Abstract
Skin that is exposed to radiation has an impaired ability to heal wounds. This is especially true for whole body irradiation, where even moderate non-lethal doses can result in wound healing deficits. Our previous attempts to administer dermal cells locally to wounds to correct radiation-induced deficits were hampered by poor cell retention. Here we improve the outcome by using biodegradable fibrin microbeads (FMB) to isolate a population of mesenchymal marrow-derived stromal cells (MSC) from murine bone marrow by their specific binding to the fibrin matrix, culture them to high density in vitro and deliver them as MSC on FMB at the wound site. MSC are retained and proliferate locally and assist wounds gain tensile strength in whole body irradiated mice with or without additional skin only exposure. MSC-FMB were effective in 2 different mouse strains but were ineffective across a major histocompatability barrier. Remarkably, irradiated mice whose wounds were treated with MSC-FMB showed enhanced hair regrowth suggesting indirect effect on the correction of radiation-induced follicular damage. Further studies showed that additional wound healing benefit could be gained by administration of G-CSF and AMD3100. Collagen strips coated with haptides and MSCs were also highly effective in correcting radiation-induced wound healing deficits.
Introduction
There is growing concern about the dearth of medical countermeasures for the treatment of ionizing radiation injuries in the event of either a radiological or nuclear incident (Williams and McBride, 2011). This has led to many studies directed at correcting the acute radiation syndrome (ARS). Much is known about how different tissues individually respond to radiation but less about how damage to one tissue affects healing of another and how effects are compounded by non-radiation injuries. The skin is particularly susceptible to compounded injuries.
In an ARS situation, rapid wound closure, re-epithelialization, and re-establishment of the skin tissue integrity are top clinical treatment priorities. These processes are compromised by irradiation, which seriously impedes the healing of physical or thermal skin wounds. Indeed radiation-impaired wound healing is a specific form of the general clinical challenge posed by non-healing wounds for which few effective treatment options are available (Olascoaga et al., 2008).
Healing of skin wounds involves complex, well-orchestrated interactions between different cell types and extracellular matrix materials (Wu et al., 2007b). After exposure to ionizing radiation, the time to expression of skin damage is determined by the slow turnover of dermal cells (Withers, 1967). However, trauma speed the proliferation rate of the affected cells and thereby greatly accelerates expression of any radiation-induced latent damage and wounded irradiated skin displays healing defects acutely (Gorodetsky et al., 1988). The dose and the body volume are critical variables. The dermis is relatively radiation resistant but local stem/progenitor skin cells can be damaged so that they fail to replace those lost through normal turnover or through physical or thermal injury. On the other hand, damage to the more sensitive hematopoietic system through whole or partial body radiation exposure can compromise the pool of bone marrow-derived cells that contribute to the healing process. This includes stem cells, immune cells, endothelial progenitor cells, and fibrocytes all of which can contribute to healing either structurally or as regulatory influences (Wu et al., 2007b). Sublethal whole body irradiation (WBI) therefore requires far less dose than skin only irradiation (SI) to delay healing of full thickness incisional wounds in mouse skin (Vegesna et al., 1993). In a radiological incident the whole body dose received is therefore critical to the assessment of potential deficits in wound healing. The dose received by the hematopoietic system may however be very different from that received by the skin due to proximity to the radiation source.
In recent years, multiple animal injury models, as well human studies (Garcia-Gomez et al., 2010), have shown that MSC are excellent candidates for enhancing tissue repair, including damage caused by radiation (Leclerc et al., 2011). This is enhanced by reports that they may be effective across histocompatibility barriers (Shi et al., 2010), although their true potential in this regard is still controversial.
Our approach to correcting radiation-induced wound healing problems using MSC was tempered by our previous experience. Based on an earlier study in pigs (Kruegler, 1978.), we reported that implanted neonate skin fibroblasts could partly correct radiation-induced wound healing deficits in mice (Gorodetsky et al., 1991), findings that Dantzer (Dantzer et al., 2003) later extended in a rat model using bone marrow-derived stromal cells. The primary factor responsible for our limited success at the time was that cells implanted directly into a wound site rapidly disappeared, with <1% remaining for more than a few days. To circumvent this problem, we developed a novel fibrin microbead (FMB) cell carrier (Gorodetsky, 2008; Gorodetsky et al., 1999; Gorodetsky et al., 2004). Matrix-dependent cells including MSC attach to the FMB in 3 dimensional suspension culture, allowing easy removal of the non-attaching hematopoietic and epidermal cells. Attachment is mediated by newly described cell binding homologous C-terminal short peptides on β- and γ-chains of fibrin, termed haptides (Gorodetsky et al., 1998; Gorodetsky et al., 2003; Levy-Beladev et al., 2010). MSC isolated on FMB proliferate to high density (up to 108/ml packed beads) in vitro, yielding up to a log more cells than conventional plastic adhesion-based culture methods.
When MSC-FMB are implanted in skin wounds in vivo, the FMB degrade slowly and the MSC are retained in high numbers to proliferate and differentiate normally within the target tissue. Also, FMB can support the viability of MSC for up to 10 days at room temperature, making cell transportation easy in emergencies (Gorodetsky et al., 2011).
Here, we use the incisional wound healing model to examine the ability of MSC-FMB to correct radiation-induced damage. The ability of MSC-FMB to act across an allogeneic barrier was examined, as was the effects of addition of granulocyte colony-stimulating factor (G-CSF) and Plerixafor (AMD3100). Finally, the ability of MSC on haptized collagen strips to reduce radiation wound healing deficits was assessed.
Results
Dose/time effects of WBI and SI on gain in skin WTS
A dermal wound healing model was established to examine the effect of irradiating the skin only with or without total body exposure of the hematopoietic system with the aim of examining how different doses to these different organs, as might easily happen in a radiological situation, would interact.
