Abstract
Tenascin-C (TNC), overexpressed in invasive growths, has been implicated in progression of melanoma but the source and function of this molecule are not well defined. We found TNC expression at the front of invading melanoma cells, and that adding TNC to matrices enhances individual melanoma cell migration. As TNC is a multidomain protein, we examined the role of the TNC EGF-like repeats (EGFL) as these activate motogenic signaling cascades. We overexpressed a TNC fragment containing the assembly and EGFL domains of TNC (TNCEGFL). TNCEGFL-expressing melanoma cells had lower speed and persistence in 2D migration assays due to a shift in the adhesion-contractility balance, as expression of TNCEGFL delayed melanoma cell attachment and spreading. The less adhesive phenotype was due, in part, to increased ROCK signaling concomitant with MLC2 and MYPT phosphorylation. Inhibition of ROCK activity, which drives transcellular contractility, restored adhesion of TNCEGFL expressing melanoma cells and increased their migration in 2D. In contrast to the diminished migration in 2D, TNCEGFL-expressing melanoma cells had higher invasive potential in Matrigel invasion assays, with cells expressing TNCEGFL having amoeboid morphology. Our findings suggest that melanoma-derived TNC EGFL play a role in melanoma invasion by modulating ROCK signaling and cell migration.
Introduction
Melanoma, which continues to increase in frequency, has a very low response rate to current therapies, with the tumor invading through a collagen-rich dermis to disseminate to ectopic sites. The transition from melanoma in situ and radial growth phase (RGP) to the vertical growth phase (VGP) melanoma is poorly understood. The invasive aspects of tumor progression represent complex molecular events, which involve recognition and remodeling of extracellular matrix (ECM), changes in intracellular signaling and reorganization of the cytoskeleton, allowing for enhanced motility of tumor cells (Wells 2000; Friedl et al. 2009). While there are numerous changes in both the melanoma cells and the dermis during this progression, one striking feature is the re-expression of Tenascin C (TNC) (Ilmonen et al. 2004; Kaariainen et al. 2006; Hood et al. 2010). TNC is an extracellular matrix protein linked to development and tissue regeneration along with tumor invasion (Jones et al. 2000; Midwood et al. 2011). TNC is barely detectable in normal skin, but present in substantial amounts in advanced melanoma particularly at the invasive fronts and lack of detectable TNC in primary melanoma lesions has been shown to correlate with a lower risk of developing metastases (Kaariainen et al. 2006). In the tumor microenvironment, TNC is produced by both transformed tumor cells and stromal cells (Hanamura et al. 1997; Yoshida et al. 1997; De Wever et al. 2004) and in vitro, the majority of human melanoma cell lines secrete TNC (Herlyn et al. 1991). Thus, an open question remains of whether the TNC expressed by the melanoma cells can drive tumor cell invasion.
TNC is a hexameric protein composed of 180 to 320 kDa monomers, which are disulfide linked at the N-termini. Each subunit contains: a globular N-terminal assembly domain, a domain composed of 14.5 Epidermal Growth Factor-like (EGFL) repeats, a domain composed of fibronectin type III-like (FNIII) repeats, and a fibrinogen-like sequence on the C terminus (Aukhil et al. 1993), and each domain has a potentially distinct role (Jones et al. 2000). Tenascin C has been shown to modulate many of the processes involved in cell migration and invasion (reviewed in (Orend 2005; Orend et al. 2006). The majority of cancer cell lines fail to attach on TNC coated surfaces (Huang et al. 2001), with some exceptions where TNC is adhesive (Paron et al. 2011), while endothelial cells attach and spread on TNC (Sriramarao et al. 1993). In addition, soluble and substratum-adsorbed TNC have distinct effects on cell adhesion and proliferation (Orend et al. 2000). These seemingly discrepant findings are in part traceable to the different signaling propensities of the multiple domains of TNC being impacted by the presence of other matrix components and the potentially differential effects of signals in two- versus three-dimensions.
We have previously reported that distinct EGFL repeats of TNC bind and signal through the EGF receptor in a novel manner as ultra low affinity/high avidity ligands (Swindle et al. 2001; Iyer et al. 2007). The ‘staccato’ nature of the individual EGFL repeat binding to the receptor results in an anti-adhesive phenotype that must be balanced by the adhesive matrix proteins or even the fibronectin-like repeats of TNC. In the presence of adequate pro-adhesive moieties, these EGFL repeats restrict signaling to the plasma membrane and preferentially activate a motogenic signaling cascade (Iyer et al. 2008). As little is known about TNC matrikine role in melanoma we tested the role of EGFL repeats in melanoma motility and invasion. Herein we report that TNC promotes melanoma cell invasion and that this activity can be localized to the EGFL repeats.
