Abstract
Objective
Older adults have exaggerated postprandial lipemia (PPL), which increases their risk for cardiovascular disease. We sought to determine the effects of increased plasma L-arginine availability on the oxidation of ingested fat (enriched with [1,1,1-13C]-triolein) and plasma triglyceride (TG) concentrations during the postprandial period in older subjects.
Methods
On one day, eight healthy subjects (67.8 ± 1.3 years old) received an intravenous infusion of L-arginine during the first hour of the postprandial period (L-ARG), while on a separate day they received saline (control trial; CON).
Results
The 8-h area under the curve (AUC0–8h) describing the postprandial plasma TG concentrations was considerably lower in the L-ARG trial than the CON trial (−4 ± 21 vs 104 ± 21 mg·dL−1·h; P < 0.01). The rate of the postprandial oxidation of the ingested lipid was not different between the trials, but the average contribution of ingested-oleate to the oleate of TG of the plasma small TG-rich lipoproteins (TRL; Sf = 20–400) was lower in the L-ARG trial (11 ± 1 vs 18 ± 2%; P < 0.01). L-arginine infusion decreased also the AUC0–8h of the plasma free fatty acid concentrations derived from the ingested fat when compared to the saline infusion (0.77±0.09 vs 1.11 ± 0.08; mmol·L−1·h; P < 0.01).
Conclusion
Increasing the plasma L-arginine availability during the postprandial period decreases the PPL in older adults, in association with a decrease in the postprandial contribution of ingested lipid into TG of the plasma small TRL.
Keywords: Elderly, Fatty acids, Triglycerides, Stable isotope tracers, Fat meal
Introduction
Cardiovascular disease (CVD) secondary to atherosclerosis is the main cause of death and a major cause of disability in developed societies [1]. Current evidence suggests that impaired lipid metabolism documented as increased fed-state plasma triglyceride (TG) concentrations is an independent predictor for atherosclerosis, and that exaggerated postprandial plasma TG response is positively associated with increased risk for CVD [2–5]. Both the magnitude and duration of postprandial lipemia (PPL) are increased in older adults [6–9], which can contribute to the increased risk for CVD in the older population. Approaches that effectively decrease the magnitude of PPL are thus particularly important for older individuals in an effort to prevent or retard metabolic processes that increase the risk for CVD in this segment of the population.
Recent studies suggest that increasing the amino acids in plasma, specifically L-arginine, may be such an approach. In young healthy subjects, increasing the amino acids in plasma by protein ingestion attenuates the postprandial increase in plasma TG concentrations [10]. In older subjects, Borsheim et al. [11] have recently shown that supplementation with essential amino acids and L-arginine decreases the plasma TG concentrations in the postabsorptive state. However, such evidence, related to the metabolism of endogenous lipid in the fasting state, cannot be directly translated into responses associated with the metabolism of exogenous (i.e. dietary) lipid in the postprandial state. Nevertheless, this overall evidence provides intriguing support for a role of plasma L-arginine as a unique amino acid in improving the postprandial plasma lipid metabolism in older individuals.
We have recently shown that the postprandial oxidation of the ingested fat at whole-body level is impaired in older adults [9]. Muscle contributes considerably to whole-body lipid oxidation and decreased capacity for substrate oxidation in muscle mitochondria with aging [12], may impair the postprandial oxidation of the ingested fat and contribute to the increase in PPL in these individuals. Indeed, decreased lipid oxidation during the postprandial period has been shown to contribute to increased PPL [13]. In vitro experiments have documented an effect of L-arginine on increasing the fatty acid oxidation at the level of mitochondria isolated from skeletal muscle [14]. Based on such evidence, increasing the plasma L-arginine availability may provide the means to improve whole-body oxidative disposal of ingested lipid, particularly in a metabolic circumstance associated with accumulation of lipid in plasma such as the postprandial period, and thus attenuate the PPL in older adults.
The current study was, therefore, undertaken to investigate the effects of an acute increase in plasma L-arginine availability on PPL in apparently healthy older subjects, with a special focus on the effects of L-arginine on enhancing the postprandial oxidative disposal of the ingested fat during the 8-h postprandial period. We used a standardized protocol of an intravenous bolus-infusion of L-arginine that has been traditionally employed to study the effects of L-arginine on various physiologic and metabolic parameters [15–18]. The 1-h bolus-infusion of L-arginine rapidly increases (~45-fold) the plasma L-arginine concentrations [15]. After the end of the L-arginine infusion, and for the following several hours (i.e., 7 hrs), the plasma L-arginine concentration remains between 3- and 7-fold higher than that in the postabsorptive state [19,20]. Intravenous, as compared to oral, administration of L-arginine allows standardizing the availability of L-arginine in plasma, given the considerable wide range (i.e. 20% to 70%) in the bioavailability of orally administered L-arginine across individuals [21].
Materials and Methods
Subjects
Eight healthy Caucasian older males participated in this study after the purpose, procedures, and the risks associated with the experiments had been explained and informed written consent obtained from each subject. All subjects participating in the study were determined to be healthy based on medical history report, physical examination, resting electrocardiogram, and routine blood and urine tests. Exclusion criteria included smoking, body mass index > 30 kg/m2, hypertension, diabetes, heart disease, peripheral vascular disease, history of liver or kidney disease and use of any prescribed or over-the-counter medications. The physical and clinical characteristics of the subjects are presented in Table 1. Percent body fat was determined using Bioelectrical Impedance Analysis. The study protocol was approved by the Institutional Review Board at Arizona State University.