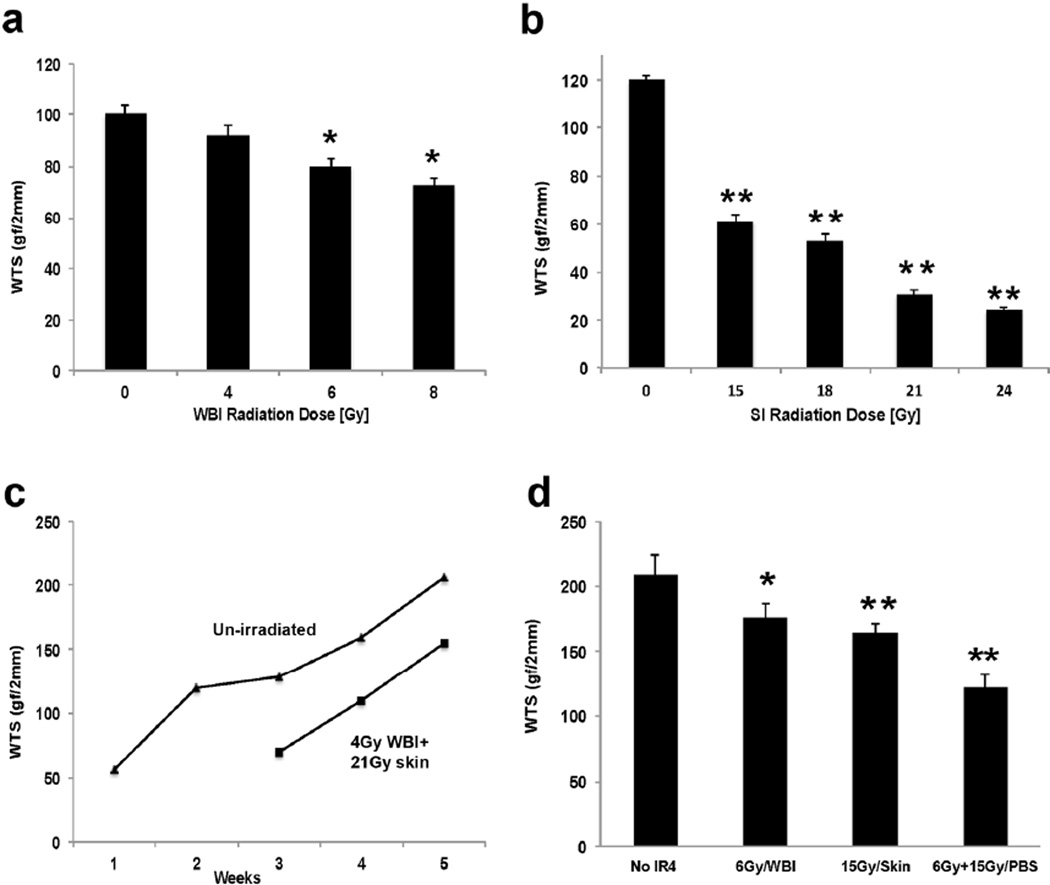
Sublethal WBI of C3H mice using gamma rays compromised the ability of wounds in their skin to gain WTS by 2 weeks (Fig 1a). After 4 Gy WTS was approximately 10% lower; after 6 Gy 20%; and after 8 Gy 30%. These data agree closely with what we found previously (Vegesna et al., 1993). In contrast, doses of around 15 Gy 150kVp X-rays to the skin only (SI) were required to reduce the gain in WTS by 50% at 2 weeks (Fig 1b), rather more than the 13 Gy that we first reported 20 years ago, but considering that the irradiator and tensiometer were different, the reproducibility of radiation effects on WTS measurements is remarkable.
Figure 1. Effects of WBI and SI on wound healing in C3H mice.
a) WTS was measured 2 weeks after varying doses of WBI exposure b) WTS measured at 2 weeks after varying doses of SI exposure. c) The effect of time on gain in WTS was assessed in un-irradiated skin (triangles) and skin exposed to 4Gy/WBI + 21Gy/SI (squares). WTS measurements at 2 weeks are unreliable within these high SI doses as the first “phase” of wound healing is most affected by radiation d) WTS was measured 4 weeks after 6Gy WBI or 15Gy SI, or both combined. Error bars indicate mean+/-SEM, n=4. Significance: *P<0.05, **P<0.01 by Student’s t-test. (WTS: wound tensile strength; WBI: whole body irradiation; SI: Skin only irradiation)
The gain in WTS with time after radiation was also re-examined. We confirmed our earlier finding that WTS increased in unirradiated C3H skin in two phases with nearly half normal strength being recovered within 2 weeks (Fig 1c) and 90% by 4–5 weeks (Fig 1c) (Gorodetsky et al., 1988). C57Bl/6 skin responded in a very similar manner though with slightly higher WTS values (not shown), which could have been due to sex or strain differences. The combination of 4 Gy WBI and 21 Gy SI, a scenario that mimics the expected scenario of a radiological incident where the skin might be compromised by a high dose while the whole body might receive a more moderate dose, resulted in a fairly consistent delay of around 10 days to gain the unirradiated level of WTS in C3H mice at 3 or 4 weeks (Fig 1c). The first “phase” of wound healing is most affected by radiation exposure (Gorodetsky et al., 1988), so much so that the 2 week values are too low to be reliable after these doses. We therefore chose to measure the effects of 6Gy WBI and 15Gy SI on WTS measured at 4 weeks, the combined WBI and SI deficit in WTS being more than additive (Fig 1d).
MSC-FMB compared to plastic adherent MSC populations
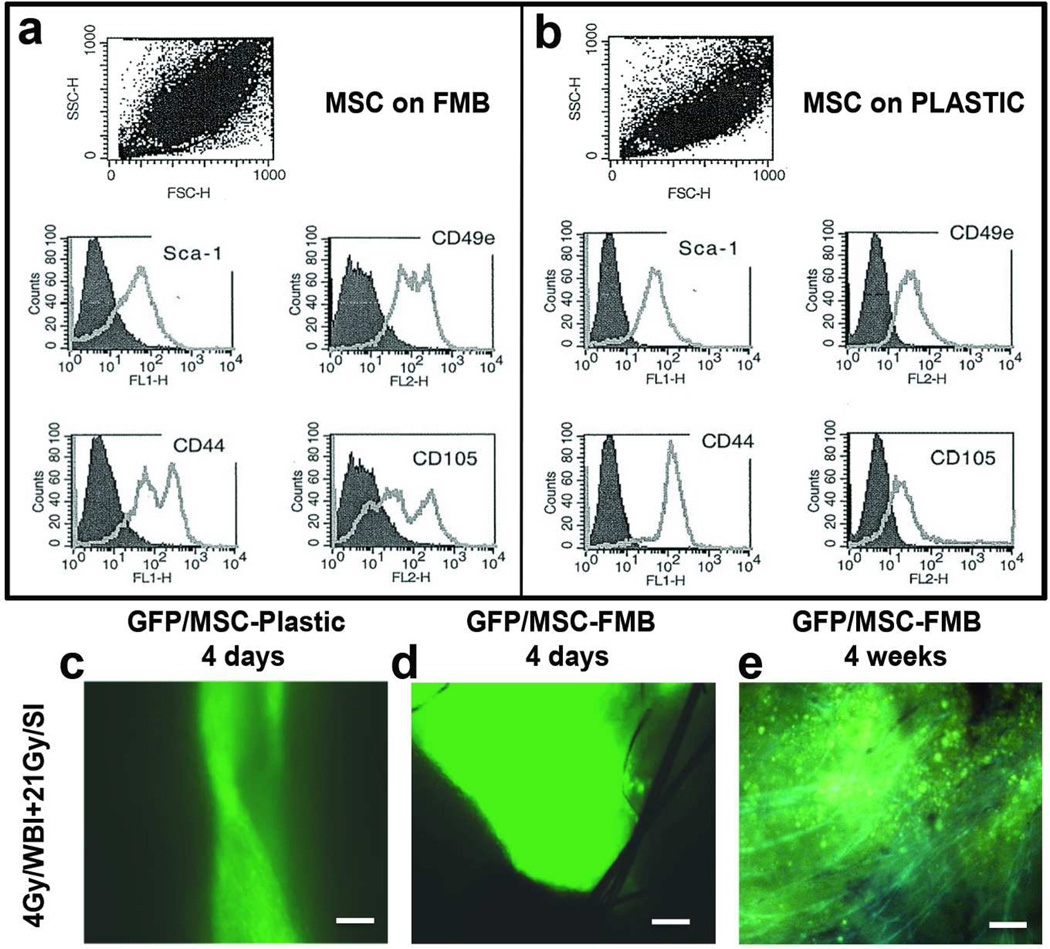
FMB were used as a substrate for culture of MSC to allow their rapid purification from hematopoietic cells and high-density expansion (Gorodetsky, 2008). Because culture on FMB may change the MSC phenotype, we have previously compared the flow cytometric profiles of MSC downloaded from FMB to those cultured on plastic (Rivkin et al., 2007) using a wide range of putative MSC markers which were more characteristic to a pure population of MSC than the plastic isolated cells. For these experiments, the major differences were that MSC cultured on FMB (Fig 2a) showed bimodal distributions for CD44, CD49e and CD105 with increases in expression of CD49e and CD105. The bimodal distribution was largely contributed to by Sca1+, CD44lo, CD49ehi, CD105hi cells of smaller size (not shown). CD45+ and CD19+ cells were absent indicating purification from hematopoietic cells, which was not the case for cells grown on plastic, while the proportions of CD29 and CD31 positive cells were identical in the two populations (Rivkin et al., 2007).
