Abstract
Background
Simultaneous adherence with multiple self-care instructions among heart failure (HF) patients is not well described.
Methods
Patient-reported adherence to eight recommendations related to exercise, alcohol, medications, smoking, diet, weight, and symptoms was assessed among 308 HF patients using the Medical Outcomes Study Specific Adherence Scale questionnaire (0=‘never’, 5=‘always’; maximum score=40). A baseline cumulative score of ≥32/40 (average ≥80%) defined good adherence. Clinical events (death/transplantation/ventricular assist device), resource utilization, functional capacity (6-minute walk distance), and health status (Kansas City Cardiomyopathy Questionnaire [KCCQ]) were compared among patients with and without good adherence.
Results
Mean follow-up 2.0±1.0 years. Adherence ranged from 26.3% (exercise) to 89.9% (medications). A cumulative score indicating good adherence was reported by 35.7%, whereas good adherence with every behavior was reported by 9.1% of patients. Good adherence was associated with fewer hospitalizations (all-cause 87.8 vs. 107.6; P=0.018; HF 29.6 vs. 43.8; P=0.007), and hospitalized days (all-cause 422 vs. 465; P=0.015; HF 228 vs. 282; P<0.001) per 100 person-years; and better health status (KCCQ overall score 70.1±24.6 vs. 63.8±22.8; P=0.011). Adherence was not associated with clinical events or functional capacity.
Conclusions
Patient-reported adherence with HF self-care recommendations is alarmingly low and selective. Good adherence was associated with lower resource utilization and better health status.
Keywords: Cardiovascular, compliance, outcomes
Heart failure (HF) prevalence continues to rise and is expected to worsen as the proportion of elderly population increase.[1] Despite advances in therapy, absolute outcomes for these patients remain sub-optimal.[2] Heart failure is the leading cause of hospitalization, with nearly half of all admitted patients being readmitted within six months of discharge.[3] Approximately half the HF readmissions are considered preventable, and poor adherence with recommended self-care is identified as a contributing factor in many cases.[4, 5] Advances in therapy have resulted in an increased number of prescribed medications requiring complex daily dosing schedules.[6] Most HF patients are elderly with multiple co-morbidities, and may ultimately be responsible for taking more than ten daily treatment doses.[7] In addition to complex medication regimen, the current guidelines include diet, exercise, and lifestyle recommendations that can be challenging for patients.[8, 9] Adherence to some of these recommendations however, e.g. medications and low salt diet, may reduce readmissions and mortality rates.[10–12] Previous studies have demonstrated variable adherence to self-care depending on patient population, specific recommendation, and the method of assessing adherence.[9, 13] However, many prior investigations assessing adherence with HF self-care have studied only select recommendations.[14, 15] This is concerning as many patients demonstrate selective adherence to some self-care recommendations at the expense of others.[16] It is possible that benefit from adherence with one, e.g. medications, is neutralized by poor follow-through with other, e.g. low sodium diet, self-care behavior. In this study we sought to comprehensively assess patient-reported adherence to eight HF self-care recommendations, predictors of adherence, and its association with outcomes.