Table 1.
Physical and clinical characteristics of the subjects (n = 8)
| Age, y | 67.8 ± 1.3 |
| Weight, kg | 88.4 ± 2.7 |
| Height, cm | 181.3 ± 1.6 |
| Body fat, % | 26.7 ± 1.0 |
| Plasma lipids, mg·dL−1 | |
| Triglycerides | 81.9 ± 11.5 |
| Total Cholesterol | 182.4 ± 16.1 |
| HDL-C | 52.9 ± 4.4 |
| LDL-C | 96.4 ± 15.5 |
| Plasma glucose, mg·dL−1 | 92.3 ± 2.8 |
| Plasma insulin, uIU·mL−1 | 6.6 ± 1.1 |
| ALT, IU·L−1 | 26.1 ± 4.9 |
| AST, IU·L−1 | 30.0 ± 6.7 |
| SBP, mmHg | 123.8 ± 4.1 |
| DBP, mmHg | 75.9 ± 1.9 |
Values are means ± SEM; HDL-C, High Density
Lipoprotein-Cholesterol; LDL-C, Low Density
Lipoprotein-Cholesterol; ALT, Alanine
Aminotransferase; AST, Aspartate
Aminotransferase; SBP, Systolic Blood Pressure.
Experimental protocol
All subjects underwent two lipid challenge studies with whipping cream ingestion. The studies were carried out on two different days separated by at least a week and were performed in a randomized, cross-over fashion. On one occasion subjects received an intravenous infusion of L-arginine (L-ARG trial) following the ingestion of whipping cream, while on another occasion they received saline infusion instead of L-arginine as a control (CON trial). On both occasions subjects were instructed to abstain from any form of exercise, maintain their regular diet, and avoid alcohol consumption for three days prior to the infusion study.
Subjects were admitted to the Clinical Research Unit at Arizona State University in the morning at ~ 6:30 AM, and after at least a 9-h overnight fast. After compliance with the instructions had been verified, subjects were laid in bed, and an intravenous catheter was inserted into an antecubital vein of each arm for blood sampling and infusions, respectively. An hour later, at ~ 8 AM, a blood sample was collected for baseline measurements, after which subjects ingested fat in the form of whipping cream (0.4 grams fat·kg body weight−1), enriched with [1,1,1-13C]-triolein (4 mg·kg body weight−1; Cambridge Isotope Laboratories, Inc, Andover, MA) over 15 minutes. On average, the participants ingested ~35 grams of fat (range 31 – 41 grams). The macronutrient composition of whipping cream was as follows (100 g): calories, 345 kcal; protein, 2.1 g; carbohydrate, 2.8 g; and fat, 37.0 g (saturated fat, 23.0 g; monounsaturated fat, 10.7 g; polyunsaturated fat, 1.4 g). Immediately after the fat ingestion, subjects received an one-hour infusion of either L-arginine (0.5 g·min−1; R-Gene® 10, 10% Arginine HCl Injection; Pharmacia & Upjohn Co, NY) designed to mimic the pattern in plasma L-arginine response following L-arginine ingestion [21], or saline.
Blood samples were collected at hourly intervals for eight hours following the ingestion of the fat for the measurement of plasma concentrations of TGs, free fatty acids (FFA), 3-hydroxybutyrate (3-HB), and insulin. Breath samples for the determination of the rate of oxidation of ingested fat and blood samples for the determination of labeled lipid in TG of TG-rich lipoprotein (TRL) fractions were collected at two hours intervals during the postprandial period. Plasma was immediately separated by centrifugation (1500 × g for 15 min at 4°C). TRL sub-fractions were then isolated from plasma within 48 hours for the determination of the 13C enrichment in TG of plasma TRL with Svedberg flotation index (Sf) > 400 (i.e., large TRL fraction) that contains primarily chylomicrons, and TRL with Sf = 20–400 (i.e., small TRL fraction) that contains predominately very-low-density-lipoproteins. The remaining plasma was stored at −80°C, and used for the measurement of the blood chemistry parameters indicated above, as well as 13C enrichment of oleate in plasma FFA. For the determination of the rate of oxidation of ingested fat, rates of expired CO2 were measured for 20 min using a metabolic cart (TrueMax 2400, Parvo Medics, Salt Lake City, UT) immediately prior to the ingestion of fat and at the 2-h intervals during the postprandial period by having the subjects breathe under a ventilated hood. After each of these measurements, a breath sample was collected into an Exetainer tube for the determination of 13CO2 enrichment in the expired CO2.
