Abstract
A therapeutic intervention that could decrease tumor burden and increase sensitivity to chemotherapy would have a significant impact on the high morbidity rate associated with ovarian cancer. MicroRNAs (miRNAs) have emerged as potential therapeutic candidates due to their ability to down regulate multiple targets involved in tumor progression and chemoresistance. MiR-200c is down regulated in ovarian cancer cell lines and stage III ovarian tumors, and low miR-200c correlates with poor prognosis. MiR-200c increases sensitivity to taxanes in vitro, by targeting TUBB3, a tubulin known to mediate chemoresistance. Indeed, we find that patients with tumors with low TUBB3 had significantly prolonged survival (average survival 52.73 ± 4.08 months) compared to those with high TUBB3 (average survival 42.56 ±3.19 months). MiR-200c also targets TrkB, a mediator of resistance to anoikis. We demonstrate that restoration of miR-200c to ovarian cancer cells results in increased anoikis sensitivity and reduced adherence to biological substrates in vitro. Since both chemo- and anoikis-resistance are critical steps in the progression of ovarian cancer, we sought to determine how restoration of miR-200c affects tumor burden and chemosensitivity in an in vivo preclinical model of ovarian cancer. Restoration of miR-200c in an intraperitoneal xenograft model of human ovarian cancer, results in decreased tumor formation and tumor burden. Furthermore, even in established tumors, restoration of miR-200c, alone or in combination with paclitaxel, results in significantly decreased tumor burden. Our study suggests that restoration of miR-200c immediately prior to cytotoxic chemotherapy may allow for a better response or lower effective dose.
Keywords: miR-200, Hey cells, chemotherapy, IVIS, paclitaxel
INTRODUCTION
Epithelial ovarian cancer (EOC), which accounts for 90% of ovarian cancer, is a heterogeneous disease divided into histologic subtypes: serous, mucinous, endometrioid, clear cell, Brenner, and undifferentiated carcinomas. The serous type represents 75%–80% of EOCs and accounts for the majority of deaths from gynecological malignancies (1–4). Even with optimal treatment consisting of surgical cytoreduction (debulking) followed by platinum and taxane-based chemotherapy, the 5-year survival for women with advanced stage disease is only 46% at best (5). Currently there is no molecularly targeted therapy for ovarian cancer, and over 50% of women with tumor that respond to initial treatment relapse within 18 to 24 months (5). In ovarian cancer, metastasis primarily occurs by a mechanism termed direct seeding, which involves shedding of tumor cells from the primary site into the peritoneal cavity. For this journey, ovarian cancer cells must acquire resistance to anoikis (apoptosis initiated upon loss of attachment to native extracellular matrix) to allow survival in suspension in ascitic fluid before attaching to the peritoneum. A targeted therapy that could reduce tumor burden by simultaneously affecting multiple factors critical for ovarian cancer progression such as anoikis resistance, chemoresistance and attachment to sites in the peritoneal cavity would be a revolutionary breakthrough for this aggressive gynecologic malignancy.
A recent study demonstrated that micro-RNA 200c (miR-200c) targets ZEB1 and ZEB2 in ovarian cancer cells and thereby restores E-cadherin and reduces motility (6). Subsequently, we identified class IIIβ-tubulin gene (TUBB3) as an additional direct miR-200c target and demonstrated that decreasing TUBB3 was the molecular mechanism whereby restoration of miR-200c increases sensitivity to paclitaxel in endometrial and ovarian cancer cells (7, 8). TUBB3 is not expressed in normal epithelial cells, but is often overexpressed in clinical specimens and cell lines of taxane-resistant carcinomas (9–12) including ovarian cancer (13, 14). Indeed TUBB3 expression is considered to be one of the main mechanisms of resistance to taxanes in general in many types of carcinomas (9) and in ovarian cancer (13–16). The mechanism by which overexpression of TUBB3 is thought to result in resistance to taxanes is by enhancement of the dynamic instability of microtubules, thereby counteracting the activity of microtubule targeting agents. A recent study suggests additional novel mechanisms by which TUBB3 contributes to drug resistance (17).
Another important direct target of miR-200c that we recently identified is NTRK2, the gene encoding TrkB (18) a tyrosine kinase receptor normally expressed in neurons, but co-opted by various types of cancer including ovarian cancer to achieve anoikis resistance (19–23). Since chemo-and anoikis resistance represent critical steps in ovarian cancer progression, we sought to determine how restoration of miR-200c would affect ovarian cancer tumor burden and chemosensitivity in vivo. Here we demonstrate that restoration of miR-200c to ovarian cancer cell lines enhances anoikis sensitivity, decreases attachment to biological substrates, decreases tumor burden and enhances chemosensitivity in vitro and in vivo.
MATERIALS AND METHODS
Cell lines and tissue culture
Ovarian cell lines HEY, SKOV3, OVCA 420, OV 1847, OVCA 433 were grown in RPMI with 10% FBS, L-glutamine and penicillin/ streptomycin. Cell lines were obtained from Monique A. Spillman, who in collaboration with the University of Colorado DNA Sequencing and Analysis Core verified the identity by DNA fingerprinting (24). All cells were used within 20 passages from receipt and less than 6 months from authentication.
Generation of stable cell lines
Stable overexpression of miR-200c was obtained with lentiviral vectors expressing miR-200c precursor sequence (pMIRNA, System biosciences, CA).
Generation of inducible system for miR-200c expression
Hey cells were transduced with GFP-TGL-luciferase (pGL-luc retroviral vector) and selected by GFP expression. HeyTGL cells were transduced with inducible lentiviral vector pTRIPz-RFP encoding or not the precursor sequence for miR-200c (p-TRIPz-EV or pTRIPz-200c). Stable expression was selected using puromycin, and multiple clones were tested to identify those with low/absent background expression of miR-200c and at least 500 fold expression miR-200c in the presence of doxycycline inducer.
Western blot and protein expression quantification
Total cell lysates were prepared and analyzed by western blot exactly as described elsewhere [12]. Primary antibodies used for western blot included mouse-monoclonal anti-β-III-tubulin (Sigma-Aldrich, St. Louis, MO, 1:1000 dilution); mouse monoclonal anti α-tubulin (Sigma-Aldrich, St. Louis, MO, USA, clone B-5-1-2, 1:30,000); rabbit polyclonal anti-ZEB1 (from Dr. Doug Darling, University of Louisville, Louisville, KY, USA; 1:1,500 dilution). Secondary antibodies were Alexa-fluor 680 Conjugated Affinity Purified Anti-Rabbit or Anti-Mouse IgG (Invitrogen, Garland, CA) detected using an Odyssey Infrared Imaging System (Licor Biosciences, Lincoln, NE). Bands at the expected molecular weights were cropped for presentation purposes and independent blots are shown surrounded by black boxes. Protein levels were measured by obtaining the integrated intensity of the target band relative to the integrated intensity of a loading control (i.e. α-tubulin) using the Odyssey quantification tools.