METHODS
Patient Population
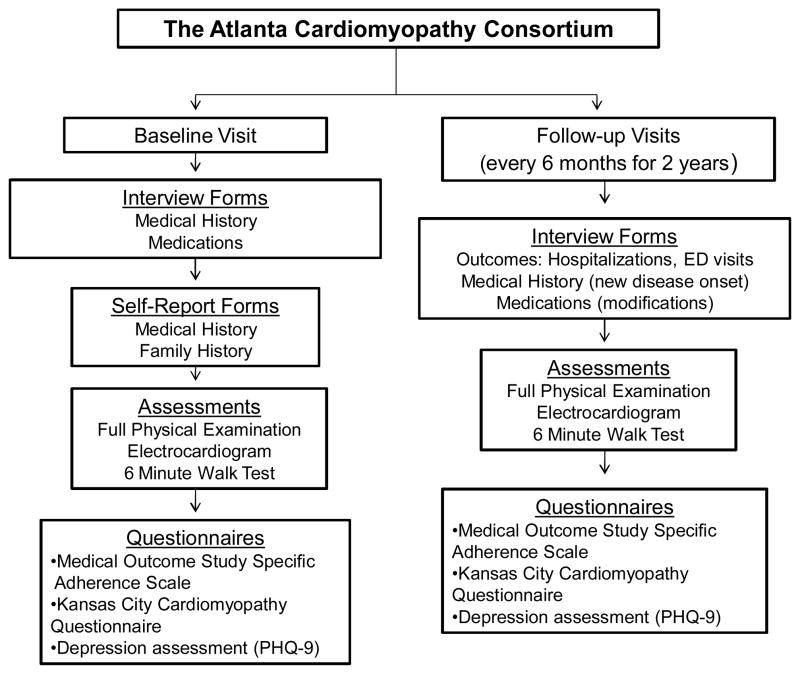
The Atlanta Cardiomyopathy Consortium is a prospective cohort study enrolling outpatients with HF from three university-affiliated hospitals in the greater metropolitan Atlanta area (Figure 1). Inclusion criteria included age >18 years, able to understand and sign written informed consent and participate, and a diagnosis of HF with either reduced or preserved ejection fraction. The diagnosis of HF with preserved ejection fraction required, in addition to clinical diagnosis of HF, elevated B-type natriuretic peptide level >200 pg/dl and/or an echocardiogram evidence of diastolic dysfunction.[17] Exclusion criteria included congenital heart disease, previous heart transplantation or awaiting transplant, known cardiac infiltrative disease (e.g., amyloidosis), previous other solid organ transplantation, and end-stage HF requiring outpatient continuous inotrope infusion.
Figure 1.
The Atlanta Cardiomyopathy Consortium: Study Design
Study Procedures
All patients undergo past history surveys, history and physical examination, electrocardiogram, 6-minute walk test, several questionnaires, and collection of blood and urine samples at baseline. Race is self-reported. Education level assessed as number of school years completed. Depression was determined based on Patient Health Questinnaire-9 (PHQ-9), defined below. Every six months, the patients are contacted to assess medication changes, procedures, new diagnoses, and hospitalizations. Mortality data are collected through medical record review, information from family members, and Social Security Death Index query. Hospitalization data are obtained from electronic health records review, outpatient notes from any specialty encounter for any admission to an outside hospital, and direct patient inquiry during follow-up. The Institutional Review Board has approved the study. Informed consent was obtained from all patients prior to enrollment. At the time of this analysis, a total of 321 patients were enrolled; of these, 308 (96.0%) completed the Medical Outcomes Study Specific Adherence Scale (MOS-SAS) for self-care behaviors at baseline and were included in the study.
Adherence to Self-care
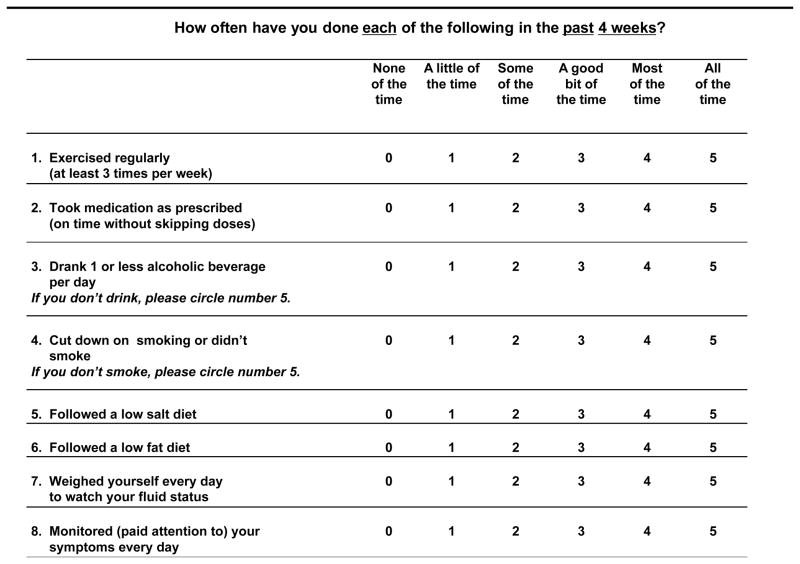
Patient-reported self-care adherence was measured using the MOS-SAS questionnaire (Figure 2), an eight-item scale that has been successfully used to measure adherence in HF, demonstrating adequate reliability and validity.[18–21] The self-care behaviors assessed include, regular exercise, taking medication as prescribed, consuming one or less alcoholic beverage per day, cutting down on smoking or not smoking, following a low salt diet, a low fat diet, weighing daily, and monitoring and paying attention to symptoms. Patients were asked how often they adhere to each behaviors in the previous four weeks (0 “none of the time”, 1 “a little of the time”, 2 “some of the time”, 3 “a good bit of the time”, 4 “most of the time”, or 5 “all of the time”).