Figure 2. Phenotypic profile of C57Bl/6 marrow stromal cells –FMB.
(a,b) Flow cytometric analysis of MSC markers Sca-1, CD49e, CD44, and CD105 of MSC grown on FMB or on plastic c,d) MSC/GFP grown on plastic or loaded on FMB were implanted in pre-irradiated wounds in C57Bl/6 mice. Scale bar: 200μm e) Four weeks after implantation, MSC/GFP-FMB were still clearly detectable in healing wounds but not in skin wounds implanted with MSC isolated and grown on plastic (not shown). Combination of fluorescence with dim light to demonstrate the fluorescence with the outline structures of the intact skin. Scale bar: 200mm (MSC: Marrow Stromal Cells; FMB: Fibrin Microbeads; WBI: whole body irradiation; SI: Skin only irradiation)
WBI, but not SI, impairs normal cell infiltration into wounds (Vegesna et al., 1993), suggesting dependency of optimal wound healing on bone marrow-derived cells and that MSC may be a useful therapeutic intervention in combined radiation/wound healing scenarios. We considered that FMB-MSC implantation into the wound may additionally overcome the rapid loss of cells from the wound site by allowing MSC to download slowly from the FMB into the damaged tissue where they could proliferate and differentiate under local influences within the wound microenvironment. Importantly, if GFP-MSC from transgenic mouse bone marrow that were highly positive for GFP by flow cytometry were cultured and implanted on FMB into wounds in WBI+SI treated mice they could be detected after 4 days at higher numbers than if they came from plastic adherent cultures (Fig 2c,d). Additionally, they integrated in the repaired dermis and persisted for at least 4 weeks post-implantation in the healing wounds (Fig 2e) when the plastic isolated cells that were injected could not be detected.
Syngeneic MSC-FMB correct radiation-induced deficits in WTS
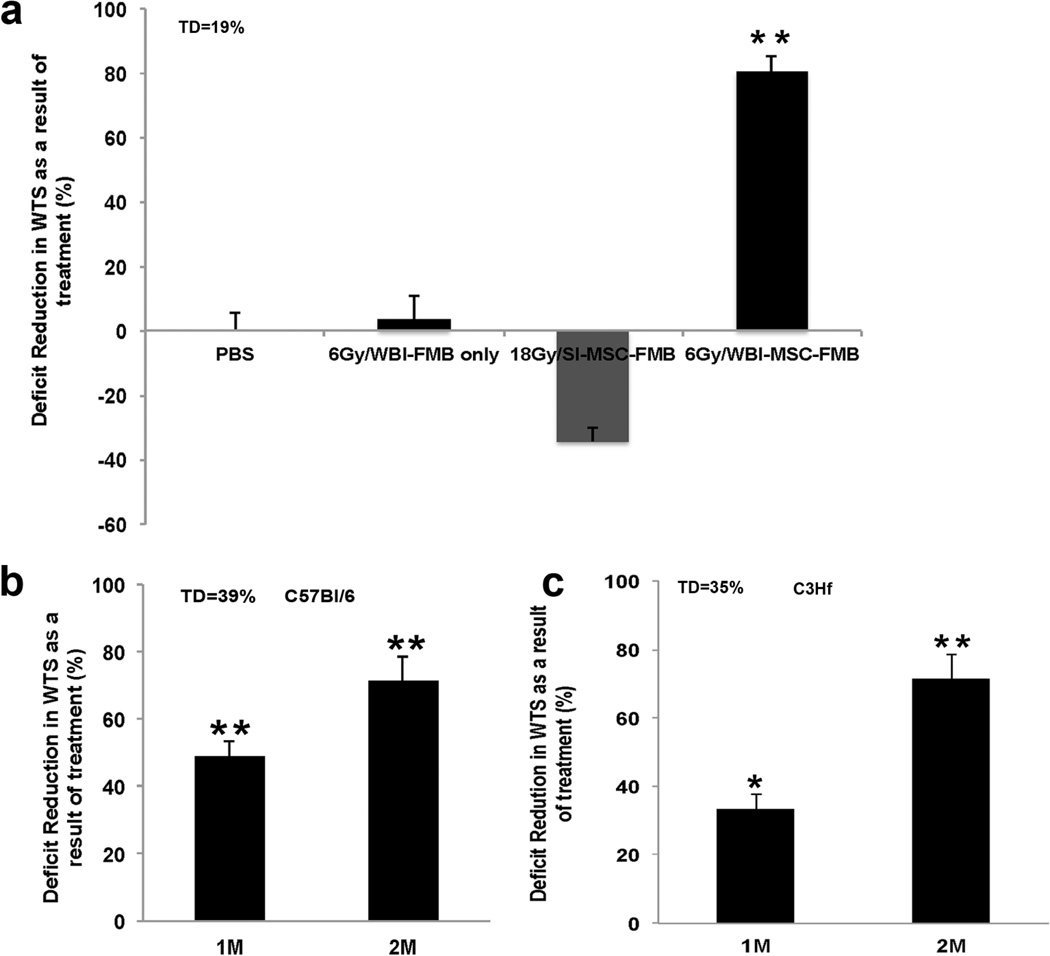
Repeated experiments consistently showed that implantation of FMB devoid of cells did not affect the gain in WTS in unirradiated (not shown) or irradiated (Fig 3a) skin. MSC-FMB implanted into wounds in SI mice also failed to affect gain in WTS (Fig 3a), as would be expected since the infiltrate is still effective. However, MSC-FMB loaded with approximately 106 syngeneic cells corrected a 19% WTS total deficit caused by 6 Gy WBI by around 80% at 4 weeks (Fig 3a). Pilot experiments showed that although improvement was evident at 2 weeks after wounding (not shown), it was markedly less dramatic than at 4 weeks, suggesting the implanted MSC delivered with FMB proliferated or differentiated in the site, as was also suggested by the data shown in Fig 2e.