Anoikis assays
Poly-hydroxyethyl methacrylate (poly-HEMA, Sigma- Aldrich) was reconstituted in 95% ethanol to a concentration of 12 mg/mL. To prepare poly-HEMA coated plates, 0.5 mL of 12 mg/mL solution was added to each well of a 24-well plate and allowed to dry overnight in a laminar flow tissue culture hood. Cells were transfected with transfection reagent alone, 50nM negative control or 50nM miR-200c mimics. Twenty-four hours later, 50,000 cells were plated in poly-HEMA coated 24- well plates in the presence of 10µM EDTA. Media was collected at 8, 24 and 48 hr and floating cells were pelleted and lysed for apoptosis analyses. For the inducible miR-200c system, the pTRIPz-200c cells were pre-treated with 1µg/ml dox for 24h, then plated (15,000 cells/well) in 96-well plates in either adherent or suspension conditions. After 24h, cells were pelleted and lysed. Apoptosis was measured using the cell death ELISA kit (Roche) following manufacturer instructions.
MTT assay
Cells were plated (1000/well) and treated or not with 1µg/ml doxycycline for 24, 48, 72 and 96h and cell proliferation measured as a function of metabolism by 3-(4,4-dimenthylthiazol-2-ul)-2,5-diphenyltetrazolium bromide (MTT, Sigma, St. Louis MO) assay Briefly, at the indicated times, cells were incubated with 100µl of media containing 1µg/ml MTT for 4 hr. Formazan crystals were dissolved in 100µl DMSO and absorbance at 490nm was recorded.
Cell-adhesion assay
Cell adhesion to vitronectin, collagen IV, fibronectin and basement membrane complex (BMC) was performed using the Innocyte ECM cell adhesion assay (Calbiochem, Merck KGaA, Darmstadt, Germany) according to manufacturer's instructions.
RNA Extraction and qRT-PCR
Total RNA from cultured cell lines and tumors was isolated using Trizol. cDNA from mature miR-200c was synthesized from 50 ng of total RNA using the TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Life technologies, NJ) as described by the manufacturer. qRT-PCR was performed using TaqMan MicroRNA hsa-miR-200c Assay (Applied Biosystems, Life technologies, NJ) and the TaqMan Universal PCR Master Mix, No Amperase UNG (Applied Biosystems, Life technologies, NJ) as described by the manufacturer. RNU6 was used for normalization. At least 3 biological replicates and triplicate PCRs were used to calculate relative expression. The relative mRNA or miRNA levels were calculated using the comparative Ct method (ΔΔCt). Briefly, the Ct (cycle threshold) values for the normalization gene were subtracted from Ct values of the target gene to achieve the ΔCt value. The 2−ΔCt was calculated for each sample and then each of the values was divided by a control sample to achieve the relative mRNA or miRNA levels (ΔΔCt).
In vitro bioluminescent assays
Cell expressing luciferase reporter (HeyTGL-EV or Hey-TGLmiR-200c) were plated in 6-well plates and treated for indicated times with 1µg/mL doxycycline. Ten minutes before imaging, cells were added 150µg/mL D-luciferin (Xenogen #XR-1001) as substrate, and total-photon flux per second was measured at 0.5, 1, 5 and 10 sec.
Ovarian tumor xenograft and luminescent imaging in mice
Tumor xenografts were developed by injecting 100.000 cells diluted in 100µl PBS into the peritoneal cavity of female non-obese diabetic (NOD)/SCID mice (Charles-River Laboratories). MiR-200c expression was activated with 2g/L doxycycline in drinking water. Tumor burden was measured using IVIS 200 Optical Imaging system (Xenogen, Caliper Life Sciences) after intraperitoneal (IP) injection of 200µl of luciferin/PBS (150mg/kg body-weight). Images were obtained 10 minutes after luciferin injection. Living Image 2.60.1 (Caliper Life Sciences) software was used for quantitative analysis at 0.5-, 1-, 5 and 10-second time points. Images from the prone and supine position were taken and total-photon flux/second per each view, from a fixed ROI covering the abdominal area, was recorded. Total tumor burden included the total flux/second at the prone and supine positions. At termination of the experiment mice were euthanized by CO2 asphyxiation and tumors were excised. The University of Colorado Institutional Animal Care and Use Committee approved these experiments.
Immunohistochemistry for TUBB3 in clinical samples of serous ovarian carcinomas and correlation with clinical outcome
Formalin-fixed, paraffin embedded slides obtained from 129 surgical specimens from MD Anderson Cancer Center described previously (25) were stained for TUBB3 protein by immunohistochemistry. Tissue was then stained with neuronal class III B-tubulin polyclonal antibody (PRB-435P, Covance, Emeryville, CA). A positive control tissue was used for normalization between staining batches.
Tumor and associated stromal TUBB3 staining were scored independently in a blinded fashion by pathologist (MP) in categories of 0, 1, 2, 3, and 4 that combined percentage of tumor cells staining and intensity of staining as follows: Score 0: staining absent in tumor or weak staining in less than 10% of tumor cells (n=20); Score 1: 1 to 10% of tumor with weak or moderate staining (n=52); Score 2: more than 75% of tumor with weak staining, or less than 10% tumor with moderate to strong staining (n=13); Score 3: more than 75% tumor with moderate staining or 10 to 75% tumor with strong staining (n=35); Score 4: more than 75% tumor with strong staining (n=9). Stromal cells served as an internal positive control.
For survival analysis, high TUBB3 expression was defined as tumors scores of 3 or 4 moderate to high intensity staining and greater than 10% positive tumor cells (n=44) and low TUBB3 staining was defined as weak or moderate intensity staining in less than 10% of tumor cells (n=52). Overall survival time was defined as the interval between initial surgical treatment and date of last follow-up or death. Association between death and TUBB3 scores was analyzed using a Cox proportional hazards model.
Statistical Analysis
Survival analysis regarding the association between TUBB3 expression and death was performed by the University of Colorado Cancer Center Biostatistics and Bioinformatics Core using SAS/BASE and SAS/STAT software, Version 9.2 of the SAS System for Windows (SAS Institute Inc., Cary, NC, USA) using a Cox proportional hazards model after confirming that the proportional hazards assumption was satisfied. The University of Colorado Department of Pathology using GraphPad Prism 5.0 software performed all other statistical analyses. Two-tailed Student’s t- tests (two groups) or ANOVA followed by Tukey post hoc test (three or more groups) were used. Results with P<0.05 were considered statistically significant.