Analyses of samples
Large and small TRL sub-fractions were isolated from plasma by density gradient ultracentrifugation, as we have previously described [9]. These plasma TRL sub-fractions were stored at −80°C until analysis. Blood glucose concentrations were determined using an automated glucose analyzer (YSI 2300, Yellow Springs, OH). Commercially available kits were used for the measurement of the concentrations of plasma TG (Sigma-Aldrich, St. Louis, MO), FFA and 3-HB (Wako Chemicals, Richmond, VA), as well as insulin (ALPCO Diagnostics, Windham, NH). For consistency across variables, these chemistry parameters were measured at the 2-h plasma samples, unless otherwise noted. For the measurement of 13C-oleate enrichment in plasma lipids, TG in large and small TRL sub-fractions and FFA in plasma were isolated using thin-layer chromatography as we have previously described [9]. The 13C-oleate enrichment in the above plasma lipids (i.e., their fatty acid methyl esters) was determined using gas chromatography-mass spectrometry (Thermo Scientific Trace GC Ultra-DSQ GC/MS system; Thermo Scientific, West Palm Beach, FL) by selected ion monitoring of mass-to-charge ratio (m/z) 296 and 297, and expressed as tracer-to-tracee ratio (TTR). Breath samples were analyzed for isotopic enrichment of 13CO2 using a Finnigan BreathMat gas isotope ratio mass spectrometer by Metabolic Solutions (Metabolic Solutions, Inc., Nashua, NH).
Calculations
The contributions of ingested-oleate to the total oleate in plasma TG of the small TRL fraction and FFA were calculated by dividing the measured 13C-oleate TTR in plasma TG of the small TRL fraction and FFA, respectively, by the 13C-oleate TTR in the ingested fat, and expressed as a percent [22]. The oleate enrichment of the ingested fat was calculated using the exact weighted amounts of whipping cream, which contains 9.3% oleate, and the [1,1,1-13C]-triolein added into the whipping cream and ingested during each trial. The concentration of plasma FFA from the ingested fat (FFAi) was calculated based on the percent contribution of the ingested lipid to the total plasma FFA (FFAt), which was in turn based on the percent contribution of ingested-oleate to the total oleate in plasma FFA. The concentration for plasma FFA from endogenous sources (FFAe) was calculated as the difference between FFAt and FFAi. The rate of oxidation of ingested lipid was calculated from the 13C enrichment in expired CO2 and the rate of CO2 production as we have previously described [9].
Areas under the plasma concentration-time curves (AUC) for the variables of interest were calculated for the 8-h postprandial period (AUC0–8h) by using the trapezoidal rule, and they were compared between the two trials. AUC is reported as the incremental AUC, which was computed by subtracting the plasma/blood concentration of the postabsorptive state from each of the respective concentrations measured in the postprandial period prior to the calculation of the AUC. Responses were also compared between trials during the early (0 – 4 h; AUC0–4h) and late (4 – 8 h; AUC4–8h) parts of the postprandial period because aging results in more pronounced differences in plasma lipid responses during the late part of the postprandial period (i.e. 4–8 h) [9].
Statistical analyses
Data between trials were compared using the paired student’s t-test. Results are expressed as means ± SEM. The Minitab® 15.1 statistical software (Minitab Inc., State College, PA) was used for all the statistical analyses. Statistical significance was set at P ≤ 0.05
Results
Triglycerides
Baseline concentrations of plasma TG were not different between the two infusion trials (P > 0.05). After fat ingestion, the peak plasma TG concentration describing the average response was observed at 4 h in the CON trial, whereas it was observed earlier (i.e. at 2 h) in the L-ARG trial (Fig. 1A). In the latter trial, the average plasma TG concentration appeared to return to postabsorptive value by approximately 4 h. The overall plasma TG response (AUC0–8h) was significantly lower in the L-ARG trial than the CON trial (P < 0.01; Fig. 1B). This response was the result of lower AUC for plasma TG concentrations with the L-arginine infusion in both the early (0 – 4 h) and the late (4 – 8 h) parts of the postprandial period (Table 2).
Fig. 1.
Change in plasma triglyceride concentrations following fat ingestion at time 0 (A). Area under the plasma concentration-time curve (AUC) for triglyceride (TG), calculated after subtracting the postabsorptive plasma TG concentration from the postprandial plasma TG concentrations (i.e., incremental AUC), during the 8-h postprandial period following the fat ingestion (B). L-arginine (L-arginine) or saline (Control) were infused during the first hour following the fat ingestion. *Significant difference between trials (P < 0.01).
Table 2.
Incremental area under the curve (AUC; area under the curve after subtracting the postabsorptive value) calculated for the early (0 – 4 hours; AUC0–4h) and late (4 – 8 hours; AUC4–8h) parts of the postprandial period for blood chemistry parameters associated with lipid metabolism.
| AUC0–4h | AUC4–8h | |||||
|---|---|---|---|---|---|---|
| Control | L-Arginine | P value | Control | L-Arginine | P value | |
| TG, mg·dL−1·h | 81 ± 16 | 33 ± 9 | 0.004 | 23 ± 10 | −37 ± 15 | 0.013 |
| FFAt, mmol·L−1·h | 0.30 ± 0.15 | −0.15 ± 0.09 | 0.038 | 0.46 ± 0.17 | 0.40 ± 0.13 | 0.652 |
| FFAi, mmol·L−1·h | 0.51 ± 0.04 | 0.30 ± 0.04 | 0.001 | 0.60 ± 0.05 | 0.47 ± 0.05 | 0.026 |
| FFAe, mmol·L−1·h | −0.20 ± 0.15 | −0.45 ± 0.07 | 0.134 | −0.14 ± 0.20 | −0.07 ± 0.11 | 0.618 |
| 3-HB, umol·L−1·h | 376 ± 49 | 298 ± 60 | 0.256 | 695 ± 173 | 957 ± 134 | 0.063 |
Values are means ± SEM; n = 8; TG, plasma triglycerides; FFAt, total plasma free fatty acids; FFAi, plasma free fatty acids from the ingested fat; FFAe, plasma free fatty acids from endogenous sources; 3-HB, plasma 3-hydroxybutyrate.