Figure 2.
Medical Outcomes Study Specific Adherence Scale
Adherence Definition
Currently there is no accepted standard to grade adherence for HF self-care. However, 80% level has been used to define “good” adherence to medications and is associated with an acceptable sensitivity and specificity in medication adherence studies.[22] We therefore used this threshold to define good adherence. Since our aim was to assess simultaneous adherence with eight self-care measures, good adherence was defined as achieving a cumulative score of ≥80% (32/40 points derived from 8 questions having answer choices ranging from 0 to 5), regardless of score on individual questions. Therefore, patients could still be considered as having good adherence even if adherence was not good for any given individual behavior, as long as the overall score was ≥80%. We however also performed a secondary analysis to assess the proportion of patients reporting ≥ 80% adherence for each of the eight individual behaviors. For the purposes of this study, scores of 4 or 5 (“most of the time” and “all of the time”) were combined and considered as ‘adherent’ for any given question.
PHQ-9
Patient Health Questinnaire-9 (PHQ-9), is an established reliable and valid depression-screening tool, which scores each of the 9 standard (DSM-IV) criteria of depression as “0” (not at all) to “3” (nearly every day). PHQ-9 score ≥10 has 88% sensitivity and 88% specificity for major depression.[23]
Self-Care Education
As part of the routine HF clinic practice, patients are educated regarding self-care behaviors at the initial consultation and each subsequent clinic visit by the nurses. During initial visit, patients and family members also watch a video describing the importance of self-care and how to best comply with it, including practical daily tips and resources. Each patient also receives a copy of the patient education brochure, A Stronger Pump: A Guide for People with All Types of Heart Failure (Patient Education Solutions, Pritchett and Hull Associates Inc., Atlanta, GA). Additional printed reading material is given to the patients regarding low salt diet and the sodium content in the common food items. Patients are asked about self-care at each visit, are given the opportunities to ask questions regarding HF self-care; further education is provided as needed.
Outcomes
Clinical event was defined as a composite of death, heart transplantation, or left ventricular assist device placement. Resource utilization was assessed as emergency department visits, all-cause and HF hospitalizations, and total number of days hospitalized per 100 person-year follow-up. Functional capacity was determined using 6-minute walk test, a simple measure of functional capacity in HF patients.[24] Two red cones were placed 50 feet apart in a hallway adjacent to the HF clinic. Following a two-minute rest period, baseline vital signs (heart rate, blood pressure, respiratory rate and pulse oximetry) were taken in the right arm immediately before the test. Patients were then instructed to walk at their own pace for a total of 6 minutes and were alerted at the 3-minute mark. At the 6-minute mark, vital signs were again taken and the supervising nurse measured the distance walked and recorded the data in both meters and feet. Health status was assessed with the Kansas City Cardiomyopathy Questionnaire (KCCQ), a 23-item tool that quantifies several health status domains that include physical limitations, symptoms (frequency, severity, and recent change over time), self-efficacy, social function, and quality of life.[25] The KCCQ has been established as a valid, reliable, and responsive health status measure for HF.[26] Each scale is transformed to a score of 0 to 100, with higher scores reflecting better overall functioning, fewer symptoms or better quality of life. The KCCQ is also summarized into a single overall summary score ranging from 0–100 that reflects overall health status. It has been established that a 5-point change in the KCCQ overall summary score represents an important difference as it is related to clinical outcomes.[27]
Statistical Analysis
Values are expressed as mean ± standard deviation (SD) for continuous and N (%) for categorical variables. Normality for continuous variables was assessed with normal distribution quantile graphs; non-normal variables were expressed as median and interquartile range (IQR). Descriptive analyses were performed for individual behavior as well as simultaneous adherence to all recommendations. Clinical event rates are expressed as annualized rates (total events divided by total time at risk in years) and resource utilization rates are expressed as events per 100 patient-years (total events divided by total time at risk in years multiplied by 100) to standardize for at-risk time. To identify predictors of good adherence at baseline, we first examined the association of patient characteristics with adherence in univariate logistic regression models. We then entered all univariate predictors with a P value of <0.1 in multivariate models and used backwards elimination to identify independent predictors of good adherence. The association of baseline adherence with clinical events (death, heart transplantation, or left ventricular assist device placement) was examined with Cox proportional hazards models. The proportionality of hazards was examined using the Schoenfeld residuals. The association of adherence with healthcare resource utilization count data (all-cause and HF-related admissions, emergency department visits, and days in the hospital) was examined in Poisson models with time since enrollment as the exposure variable. KCCQ scores and six-minute walk distance was compared between adherence-based groups with the nonparametric Mann-Whitney rank-sum test. A two-sided p <0.05 was considered to be statistically significant. All analyses were performed using STATA version 11.2 (StataCorp, College Station, TX).