Figure 3. MSC-FMB correct deficits in gain in WTS caused by WBI.
a) Syngeneic MSC-FMB were implanted in pre-irradiated C57Bl/6 mice and deficit reduction in WTS was assessed after 4 weeks of healing. Mice were exposed to either 18Gy SI or to 6Gy WBI. FMB alone were implanted as a control. b) 1×106 MSC-FMB and 2×106 MSC-FMB were implanted in pre-irradiated wounds of C57Bl/6 and C3H mice. Deficit reduction in WTS was assessed after 4 weeks of exposure to 6 Gy/WBI+ 15Gy SI. Error bars indicate mean+/−SEM, n=4. Significance: *P<0.05, **P<0.01 by Student’s t-test. (WTS: wound tensile strength; WBI: whole body irradiation; SI: Skin only irradiation; MSC: Marrow Stromal Cells; FMB: Fibrin Microbeads; TD: Total deficit)
The efficacy of syngeneic MSC-FMB at reducing the WTS deficit depended upon the number of MSC-FMB implanted but not on the strain of mice (Fig 3b,c). If mice were given 6 Gy WBI followed immediately by 15 Gy SI, 106 syngeneic MSC-FMB corrected a 39% total deficit in C57Bl/6 mice by 49%, and a 35% total deficit in C3H mice by 34% after 4 weeks of healing. If the dose of MSC-FMB was doubled, the degree of correction increased to 71% in both strains. Because of limitations in the volume that can be implanted into an incisional wound, it was not possible to test higher numbers of MSC-FMB.
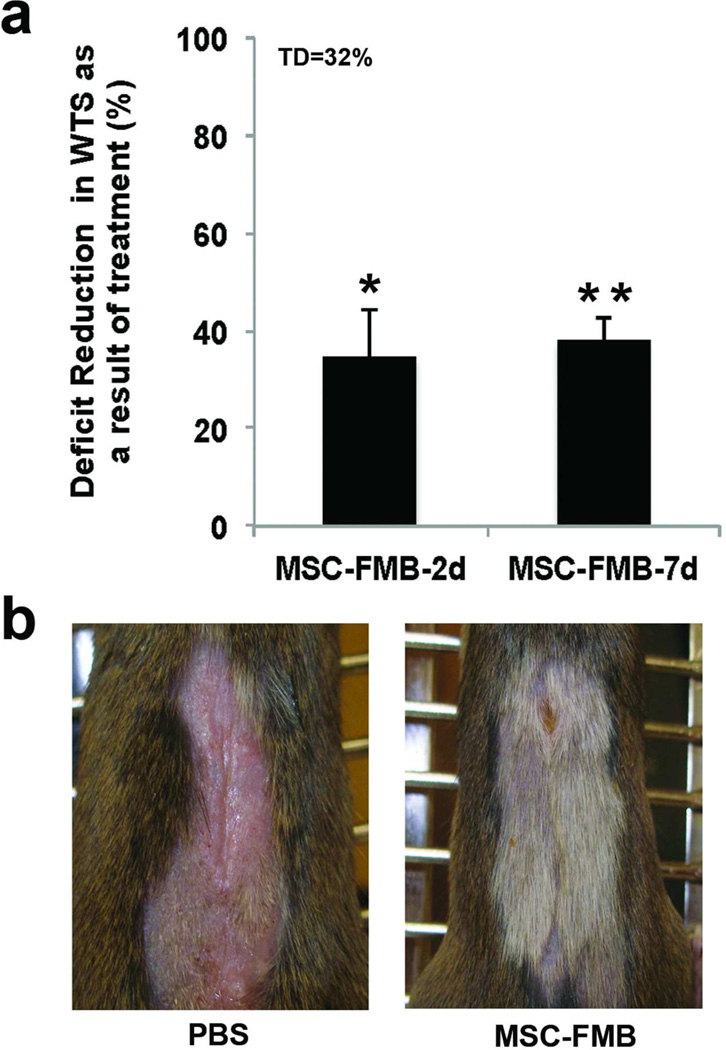
The experimental schedule for the preparation of MSC-FMB was chosen as 2 days of rotational culture to allow MSC bone marrow cells to load onto FMB and a further 5 days to allow them to proliferate to a high density. While this was a reasonable experimental schedule, in an emergency setting a shorter preparation time might be needed. We therefore examined the efficacy of MSC-FMB implanted immediately after 2 days loading with similar cells loaded and cultured for the additional 5 days in vitro prior to implantation. Figure 4a shows that MSC-FMB implanted 2 days after loading were almost as effective in reducing radiation-induced wound healing deficits at 4 weeks as MSC that had been expanded on FMB in culture for 5 more days, suggesting that MSC proliferate in the wound at least as well as they can on FMB in vitro.
Figure 4. MSC-FMB implantation times and hair regrowth.
a) 1.5×106 MSC-FMB were implanted into C3H mice wounds immediately after 2 days of loading onto FMB or after being expanded for a further 5 days in vitro. C3H mice were pre-irradiated with 4Gy/WBI + 21Gy/SI and WTS was assessed after 4 weeks of healing. Error bars indicate mean+/−SEM, n=4. Significance: *P<0.05, **P<0.01 by Student’s t-test. b) Skin in C3H mice irradiated with 4Gy/WBI + 21Gy/SI and implanted with MSC-FMB regrew hair by 3 weeks after treatment, although color was not restored. (WTS: wound tensile strength; MSC: Marrow Stromal Cells; FMB: Fibrin Microbeads; TD: Total deficit)
Consistent findings in all experiments were that irradiation with WBI and SI prevented hair from regrowing in the shaved skin and that, remarkably, MSC-FMB implantation into the wound promoted hair regrowth as early as 3 weeks after exposure, although hair color was not restored (Fig 4b).
Attempts to improve MSC-FMB efficacy
Our studies clearly showed that syngeneic MSC-FMB implants were effective at correcting radiation-induced deficits in wound healing. Since a limitation of an incisional wound system is the volume that can be added, we explored in parallel other possible strategies to enhance wound healing efficacy in pre-irradiated skin.