Results
Advanced melanoma cell lines express TNC and increase migration in response to exogenous TNC
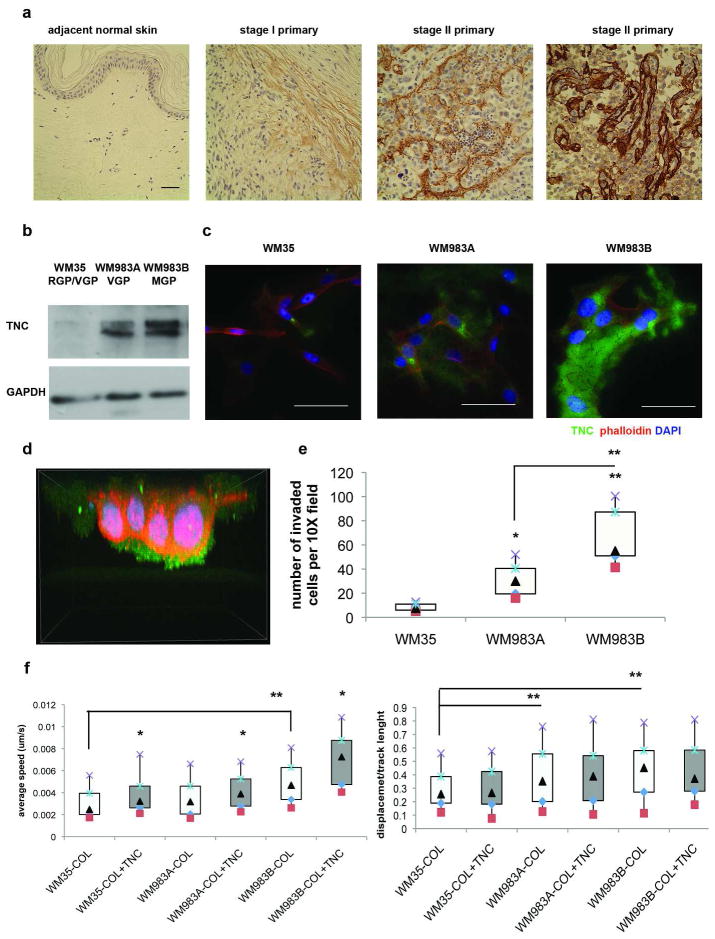
In melanoma, TNC is present at high concentrations in the dermal matrix having increased from negligible levels in normal dermis (Tuominen et al. 1994; Kaariainen et al. 2006; Hood et al. 2010). We examined TNC expression in human melanoma tissue microarray with the antibody specific for the EGFL repeats of Tenascin C (ab6393) and observed that it’s expression increases with melanoma progression (Figure 1A). To test whether melanoma cell derived TNC could be involved in dissemination we first examined expression levels in three melanoma cell lines derived form different stages. The WM35 cells derived from superficial spreading melanoma (RGP/VGP) expressed little if any TNC, similar to human melanocytes (Hood et al. 2010), while VGP WM983A cells and metastatic growth phase (MGP) cells derived from the same patient WM983B expressed high levels of TNC ( Figure 1B). This TNC was incorporated into the cell-generated matrix in the extracellular space and, of greater interest, the melanoma-derived TNC was being secreted asymmetrically at the front of the invading melanoma cells, as observed by confocal microscopy during the invasion of a Matrigel matrix (Figure 1C and D). In fact, we found that invasiveness of the three melanoma cell lines directly correlated to the TNC expression levels (R2=0.83) (Figure 1E). These data strongly suggest a role for TNC, derived from the melanoma itself as driving transmigration.
Figure 1. Expression of TNC in human melanoma samples and cell lines and its influence on melanoma cell invasion and migration.
(A) TMA (tissue micro-array) stained with the antibody recognizing EGFL repeats of TNC (ab6393) (brown) and counterstained with hematoxylin. Scale bar 50μm. Shown are representative primary tumor samples. (B) Immunoblot and (C) immunostaining analyses show increased TNC protein expression and extracellular deposition in VGP and MGP phase melanoma cell lines compared to a cell line derived from superficially spreading melanoma. Scale bars are 50μm. Images are representative of at least three independent experiments. (D) During invasion in Matrigel, TNC is deposited at the fronts of WM983A cells. The size of the 3D model is: width 83μm, height 85μm, depth 63μm. (E) Melanoma cell lines expressing higher amounts of TNC invade to a greater extent in Matrigel invasion assays (R2=0.83). Shown is the mean ± SD of three experiments. (F) Melanoma cell speed (left panel) increases on surfaces coated with Collagen I -Tenascin C mixture compared to Collagen I alone, while cell track straightness (displacement divided by total track length, right panel) correlates with the levels of endogenous TNC expression (R2=0.99) but does not change upon exogenous addition of TNC. Box and whisker plots summarize average from three independent experiments, N > 50 tracks per treatment in repeated experiments. *p < 0.05, ** p < 0.01 (box encompasses 25–75%, with bars 10 (square)-90 (X)%, median is the triangle).
As motility is a predictive aspect of tumor invasion (Wells 2000; Kassis et al. 2001) and consistent with reports of TNC being motogenic (Chung et al. 1996; Swindle et al. 2001; Nishio et al. 2005; Iyer et al. 2008; Paron et al. 2011), melanoma cell lines expressing higher levels of TNC migrated faster in live cell tracking experiments and had greater directional persistence, as measured by cell track straightness (Figure 1F, white bars). That the increase in cell speed was due to the TNC incorporated into the substratum was shown by adding TNC to the Collagen-I coating on tissue culture dishes; this further increased melanoma migration of all three cell lines tested (Figure 1F, gray bars). Additionally, there was high correlation (R2=0.99) between levels of TNC being expressed by melanoma cells, but not with exogenously added TNC, and cell track straightness. Cell track straightness is a measure of directionality of cell movement and is a ratio between displacement of a cell and the track length for a set period of time implying a directional component to the endogenously expressed TNC. These data suggest that both endogenously produced and exogenously presented TNC contribute to melanoma migration, but that the cells asymmetrically place the endogenously produced TNC to promote directional translocation.
TNCEGFL expression impairs migration of melanoma cells in 2D
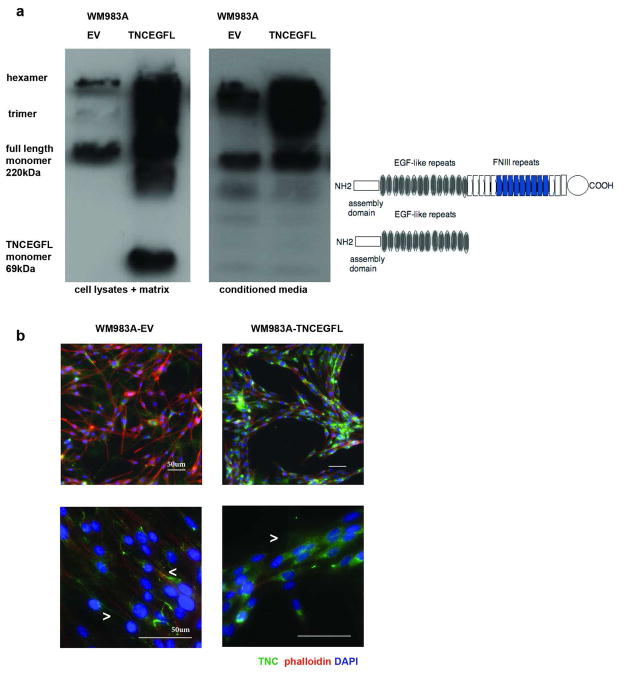
TNC is distinguished from most of the other Tenascin family members by the inclusion and/or extent of the EGF-like repeat region. We have reported that at least some of these EGFL repeats function as cryptic growth factors that preferentially promote motility (Swindle et al. 2001; Iyer et al. 2007). As multivalent concatamers of EGF-like repeats are difficult to synthesize and pose challenges to incorporating into matrices, we over-expressed the Tenascin C EGF-like repeats transcript in the WM983A cell line (WM983A-TNCEGFL), a cell line already expressing substantial amounts of endogenous full length TNC, but responding to additional exogenous TNC (Figure 1). A vertical growth phase melanoma cell line was chosen as a model system as an intermediate phenotype of the cell lines tested regarding motility in 2D and 3D assays, so that changes in both directions can be observed. The TNCEGFL monomer ran in PAGE analyses at the predicted size of 69kDa and, as assessed under non-reducing conditions, assembled in polymers with the endogenous full length TNC (Figure 2A). Staining with antibody that recognized the EGFL repeats (MAB2138, R&D Systems) revealed that TNCEGFL formed punctuated extracellular network, compared to WM983A cells transfected with an empty vector (WM983A-EV), which formed prominent fibrilar TNC mesh, supporting the role of TNC FN repeats in organization of TNC extracellular network (Ramos et al. 1998) (Figures 2B, lower panel and 4B). Interestingly, while WM983A-EV cells arranged themselves in a distributed mesh, the WM983A-TNCEGFL cells aligned to form cords (Figure 2B, upper panel).
