser-831 and Ser-845 facilitates synaptic deliver of AMPA receptors (Fig. 1).
Decreased level of phospho-GluR1 resulting from CUS was previously demonstrated in the PFC and hippocampus of adult rats and PFC of young rats (Toth et al., 2008). Reduced expression of unphosphorylated GluR1 in synaptosomal preparations has also been previously demonstrated by Li et al. (2011) indicating reduced expression of synaptic pool of GluR1 protein. In a similar vein, reduced level of phospho-GluR1 seen in our study could indicate reduced level of synaptic GluR1, given that the phosphorylation process is necessary to deliver AMPA receptors into synapses.
In summary, our study provides evidence that an eight-week CUS exposure produces deficits in the mTOR signaling pathway components in the amygdala. Because of the complex nature of depression with altered neurochemistry across many brain regions, our results showing deficits in mTOR signaling pathway components in the amygdala could indicate that the effects of CUS are time dependent considering the dynamic nature of phosphorylation events. Therefore, studying different time points in a CUS paradigm will be very important in understanding the role mTOR and its signaling partners play in depression. These findings could lead to the development of faster acting antidepressant medications.
Highlights.
We used CUS model to examine mTOR signaling in the rat brain
Reductions in phosphorylation of mTOR, p70S6K, S6 were identified in the amygdala.
Reduced phosphorylation of Akt1 and ERK1/2 was also seen in the amygdala
No changes were detected in the frontal cortex, hippocampus and dorsal raphe
Specific dysregulation of mTOR signaling is evident in stress model of depression
Acknowledgements
This study was supported by grant from the National Center of Research Resources (NCRR, RP17701), a component of NIH.
Abbreviation
- Akt
Akt/protein kinase-B
- AMPA
α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionic acid receptor
- CUS
chronic unpredictable stress
- eIF-4B
eukaryotic translation initiation factor 4B
- eIF-4E
eukaryotic translation initiation factor 4E
- ERK
extra cellular signal-regulated kinase
- GluR1
α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionic acid receptor subunit 1
- MDD
major depressive disorder
- mTOR
mammalian target of rapamycin
- NMDA
N-methyl-D-aspartate receptor
- mGluR5
metabotropic glutamate receptor subtype 5
- PDK1
phosphoinositide – dependent kinase 1
- PI3K
phosphoinositide-3 kinase
- PFC
prefrontal cortex
- PSD-95
postsynaptic density protein-95 kDa
- p70S6K
70-kDa ribosomal protein S6 kinase
- S6
ribosomal protein S6
- TrkB
Tyrosine kinase-B receptor
- 4E-BP
eukaryotic translation initiation factor 4E-binding protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banasr M, Duman RS. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol. Psychiatry. 2008;64:863–870. doi: 10.1016/j.biopsych.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charest DL, Mordret G, Harder KW, Jirik F, Pelech SL. Molecular cloning, expression, and characterization of the human mitogen-activated protein kinase p44erk1. Mol Cell Biol. 1993;13:4679–90. doi: 10.1128/mcb.13.8.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkach V, Barria A, Soderling TR. Ca2+/calmodulin-kinase II enhances channel conductance of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc Natl Acad Sci U S A. 1999;96:3269–74. doi: 10.1073/pnas.96.6.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007;8:101–13. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- Deschwanden A, Karolewicz B, Feyissa AM, Treyer V, Ametamey SM, Johayem A, Burger C, Auberson YP, Sovago J, Stockmeier CA, Buck A, Hasler G. Reduced metabotropic glutamate receptor 5 density in major depression determined by [(11)C]ABP688 PET and postmortem study. Am J Psychiatry. 2011;168:727–34. doi: 10.1176/appi.ajp.2011.09111607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Conley RR, Pandey GN. ERK MAP kinase signaling in post-mortem brain of suicide subjects: differential regulation of upstream Raf kinases Raf-1 and B-Raf. Mol Psychiatry. 2006;11:86–98. doi: 10.1038/sj.mp.4001744. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Roberts RC, Conley RC, Tamminga CA, Pandey GN. Reduced activation and expression of ERK1/2 MAP kinase in the post-mortem brain of depressed suicide subjects. J Neurochem. 2001;77:916–28. doi: 10.1046/j.1471-4159.2001.00300.x. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Zhang H, Roberts RC, Conley RR, Pandey GN. Modulation in activation and expression of phosphatase and tensin homolog on chromosome ten, Akt1, and 3-phosphoinositide-dependent kinase 1: further evidence demonstrating altered phosphoinositide 3-kinase signaling in postmortem brain of suicide subjects. Biol Psychiatry. 2010;67:1017–25. doi: 10.1016/j.biopsych.2009.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer JM, Lepack AE, Duman RS. mTOR activation is required for the antidepressant effects of mGluR2/3 blockade. Int J Neuropsychopharmacol. 2012;15:429–34. doi: 10.1017/S1461145711001702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban JA. AMPA receptor trafficking: a road map for synapticplasticity. Mol Interv. 2003;3:375–385. doi: 10.1124/mi.3.7.375. [DOI] [PubMed] [Google Scholar]
- Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:70–75. doi: 10.1016/j.pnpbp.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyissa AM, Woolverton WL, Miguel-Hidalgo JJ, Wang Z, Kyle PB, Hasler G, Stockmeier CA, Iyo AH, Karolewicz B. Elevated level of metabotropic glutamate receptor 2/3 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:279–83. doi: 10.1016/j.pnpbp.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Gil-Ad I, Taler M, Tarasenko I, Novak N, Weizman A. The effects of fluoxetine treatment in a chronic mild stress rat model on depression-related behavior, brain neurotrophins and ERK expression. J Mol Neurosci. 2011;45:246–55. doi: 10.1007/s12031-011-9515-5. [DOI] [PubMed] [Google Scholar]
- Gourley SL, Wu FJ, Kiraly DD, Ploski JE, Kedves AT, Duman RS, Taylor JR. Regionally specific regulation of ERK MAP kinase in a model of antidepressant-sensitive chronic depression. Biol Psychiatry. 2008;63:353–9. doi: 10.1016/j.biopsych.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K. Role of the mTOR signaling pathway in the rapid antidepressant action of ketamine. Expert Rev Neurother. 2011;11:33–6. doi: 10.1586/ern.10.176. [DOI] [PubMed] [Google Scholar]
- Hoeffer CA. Klann E mTOR signaling: At the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan CS, Goswami DB, Austin MC, Iyo AH, Chandran A, Stockmeier CA, Karolewicz B. The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1774–9. doi: 10.1016/j.pnpbp.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karege F, Perroud N, Burkhardt S, Schwald M, Ballmann E, La Harpe R, Malafosse A. Alteration in kinase activity but not in protein levels of protein kinase B and glycogen synthase kinase-3beta in ventral prefrontal cortex of depressed suicide victims. Biol Psychiatry. 2007;61:240–5. doi: 10.1016/j.biopsych.2006.04.036. [DOI] [PubMed] [Google Scholar]
- Kim SH, Park HG, Kim HS, Ahn YM, Kim YS. Effects of neonatal MK-801 treatment on p70S6K-S6/eIF4B signal pathways and protein translation in the frontal cortex of the developing rat brain. Int J Neuropsychopharmacol. 2010;13:1233–46. doi: 10.1017/S1461145709991192. [DOI] [PubMed] [Google Scholar]
- Kim H, Whang WW, Kim HT, Pyun KH, Cho SY, Hahm DH, Lee HJ, Shim I. Expression of neuropeptide Y and cholecystokinin in the rat brain by chronic mild stress. Brain Res. 2003;983:201–8. doi: 10.1016/s0006-8993(03)03087-7. [DOI] [PubMed] [Google Scholar]
- Koike H, Iijima M, Chaki S. Involvement of the mammalian target of rapamycin signaling in the antidepressant-like effect of group II metabotropic glutamate receptor antagonists. Neuropharmacology. 2011;61:1419–23. doi: 10.1016/j.neuropharm.2011.08.034. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Mazei-Robison M, Iñiguez SD, Ables JL, Vialou V, Berton O, Ghose S, Covington HE, 3rd, Wiley MD, Henderson RP, Neve RL, Eisch AJ, Tamminga CA, Russo SJ, Bolaños CA, Nestler EJ. AKT signaling within the ventral tegmental area regulates cellular and behavioral responses to stressful stimuli. Biol Psychiatry. 2008;64:691–700. doi: 10.1016/j.biopsych.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Takamiya K, He K, Song L, Huganir RL. Specific roles of AMPA receptor subunit GluR1 (GluA1) phosphorylation sites in regulating synaptic plasticity in the CA1 region of hippocampus. J Neurophysiol. 2010;103:479–89. doi: 10.1152/jn.00835.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–64. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biological Psychiatry. 2011;69:754–61. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsetti M, Canonico PL, Dellarole A, Colella L, Di Brisco F, Ghi P. Quetiapine prevents anhedonia induced by acute or chronic stress. Neuropsychopharmacology. 2007;32:1783–90. doi: 10.1038/sj.npp.1301291. [DOI] [PubMed] [Google Scholar]
- Sowa-Kućma M, Legutko B, Szewczyk B, Novak K, Znojek P, Poleszak E, Papp M, Pilc A, Nowak G. Antidepressant-like activity of zinc: further behavioral and molecular evidence. J Neural Transm. 2008;115:1621–8. doi: 10.1007/s00702-008-0115-7. [DOI] [PubMed] [Google Scholar]
- Qi X, Lin W, Li J, Li H, Wang W, Wang D, Sun M. Fluoxetine increases the activity of the ERK-CREB signal system and alleviates the depressive-like behavior in rats exposed to chronic forced swim stress. Neurobiol Dis. 2008;31:278–85. doi: 10.1016/j.nbd.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Toth E, Gersner R, Wilf-Yarkoni A, Raizel H, Dar DE, Richter-Levin G, Levit O, Zangen A. Age-dependent effects of chronic stress on brain plasticity and depressive behavior. J Neurochem. 2008;107:522–32. doi: 10.1111/j.1471-4159.2008.05642.x. [DOI] [PubMed] [Google Scholar]
- Yuan P, Zhou R, Wang Y, Li X, Li J, Chen G, Guitart X, Manji HK. Altered levels of extracellular signal-regulated kinase signaling proteins in postmortem frontal cortex of individuals with mood disorders and schizophrenia. J Affect Disord. 2010;124:164–9. doi: 10.1016/j.jad.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]