RESULTS
Restoration of miR-200c to ovarian cancer cells increases anoikis sensitivity and decreases adherence to components of the extracellular matrix
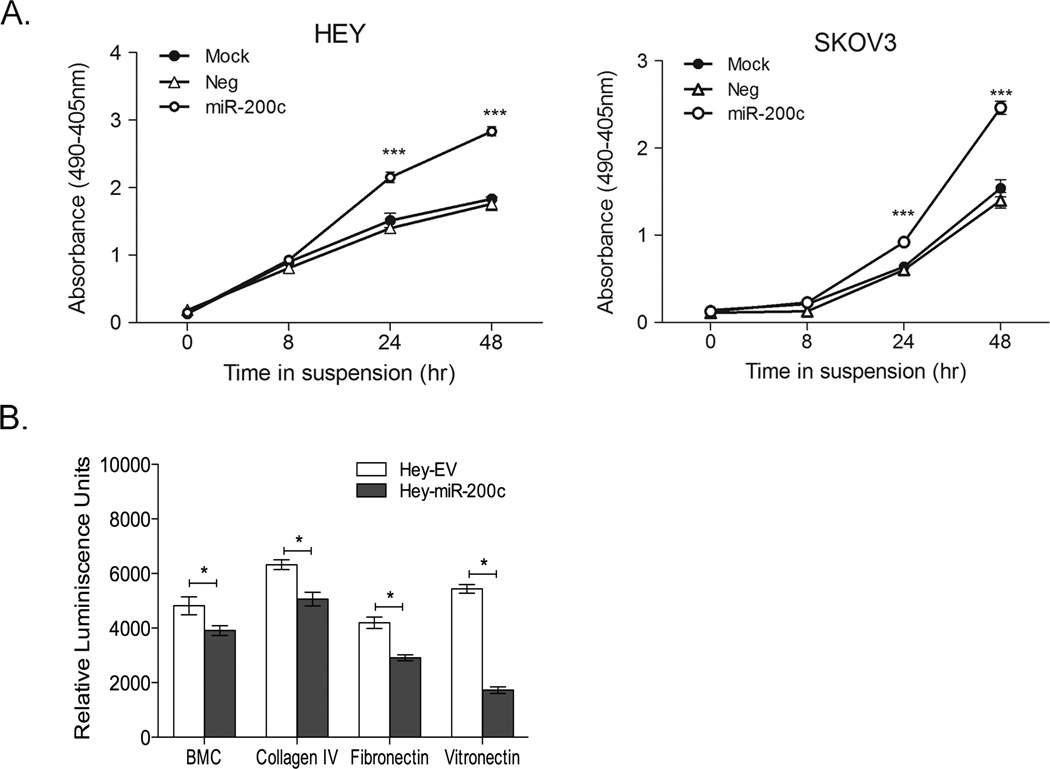
To determine whether miR-200c restoration could render ovarian cancer cells sensitive to anoikis, Hey and SKOV3 cells were transiently transfected with either control or miR-200c mimics then cultured under non-adherent conditions. Time course experiments demonstrate that miR-200 restoration significantly increases cell death in Hey and SKOV3 cells forced to grow in suspension as compared to cells transfected with a scrambled negative control mimic or mock-transfected cells under the same conditions (Figure 1A). Relevant to attachment to various sites in the peritoneal cavity, restoration of miR-200c to Hey cells decreases the ability to bind to components of the extracellular matrix such as BMC, collagen IV, fibronectin and vitronectin (Figure 1B). Thus, miR-200c restoration results in increased anoikis sensitivity and reduced ability to adhere to biological substrates.
Figure 1. Restoration of miR-200c to ovarian cancer cells decrease adhesion to substrates and increases sensitivity to anoikis.
A) Ovarian cancer cells were mock-transfected or transfected with 50 nM negative control (neg) or miR-200c mimic (200c) and 48 hrs later plated on poly-HEMA coated plates in the presence of 10 µM EDTA and harvested at times indicated for Cell Death ELISAs. B) Hey cells transduced with empty vector (EV) or miR-200c (miR-200c) were assayed in a fluorescent adhesion assay to BCM (basement membrane complex), collagen type IV, fibronectin and vitronectin. Mean of 3 biological replicates; bars= SEM * p < 0.01 ANOVA.
An inducible system for restoration of miR-200c
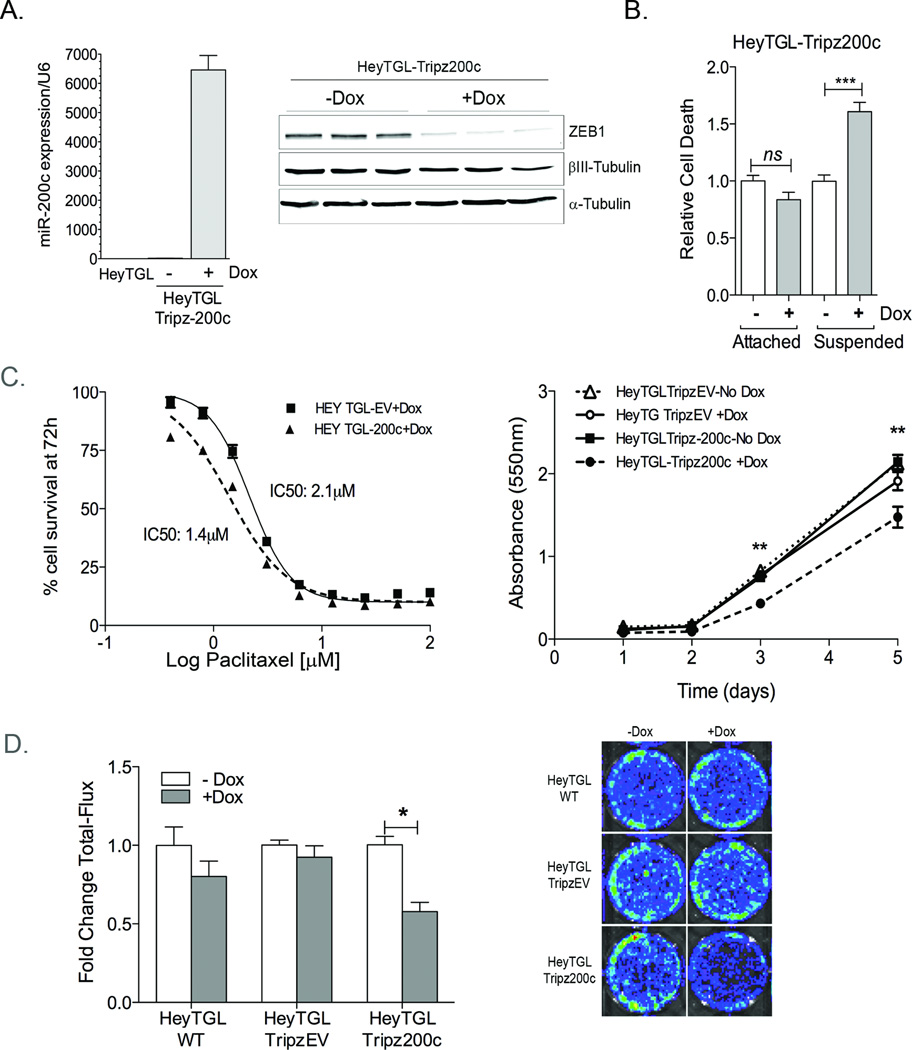
Since transient miR-200c restoration has profound effects on survival of ovarian cancer cells in vitro, we sought to determine whether miR-200c restoration could affect the establishment and progression of ovarian cancer in an in vivo model. To address these questions, we engineered Hey cells to express a luciferase reporter (TGL) and an inducible miR-200c lentiviral vector (Hey-TGL-pTRIPz-200c) or empty vector (HeyTGL-pTRIPz-EV). Doxycycline (dox) addition to Hey-TGL-TRIPz-200c cells results in a significant increase in miR-200c expression (Figure 2A, left) and a decrease in the miR-200c targets ZEB1 (reduced to 19.5% ±2.7, P<0.001) and TUBB3 (βIII–Tubulin, reduced to 65.9% ±6.6, P<0.05) (Figure 2A, right). Dox does not affect miR-200c, ZEB or TUBB3 protein levels in the pTRIPz- EV control (data not shown). Dox significantly increases cell death in Hey-TGL-Tripz-200c cells growing in suspension, but not in attached cells (Figure 2B), confirming that miR-200c activation induces increased sensitivity to anoikis. Consistent with our previous findings, dox activation in the pTRIPz-200c containing cells results in increased sensitivity to paclitaxel within the first 72h compared to dox treated TRIPz-EV, as demonstrated by a shift in the IC50 from 2.1nM (EV) to 1.4nM (Tripz-200c) (Figure 2C, left). Additionally, MTT assays demonstrate that long-term dox exposure (5 days) decreased cell survival in the pTRIPz-200c cells but not the empty vector control (Figure 2C, right). Taken together, this data suggests that miR-200c restoration results in an increase in chemosensitivity even though cell proliferation is decreased. Since we engineered these cells to express a luciferase reporter, we determined whether the miR-200c-mediated decrease in cell survival was accurately reflected by luciferase activity using in vivo imaging system (IVIS). Dox exposure did not affect luciferase activity in wild type Hey cells or HeyTGL-Tripz-EV expressing cells, but it significantly decreased the luciferase activity of HeyTGL-Tripz-200c, indicating that miR-200c-mediated decrease in cell survival can be accurately monitored through the luciferase based /IVIS imaging system (Figure 2D). These data suggest that the HeyTGL-TRipz-200c cells constitute a reliable model for studying miR-200c restoration in vivo.