Oxidation of ingested lipid and its incorporation in plasma TG of TRL sub-fractions and FFA
Whole-body oxidation of the ingested lipid increased progressively and similarly in both trials after fat ingestion, reaching peak values at approximately 6 h (Fig. 2). Throughout the 8-h postprandial period, 42 ± 6 % and 40 ± 5 % of the ingested fat was oxidized in the L-ARG and the CON trials, respectively (P = 0.66).
Fig. 2.
Whole-body rate of oxidation of ingested lipid following fat ingestion at time 0. L-arginine (L-arginine) or saline (Control) were infused during the first hour following the fat ingestion. 8-h incremental AUC values for whole-body rate of oxidation of the ingested lipid (mg·kgFFM−1·h) were 240 ± 32 and 227 ± 31 in the L-arginine and Control trials, respectively (P = 0.69).
Postprandial 13C-oleate enrichment of TG in large TRL particles increased rapidly and similarly in both trials. In contrast, 13C-oleate enrichment of TG in small TRL particles increased to a lesser degree and was consistently lower in the L-ARG trial than the CON trial throughout the postprandial period, such that ingested-oleate contributed on average 11 ± 1 % and 18 ± 2 % to the oleate of TG of the small TRL particles in the L-ARG and CON trials, respectively (Fig. 3). Postprandial 13C-oleate enrichments of TG in large and small TRL particles are presented in Fig. 4.
Fig. 3.
Contribution of ingested-oleate to oleate of TG of plasma small triglyceride-rich lipoprotein (TRL) particles (Sf 20–400) during the 8-h postprandial period after fat ingestion. L-arginine (L-arginine) or saline (Control) were infused during the first hour following the fat ingestion. *Significant difference between trials (P < 0.01).
Fig. 4.
Change in 13C-oleate enrichment in plasma TG of plasma large triglyceride-rich lipoprotein (TRL) particles (Sf > 400) (A), free fatty acids (B), and small TRL particles (Sf = 20–400) (C) following ingestion of fat enriched with 13C-triolein. 8-h incremental AUC values for 13C-oleate enrichment (%·h) were 12.8 ± 2.3 and 13.4 ± 2.5 for plasma TRL particles with Sf > 400 (P = 0.84), 6.6 ± 0.7 and 8.6 ± 0.8 for plasma free fatty acids (P < 0.05), and 4.2 ± 0.7 and 7.1 ± 1.8 for plasma TRL particles with Sf = 20–400 (P = 0.05), in the L-arginine and Control trials, respectively.
In both trials, 13C-oleate enrichment of plasma FFA increased to peak values at approximately 4 hours following the ingestion of fat, and subsequently decreased to values that remained above baseline until the end of the 8-h postprandial period (Fig. 4). However, the overall 13C-oleate enrichment of plasma FFA was considerably lower in the L-ARG trial compared to the CON trial, such that the contribution of ingested-oleate to the plasma oleate during the entire postprandial period was about 23% lower in the former trial (17 ± 2 % vs 22 ± 2 %; P < 0.05).
Insulin, glucose, FFA and 3-hydroxybutyrate
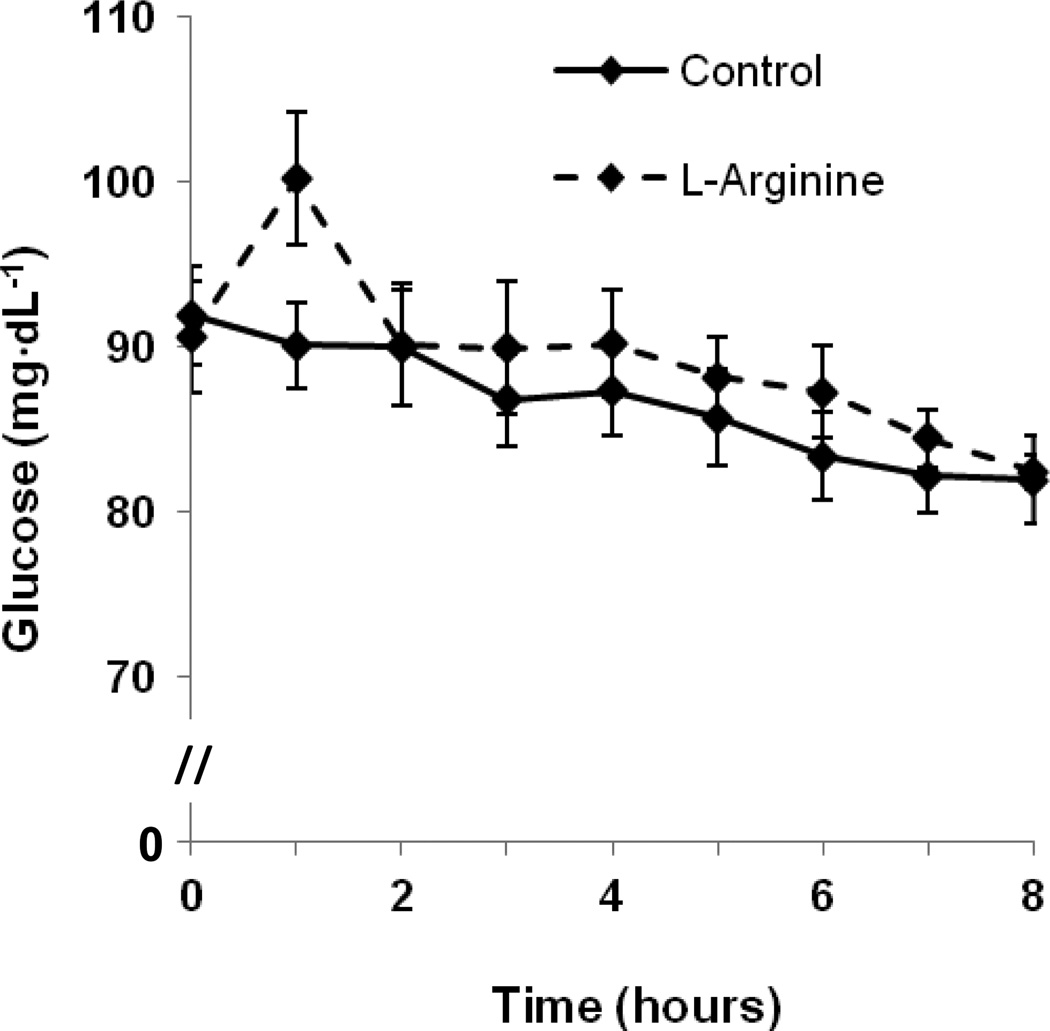
Compared to the CON trial, plasma insulin concentrations increased during the one-hour L-arginine infusion, but subsequently returned to levels similar to those in the CON trial by 2 hours postprandially (Fig. 5). Blood glucose concentrations also appeared to increase during the L-arginine infusion (Fig 6).
Fig. 5.