Figure 2. Expression of TNCEGFL in WM983A cell line.
(A) Immunoblotting of TNC under non-reducing conditions with the antibody recognizing EGFL repeats (R&D Systems): whole cell lysates and matrix are shown in the left and conditioned melanoma media are in the right. Graphic depiction of the full length TNC and TNCEGFL construct is provided. (B) Immunostaining of TNC: endogenous TNC forms fibrilar mesh in the ECM while TNCEGFL is punctuate (lower panel 60X, arrow heads point to the TNC fibers and punctae, respectively), WM983A-TNCEGFL forms organized cords compared to unorganized WM983A-EV cell distribution (upper panel 20X). Scale bars 50μm.
Of interest, and unexpectedly, WM983A-TNCEGFL cells migrated significantly slower in a 2D wound healing assay that measures directional collective 2D migration (Figure 3A) and presented individually at a significantly reduced average speed in live cell tracking experiments (3.9 nm/s) compared to WM983A-EV cells (4.5 nm/s) (Figure 3B). Moreover, cell track straightness was significantly reduced (Figure 3B right panel) in WM983A-TNCEGFL cells, suggesting that the loss in cell movement directionality in 2D is contributing to decrease in collective cell migration. The phenotype was confirmed in WM35 cell line, which expresses little if any endogenous TNC. Upon transient expression of either full length TNC or TNCEGFL average cell speed and cell track straightness significantly decreased in 2D live cell tracking experiments with the TNCEGFL cells migrating the slowest (Figure S1). This confirms the findings with the overexpression of TNC constructs in the invasive melanoma cells.
Figure 3. WM983A-TNCEGFL cells present impaired 2D cell migration.
(A) WM983A-TNCEGFL cells migrate significantly slower in wound healing assays. Scale bar 200μm. The graph shows mean ± SEM of three experiments each in triplicate. (B) Individual cell speed and track straightness of WM983A-TNCEGFL cells are significantly decreased compared to WM983A-EV cells. N > 50 tracks per phenotype (box encompasses 25–75%, with bars 10 (square)-90 (X)%, median is the triangle). * p < 0.05, ** p < 0.01.
TNCEGFL expression impairs melanoma cell attachment
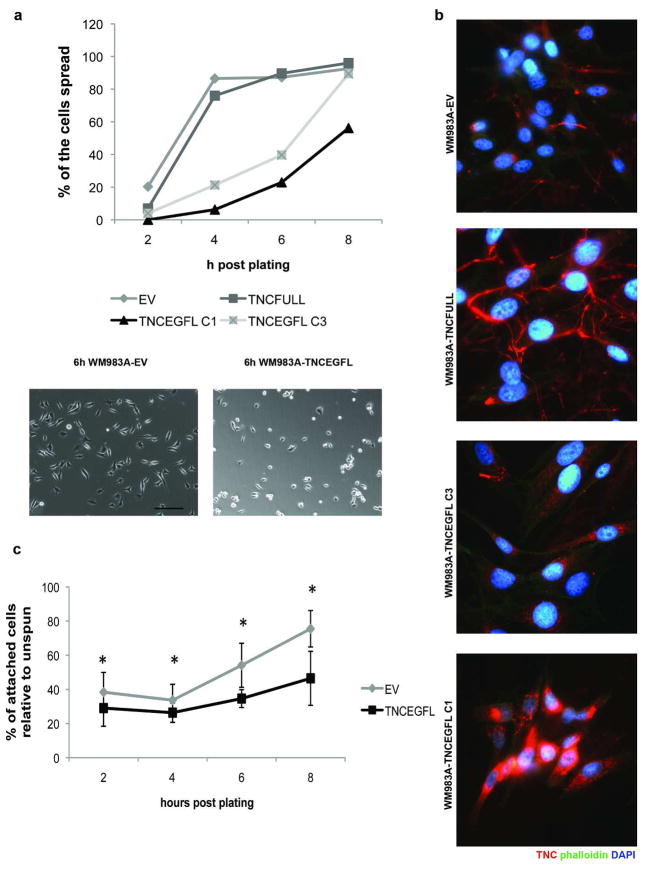
Migration speed in 2D results from a balance in adhesion and contractility in a biphasic manner (DiMilla et al. 1991; DiMilla et al. 1993; Lauffenburger et al. 1996). Decrease in adhesiveness could alter the cell speed in increasing or decreasing manner, depending on the initial adhesiveness to substratum. Examining this aspect, we found that TNCEGFL expressing cells were slower to adhere to and spread on substrata (Figure 4A). In cell spreading assays, TNCEGFL expressing cells started spreading with a 4h lag compared to cells expressing an empty pcDNA3.1 vector (Figure 4A). Furthermore, attachment of WM983A cells was inversely correlated to the amount of TNCEGFL repeats being expressed (Figures 4A and B). While over-expression of the full length of TNC (TNCFULL) did not impair cell attachment, expression of the EGFL repeats suppressed attachment in dose dependent manner. This phenotype was intrinsic as the difference in cell spreading and migration between WM983A-TNCEGFL and WM983A-EV persisted on plastic culture dishes or Fibronectin-1 coated surfaces (Figure S2A). The difference in adhesiveness was not likely due to altered attachment proteins as the integrin profile of the TNCEGFL-expressing cell line remained the same as the one transfected with an empty vector, as determined by PCR Array (SABiosciences, PAHS-013A) (Table S1) nor was this an artifact of cell death as 48 hours post plating there were equal cell numbers (Figure S2B). The reduced adhesion and spreading were due to an anti-adhesive phenotype of the EGFL repeats, as quantified in inverted centrifugation assays. Adherent WM983A-TNCEGFL cells detached to a greater extent when subjected to centrifugal force than WM983A-EV cells (Figure 4C).