RESULTS
Study Participants
The baseline patient characteristics are presented in Table 1. Mean age of patients was 57±12 years (range 25–87 years); 35.1% female, and 46.4% black. The majority of patients had HF with reduced ejection fraction.
Table 1.
Baseline Patient Characteristics (N=308)
| Characteristic | Value |
|---|---|
|
Demographics
| |
| Age, years | 57±12 |
| Female, N (%) | 108 (35.1) |
| Black, N (%) | 143 (46.4) |
| Insurance, N (%) | 283 (91.9) |
| Live alone, N (%) | 62 (20.1) |
| Married, N (%) | 172 (55.8) |
| Number school years | 14±3 |
| Active smoking, N (%) | 41 (13.3) |
|
| |
|
Heart Failure Characteristics
| |
| Ischemic etiology, n (%) | 124 (40.3) |
| Left ventricular ejection fraction, % | 30.1±15.2 |
| Ejection fraction <40%, n (%) | 217 (70.4) |
|
| |
|
Comorbid Conditions
| |
| Atrial fibrillation, n (%) | 45 (14.6) |
| Cancer, n (%) | 46 (14.9) |
| Chronic kidney disease, n (%) | 93 (30.2) |
| Coronary artery bypass surgery, n (%) | 54 (17.5) |
| Diabetes, n (%) | 103 (33.4) |
| Depression, n (%)* | 80 (26.0) |
| Dyslipidemia, n (%) | 159 (51.6) |
| Hypertension, n (%) | 207 (67.2) |
| Peripheral arterial disease, n (%) | 7 (2.3) |
| Sleep apnea, n (%) | 67 (21.8) |
|
| |
|
Physical exam
| |
| Weight, kg | 92.3±24.2 |
| Waist, cm | 102±16 |
| Body mass index, kg/m2 | 31.0±7.3 |
| Systolic blood pressure, mmHg | 113±20 |
| Heart rate, beats/min | 72±11 |
|
| |
|
Laboratory Tests
| |
| Serum sodium, mEq/L | 138±3 |
| Blood urea nitrogen, mg/dL† | 18 (13 to 26) |
| Creatinine, mg/dL† | 1.2 (1.0 to 1.5) |
| Hemoglobin, gm/dL | 13.1±1.8 |
| B-type natriuretic peptide | 208 (69, 658)† |
|
| |
|
Therapy
| |
| Overall | |
| ACE inhibitor or angiotensin receptor blocker, N (%) | 242 (78.6) |
| Beta-blocker, N (%) | 288 (93.5) |
| Diuretics, N (%) | 259 (84.1) |
| Aldosterone Antagonists, N (%) | 134 (43.5) |
| Hydralazine &/or Isosorbide Dinitrate, N (%) | 78 (25.3) |
| Defibrillator and/or biventricular pacemaker, N (%) | 186 (60.4) |
| Patients with Ejection Fraction <40% | |
| ACE inhibitor or angiotensin receptor blocker, N (%) | 178 (82.0) |
| Beta-blocker, N (%) | 208 (95.8) |
| Diuretics, N (%) | 186 (85.7) |
| Aldosterone Antagonists, N (%) | 101 (46.5) |
| Hydralazine &/or Isosorbide Dinitrate, N (%) | 60 (27.6) |
| Defibrillator and/or biventricular pacemaker, N (%) | 152 (70.0) |
Depression based on Patient Health Questionnaire-9 score ≥10
Median, 25th – 75th percentile
Adherence
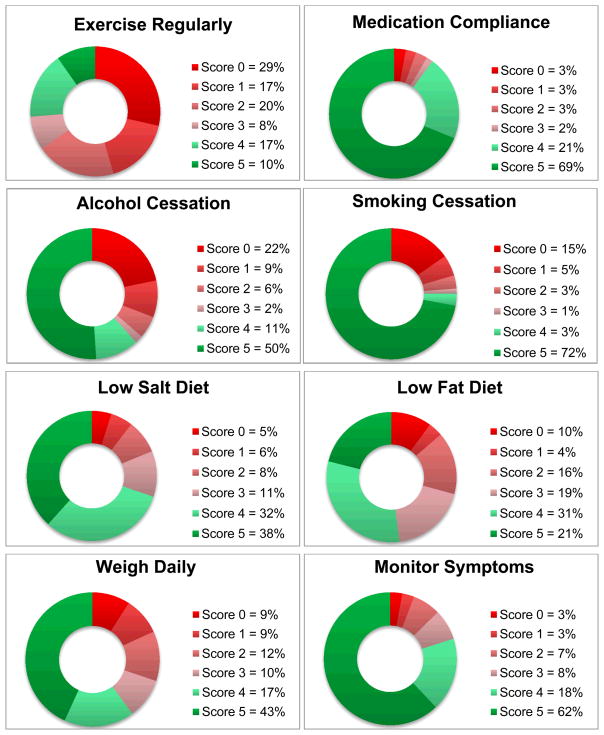
The highest adherence was reported with medications (89.9%), followed by symptom monitoring (79.9%). The lowest adherence was noted for exercise (26.3%). Good adherence was reported by 110/308 patients (35.7%). Adherence >80% to every single measure was reported by 28/308 (9.1%) of patients. Figure 3 highlights the adherence to the various self-care behaviors.
Figure 3. Adherence by Individual Self-care Recommendation.