Figure 2. An inducible system for miR-200c restoration in vitro and in vivo.
A) Left: RNA was extracted from HeyTGL wild-type (WT) and HeyTGL-Tripz200c cells after 140h +/− dox and miR-200c levels measured by qRT-PCR. Bars represent average miR-200c expression normalized to RNU6, relative to levels in WT cells, +/− SD (n=3) Right: HeyTGL-Tripz200c cells were plated in triplicate and treated (+Dox) or not (−Dox) with 1µg/ml DOX for 140h. Cell lysates were probed for expression of ZEB, βIII-Tubulin (TUBB3), and α-tubulin. B) Hey TGL-Tripz-200c cells were treated (+Dox) or not (−Dox) with 1µg/ml for 24h and then plated in either attached or suspended conditions and cell death measured by ELISA 24h later. Graph represents cell death relative to untreated-attached cells. Bars= SEM *** p < 0.001 ANOVA followed by Tukey post-hoc test. C) Left: Hey-TGL-Trip-EV or Tripz200c were plated in 96well plates, treated with 1µg/mL doxycycline for 36 hr and then treated with media containing 1µg/mL dox and increasing concentrations of Paclitaxel for additional 72h. Graph shows percentage of cell survival relative to 0nM-paclitaxel treated cells (n=6 per dose). IC50 for paclitaxel sensitivity was calculated using GraphPad sigma plot V5.0, using a sigmoidal dose-response fit analysis. P<0.0001. Right: HeyTGL-TripzEV and HeyTGL-Tripz200c were treated (+Dox) or not (−Dox) with 1µg/mL doxycycline, and cell viability measured at 24, 48, 72 and 140h using MTT assay. Data represents mean absorbance +/− SEM. ** ANOVA followed by Tukey Post-hoc test. P<0.01 Tripz200c+Dox compared to –Dox treated or EV control. D) HeyTGL WT, TripzEV or Tripz200c were plated in triplicate and treated (+Dox) or not (−Dox) for 5 days. Luciferase activity measured using IVIS. Fold change in total-photon flux was calculated relative to untreated WT cells. Bars are mean +/− SD (n=3 per treatment). *P<0.05
Stable miR-200c restoration reduces ovarian tumor formation and tumor burden
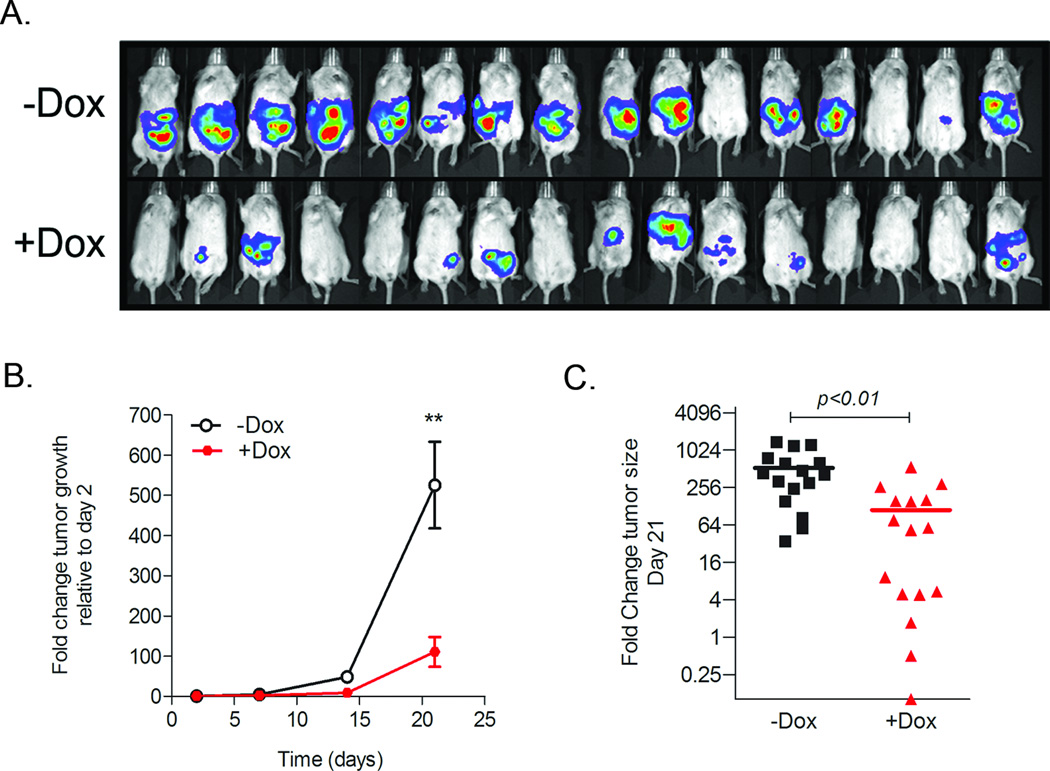
We show that miR-200c increases anoikis and results in decreased cell survival in vitro, thus we hypothesized that miR-200c might have a tumor suppressor effect in vivo in ovarian cancer. To address this question, we injected 100,000 HeyTGL-Trip-200c cells directly into the peritoneal cavity of NOD/SCID mice that had been fed (+Dox, n=16) or not (−Dox, n=16) with doxycycline-supplemented water. Mice were exposed to dox 24h prior to cell injections and constantly thereafter, to ensure the fast upregulation of miR-200c upon cell injection. Since ovarian cancer spreads through the peritoneal cavity, we used IVIS to measure the luciferase signal in both the prone and supine positions. Total tumor burden was calculated by adding the total luciferase flux in the prone and supine position for each time point. As shown in Figure 3, tumors in mice receiving miR-200c (+Dox) have less tumor burden as compared to control (−Dox) mice. We selected 3 mice with similar tumor size in each group and performed RT-PCR analyses of miR-200c levels. As shown in Supplementary Figure 1, miR-200 levels were increased from 200 to 1000 fold compared to control mice. Thus, these data demonstrate that stable miR-200c restoration decreases tumor formation and tumor burden in vivo.
Figure 3. Restoration of miR-200c decreases tumorigenicity of Hey Ovarian Cancer cells.