Figure 4. WM983A-TNCEGFL cells present impaired cell spreading and attachment that is TNCEGFL dose dependant.
(A) Cells were imaged at the indicated times after seeding by phase contrast microscopy and scored for the percentage of spread cells (upper panel). Lower panel shows the difference in cell spreading at 6h. Shown are representative of three experiments. Scale bar 100μm. (B) Immunostaining of TNC in two clones of WM983A-TNCEGFL (C1 and C3) and the full length TNC (TNCFULL) construct. Arrowheads point to TNC fibers. (C) WM983A-TNCEGFL detached to a greater extent in inverted centrifugation assays. Shown is an average ± SEM of three experiments, each in triplicate. * p < 0.05.
Expression of TNCEGFL activates ROCK signaling
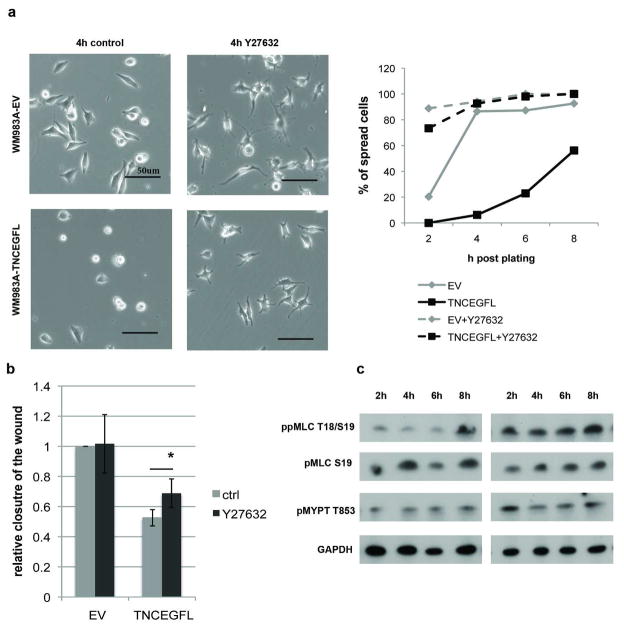
The critical aspect of both cell migration and attachment/spreading is the ratio of adhesion to transcellular contractility (Lauffenburger et al. 1996). As integrin profiles were similar and the phenotype persisted across different substrata, we focused on contractility that creates tension within the cell and determines the round cell shape (reviewed in (Sanz-Moreno et al. 2010)), mainly the Rho-associated kinase (ROCK) and myosin light chain 2 (MLC2). ROCK inhibitor Y27632 dramatically improved adhesion and spreading of WM983A-TNCEGFL cells when added at the time of plating (Figure 5A). 2D migration of WM983A-TNCEGFL cells was slightly but statistically significantly increased when 5μM Y27632 was added at the time of introducing “the wound”, compared to the WM983A-EV (Figure 5B). This is in accordance with findings that inhibition of ROCK in the absence of three-dimensional environment enhances cell movement (Sahai et al. 2003) as it allows generation of protrusions and adhesion of the leading edge.
Figure 5. Expression of TNCEGFL activates ROCK signaling.
(A) Effects of ROCK inhibitor Y27632 on WM983A cell spreading (shown at 4h post plating (left panel) and quantification (right panel)) and migration (B). Shown is mean ± SEM of three experiments each in triplicate, * p > 0.05. Scale bars 50μm. (C) Phosphorylation of the downstream ROCK effectors MLC2 and MYPT in WM983A-TNCEGFL cells during the course of attachment compared to WM983A-EV. Intensities of protein bands determined by integrating optical density over the band area using Image J software, normalized to the GAPDH levels showed increases between 1.5 to 2.0 fold across all three repeats for WM983A-TNCEGFL ppMLC and pMYPT levels compared to WM983A-EV across time points.
Activated ROCK affects the state of MLC2 phosphorylation by direct phosphorylation of MLC2 and by phosphorylation and inhibition of MLC phosphatase (Amano et al. 2000). Phosphorylation of MLC2 on Ser-19 is only partially dependent on ROCK whereas diphosphorylation on both Thr-18 and Ser-19 and myosin phosphatase phosphorylation (which is inhibitory to this contractility antagonist) on Thr-853 are ROCK dependent (Ren et al. 2004). It has been shown that upon cell detachment Rho-ROCK signal transduction is disrupted (Ren et al. 2004). Therefore, we examined the phosphorylation status of these molecules in trypsinized (detached) and replated WM983A-EV and WM983A-TNCEGFL cells during the time course of 8 hours (Figure 5C). We observed that after detachment phosphorylation levels drop and during the course of attachment WM983A-EV cells gradually increase diphosphorylation of MLC2 (ppMLC2) while TNCEGFL expressing cells have constant higher levels of ppMLC2. Monophosphorylation on Ser-19, as expected, did not differ in WM983A-TNCEGFL cells compared to WM983A-EV. Increased diphosphorylation of MLC2 may induce premature contraction and decreased spreading, the phenotype that we observe in TNCEGFL expressing cells. Additionally, in TNCEGFL expressing cells phosphorylation of Thr-853 MYPT, myosin-binding subunit of myosin phosphatase, that inhibits its phosphatase activity, was increased compared to WM983A-EV. Furthermore, transient expression of TNCFULL or TNCEGFL in WM35 cells increased basal levels of ppMLC (Figure S1A) As adhesion provides physical support for contraction and effective migration on rigid substrates, our results imply that overly activated ROCK and its effectors in TNCEGFL expressing cells lead to impaired cell attachment and movement in 2D.