Patient self-reported score 0 indicates “none of the time”, 1 “a little of the time”, 2 “some of the time”, 3 “a good bit of the time”, 4 “most of the time”, and 5 represents “all of the time.”
Predictors of Adherence
Among baseline patient characteristics, good adherence was positively associated with age, education, dyslipidemia, and history of coronary artery bypass surgery in univariate analysis (Table 2); depression and black race were negatively associated with adherence in univariate analysis. Marital status and insurance status were not associated with adherence. There were no sex (34.7% females vs. 34.0% males; p=0.91) related differences. In multivariate analysis, using backwards elimination, education (odds ratio [OR] 1.17 per school year, 95% confidence interval [CI]: 1.07–1.28; p=0.001) and age (OR: 1.05 per year; 95% CI: 1.02–1.07; p<0.001) were related to good adherence.
Table 2.
Association of Baseline Characteristics with Good Adherence* (N=308)
| Patient Characteristics | OR (95% CI) | χ2 | P |
|---|---|---|---|
| Univariate | |||
| Age, per year | 1.04 (1.02, 1.07) | 18.06 | <0.001 |
| Education level, per school year | 1.18 (1.08, 1.28) | 15.02 | <0.001 |
| Dyslipidemia | 2.10 (1.30, 3.41) | 9.36 | 0.003 |
| Black race (vs. white race) | 0.52 (0.32, 0.85) | 7.11 | 0.008 |
| Coronary artery bypass grafting | 2.07 (1.14, 3.76) | 5.72 | 0.016 |
| Depression | 0.51 (0.29, 0.90) | 5.66 | 0.021 |
| Cancer history | 2.00 (1.06, 3.77) | 4.57 | 0.032 |
| Multivariate | |||
| Age, per year | 1.05 (1.02, 1.07) | 15.56 | <0.001 |
| Education level, per school year | 1.17 (1.07, 1.28) | 11.29 | 0.001 |
Defined as ≥32/40 points (≥80%) in the Medical Outcomes Study Specific Adherence Scale
Outcomes
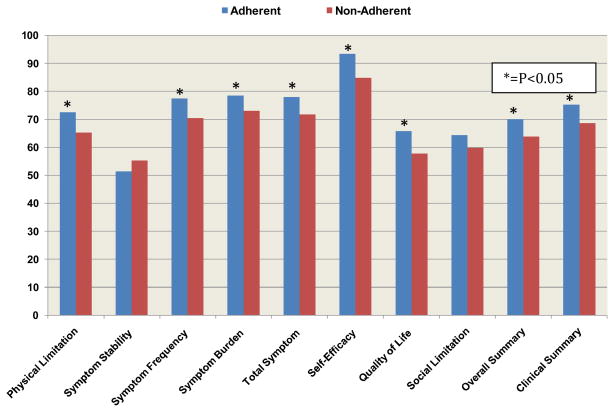
During a mean follow-up of 2.0±1.0 years (total: 627 patient-years), 33 patients died, 5 underwent transplantation, and 2 received ventricular assist devices, for a total clinical event rate of 13.0% and annualized event rate of 6.4%. Clinical event rate was not associated with good adherence (17/110 [15.5%] vs. 23/198 [11.6%]; hazard ratio 1.36, 95% CI: 0.72–2.53; p=0.34). Good adherence was associated with reduced all-cause hospitalizations, HF hospitalizations and number of days hospitalized as well as lower all-cause and HF-specific emergency department visits (Table 3). For the 270 patients who performed the 6-minute walk test, the mean distance was 354±106 meters; there was no significant difference between patients with good vs. less optimal adherence (358±107 meters vs. 351±106 meters; p=0.52). Patients with good adherence had higher KCCQ overall (70.1±24.6 vs. 63.8±22.8; p=0.011) and clinical summary (75.3±22.8 vs. 68.6±21.6; p=0.003) scores. In addition, multiple KCCQ domains including, physical limitation, symptom frequency, symptom burden, total symptom, self-efficacy, and quality of life scores were significantly better among patients with good adherence (Figure 4).