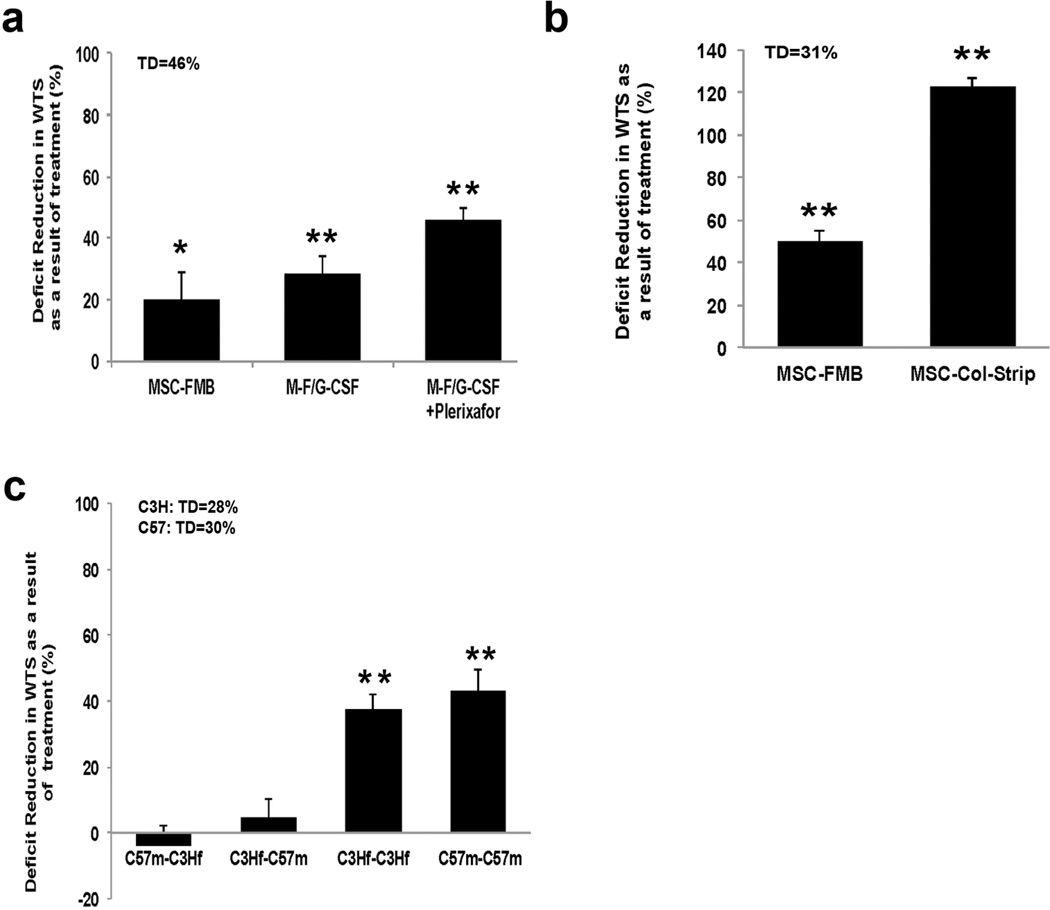
Because G-CSF is likely to be given to patients exposed to potentially lethal WBI doses in a high dose radiological incident, we examined whether G-CSF treatment of mice with and without Plerixafor (AMD3100), which enhances G-CSF-induced mobilization of hematopoietic progenitor cells, would assist or detract from MSC-FMB activity in correcting radiation-induced WTS deficiencies. MSC-FMB were 20% effective in reducing the WTS deficit and this was increased to 29% by in vivo G-CSF treatment and to 46% by an additional single injection of Plerixafor given after G-CSF (Fig 5a), indicating that hematopoietic mobilization can act in conjunction with MSC-FMB implantation to better enhance wound healing.
Figure 5. Hematopoietic mobilization, collagen sheets and allogeneic and syngeneic MSC.
a) Five daily injections of G-CSF were given s.c. to previously wounded C3H mice pre-irradiated with 4 Gy/WBI+21 Gy/SI. An additional single injection of Plerixafor was administrated on day 6 b) MSC on haptized collagen sheets were implanted in the wound of C3Hf mice previously exposed to 4Gy/WBI + 21Gy/SI. Deficit reduction in WTS was measured 4 weeks after treatment c) C3H and C57Bl/6 mice were exposed to 4Gy/WBI+21Gy/SI, allogeneic or syngeneic MSC+FMB were implanted in skin wounds, and WTS was assessed at 5 weeks. Error bars indicate mean+/−SEM, n=4. Significance: *P<0.05, **P<0.01 by Student’s t-test. (WTS: wound tensile strength; MSC: Marrow Stromal Cells; FMB: Fibrin Microbeads; G-CSF: granulocyte colony-stimulating factor; TD: Total deficit)
Additionally, collagen sponge sheets that had been coated haptides, 20mer peptides homologous to the cell binding domains on the c-termini of fibrin (“haptized”) (Marx et al., 2008) were examined as an alternative support structure. These were used to purify MSC and were implanted in the bed under the wound, and were highly successful in correcting radiation-induced WTS deficits (Fig 5b).
MSC-FMB implants across an allogeneic barrier
Literature reports indicate that MSC can be immunosuppressive, even to the extent that they may be effective across histocompatibility barriers. But contradictory data were also presented (English and Mahon, 2011). This could have important clinical consequences in radiation settings as autologous MSC might be difficult to obtain from a patient exposed to potentially lethal WBI. MSC-FMB that could be administered as a commercial product would be ideal for treatment of to a large number of individuals, especially as FMB maintain MSC viable for long periods of time without incubation (Gorodetsky et al., 2011). We therefore examined the ability of MSC-FMB to reverse radiation-induced wound healing deficits across an allogeneic barrier, but only syngeneic MSC were shown to be effective in this system (Fig 5c).
Discussion
Exposure to ionizing radiation sets in motion a train of events that can culminate in failure of one or multiple organs depending upon many factors, amongst which dose and volume are perhaps the most important. As shown in this study and elsewhere, the skin itself is moderately resistant to radiation effects (Vegesna et al., 1993; Withers, 1966), but wound healing is heavily impacted by modest doses to the whole body that damage the hematopoietic system but that are themselves non-lethal (Ran et al., 2004; Vegesna et al., 1993). WBI decreases the cellular infiltrate into wounds far more than local skin irradiation (Vegesna et al., 1993). It is not known which of the many bone marrow-derived cells that migrate into the wound site are most affected or the extent to which this effect extends to tissue repair in other non-hematopoietic organs (Wu et al., 2007b).