TNCEGFL expressing melanoma cells have increased invasion potential
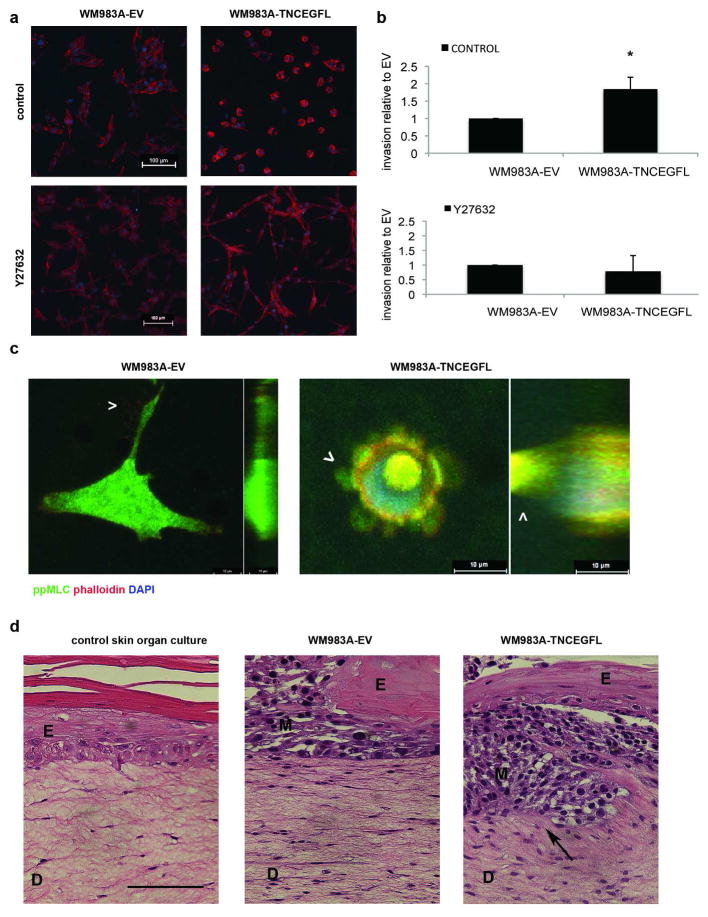
The foregoing presents a cell that is less attached to the substrata, and likely exhibits greater shape plasticity, and thus may preferentially move through a 3D matrix (Friedl et al. 1998; Wolf et al. 2003). To address the question of the role of TNCEGFL in 3D migration and invasion we performed Matrigel invasion experiments. WM983A-TNCEGFL cells had rounded morphology in Matrigel, as observed by confocal microscopy, and the phenotype could be reverted to the elongated mesenchymal type in the presence the ROCK inhibitor (Figure 6A). In addition, WM983A-TNCEGFL cells invaded to a greater extent than WM983A-EV cells (Figure 6B). Confocal imaging of individual invading cells in 3D revealed that WM983A-TNCEGFL cells had rounded blebbing morphology with high diphosphorylated MLC present in the invading blebs, compared to WM983A-EV cells which had a mesenchymal morphology with filamentous protrusions (Figure 6C). Increased invasion potential was not due to increased gelatinase activity as the activities of MMP2 and MMP9 did not change (Figure S2C). Our findings are in line with the notion that round melanoma cells are able to squeeze through gaps in ECM more readily (Gaggioli et al. 2007) as a result of strengthening of the Rho/ROCK signaling pathway as one of the mechanisms leading to mesenchymal to amoeboid migration transition (reviewed in (Friedl 2004)).
Figure 6. TNCEGFL expressing cells have rounded morphology in 3D and present higher invasion potential.
(A) Morphology of WM983A-EV and WM983A-TNCEGFL cells seeded in Matrigel in the absence or presence of Y27632 inhibitor. Scale bars 100μm. (B) Extent of invasion into Matrigel after 48h, in the absence or presence of Y27632 inhibitor, results are shown relative to the WM983A-EV invasion, average ± SD n=3, * p < 0.05. (C) Confocal stacks of individual WM983A-EV and WM983A-TNCEGFL invading cells in 3D Matrigel, immunostained for diphosphorylated MLC (Cell Signaling). Arrowheads point to actin protrusions and blebs, respectively. The left hand part of each image is the Z stack in the transverse plane through the cell, whereas the right hand part is the coronal section with the depth to the left. Scale bars 10μm. (D) Representative images of H&E stained skin organ cultures seeded without or with WM983A-EV and WM983A-TNCEGFL melanoma cells after 20 days of culture. Arrow points to invading WM983A-TNCEGFL cells. Scale bar 100μm. E-epidermis, D-dermis, M-melanoma. These results are representative of three independent experiments.
Finally, we tested the ability of WM983A-EV and WM983A-TNCEGFL cells to invade into the collagen-rich dermis of an all human skin organ cultures, a model that better captures melanoma invasion in the human skin. While WM983A-EV cells disrupted formation of stratified epidermis, they did not invade into dermis in the 20-day period. On the contrary, WM983A-TNCEGFL cells were able to penetrate in the dermis compartment of skin organ cultures (Figure 6D).
Discussion
The most important step in progression from melanoma in situ to advanced metastatic disease is acquiring invasive ability. Advanced melanomas produce substantial amounts of TNC that are thought to form fibrillar channels allowing invasion (Kaariainen et al. 2006). Down-regulating TNC in melanoma cells leads to impaired lung colonization and metastasis formation (Fukunaga-Kalabis et al. 2010); this was also observed in TNC-deficient breast cancer cells in which TNC maintains the metastatic niche (Oskarsson et al. 2011). Therefore, increased levels of TNC secreted by melanoma cells may not only promote tumor invasion but also contribute to metastatic seeding. Despite the phenomenological descriptions, the actual cellular mechanisms by which TNC contributes to invasiveness of melanoma are not defined. Herein, we show that one aspect driven by TNC is the enhanced migration through matrices signaled by the EGFL of this matrix molecule.