Change in plasma insulin concentrations following fat ingestion at time 0. L-arginine (L-arginine) or saline (Control) were infused during the first hour following the fat ingestion. 8-h incremental AUC values for plasma insulin concentrations (uIU·mL−1·h) were 40 ± 10 and 1 ± 5 in the L-arginine and Control trials, respectively (P < 0.05).
Fig. 6.
Change in blood glucose concentrations following fat ingestion at time 0. L-arginine (L-arginine) or saline (Control) were infused during the first hour following the fat ingestion. 8-h incremental AUC values for blood glucose concentrations (mg·dL−1·h) were −8 ± 17 and −43 ± 9 in the L-arginine and Control trials, respectively (P = 0.05).
FFAt concentrations were not different at baseline (P > 0.05). Although the FFAt decreased in the L-ARG trial during the early part of the postprandial period, FFAt concentrations remained similarly elevated in both trials and above the postabsorptive values during the last part of the postprandial period. Accordingly, there was a trend towards a lower AUC0–8h of plasma FFAt in the L-ARG trial compared to the CON trial (0.25 ± 0.20 mmol·L−1·h vs 0.76 ± 0.30 mmol·L−1·h; P = 0.08). This response was significantly lower in the L-ARG trial in the early part of the postprandial period (0 – 4 h; Table 2). The AUC0–8h of plasma FFAi was significantly lower in the L-ARG trial than the CON trial (0.77 ±0.09 vs 1.11 ± 0.08; mmol·L−1·h; P < 0.01). Average responses for plasma concentrations of FFAt, FFAi, and FFAe are presented in Fig. 7.
Fig. 7.
Change in total plasma free fatty acid concentrations (A), as well as plasma free fatty acid concentrations derived from ingested fat (B) and endogenous sources (C) following fat ingestion at time 0. L-arginine (L-arginine) or saline (Control) were infused during the first hour following the fat ingestion. 8-h incremental AUC values for plasma free fatty acid concentrations (mmol·L−1·h) were 0.2 ± 0.2 and 0.8 ± 0.3 for total (P = 0.08), 0.8 ± 0.1 and 1.1 ± 0.1 for those derived from ingested fat (P < 0.05), and −0.5 ± 0.2 and −0.3 ± 0.3 for those from endogenous sources (P = 0.53), in the L-arginine and Control trials, respectively.
Postabsorptive plasma 3-HB concentrations were comparable between trials (P > 0.05) and increased progressively following the fat ingestion in both trials reaching the highest values at 8 h postprandially (Fig. 8). The AUC0–8h of the plasma 3-HB was not different between trials (L-ARG, 1255 ± 171 umol·L−1·h vs CON, 1070 ± 207 umol·L−1·h, P > 0.05). However, the AUC4–8h of the plasma 3-HB was ~38% higher in the L-ARG trial than the CON trial (P = 0.06; Table 2).
Fig. 8.
Change in plasma 3-hydroxybutyrate concentrations following fat ingestion at time 0. L-arginine (L-arginine) or saline (Control) were infused during the first hour following the fat ingestion. 8-h incremental AUC values for plasma 3-hydroxybutyrate concentrations (umol·L−1·h) were 1255 ± 171 and 1071 ± 208 in the L-arginine and Control trials, respectively (P = 0.34).
Discussion
The present study reveals a clear effect of increased plasma L-arginine availability in the postprandial period on attenuating the PPL in older individuals. However, the postprandial whole-body oxidative disposal of the ingested fat, which was our primary end-point, was not stimulated further by the increase in plasma L-arginine concentrations.