MSC have been a major focus of many recent approaches aimed at improving tissue repair and regeneration (Garcia-Gomez et al., 2010) and have been shown to aid wound healing in different preclinical models as well as in humans (Chen et al., 2009; Conget et al., 2010; Hu et al., 2011; McFarlin et al., 2006; Rogers et al., 2008; Wu et al., 2007a). In bone marrow, MSC represent a small fraction of all nucleated cells: less than 0.01% (Bartmann et al., 2007)
FMB based technology is a convenient, simple and rapid technique to isolate and enrich matrix dependent mesenchymal cells that can be applied to tissue regeneration models (Ben-Ari et al., 2009). One ml of packed FMB can typically bind ~30–70×106 mesenchymal cells. Their doubling time at subconfluence in slowly rotating conditions is typically ~48hrs (Gorodetsky, 2008, Gorodetsky et al., 2011). Cell binding to FMB seems based on interaction of small fibrin domains termed "haptides" with the cell membrane (Gorodetsky et al., 2003; Levy-Beladev et al., 2010). Haptides interact with the membranes of different cell types but mesenchymal cells once anchored secrete their own extracellular matrix that allows them to adhere strongly while other cells of hematopoietic origin are shed during rotation. As a result, the populations isolated on FMB are phenotypically purer than those isolated on plastic, even if their proportion in the population is originally extremely low, (Kassis et al.,;Rivkin et al., 2007). It is notable that in the current study a subpopulation of small marker positive MSCs were found that were not seen in populations generated by plastic adherence and require further investigation. Multipotency and plasticity are well-established features of MSC as is the impact of culture methods, which makes it imperative that each system for generating MSC is assessed on its own merits.
For wound healing, FMB provide a protein-based biodegradable support lasting about 2 weeks in vivo after implantation that carries non-trypsinized intact MSC, retains them in the wound site and allows their integration into the ongoing healing processes. MSC-FMB were effective in correcting deficits in healing of incisional wounds in mice whose hematopoietic system had been compromised by WBI exposure but did little to assist if only the skin was irradiated, suggesting that the MSC may have replaced some of the effects of a natural bone marrow-derived population that was required for optimal wound healing. This contrasts with the recent study by Focheron et al. (2012) who showed that adipose-derived stromal cells could heal the skin of minipigs irradiated to 50 Gy which normally would develop necrosis. This difference may be ascribed to differences in the dose and model but more likely to the fact that our studies on MSC-FMB were somewhat limited by the wound incision model, where the area in which therapeutics can be delivered is small. We were however able to show that treatment of mice with G-CSF with or without Plerixafor could augment the efficacy of MSC-FMB and that haptized collagen sponges are also a promising new delivery system for MSC that is not based on whole fibrin protein that may be a more flexible system for future investigations. It has been shown in many systems that MSC may function indirectly to improve tissue regeneration, and that the effect could be related to modification of inflammatory responses (Garcia-Gomez et al., 2010; Chamberlain et al. 2007; Dominici et al., 2006; Keyzer et al., 2007). Allogeneic MSC can therefore be effective across histocompatibility barriers as they have low immunogenicity and they do not need to survive long (Griffin et al., 2010), as they most likely often act by secreting immunosuppressive factors (Tolar et al., 2010). In our MSC-FMB system we were unable to demonstrate production of immunosuppressive cytokines (not shown) and further experimentation is needed to determine if this is an effect of the wound healing/radiation model or of the growth promoting FMB culture conditions (Gorodetsky et al., 1998).
An unexpected impressive observation of this investigation was the recovery of hair follicles in the high dose irradiated skin as a result of subdermal MSC-FMB implantation into irradiated wounds. Hair follicles consist of many different epithelial and mesenchymal cell types geared towards the production of hair and may provide an additional source of stem cells, which appears in continuity with the basal stem cell layer and that appear to reside in the “bulge” region of the follicle shaft (Cotsarelis, 2006; Sun et al., 2007; Yang and Cotsarelis, 2010). Since the mesenchymal cells are not expected to trans-differentiate to form hair follicles, these findings hint for an indirect secretory effect on these cells on the regeneration of a wide range of cell types in the injected area.
Materials and Methods
Mice, Skin Wounds, and Irradiations
C3Hf/Kam (H2-k) female and C57Bl/6 (H2-b) male gnotobiotic mice were used for wound healing studies at 8–10 weeks of age. They were bred at UCLA. MSC-GFP were cultured from bone marrow from C57Bl/6-Tg(ACTB-EGFP)1Osb/J mice obtained from the Jackson Labs (Maine) and maintained at Hebrew University. All experiments were approved by the UCLA IACUC.
WBI was delivered to 8 mice at a time in a well-ventilated Lucite chamber without anesthesia using a γ -ray irradiator (137 Cs; AEC) at a dose rate of 67cGy/min. For SI, 150 kVp/20mA X-rays (Gulmay) and lead shielding were used to reduce the whole body dose. Mice were anesthetized with ketamine and xylazine (Bedford, MA) and placed in a lead box (1 mm thick) with a flap of dorsal skin pulled through a slit and held loosely using tape. The dose rate was 3.623 Gy/min, as determined using thermoluminescent dosimeters on the skin surface.
Mice were irradiated one day before wounding (Gorodetsky et al., 1988). WBI plus SI were given sequentially with less than 15 minutes in between. Full thickness wounds approximately 2.5 cm long were made in the shaved dorsal skin of anesthetized mice (Gorodetsky et al., 1988), with ketamine/xylazine as anesthetic. Cells, FMBs and solutions were implanted into the wound site in 50 µl volumes. Wounds were closed with 3~4 clips which were removed after 2 days. In some experiments 5 μg (micrograms) G-CSF (GenScript, NJ) or sterile phosphate-buffered saline (PBS) was given s.c. to C3H mice, once a day for 5 days followed on day 6 by 5 mg/kg AMD3100 (Plerixafor; Sigma-Aldrich, MO) or PBS.
FMB and MSC
FMB were made from fibrinogen and thrombin as described previously (Gorodetsky et al., 2004). They were sterilized in 70% ethanol for 8 hours and dried. Meshing was used to select FMB with diameters ranging from 108-180µm. Before use they were hydrated in PBS, which expands their size by ~50%.
Bone marrow (BM) cells were collected by flushing the femurs and tibias of euthanized mice with Minimum Essential Medium (Invitrogen/GIBCO, NY) containing 1% antibiotics (penicillin/streptomycin/Amphotericin B; Mediatech, VA). Red cells were lysed using ACK buffer (Lonza, Basel, Switzerland) and cells were resuspended in MEM + 20%FBS (Invitrogen, Ca.) with antibiotics. For isolation of MSC on plastic, 108 BM cells were plated onto 100mm × 20mm plates. After 2 days in culture with 7% CO2 at 37°C, the non-adherent cells were rinsed away and the remaining cells expanded for 5 more days with medium changes. The enriched population of MSC was harvested using 0.25% trypsin-EDTA (Invitrogen, Ca).
For MSC-FMB preparation, 100 μl of sterile hydrated FMB (equivalent to ~50 mg of dry FMB) were washed with PBS and mixed with a total of 108 BM cells in culture medium. These were put in a 50ml CultiFlask disposable bioreactor tube with vented cap that allowed gas exchange and reduced water loss by evaporation (Sartorius Stedim Biotech, Aubagne, France). The tube was rotated slowly in ~10–20 rpm at an angle of 20 degree (Gorodetsky et al., 2004) for 2 days in an incubator. The non-adherent cells were removed by washing, and the MSC-FMB were cultured for another 5 days (unless otherwise indicated) by which time the beads were totally covered by MSC. This was confirmed by nuclear count using propidium iodide staining performed as described previously (Rivkin et al., 2007). Details of the cell yield, purity, marker expression and other technical issues have been described extensively in previous publications (Gorodetsky et al., 1999; Gorodetsky et al., 2004; Gorodetsky et al., 2008).