In this report we show that advanced melanoma cell lines themselves express large amounts of TNC that is incorporated into the extracellular matrix, and further respond to exogenous TNC by increasing migration speed. We observe that asymmetrical placement of TNC at the cells’ front may promote directional translocation during melanoma cell invasion. As TNC is susceptible to proteolytic degradation by MMPs (Imai et al. 1994; Siri et al. 1995) and EGFL repeats can be released or ‘uncovered’ by this cleavage (Wallner et al. 2004), we focused in particular on the possible impact of TNCEGFL repeats on melanoma cell migration and invasion. We show that overexpressing TNCEGFL impairs TNC extracellular network organization and confers intrinsic anti-adhesive phenotype to melanoma cells, which is EGFL-dose dependent. We find that TNCEGFL-expressing cells migrate slower in 2D motility assays but move and have increased invasion potential in 3D systems. Invading TNCEGFL expressing cells have rounded cell morphology, consistent with reports that melanoma cells can shift to a less adhesive amoeboid mode of cell movement (Sahai et al. 2003). This dichotomy of migration potential in 2D and 3D matrices has been noted previously with a shift in the adhesion-contractility towards a lesser overall value promoting speed through 3D matrices while diminishing the locomotion on 2D surfaces (Zaman et al. 2006) The mechanism of this shape plasticity in presence of TNCEGFL points to ROCK signaling that has been shown to allow melanoma switching between mesenchymal and ameboidal types of cell movement (Sahai et al. 2003; Sanz-Moreno et al. 2009). This is consistent with signaling of the EGFL repeats via the EGFR. However, we could not definitively demonstrate such a pathway as EGFL repeats being at ultra-low affinity (Swindle et al. 2001) and melanoma cells expressing low levels of steady state EGFR; further, the melanoma cells did not survive in the face of EGFR inhibition (data not shown). Nevertheless, Proximity Ligation Assays (PLA) (Soderberg et al. 2006) implicate a close physical association (<40nm) of TNC and EGFR in these melanoma cells (Figure S3). Still in the absence of definitive evidence, it is possible that the EGFL repeats signal via other surface receptors.
It has been described that amoeboid-like melanoma cells that have high ROCK activity are found at the invasive fronts of melanomas, while mesenchymal elongated cells comprise the body of the tumors (Sanz-Moreno et al. 2011). We find that TNCEGFL-expressing cells have increased ROCK signaling, as observed by increased phosphorylation of its targets: biphosphorylation of MLC2, that increases transcellular contractility, and phosphorylation of MYPT, that negates the inhibition of actin cytoskeleton contraction. This is validated by the loss of the anti-adhesive phenotype and rounded morphology in presence of the ROCK inhibitor Y27632 on both rigid 2D substrates and in 3D Matrigel. Furthermore, in the blebs of TNCEGFL expressing cells invading the 3D matrices, diphosphorylated MLC2 co localizes with the actin cytoskeleton; this is characteristic of contractile amoeboid type of movement (reviewed in (Lammermann et al. 2009)). Finally, we show that TNCEGFL expressing cells are more efficient in invading into a human skin organ culture, a setting that better resembles the first steps of actual melanoma invasion in patients.
Supporting our signaling model, it has been shown that recombinant TNC construct Ten70 which does not contain EGFL repeats, suppresses Rho A activation while maintaining the level of active Cdc42 thus preventing stress fiber formation (Wenk et al. 2000). Cells seeded on Ten70 form prominent filopodia, and that exact feature is lost in TNCEGFL-expressing melanoma cells. By overexpressing EGFL repeats, the balance of endogenous signaling of TNC is shifted and activation of ROCK enables amoeboid morphology. Our results are in concordance with observation that distinct modes of cell motility have different requirements for ROCK activity (Sahai et al. 2003), as TNCEGFL expressing cells perform better in 3D migration assays and have impaired migration in 2D assays compared to melanoma cells transfected with an empty vector. It is interesting to speculate that TNC, highly expressed at the invasion fronts of melanoma (Kaariainen et al. 2006) and at the front of invading melanoma cells promotes activation of ROCK and amoeboid cell morphology observed at the fronts of invasion in melanoma tumors (Sanz-Moreno et al. 2011).
Taken together, our data demonstrate that over expression of TNCEGFL repeats alters both adhesive and migratory states of melanoma cells and the composition of the extracellular matrix making it more permissive for melanoma cell invasion. The rounded morphology and enhanced blebbing of the amoeboid form of motility allows for cells to more easily penetrate smaller sized pores in matrices and thus are less dependent on proteolytic ‘opening’ of channels in a collagen-rich matrix such as the dermis (Friedl 2004). Our findings also reinforce the notion that cells utilize different signaling cascades to effect migration dependent on the 2D versus 3D matrix context.
Materials and Methods
Cell culture
Melanoma cell line WM35, obtained from Coriell Institute for Medical Research (Camden, NJ), was maintained in MCDB153: L15 4:1 medium mixture with addition of 5% FBS, 5μg/ml insulin and 2mM CaCl2. WM983A and WM983B were obtained from Wistar Institute (Philadelphia, PA) and cultured in DMEM: L15 3:1 mixture with addition of 10% FBS (Herlyn et al. 1991). Human epidermal keratinocytes were obtained from Invitrogen and cultured on collagen-coated dishes in Epilife medium with growth supplements (Invitrogen, Grand Island, NY). Human neonatal fibroblasts were cultured in DMEM with 10% FBS. Primary cells were used in skin organ cultures under 6th passage.
Generation of TNCEGFL and TNCFULL constructs and TNCEGFL expressing melanoma cell lines
TNCEGFL and full length TNC (TNCFULL) constructs were generated by PCR amplification from the X78565 full length TNC in pNUT vector kindly provided by Dr. Harold Erickson (Duke University, Durham, NC). Detailed information about the primer sequences and enzymes used can be found in the supplementary material section. WM983A and WM35 cell lines were transfected with TNCEGFL or TNCFULL using Lipofectamine (Invitorgen). WM35 cell line was used 24h after transfection and WM983A clones stably expressing TNCEGFL or TNCFULL were selected with G418.