Oxidation of lipid to CO2 provides a mechanism for the complete oxidative disposal of ingested fat during the postprandial period, and changes in lipid oxidation during the postprandial period are inversely associated to those in PPL [13]. Cumulative oxidation of the ingested fat in the L-ARG trial was not different than that in the CON trial, indicating that increased plasma L-arginine availability does not stimulate further the oxidation of the ingested fat in the immediate postprandial period. Therefore, stimulation of lipid oxidation by L-arginine in vitro [14] does not appear to be translated into stimulation of ingested-lipid oxidation in vivo, at least in the older population.
An increase in plasma L-arginine concentration stimulates increases in both plasma insulin and glucose concentrations [23]. Both of these effects are clearly documented in the present study (Figs. 5 and 6). Given the well-known role of plasma insulin in the regulation of lipid metabolism, it is reasonable to attempt to explain the observed postprandial responses in plasma lipids in the context of the L-arginine-mediated increase in plasma insulin concentrations in the L-ARG trial. Accordingly, the increase in plasma insulin concentration has probably mediated the decrease in plasma FFAt observed in the early part of the postprandial period (0–4 h; Table 2) through the suppressive effects of insulin on adipose tissue lipolysis and the decrease in the rate of release of FFAe into plasma [24]. This down-regulation of the FFAe release may have also allowed for increased uptake of lipoprotein lipase-liberated fatty acids delivered to tissues in the form of chylomicron-TG, thus reducing the rate of the spillover of the diet-derived fatty acids and their accumulation in plasma during the postprandial period in the L-ARG trial. Decreased accumulation of FFAi in plasma during the postprandial period is possible that has mediated the decreased contribution of ingested-oleate into the plasma small TRL-TG-oleate (Fig. 3). This is because TRLs secreted by the liver, and which constitute the vast majority of TRLs in the plasma small TRL pool during the postprandial period [25], continuously incorporate plasma FFAi during this period. Finally, the L-arginine-mediated plasma insulin response is expected to have likely played a role in decreasing hepatic TG secretion, secondary to decreased fatty acid substrate availability (i.e., plasma FFAe and FFAi) for incorporation into hepatic TG (indirect mechanism), and/or by directly inhibiting TRL secretion by the liver [26].
Plasma insulin inhibits hepatic ketogenesis [27], and plasma 3-HB concentrations decrease below their postabsorptive levels following mixed-meal ingestion in parallel with a decrease in the plasma FFA concentrations [28]. However, there was no apparent decrease in the plasma 3-HB concentrations in the present study. Although not measured, a bolus-infusion of L-arginine similar to that used in the present study increases the concentration of plasma glucagon [16], which, contrary to the effects of plasma insulin on hepatic lipid metabolism, stimulates ketogenesis [29]. L-arginine infusion increases also the concentration of plasma growth hormone [16], which also stimulates ketogenesis [30]. Therefore, L-arginine-mediated effects on increasing plasma glucagon and growth hormone concentrations may have counteracted the effects of insulin on decreasing the 3-HB concentrations early in the postprandial period and may have also mediated the trend for increased production of 3-HB during the late part of the postprandial period. However, given that the kinetics of 3-HB concentrations were not measured, it is not clear the extent to which removal rather than production of 3-HB contributed to the apparent increase in 3-HB concentrations in the present study (Table 2).
L-arginine infusion under experimental conditions comparable to the ones of the present study increases both muscle capillary blood flow [31] and muscle bulk blood flow [15,32]. Specifically in the older individuals, L-arginine infusion reverses an age-related impairment in muscle microvascular blood flow [33]. An increase in muscle bulk blood flow combined with an increase in capillary blood flow are expected to increase the overall delivery and disposal of chylomicron-TG lipid into muscle. Such redistribution of blood flow towards the skeletal muscle is associated with a reduction in PPL [34].
The L-arginine-mediated hormonal responses (e.g., increase in plasma insulin concentration), constitute a limiting factor in any effort to attribute the observed attenuation of PPL to hormonal-independent metabolic processes. Experimental abolishment of hormonal responses associated with the L-arginine infusion (i.e., by somatostatin adminstration) [16], can possibly provide better insight into effects of increased plasma L-arginine availability on plasma lipid metabolism. Furthermore, the extent to which similar effects of L-arginine on PPL are observed after oral administration of L-arginine, or when combined with mixed-meal ingestion, as well as the dose of orally-administered L-arginine necessary to observe these effects, remain to be determined.
Conclusion
Increasing the availability of L-arginine in plasma markedly blunts the postprandial rise in plasma TG concentrations in association with decreased postprandial contribution of ingested lipid into TG of the plasma small TRL in older adults. These responses are not mediated by increased postprandial oxidative disposal of the ingested fat. The precise biochemical mechanisms accounting for the observed L-arginine-mediated postprandial lipid responses deserve further investigation.