1 × 105 HeyTGL-Tripz-200c cells were injected IP (n=16/group) in mice treated (+Dox) or not (−Dox) for activation of miR-200c expression. Tumor growth was calculated as fold change relative to initial signal at day 2 after cell injection. A) Imaging of mice at day 21 in the supine position. B) Tumor growth over time (Line =mean +/− SE). *P<0.05 Student t-test at day 21. C). Scatter plot shows tumor sizes at the end of the study (Day 21) in control and dox-treated mice (n=16 per group). Y-axis is plotted in Log2 scale to demonstrate the full range of each mouse tumor burden in both groups. Line= mean. *P<0.01 Student t-test at day 21.
miR-200c restoration in established tumors delays tumor progression and increases sensitivity to paclitaxel
Since miR-200c restoration as a therapeutic in ovarian cancer patients would likely occur as a neoadjuvant or adjuvant treatment with current chemotherapies, we tested the effect of miR-200c restoration on established tumors alone or in combination with paclitaxel (8). Transient restoration of miR-200c using miRNA mimics results in a significant decrease in TUBB3 levels in not only the Hey cells, but two additional ovarian cancer lines that also inappropriately express TUBB3 (Supplementary Figure 2).
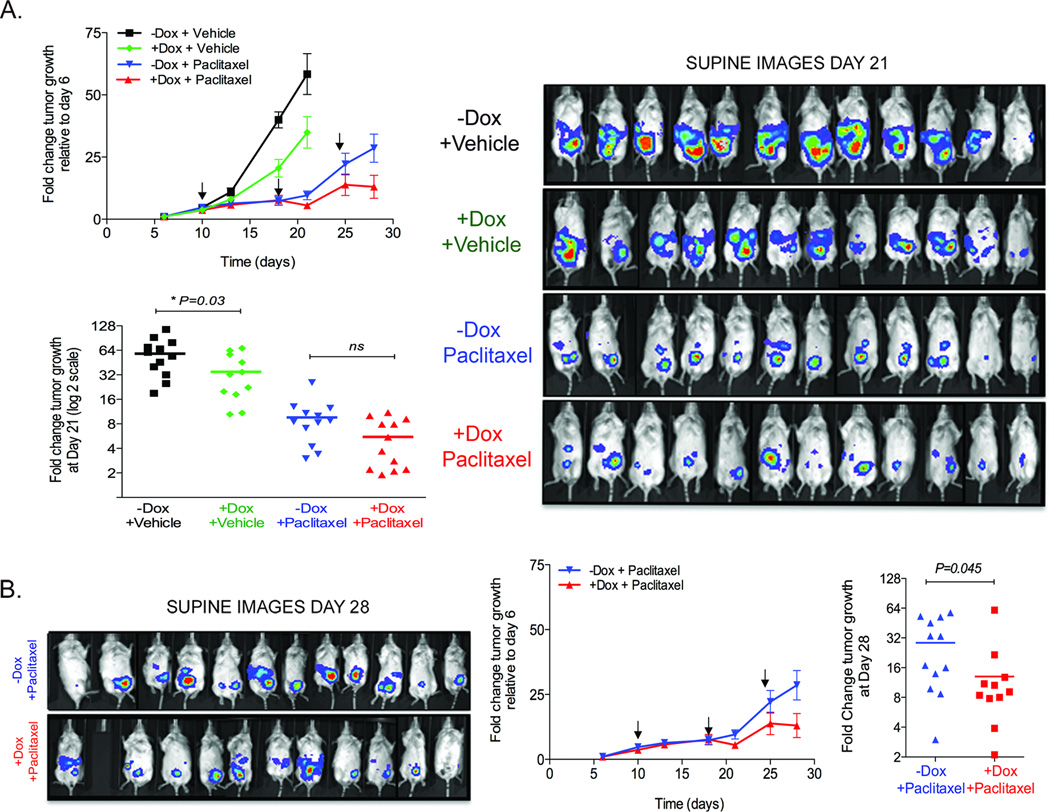
We injected mice with HeyTGL-TRIPz-200c cells and allowed tumors to establish for 6 days. At this time, tumor size was measured and mice with equivalent tumor burden were matched and separated into 4 groups (Supplementary figure 3A). Mice were continuously supplemented with water containing doxycycline or not (+Dox or −Dox groups respectively), and injected intraperitoneally with either vehicle or 10mg/kg body weight paclitaxel (n=12 per group) at days 10, 18 and 24 as indicated. Tumors from mice that received +dox+vehicle (increased miR-200 expression only) showed an overall delay in tumor growth compared to mice where miR-200c expression was not activated (Figure 4A). Mice in these groups were sacrificed at day 21 when the signal in the control group reached saturation (Figure 4A). Tumors from mice receiving paclitaxel showed an overall reduction in tumor burden compared to untreated tumors (Figure 4A, B), but progressed despite treatment with chemotherapy. Importantly, mice given dox to induce miR-200c in combination with paclitaxel, showed a significant decrease in tumor burden by day 28 as compared with mice receiving paclitaxel alone (Figure 4B). Since none of the mice reached complete remission, we tested whether miR-200c restoration had been successful in all mice. MiR-200c expression was significantly increased while ZEB expression was reduced in all groups receiving dox (Supplementary Figure 3B,C), indicating that miR-200 was indeed restored in these tumors.
Figure 4. Restoration of miR-200c in established tumors delays tumor proliferation and increases sensitivity to paclitaxel.
1 × 105 Hey TGL-Tripz200c cells were injected IP. Tumor burden was measured after 6 days and equivalent intraperitoneal tumor burdens were matched to four different groups (n=12 per group). MiR-200c was induced with doxycycline from day 9, and either vehicle or 10 mg/kg paclitaxel/Kg body weight were injected IP at indicated times (arrows). Tumor burden was calculated as fold change relative to values at Day 6. A) Top: Tumor growth over time for all groups. Graph shows mean +/− SEM. Bottom: Scatter plot shows tumor burden in individual mice for all groups at day 21. Tumor size is significantly smaller in and miR-200c expressing (+Dox+Vehicle) group compared to untreated (−Dox+Vehicle) tumors at day 21 (P<0.05 Student T-test). Tumor size is not statistically different in paclitaxel-treated groups (ns) by day 21. Right: Image of mice in the supine position at day 21. B) Left: Image of mice in the supine position at day 28 in the paclitaxel-treated groups. Middle: Tumor growth over time for paclitaxel-treated groups. Arrows indicate paclitaxel injection. Right: Scatter plot of tumor sizes in the paclitaxel-treated (−Dox+Paclitaxel) and miR-200c+Paclitaxel treated (+Dox+Paclitaxel) groups at the end of the experiment (day 28). Line=mean. P<0.05 Student t-test. Y-axis is plotted in Log2 scale.