Confocal imaging of Matrigel invading cells
Matrigel invasion assay was performed in BD BioCoat Matrigel Invasion Chamber per manufacturer instructions (354480, BD Biosciences, Bedford, MA). 48h after cell seeding gels were fixed in 4% formaldehyde 0.25% glutaraldehyde in PBS and stained. Membranes with gels were excised and mounted on glass slides with PBS. Detailed information about staining procedure and antibodies used can be found the supplementary materials section. Images were taken on Nikon Sweptfield Confocal Microscope (TSI inverted) using 10X and 60X 1.4 NA objectives. 3D volume representations of Z-stacks were made using Nikon Elements software.
Other methods
Further detailed information about cell spreading quantification, inverted centrifugation assays, 2D migration and tracking and skin organ cultures is provided in the supplementary materials section.
Supplementary Material
Acknowledgments
We would like to thank Taiki Hakozaki for assistance with the cell attachment experiments, Diane George for technical assistance and Dr. Harold Erickson (Duke University, Durham, NC) for the TNC construct in pNUT vector. A VA Merit Award and RO1 award to A. W. supported these studies.
Footnotes
Conflict of interest
The authors state no conflict of interest.
References
- Amano M, Fukata Y, Kaibuchi K. Regulation and functions of Rho-associated kinase. Exp Cell Res. 2000;261(1):44–51. doi: 10.1006/excr.2000.5046. [DOI] [PubMed] [Google Scholar]
- Aukhil I, Joshi P, Yan Y, et al. Cell- and heparin-binding domains of the hexabrachion arm identified by tenascin expression proteins. J Biol Chem. 1993;268(4):2542–2553. [PubMed] [Google Scholar]
- Chung CY, Murphy-Ullrich JE, Erickson HP. Mitogenesis, cell migration, and loss of focal adhesions induced by tenascin-C interacting with its cell surface receptor, annexin II. Mol Biol Cell. 1996;7(6):883–892. doi: 10.1091/mbc.7.6.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wever O, Nguyen QD, Van Hoorde L, et al. Tenascin-C and SF/HGF produced by myofibroblasts in vitro provide convergent pro-invasive signals to human colon cancer cells through RhoA and Rac. FASEB J. 2004;18(9):1016–1018. doi: 10.1096/fj.03-1110fje. [DOI] [PubMed] [Google Scholar]
- DiMilla PA, Barbee K, Lauffenburger DA. Mathematical model for the effects of adhesion and mechanics on cell migration speed. Biophys J. 1991;60(1):15–37. doi: 10.1016/S0006-3495(91)82027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMilla PA, Stone JA, Quinn JA, et al. Maximal migration of human smooth muscle cells on fibronectin and type IV collagen occurs at an intermediate attachment strength. J Cell Biol. 1993;122(3):729–737. doi: 10.1083/jcb.122.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P. Prespecification and plasticity: shifting mechanisms of cell migration. Curr Opin Cell Biol. 2004;16(1):14–23. doi: 10.1016/j.ceb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10(7):445–457. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- Friedl P, Zanker KS, Brocker EB. Cell migration strategies in 3-D extracellular matrix: differences in morphology, cell matrix interactions, and integrin function. Microsc Res Tech. 1998;43(5):369–378. doi: 10.1002/(SICI)1097-0029(19981201)43:5<369::AID-JEMT3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Fukunaga-Kalabis M, Martinez G, Nguyen TK, et al. Tenascin-C promotes melanoma progression by maintaining the ABCB5-positive side population. Oncogene. 2010;29(46):6115–6124. doi: 10.1038/onc.2010.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaggioli C, Sahai E. Melanoma invasion - current knowledge and future directions. Pigment Cell Res. 2007;20(3):161–172. doi: 10.1111/j.1600-0749.2007.00378.x. [DOI] [PubMed] [Google Scholar]
- Hanamura N, Yoshida T, Matsumoto E, et al. Expression of fibronectin and tenascin-C mRNA by myofibroblasts, vascular cells and epithelial cells in human colon adenomas and carcinomas. Int J Cancer. 1997;73(1):10–15. doi: 10.1002/(sici)1097-0215(19970926)73:1<10::aid-ijc2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Herlyn M, Graeven U, Speicher D, et al. Characterization of tenascin secreted by human melanoma cells. Cancer Res. 1991;51(18):4853–4858. [PubMed] [Google Scholar]
- Hood BL, Grahovac J, Flint MS, et al. Proteomic analysis of laser microdissected melanoma cells from skin organ cultures. J Proteome Res. 2010;9(7):3656–3663. doi: 10.1021/pr100164x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Chiquet-Ehrismann R, Moyano JV, et al. Interference of tenascin-C with syndecan-4 binding to fibronectin blocks cell adhesion and stimulates tumor cell proliferation. Cancer Res. 2001;61(23):8586–8594. [PubMed] [Google Scholar]
- Ilmonen S, Jahkola T, Turunen JP, et al. Tenascin-C in primary malignant melanoma of the skin. Histopathology. 2004;45(4):405–411. doi: 10.1111/j.1365-2559.2004.01976.x. [DOI] [PubMed] [Google Scholar]
- Imai K, Kusakabe M, Sakakura T, et al. Susceptibility of tenascin to degradation by matrix metalloproteinases and serine proteinases. FEBS Lett. 1994;352(2):216–218. doi: 10.1016/0014-5793(94)00960-0. [DOI] [PubMed] [Google Scholar]
- Iyer AK, Tran KT, Borysenko CW, et al. Tenascin cytotactin epidermal growth factor-like repeat binds epidermal growth factor receptor with low affinity. J Cell Physiol. 2007;211(3):748–758. doi: 10.1002/jcp.20986. [DOI] [PubMed] [Google Scholar]
- Iyer AK, Tran KT, Griffith L, et al. Cell surface restriction of EGFR by a tenascin cytotactin-encoded EGF-like repeat is preferential for motility-related signaling. J Cell Physiol. 2008;214(2):504–512. doi: 10.1002/jcp.21232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PL, Jones FS. Tenascin-C in development and disease: gene regulation and cell function. Matrix Biol. 2000;19(7):581–596. doi: 10.1016/s0945-053x(00)00106-2. [DOI] [PubMed] [Google Scholar]