ACKNOWLEDGMENTS
Conception and design of the study: C.M., L.J.M., and C.S.K; generation, collection, assembly, analysis and interpretation of data: G.P., C.M., L.J.M., and C.S.K.; drafting and revision of the manuscript: G.P., C.M., and C.S.K.; approval of the final version of the manuscript: G.P., C.M., L.J.M., and C.S.K. The authors thank the nurses at the Clinical Research Unit at Arizona State University, as well as Christine Roberts, PhD, Clinical Research Unit Director. We also thank Ken Kirschner, MS, for skillful technical assistance with the GC/MS measurements, and Jeffrey L. Alexander, PhD, A.T. Still University, for assistance with the screening of the subjects. We gratefully acknowledge the help of Mitchell Harman, MD, PhD at Kronos Longevity Research Institute in recruiting subjects. This project has been funded in part by Arizona State University start-up funds (C.S.K.) and by NIH (R21DK082820) and American Diabetes Association (1-09-CR-39) grants (C.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mensah GA, Brown DW. An overview of cardiovascular disease burden in the United States. Health Aff (Millwood) 2007;26:38–48. doi: 10.1377/hlthaff.26.1.38. [DOI] [PubMed] [Google Scholar]
- 2.Bansal S, Buring JE, Rifai N, Mora S, Sacks FM, Ridker PM. Fasting compared with nonfasting triglycerides and risk of cardiovascular events in women. JAMA. 2007;298:309–316. doi: 10.1001/jama.298.3.309. [DOI] [PubMed] [Google Scholar]
- 3.Langsted A, Freiberg JJ, Nordestgaard BG. Fasting and nonfasting lipid levels: influence of normal food intake on lipids, lipoproteins, apolipoproteins, and cardiovascular risk prediction. Circulation. 2008;118:2047–2056. doi: 10.1161/CIRCULATIONAHA.108.804146. [DOI] [PubMed] [Google Scholar]
- 4.Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 2007;298:299–308. doi: 10.1001/jama.298.3.299. [DOI] [PubMed] [Google Scholar]
- 5.Rivellese AA, Bozzetto L, Annuzzi G. Postprandial lipemia, diet, and cardiovascular risk. Curr Cardiovasc Risk Rep. 2009;3:5–11. [Google Scholar]
- 6.Cassader M, Gambino R, Ruiu G, Marena S, Bodoni P, Pagano G. Postprandial triglyceride-rich lipoprotein changes in elderly and young subjects. Aging (Milano) 1996;8:421–428. doi: 10.1007/BF03339605. [DOI] [PubMed] [Google Scholar]
- 7.Cohn JS, McNamara JR, Cohn SD, Ordovas JM, Schaefer EJ. Postprandial plasma lipoprotein changes in human subjects of different ages. J Lipid Res. 1988;29:469–479. [PubMed] [Google Scholar]
- 8.Issa JS, Diament J, Forti N. Postprandial lipemia: influence of aging. Arq Bras Cardiol. 2005;85:15–19. doi: 10.1590/s0066-782x2005001400004. [DOI] [PubMed] [Google Scholar]
- 9.Puga GM, Meyer C, Everman S, Mandarino LJ, Katsanos CS. Postprandial lipemia in the elderly involves increased incorporation of ingested fat in plasma free fatty acids and small (Sf 20–400) triglyceride-rich lipoproteins. Am J Physiol Endocrinol Metab. 2011;301:E356–E361. doi: 10.1152/ajpendo.00670.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westphal S, Taneva E, Kastner S, Martens-Lobenhoffer J, Bode-Boger S, Kropf S, et al. Endothelial dysfunction induced by postprandial lipemia is neutralized by addition of proteins to the fatty meal. Atherosclerosis. 2006;185:313–319. doi: 10.1016/j.atherosclerosis.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Borsheim E, Bui QU, Tissier S, Cree MG, Ronsen O, Morio B, et al. Amino acid supplementation decreases plasma and liver triacylglycerols in elderly. Nutrition. 2009;25:281–288. doi: 10.1016/j.nut.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phielix E, Szendroedi J, Roden M. Mitochondrial Function and Insulin Resistance during Aging - A Mini-Review. Gerontology. 2011;57:387–396. doi: 10.1159/000317691. [DOI] [PubMed] [Google Scholar]
- 13.Landry N, Bergeron N, Archer R, Samson P, Corneau L, Bergeron J, et al. Whole-body fat oxidation rate and plasma triacylglycerol concentrations in men consuming an ad libitum high-carbohydrate or low-carbohydrate diet. Am J Clin Nutr. 2003;77:580–586. doi: 10.1093/ajcn/77.3.580. [DOI] [PubMed] [Google Scholar]
- 14.Le Gouill E, Jimenez M, Binnert C, Jayet PY, Thalmann S, Nicod P, et al. Endothelial nitric oxide synthase (eNOS) knockout mice have defective mitochondrial beta-oxidation. Diabetes. 2007;56:2690–2696. doi: 10.2337/db06-1228. [DOI] [PubMed] [Google Scholar]
- 15.Bode-Boger SM, Boger RH, Alfke H, Heinzel D, Tsikas D, Creutzig A, et al. L-arginine induces nitric oxide-dependent vasodilation in patients with critical limb ischemia. A randomized, controlled study. Circulation. 1996;93:85–90. doi: 10.1161/01.cir.93.1.85. [DOI] [PubMed] [Google Scholar]