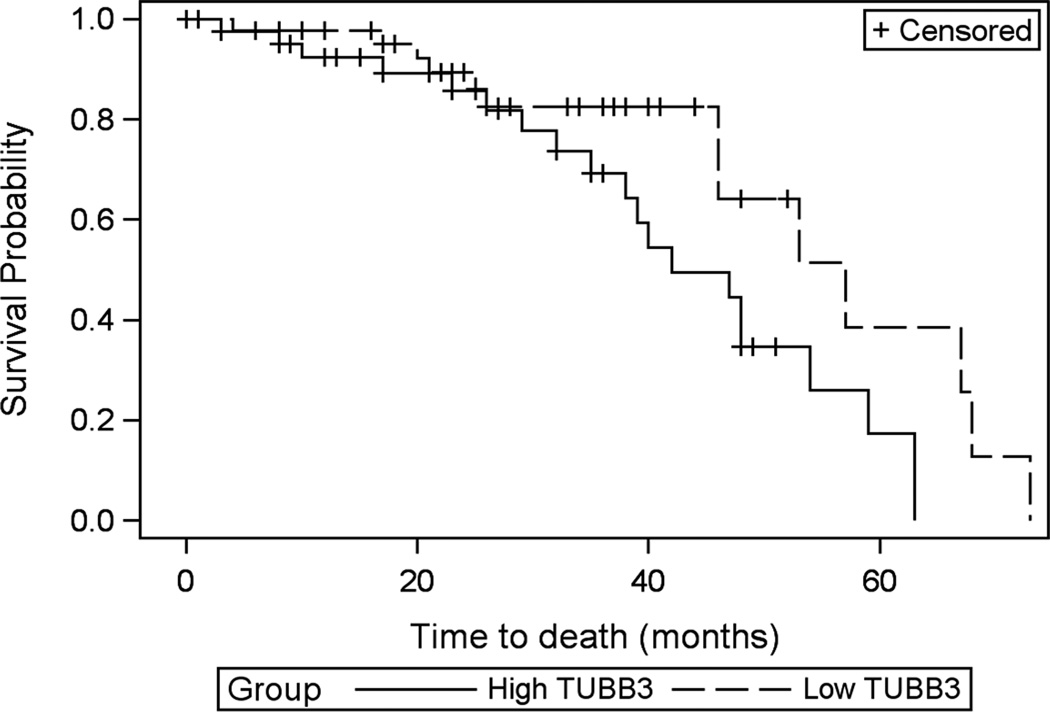
Protein expression of TUBB3 significantly correlates with shorter survival
We performed immunohistochemistry for TUBB3 on a cohort of 129 primary high-grade (stage III or IV) serous ovarian carcinomas from MD Anderson Cancer Center (described previously) (25). Patients with tumors with a low tumor TUBB3 score (1; weak staining in 1–10% of tumor) had significantly prolonged survival (average survival 52.73 ± 4.08 months) compared to patients with tumors with high TUBB3 score (3 or 4; moderate to strong staining in more than 10% of tumor (average survival 42.56 ±3.19 months) (Figure 5). The effect on survival is no longer significant when tumors with the intermediate TUBB3 score of 2 are included in the analysis (data not shown).
Figure 5. Kaplan Meier curves of overall survival in high vs. low TUBB3 expression in a cohort of ovarian cancer patients.
High TUBB3 expression is related to shorter survival compared to low TUBB3 expression. Unadjusted P=0.0438, unadjusted CI (95%): (1.022–4.720). High TUBB3 expression was defined as tumors containing moderate to high intensity staining in more than 10% positive tumor cells (n=44) and low TUBB3 staining was defined as weak or moderate intensity staining in 1 to 10% of the tumor cells (n=52).
DISCUSSION
In breast and several other carcinomas, it is well established that expression of miR-200c and family members is lower in tumor versus normal tissue and that low miR-200c is clearly linked with tumor progression. In ovarian cancer, this relationship is not clear. Some studies have indicated high miR-200 family members in ovarian tumor versus normal samples (26–30). Other studies have shown no differences in miR-200 family expression in ovarian tumors (31, 32). However, some of these studies compared ovarian tumors with whole normal ovarian tissue as a control, which contains few surface epithelial cells relative to stromal cells. Only a few studies isolated “normal” human immortalized ovarian surface epithelial cells (HOSE) in culture and in most cases the HOSE cells were generated by various immortalization methods which could potentially affect miR-200c levels. It is also not clear that OSE are always the appropriate control, as some ovarian cancers originate from fallopian tubes (33–36).
Additional complexity arises from the fact that in contrast to breast cancer, in ovarian cancer studies, it is most often metastatic-tumor that is harvested and profiled, not primary tumor. Ovarian cancer cells are thought to undergo EMT to facilitate formation of spheroids in the ascitic fluid and survival prior to attachment at metastatic sites(37). The miR-200 family has been demonstrated to facilitate this process because forcing expression of miR-200 members abrogates the capacity of cancer cells to form spheroids (38). Expression of miR-200 is higher in stage I EOC compared to stage III EOC (39) indicating a downregulation of miR-200 in widespread metastasis. Interestingly, the miR-200 target, ZEB2, is expressed in cells isolated from effusions indicating that loss of miR-200 has allowed expression of this target (40, 41). Regardless of the difference in miR-200 between tumor and the appropriate “normal” control, two recent studies found that in both early (stage 1) and advanced (stage III) ovarian cancers, low miR-200 expression is significantly associated with recurrence and poor overall survival (42, 43).
Following our report that restoration miR-200c increases sensitivity to taxanes in vitro, by directly targeting TUBB3 (7, 8), two independent studies demonstrated that low tumoral miR-200c expression is significantly associated with high TUBB3 protein expression in advanced stage serous carcinomas (15, 16). Tumors of patients with minimal response to chemotherapy and low overall survival had lower miR-200c (and higher TUBB3 protein) compared to those with complete response (16). Interestingly, patients with TUBB3 protein expression in greater than 25% of tumor cells in effusions from advanced-stage serous ovarian carcinomas had a mean overall survival of 32 months as compared to 53 months for patients with tumors with less than 25% of tumor cells staining and high TUBB3 staining also correlated with primary chemoresistance (15). Similarly, we observe a relationship between high TUBB3 protein and shorter survival in a cohort of advanced stage (III or IV) high-grade serous ovarian carcinomas. It may be of biological significance that the results were more dramatic and more strongly predictive of outcome in the study examining tumor cells from effusions (15). We previously demonstrated that lack of miR-200c expression is associated with anoikis resistance and restoration of miR-200c restores anoikis sensitivity in breast and endometrial cancer cells (18) and here we observe the same to be true of ovarian cancer cells. This could indicate that TUBB3 protein may be even higher in malignant effusions (where miR-200 is even lower) as compared to surgical specimens and may therefore be a more useful biomarker in malignant effusions.
It is highly likely that increases in other miR-200 targets in addition to ZEB1/2 and TUBB3 may play a role in tumor progression and relapse. In ovarian cancer, metastases primarily occur by direct seeding and ovarian cancer cells or spheroids must therefore acquire resistance to anoikis in order to survive in ascitic fluid before attaching to the peritoneum. Recently, we have shown that miR-200c restores anoikis sensitivity in aggressive breast and endometrial cancer cells by directly targeting TrkB (18). TrkB has been found to be expressed in ovarian cancer and implicated in anoikis resistance (22, 23, 44–46). Here we demonstrate that restoration of miR-200c indeed enhances anoikis sensitivity in ovarian cancer cells. Importantly, our data demonstrate that restoration of miR-200c at the onset of tumor formation significantly diminished tumor burden in vivo. This finding supports the idea that increased anoikis sensitivity upon restoration of miR-200c via repression of TrkB or other additional targets, and/or decreased attachment to the peritoneum may contribute to the ability of this miRNA to decrease tumor burden in vivo. When we restored miR-200c to established tumors, the effect was significant, but less dramatic, suggesting that the ability of miR-200c to affect the solid tumor is slighter than its ability to reduce tumor burden when given at stages where it can increase anoikis, potentially resulting in less tumor attachment.