Unless otherwise stated, 15 µl volumes of MSC-FMB in a total of 50 µl PBS containing approximately 106 MSC were implanted into each wound prior to its closure.
Flow Cytometry
MSC markers were assessed by flow cytometry as before (Rivkin et al., 2007) (FACSCalibur; BD Biosciences, CA). The markers used were CD105, Sca-1, CD44, CD49e (Clone 5H10-27, MFR5; BD),CD29, CD45 and CD31.
Collagen Sponges
Highly crosslinked strip sheets of collagen sponge with covalently bound cell binding 20mer Haptide sequences (Hapto Biotech, Israel) were sterilized with ethanol 70% for 8 hours, rinsed with PBS, and put under a vacuum for 30 minutes. 107 bone marrow cells were loaded onto 20mm long collagen sponge strips in 5 ml medium in 50 ml tubes and treated as for FMB. After 2 days, the non-adhered cells were rinsed off and after 5 more days of incubation, the strips were implanted into the sub dermal area of the wounds. P.I. staining confirmed a confluent layer of MSC attached to the collagen substrate.
GFP labeled MSC
For the follow-up of survival and integration of MSC implants in the wounds, MSC were isolated from bone marrow of GFP+ C57BL mice by incubation with FMB and implanted with FMB to WT C57BL mice.
Tensile Strength Measurement
At stated times after wounding, a square of skin containing the wound was removed from euthanized mice and cut into seven 2mm strips of 20mm in length with a multi-blade device so that each 2mm wide strip contained a horizontal wound sample (Gorodetsky et al., 1988). The strips were spread on filter papers soaked in ice-cold PBS in covered petri dishes till WTS measurement as previously described (Gorodetsky 1988) using an Instron tensiometer (Model 3342, Instron, Norwood, MA). The skin strips were stretched at a rate of 1 cm/min to breaking point to obtain the peak WTS in gram force/2mm.
Since different radiation doses and times are used, for simplicity and comparison between experiments, the efficacy of the treatment can be presented as Deficit Reduction (DR), which is the reduction in the radiation-induced deficit due to the treatment,
and as Total Deficit (TD):
Student’s t test was used to assess statistical significance.
Acknowledgements
We thank John DeMarco and Kei Iwamoto (UCLA Department of Radiation Oncology) for all dosimetric calculations. This work was supported by NIH/NIAID RC1 AI-081287 and NIH 5U19AI067769.
Abbreviations
- FMB
Fibrin MicroBeads
- MSC
Marrow-Derived Stromal Cells
- WTS
Wound Tensile Strength
- WBI
Whole Body Irradiation
- SI
Skin Only Irradiation
- BM
Bone Marrow
- G-CSF
Granulocyte Colony-Stimulating Factor
- AMD3100
Plerixafor
Footnotes
Conflict of interest
The authors state no conflict of interest.
References
- Bartmann C, Rohde E, Schallmoser K, et al. Two steps to functional mesenchymal stromal cells for clinical application. Transfusion. 2007;47:1426–1435. doi: 10.1111/j.1537-2995.2007.01219.x. [DOI] [PubMed] [Google Scholar]
- Ben-Ari A, Rivkin R, Frishman M, Gaberman E, Levdansky L, Gorodetsky R. Isolation and implantation of bone marrow-derived mesenchymal stem cells with fibrin micro beads to repair a critical-size bone defect in mice. Tissue Eng Part A. 2009;15:2537. doi: 10.1089/ten.tea.2008.0567. [DOI] [PubMed] [Google Scholar]
- Chamberlain G, Fox J, Ashton B, et al. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem cells. 2007;25:2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- Chen L, Tredget EE, Liu C, et al. Analysis of allogenicity of mesenchymal stem cells in engraftment and wound healing in mice. PLoS One. 2009;4:e7119. doi: 10.1371/journal.pone.0007119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conget P, Rodriguez F, Kramer S, et al. Replenishment of type VII collagen and re-epithelialization of chronically ulcerated skin after intradermal administration of allogeneic mesenchymal stromal cells in two patients with recessive dystrophic epidermolysis bullosa. Cytotherapy. 2010;12:429–431. doi: 10.3109/14653241003587637. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G. Epithelial stem cells: a folliculocentric view. The Journal of investigative dermatology. 2006;126:1459–1468. doi: 10.1038/sj.jid.5700376. [DOI] [PubMed] [Google Scholar]
- Dantzer D, Ferguson P, Hill RP, et al. Effect of radiation and cell implantation on wound healing in a rat model. Journal of surgical oncology. 2003;83:185–190. doi: 10.1002/jso.10242. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- English K, Mahon BP. Allogeneic mesenchymal stem cells: Agents of immune modulation. Journal of cellular biochemistry. 2011;112:1963–1968. doi: 10.1002/jcb.23119. [DOI] [PubMed] [Google Scholar]
- Forcheron F, Agay D, Scherthan H, et al. Autologous adipocyte derived stem cells favour healing in a minipig model of cutaneous radiation syndrome. PloS one. 2012;7(2):e31694. doi: 10.1371/journal.pone.0031694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gomez I, Elvira G, Zapata AG, et al. Mesenchymal stem cells: biological properties and clinical applications. Expert opinion on biological therapy. 2010;10:1453–1468. doi: 10.1517/14712598.2010.519333. [DOI] [PubMed] [Google Scholar]
- Gorodetsky R. The use of fibrin based matrices and fibrin microbeads (FMB) for cell based tissue regeneration. Expert opinion on biological therapy. 2008;8:1831–1846. doi: 10.1517/14712590802494576. [DOI] [PubMed] [Google Scholar]
- Gorodetsky R, Clark RA, An J, et al. Fibrin microbeads (FMB) as biodegradable carriers for culturing cells and for accelerating wound healing. The Journal of investigative dermatology. 1999;112:866–872. doi: 10.1046/j.1523-1747.1999.00600.x. [DOI] [PubMed] [Google Scholar]
- Gorodetsky R, Levdansky L, Gaberman E, et al. Fibrin microbeads (FMB) loaded with mesenchymal cells support their long term survival while sealed at room temperature. Tissue Eng Part C Methods. 2011;17(7):745–755. doi: 10.1089/ten.tec.2010.0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorodetsky R, McBride WH, Withers HR. Assay of radiation effects in mouse skin as expressed in wound healing. Radiation research. 1988;116:135–144. [PubMed] [Google Scholar]
- Gorodetsky R, McBride WH, Withers HR, et al. Effect of fibroblast implants on wound healing of irradiated skin: assay of wound strength and quantitative immunohistology of collagen. Radiat Res. 1991;125:181–186. [PubMed] [Google Scholar]