- 16.Bode-Boger SM, Boger RH, Loffler M, Tsikas D, Brabant G, Frolich JC. L-arginine stimulates NO-dependent vasodilation in healthy humans--effect of somatostatin pretreatment. J Investig Med. 1999;47:43–50. [PubMed] [Google Scholar]
- 17.Bode-Boger SM, Boger RH, Creutzig A, Tsikas D, Gutzki FM, Alexander K, et al. L-arginine infusion decreases peripheral arterial resistance and inhibits platelet aggregation in healthy subjects. Clin Sci (Lond) 1994;87:303–310. doi: 10.1042/cs0870303. [DOI] [PubMed] [Google Scholar]
- 18.McConell GK, Huynh NN, Lee-Young RS, Canny BJ, Wadley GD. L-Arginine infusion increases glucose clearance during prolonged exercise in humans. Am J Physiol Endocrinol Metab. 2006;290:E60–E66. doi: 10.1152/ajpendo.00263.2005. [DOI] [PubMed] [Google Scholar]
- 19.Tangphao O, Grossmann M, Chalon S, Hoffman BB, Blaschke TF. Pharmacokinetics of intravenous and oral L-arginine in normal volunteers. Br J Clin Pharmacol. 1999;47:261–266. doi: 10.1046/j.1365-2125.1999.00883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bode-Boger SM, Boger RH, Galland A, Tsikas D, Frolich JC. L-arginine-induced vasodilation in healthy humans: pharmacokinetic-pharmacodynamic relationship. Br J Clin Pharmacol. 1998;46:489–497. doi: 10.1046/j.1365-2125.1998.00803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bode-Boger SM. Effect of L-arginine supplementation on NO production in man. Eur J Clin Pharmacol. 2006;62(Suppl 1):91–99. [Google Scholar]
- 22.Barrows BR, Timlin MT, Parks EJ. Spillover of dietary fatty acids and use of serum nonesterified fatty acids for the synthesis of VLDL-triacylglycerol under two different feeding regimens. Diabetes. 2005;54:2668–2673. doi: 10.2337/diabetes.54.9.2668. [DOI] [PubMed] [Google Scholar]
- 23.Floyd JC, Jr, Fajans SS, Conn JW, Knopf RF, Rull J. Stimulation of insulin secretion by amino acids. J Clin Invest. 1966;45:1487–1502. doi: 10.1172/JCI105456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meek SE, Nair KS, Jensen MD. Insulin regulation of regional free fatty acid metabolism. Diabetes. 1999;48:10–14. doi: 10.2337/diabetes.48.1.10. [DOI] [PubMed] [Google Scholar]
- 25.Nakajima K, Nakano T, Tokita Y, Nagamine T, Inazu A, Kobayashi J, et al. Postprandial lipoprotein metabolism: VLDL vs chylomicrons. Clin Chim Acta. 2011;412:1306–1318. doi: 10.1016/j.cca.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis GF, Uffelman KD, Szeto LW, Weller B, Steiner G. Interaction between free fatty acids and insulin in the acute control of very low density lipoprotein production in humans. J Clin Invest. 1995;95:158–166. doi: 10.1172/JCI117633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keller U, Gerber PP, Stauffacher W. Fatty acid-independent inhibition of hepatic ketone body production by insulin in humans. Am J Physiol. 1988;254:E694–E699. doi: 10.1152/ajpendo.1988.254.6.E694. [DOI] [PubMed] [Google Scholar]
- 28.Roberts R, Bickerton AS, Fielding BA, Blaak EE, Wagenmakers AJ, Chong MF, et al. Reduced oxidation of dietary fat after a short term high-carbohydrate diet. Am J Clin Nutr. 2008;87:824–831. doi: 10.1093/ajcn/87.4.824. [DOI] [PubMed] [Google Scholar]
- 29.Fukao T, Lopaschuk GD, Mitchell GA. Pathways and control of ketone body metabolism: on the fringe of lipid biochemistry. Prostaglandins Leukot Essent Fatty Acids. 2004;70:243–251. doi: 10.1016/j.plefa.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Keller U, Schnell H, Girard J, Stauffacher W. Effect of physiological elevation of plasma growth hormone levels on ketone body kinetics and lipolysis in normal and acutely insulin-deficient man. Diabetologia. 1984;26:103–108. doi: 10.1007/BF00281115. [DOI] [PubMed] [Google Scholar]
- 31.Schellong SM, Boger RH, Burchert W, Bode-Boger SM, Galland A, Frolich JC, et al. Dose-related effect of intravenous L-arginine on muscular blood flow of the calf in patients with peripheral vascular disease: a H215O positron emission tomography study. Clin Sci (Lond) 1997;93:159–165. doi: 10.1042/cs0930159. [DOI] [PubMed] [Google Scholar]
- 32.Meneilly GS, Battistini B, Floras JS. Contrasting effects of L-arginine on insulin-mediated blood flow and glucose disposal in the elderly. Metabolism. 2001;50:194–199. doi: 10.1053/meta.2001.20182. [DOI] [PubMed] [Google Scholar]
- 33.Chauhan A, More RS, Mullins PA, Taylor G, Petch C, Schofield PM. Aging-associated endothelial dysfunction in humans is reversed by L-arginine. J Am Coll Cardiol. 1996;28:1796–1804. doi: 10.1016/s0735-1097(96)00394-4. [DOI] [PubMed] [Google Scholar]
- 34.Hurren NM, Balanos GM, Blannin AK. Is the beneficial effect of prior exercise on postprandial lipaemia partly due to redistribution of blood flow? Clin Sci (Lond) 2011;120:537–548. doi: 10.1042/CS20100460. [DOI] [PubMed] [Google Scholar]