Here we utilize an inducible in vivo model to directly test whether restoration of miR-200c would enhance the efficacy of paclitaxel in vivo. We find that restoration of miR-200c not only significantly decreases tumor burden, but also significantly enhances the response of established tumor to paclitaxel. Our study suggests that restoration of miR-200c immediately prior to treatment with paclitaxel could enhance response or allow for a lower effective dose initially. Alternatively, miR-200c given with paclitaxel as second line therapy upon relapse might improve response. Further pre-clinical evaluations along these lines and optimization of non-viral delivery methods are warranted.
Supplementary Material
ACKNOWLEDGMENTS
We thank the University of Colorado Small Animal Imaging shared resource facility Center for Comparative Medicine at University of Colorado Anschutz Medical Campus for veterinarian support and animal care. We acknowledge Ms. Connie Liu, University of Colorado Summer Fellow for her contributions and the University of Colorado Cancer Center DNA Sequencing and Analysis and the Animal Imaging Shared Resource Facility supported by P30CA046934.
GRANT SUPPORT
NIH P50CA83639 SPORE in Ovarian Cancer (RRB); Cancer League of Colorado (JKR); Colorado Women’s Reproductive Health Research Career Development Center (K12 HD001271) (ID) and Department of Obstetrics and Gynecology, UC Denver supported this work.
Abbreviations
- TUBB3
class IIIβ-tubulin
- EOC
Epithelial ovarian cancer
- Dox
doxycycline
- miR-200c
microRNA-200c
- IVIS
in vivo imaging system
Footnotes
Conflict of Interest: The authors declare that they have no competing interests.
AUTHOR’S CONTRIBUTIONS
DMC (most experiments), ID (adhesion assays, in vivo imaging); ENH (anoikis experiments); NS, AJ (IHC); RRB (provided clinical samples), MDP (pathology scores), XL (statistical analysis clinical data); MAS, ID, RRB and MP provided intellectual input regarding clinical aspects of ovarian cancer. DMC, ID, ENH, DRC, MAS, RRB and JKR contributed intellectual input towards the design, implementation and interpretation of results. DMC and JR wrote the manuscript. All authors read and approved the final manuscript.
REFERENCES
- 1.Hennessy BT, Coleman RL, Markman M. Ovarian cancer. Lancet. 2009;374:1371–1382. doi: 10.1016/S0140-6736(09)61338-6. [DOI] [PubMed] [Google Scholar]
- 2.Kurman RJ, Shih Ie M. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–443. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177:1053–1064. doi: 10.2353/ajpath.2010.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levanon K, Crum C, Drapkin R. New insights into the pathogenesis of serous ovarian cancer and its clinical impact. J Clin Oncol. 2008;26:5284–5293. doi: 10.1200/JCO.2008.18.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hope JM, Blank SV. Current status of maintenance therapy for advanced ovarian cancer. Int J Womens Health. 2010;1:173–180. doi: 10.2147/ijwh.s4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cochrane DR, Howe EN, Spoelstra NS, Richer JK. Loss of miR-200c: A Marker of Aggressiveness and Chemoresistance in Female Reproductive Cancers. J Oncol. 2010;2010:821717. doi: 10.1155/2010/821717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cochrane DR, Spoelstra NS, Howe EN, Nordeen SK, Richer JK. MicroRNA-200c mitigates invasiveness and restores sensitivity to microtubule-targeting chemotherapeutic agents. Mol Cancer Ther. 2009;8:1055–1066. doi: 10.1158/1535-7163.MCT-08-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kavallaris M. Microtubules and resistance to tubulin-binding agents. Nat Rev Cancer. 2010;10:194–204. doi: 10.1038/nrc2803. [DOI] [PubMed] [Google Scholar]
- 10.Kavallaris M, Annereau JP, Barret JM. Potential mechanisms of resistance to microtubule inhibitors. Semin Oncol. 2008;35:S22–S27. doi: 10.1053/j.seminoncol.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Pasquier E, Kavallaris M. Microtubules: a dynamic target in cancer therapy. IUBMB Life. 2008;60:165–170. doi: 10.1002/iub.25. [DOI] [PubMed] [Google Scholar]
- 12.Pusztai L. Markers predicting clinical benefit in breast cancer from microtubule-targeting agents. Ann Oncol. 2007;18(Suppl 12):xii15–xii20. doi: 10.1093/annonc/mdm534. [DOI] [PubMed] [Google Scholar]
- 13.Mozzetti S, Ferlini C, Concolino P, Filippetti F, Raspaglio G, Prislei S, et al. Class III beta-tubulin overexpression is a prominent mechanism of paclitaxel resistance in ovarian cancer patients. Clin Cancer Res. 2005;11:298–305. [PubMed] [Google Scholar]
- 14.Umezu T, Shibata K, Kajiyama H, Terauchi M, Ino K, Nawa A, et al. Taxol resistance among the different histological subtypes of ovarian cancer may be associated with the expression of class III beta-tubulin. Int J Gynecol Pathol. 2008;27:207–212. doi: 10.1097/PGP.0b013e318156c838. [DOI] [PubMed] [Google Scholar]
- 15.Hetland TE, Hellesylt E, Florenes VA, Trope C, Davidson B, Kaern J. Class III beta-tubulin expression in advanced-stage serous ovarian carcinoma effusions is associated with poor survival and primary chemoresistance. Hum Pathol. 2011;42:1019–1026. doi: 10.1016/j.humpath.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 16.Leskela S, Leandro-Garcia LJ, Mendiola M, Barriuso J, Inglada-Perez L, Munoz I, et al. The miR-200 family controls beta-tubulin III expression and is associated with paclitaxel-based treatment response and progression-free survival in ovarian cancer patients. Endocr Relat Cancer. 2011;18:85–95. doi: 10.1677/ERC-10-0148. [DOI] [PubMed] [Google Scholar]
- 17.De Donato M, Mariani M, Petrella L, Martinelli E, Zannoni GF, Vellone V, et al. Class III beta-tubulin and the cytoskeletal gateway for drug resistance in ovarian cancer. J Cell Physiol. 2012;227:1034–1041. doi: 10.1002/jcp.22813. [DOI] [PubMed] [Google Scholar]
- 18.Howe EN, Cochrane DR, Richer JK. Targets of miR-200c mediate suppression of cell motility and anoikis resistance. Breast Cancer Res. 2011;13:R45. doi: 10.1186/bcr2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Douma S, Van Laar T, Zevenhoven J, Meuwissen R, Van Garderen E, Peeper DS. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature. 2004;430:1034–1039. doi: 10.1038/nature02765. [DOI] [PubMed] [Google Scholar]
- 20.Geiger TR, Peeper DS. Critical role for TrkB kinase function in anoikis suppression, tumorigenesis, and metastasis. Cancer Res. 2007;67:6221–6229. doi: 10.1158/0008-5472.CAN-07-0121. [DOI] [PubMed] [Google Scholar]