- Kaariainen E, Nummela P, Soikkeli J, et al. Switch to an invasive growth phase in melanoma is associated with tenascin-C, fibronectin, and procollagen-I forming specific channel structures for invasion. J Pathol. 2006;210(2):181–191. doi: 10.1002/path.2045. [DOI] [PubMed] [Google Scholar]
- Kassis J, Lauffenburger DA, Turner T, et al. Tumor invasion as dysregulated cell motility. Semin Cancer Biol. 2001;11(2):105–117. doi: 10.1006/scbi.2000.0362. [DOI] [PubMed] [Google Scholar]
- Lammermann T, Sixt M. Mechanical modes of ‘amoeboid’ cell migration. Curr Opin Cell Biol. 2009;21(5):636–644. doi: 10.1016/j.ceb.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84(3):359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- Midwood KS, Hussenet T, Langlois B, et al. Advances in tenascin-C biology. Cell Mol Life Sci. 2011;68(19):3175–3199. doi: 10.1007/s00018-011-0783-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio T, Kawaguchi S, Yamamoto M, et al. Tenascin-C regulates proliferation and migration of cultured astrocytes in a scratch wound assay. Neuroscience. 2005;132(1):87–102. doi: 10.1016/j.neuroscience.2004.12.028. [DOI] [PubMed] [Google Scholar]
- Orend G. Potential oncogenic action of tenascin-C in tumorigenesis. Int J Biochem Cell Biol. 2005;37(5):1066–1083. doi: 10.1016/j.biocel.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Orend G, Chiquet-Ehrismann R. Adhesion modulation by antiadhesive molecules of the extracellular matrix. Exp Cell Res. 2000;261(1):104–110. doi: 10.1006/excr.2000.5041. [DOI] [PubMed] [Google Scholar]
- Orend G, Chiquet-Ehrismann R. Tenascin-C induced signaling in cancer. Cancer Lett. 2006;244(2):143–163. doi: 10.1016/j.canlet.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Oskarsson T, Acharyya S, Zhang XH, et al. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nat Med. 2011;17(7):867–874. doi: 10.1038/nm.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paron I, Berchtold S, Voros J, et al. Tenascin-C Enhances Pancreatic Cancer Cell Growth and Motility and Affects Cell Adhesion through Activation of the Integrin Pathway. PLoS One. 2011;6(6):e21684. doi: 10.1371/journal.pone.0021684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos DM, Chen B, Regezi J, et al. Tenascin-C matrix assembly in oral squamous cell carcinoma. Int J Cancer. 1998;75(5):680–687. doi: 10.1002/(sici)1097-0215(19980302)75:5<680::aid-ijc4>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Ren XD, Wang R, Li Q, et al. Disruption of Rho signal transduction upon cell detachment. J Cell Sci. 2004;117(Pt 16):3511–3518. doi: 10.1242/jcs.01205. [DOI] [PubMed] [Google Scholar]
- Sahai E, Marshall CJ. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol. 2003;5(8):711–719. doi: 10.1038/ncb1019. [DOI] [PubMed] [Google Scholar]
- Sanz-Moreno V, Gaggioli C, Yeo M, et al. ROCK and JAK1 Signaling Cooperate to Control Actomyosin Contractility in Tumor Cells and Stroma. Cancer Cell. 2011;20(2):229–245. doi: 10.1016/j.ccr.2011.06.018. [DOI] [PubMed] [Google Scholar]
- Sanz-Moreno V, Marshall CJ. Rho-GTPase signaling drives melanoma cell plasticity. Cell Cycle. 2009;8(10):1484–1487. doi: 10.4161/cc.8.10.8490. [DOI] [PubMed] [Google Scholar]
- Sanz-Moreno V, Marshall CJ. The plasticity of cytoskeletal dynamics underlying neoplastic cell migration. Curr Opin Cell Biol. 2010;22(5):690–696. doi: 10.1016/j.ceb.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Siri A, Knauper V, Veirana N, et al. Different susceptibility of small and large human tenascin-C isoforms to degradation by matrix metalloproteinases. J Biol Chem. 1995;270(15):8650–8654. doi: 10.1074/jbc.270.15.8650. [DOI] [PubMed] [Google Scholar]
- Soderberg O, Gullberg M, Jarvius M, et al. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat Methods. 2006;3(12):995–1000. doi: 10.1038/nmeth947. [DOI] [PubMed] [Google Scholar]
- Sriramarao P, Mendler M, Bourdon MA. Endothelial cell attachment and spreading on human tenascin is mediated by alpha 2 beta 1 and alpha v beta 3 integrins. J Cell Sci. 1993;105( Pt 4):1001–1012. doi: 10.1242/jcs.105.4.1001. [DOI] [PubMed] [Google Scholar]
- Swindle CS, Tran KT, Johnson TD, et al. Epidermal growth factor (EGF)-like repeats of human tenascin-C as ligands for EGF receptor. J Cell Biol. 2001;154(2):459–468. doi: 10.1083/jcb.200103103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen H, Kallioinen M. Increased tenascin expression in melanocytic tumors. J Cutan Pathol. 1994;21(5):424–429. doi: 10.1111/j.1600-0560.1994.tb00284.x. [DOI] [PubMed] [Google Scholar]
- Wallner K, Li C, Shah PK, et al. EGF-Like domain of tenascin-C is proapoptotic for cultured smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24(8):1416–1421. doi: 10.1161/01.ATV.0000134299.89599.53. [DOI] [PubMed] [Google Scholar]
- Wells A. Tumor invasion: role of growth factor-induced cell motility. Adv Cancer Res. 2000;78:31–101. doi: 10.1016/s0065-230x(08)61023-4. [DOI] [PubMed] [Google Scholar]
- Wenk MB, Midwood KS, Schwarzbauer JE. Tenascin-C suppresses Rho activation. J Cell Biol. 2000;150(4):913–920. doi: 10.1083/jcb.150.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K, Mazo I, Leung H, et al. Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J Cell Biol. 2003;160(2):267–277. doi: 10.1083/jcb.200209006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Matsumoto E, Hanamura N, et al. Co-expression of tenascin and fibronectin in epithelial and stromal cells of benign lesions and ductal carcinomas in the human breast. J Pathol. 1997;182(4):421–428. doi: 10.1002/(SICI)1096-9896(199708)182:4<421::AID-PATH886>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Zaman MH, Trapani LM, Sieminski AL, et al. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc Natl Acad Sci U S A. 2006;103(29):10889–10894. doi: 10.1073/pnas.0604460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.