- Gorodetsky R, Vexler A, An J, et al. Haptotactic and growth stimulatory effects of fibrin(ogen) and thrombin on cultured fibroblasts. J Lab Clin Med. 1998;131:269–280. doi: 10.1016/s0022-2143(98)90100-7. [DOI] [PubMed] [Google Scholar]
- Gorodetsky R, Vexler A, Levdansky L, et al. Fibrin microbeads (FMB) as biodegradable carriers for culturing cells and for accelerating wound healing. Methods in molecular biology. 2004;238:11–24. doi: 10.1385/1-59259-428-x:11. [DOI] [PubMed] [Google Scholar]
- Gorodetsky R, Vexler A, Shamir M, et al. New cell attachment peptide sequences from conserved epitopes in the carboxy termini of fibrinogen. Experimental cell research. 2003;287:116–129. doi: 10.1016/s0014-4827(03)00120-4. [DOI] [PubMed] [Google Scholar]
- Griffin MD, Ritter T, Mahon BP. Immunological Aspects of Allogeneic Mesenchymal Stem Cell Therapies. Hum Gene Ther. 2010;21(12):1641–1655. doi: 10.1089/hum.2010.156. [DOI] [PubMed] [Google Scholar]
- Hu G, Liu P, Feng J, et al. Transplantation with bone marrow stromal cells promotes wound healing under chemotherapy through altering phenotypes. Int J Biol Sci. 2011;7:912–926. doi: 10.7150/ijbs.7.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassis I, Zangi L, Rivkin R, Levdansky L, Samuel S, Marx G, Gorodetsky R. Isolation of mesenchymal stem cells from G-CSF-mobilized human peripheral blood using fibrin microbeads. Bone Marrow Transplant. 2006;37:967. doi: 10.1038/sj.bmt.1705358. [DOI] [PubMed] [Google Scholar]
- Keyser KA, Beagles KE, Kiem HP. Comparison of mesenchymal stem cells from different tissues to suppress T-cell activation. Cell Transplant. 2007;16:555–562. doi: 10.3727/000000007783464939. [DOI] [PubMed] [Google Scholar]
- Kruegler WWO, Goepfert H, Romsdahl M, et al. Fibroblast Implantation Enhances Wound Healing As Indicated By Breaking Strength Determinations. Otolaryngology. 1978;86:804–811. doi: 10.1177/019459987808600527. [DOI] [PubMed] [Google Scholar]
- Leclerc T, Thepenier C, Jault P, et al. Cell therapy of burns. Cell proliferation. 2011;44(Suppl 1):48–54. doi: 10.1111/j.1365-2184.2010.00727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Beladev L, Levdansky L, Gaberman E, et al. A family of cell-adhering peptides homologous to fibrinogen C-termini. Biochemical and biophysical research communications. 2010;401:124–130. doi: 10.1016/j.bbrc.2010.09.024. [DOI] [PubMed] [Google Scholar]
- Marx G, Hotovely-Salomon A, Levdansky L, et al. Haptide-coated collagen sponge as a bioactive matrix for tissue regeneration. J Biomed Mater Res B Appl Biomater. 2008;84:571–583. doi: 10.1002/jbm.b.30905. [DOI] [PubMed] [Google Scholar]
- McFarlin K, Gao X, Liu YB, et al. Bone marrow-derived mesenchymal stromal cells accelerate wound healing in the rat. Wound Repair Regen. 2006;14:471–478. doi: 10.1111/j.1743-6109.2006.00153.x. [DOI] [PubMed] [Google Scholar]
- Olascoaga A, Vilar-Compte D, Poitevin-Chacon A, et al. Wound healing in radiated skin: pathophysiology and treatment options. International Wound Journal. 2008;5:246–257. doi: 10.1111/j.1742-481X.2008.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ran X, Cheng T, Shi C, et al. The effects of total-body irradiation on the survival and skin wound healing of rats with combined radiation-wound injury. The Journal of trauma. 2004;57:1087–1093. doi: 10.1097/01.ta.0000141885.72033.c7. [DOI] [PubMed] [Google Scholar]
- Rivkin R, Ben-Ari A, Kassis I, et al. High-yield isolation, expansion, and differentiation of murine bone marrow-derived mesenchymal stem cells using fibrin microbeads (FMB) Cloning and stem cells. 2007;9:157–175. doi: 10.1089/clo.2006.0039. [DOI] [PubMed] [Google Scholar]
- Rogers LC, Bevilacqua NJ, Armstrong DG. The use of marrow-derived stem cells to accelerate healing in chronic wounds. International wound journal. 2008;5:20–25. doi: 10.1111/j.1742-481X.2007.00349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Hu G, Su J, et al. Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell research. 2010;20:510–518. doi: 10.1038/cr.2010.44. [DOI] [PubMed] [Google Scholar]
- Sun X, Fu X, Sheng Z. Cutaneous stem cells: something new and something borrowed. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2007;15:775–785. doi: 10.1111/j.1524-475X.2007.00282.x. [DOI] [PubMed] [Google Scholar]
- Tolar J, Le Blanc K, Keating A, et al. Concise review: hitting the right spot with mesenchymal stromal cells. Stem Cells. 2010;28:1446–1455. doi: 10.1002/stem.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegesna V, Withers HR, Holly FE, et al. The effect of local and systemic irradiation on impairment of wound healing in mice. Radiat Res. 1993;135:431–433. [PubMed] [Google Scholar]
- Williams JP, McBride WH. After the bomb drops: a new look at radiation-induced multiple organ dysfunction syndrome (MODS) International journal of radiation biology. 2011;87:851–868. doi: 10.3109/09553002.2011.560996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers HR. The dose-response relationship for epithelial cells of skin. Radiology. 1966;86:1110–1111. doi: 10.1148/86.6.1110. [DOI] [PubMed] [Google Scholar]
- Withers HR. The dose-survival relationship for irradiation of epithelial cells of mouse skin. Br J Radiol. 1967;40:187–194. doi: 10.1259/0007-1285-40-471-187. [DOI] [PubMed] [Google Scholar]
- Wu Y, Chen L, Scott PG, et al. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells. 2007a;25:2648–2659. doi: 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- Wu Y, Wang J, Scott PG, et al. Bone marrow-derived stem cells in wound healing: a review. Wound Repair Regen. 2007b;15(Suppl 1):S18–S26. doi: 10.1111/j.1524-475X.2007.00221.x. [DOI] [PubMed] [Google Scholar]
- Yang CC, Cotsarelis G. Review of hair follicle dermal cells. Journal of dermatological science. 2010;57:2–11. doi: 10.1016/j.jdermsci.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangi L, Rivkin R, Kassis I, et al. High-yield isolation, expansion, and differentiation of rat bone marrow-derived mesenchymal stem cells with fibrin microbeads. Tissue engineering. 2006;12:2343–2354. doi: 10.1089/ten.2006.12.2343. [DOI] [PubMed] [Google Scholar]