- 21.Thiele CJ, Li Z, McKee AE. On Trk--the TrkB signal transduction pathway is an increasingly important target in cancer biology. Clin Cancer Res. 2009;15:5962–5967. doi: 10.1158/1078-0432.CCR-08-0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu X, Liu L, Cai B, He Y, Wan X. Suppression of anoikis by the neurotrophic receptor TrkB in human ovarian cancer. Cancer Sci. 2008;99:543–552. doi: 10.1111/j.1349-7006.2007.00722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu XH, Yang YX, Cai B, Yan Q, He YY, Wan XP. [Anoikis-suppression and invasion induced by tyrosine kinase receptor B in OVCAR3 ovarian cancer cells] Zhonghua Fu Chan Ke Za Zhi. 2008;43:695–699. [PubMed] [Google Scholar]
- 24.Korch C, Spillman MA, Jackson TA, Jacobsen BM, Murphy SK, Lessey BA, et al. DNA profiling analysis of endometrial and ovarian cell lines reveals misidentification, redundancy and contamination. Gynecol Oncol. 2012 doi: 10.1016/j.ygyno.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlumbrecht MP, Xie SS, Shipley GL, Urbauer DL, Broaddus RR. Molecular clustering based on ERalpha and EIG121 predicts survival in high-grade serous carcinoma of the ovary/peritoneum. Mod Pathol. 2011;24:453–462. doi: 10.1038/modpathol.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bendoraite A, Knouf EC, Garg KS, Parkin RK, Kroh EM, O'Briant KC, et al. Regulation of miR-200 family microRNAs and ZEB transcription factors in ovarian cancer: evidence supporting a mesothelial-to-epithelial transition. Gynecol Oncol. 2010;116:117–125. doi: 10.1016/j.ygyno.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iorio MV, Visone R, Di Leva G, Donati V, Petrocca F, Casalini P, et al. MicroRNA signatures in human ovarian cancer. Cancer Res. 2007;67:8699–8707. doi: 10.1158/0008-5472.CAN-07-1936. [DOI] [PubMed] [Google Scholar]
- 28.Nam EJ, Yoon H, Kim SW, Kim H, Kim YT, Kim JH, et al. MicroRNA expression profiles in serous ovarian carcinoma. Clin Cancer Res. 2008;14:2690–2695. doi: 10.1158/1078-0432.CCR-07-1731. [DOI] [PubMed] [Google Scholar]
- 29.Wyman SK, Parkin RK, Mitchell PS, Fritz BR, O'Briant K, Godwin AK, et al. Repertoire of microRNAs in epithelial ovarian cancer as determined by next generation sequencing of small RNA cDNA libraries. PLoS ONE. 2009;4:e5311. doi: 10.1371/journal.pone.0005311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang H, Kong W, He L, Zhao JJ, O'Donnell JD, Wang J, et al. MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res. 2008;68:425–433. doi: 10.1158/0008-5472.CAN-07-2488. [DOI] [PubMed] [Google Scholar]
- 31.Dahiya N, Sherman-Baust CA, Wang TL, Davidson B, Shih Ie M, Zhang Y, et al. MicroRNA expression and identification of putative miRNA targets in ovarian cancer. PLoS ONE. 2008;3:e2436. doi: 10.1371/journal.pone.0002436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang L, Volinia S, Bonome T, Calin GA, Greshock J, Yang N, et al. Genomic and epigenetic alterations deregulate microRNA expression in human epithelial ovarian cancer. Proc Natl Acad Sci U S A. 2008;105:7004–7009. doi: 10.1073/pnas.0801615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roh MH, Yassin Y, Miron A, Mehra KK, Mehrad M, Monte NM, et al. High-grade fimbrial-ovarian carcinomas are unified by altered p53, PTEN and PAX2 expression. Mod Pathol. 2010;23:1316–1324. doi: 10.1038/modpathol.2010.119. [DOI] [PubMed] [Google Scholar]
- 34.Jarboe EA, Folkins AK, Drapkin R, Ince TA, Agoston ES, Crum CP. Tubal and ovarian pathways to pelvic epithelial cancer: a pathological perspective. Histopathology. 2009;55:619. doi: 10.1111/j.1365-2559.2009.03408.x. [DOI] [PubMed] [Google Scholar]
- 35.Crum CP. Intercepting pelvic cancer in the distal fallopian tube: theories and realities. Mol Oncol. 2009;3:165–170. doi: 10.1016/j.molonc.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crum CP, Drapkin R, Kindelberger D, Medeiros F, Miron A, Lee Y. Lessons from BRCA: the tubal fimbria emerges as an origin for pelvic serous cancer. Clin Med Res. 2007;5:35–44. doi: 10.3121/cmr.2007.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan DS, Agarwal R, Kaye SB. Mechanisms of transcoelomic metastasis in ovarian cancer. Lancet Oncol. 2006;7:925–934. doi: 10.1016/S1470-2045(06)70939-1. [DOI] [PubMed] [Google Scholar]
- 38.Gibbons DL, Lin W, Creighton CJ, Rizvi ZH, Gregory PA, Goodall GJ, et al. Contextual extracellular cues promote tumor cell EMT and metastasis by regulating miR-200 family expression. Genes Dev. 2009;23:2140–2151. doi: 10.1101/gad.1820209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eitan R, Kushnir M, Lithwick-Yanai G, David MB, Hoshen M, Glezerman M, et al. Tumor microRNA expression patterns associated with resistance to platinum based chemotherapy and survival in ovarian cancer patients. Gynecol Oncol. 2009;114:253–259. doi: 10.1016/j.ygyno.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 40.Elloul S, Silins I, Trope CG, Benshushan A, Davidson B, Reich R. Expression of E-cadherin transcriptional regulators in ovarian carcinoma. Virchows Arch. 2006;449:520–528. doi: 10.1007/s00428-006-0274-6. [DOI] [PubMed] [Google Scholar]
- 41.Elloul S, Elstrand MB, Nesland JM, Trope CG, Kvalheim G, Goldberg I, et al. Snail, Slug, and Smad-interacting protein 1 as novel parameters of disease aggressiveness in metastatic ovarian and breast carcinoma. Cancer. 2005;103:1631–1643. doi: 10.1002/cncr.20946. [DOI] [PubMed] [Google Scholar]
- 42.Hu X, Macdonald DM, Huettner PC, Feng Z, El Naqa IM, Schwarz JK, et al. A miR-200 microRNA cluster as prognostic marker in advanced ovarian cancer. Gynecol Oncol. 2009;114:457–464. doi: 10.1016/j.ygyno.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 43.Marchini S, Cavalieri D, Fruscio R, Calura E, Garavaglia D, Nerini IF, et al. Association between miR-200c and the survival of patients with stage I epithelial ovarian cancer: a retrospective study of two independent tumour tissue collections. Lancet Oncol. 2011;12:273–2785. doi: 10.1016/S1470-2045(11)70012-2. [DOI] [PubMed] [Google Scholar]
- 44.Au CW, Siu MK, Liao X, Wong ES, Ngan HY, Tam KF, et al. Tyrosine kinase B receptor and BDNF expression in ovarian cancers - Effect on cell migration, angiogenesis and clinical outcome. Cancer Lett. 2009;281:151–161. doi: 10.1016/j.canlet.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 45.Qiu L, Zhou C, Sun Y, Di W, Scheffler E, Healey S, et al. Crosstalk between EGFR and TrkB enhances ovarian cancer cell migration and proliferation. Int J Oncol. 2006;29:1003–1011. [PubMed] [Google Scholar]
- 46.Siu MK, Wong OG, Cheung AN. TrkB as a therapeutic target for ovarian cancer. Expert Opin Ther Targets. 2009;13:1169–1178. doi: 10.1517/14728220903196787. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.