Table 3.
Outcomes According to Adherence Status (N=308)
| Outcome | Good Adherence (N=110) | Not Good Adherence (N=198) | Incidence Rate Ratio (95% Confidence Interval) | P |
|---|---|---|---|---|
| Resource utilization (per 100 person years) | ||||
| All cause hospitalizations | 87.8 | 107.6 | 0.82 (0.69, 0.97) | 0.018 |
| Heart failure hospitalizations | 29.6 | 43.8 | 0.68 (0.51, 0.90) | 0.007 |
| All-cause emergency department visits | 41.4 | 66.9 | 0.62 (0.49, 0.78) | <0.001 |
| Heart failure-related emergency department visits | 8.2 | 17.2 | 0.48 (0.28, 0.80) | 0.005 |
| Total all-cause hospitalized days | 422 | 465 | 0.91 (0.84, 0.98) | 0.015 |
| Total heart failure related hospitalized days | 228 | 282 | 0.81 (0.73, 0.90) | <0.001 |
| Health Status - Kansas City Cardiomyopathy Questionnaire Scores | ||||
| Overall Summary Score | 70.1±24.6 | 63.8±22.8 | 0.011 | |
| Clinical Summary Score | 75.3±22.8 | 68.6±21.6 | 0.003 | |
Figure 4. Health Status and Self Care.
Comparison of Kansas City Cardiomyopathy Questionnaire scores between adherent and non-adherent patients; * P<0.05 using the Mann-Whitney statistic
DISCUSSION
Less than desirable adherence to individual HF self-care recommendation has been described previously.[28] This study extends the research related to HF self-care by assessing patient-reported simultaneous adherence to eight self-care behaviors, as opposed to assessing individual behaviors, by defining good adherence as overall ≥80% cumulative score. Adherence to individual behaviors ranged from 26.3% to 89.9%; however, cumulative good adherence was alarmingly low at 35.7%, and only 9.1% of patients reported good adherence with all eight self-care recommendations, indicating high rates of selective adherence. Older and more educated patients were more likely to be adherent, whereas active smokers were less likely. Good adherence was strongly associated with resource utilization and health status. Considering the high rate and cost of HF hospitalizations, these results are important. By studying adherence in a comprehensive fashion, we highlight the issue of selective adherence. Moreover, unlike previous literature that tended to focus on particular outcomes, we assessed a full spectrum of HF outcomes, including clinical events, resource utilization, health status, and functional capacity; thereby allowing us to globally assess the importance of self-care adherence in HF.
There is considerable variation among methods used to measure adherence to self-care. Medication adherence has been studied extensively with direct measurements, e.g. observing intake or measuring drug levels.[29] These methods are costly and impractical for routine practice, and importantly, cannot be used for all self-care activities. Other methods include questionnaires and self-report, electronic medication monitors, and pharmacy refill data. Of these, self-report is the most widely used method, is specific, easily employed and is associated with outcomes.[16, 30, 31] We therefore assessed patient-reported adherence, realizing that the actual adherence may be less than self-report.
Characterization of ‘adherence’ has been largely arbitrary with most studies using an 80% threshold to define medication adherence, as this threshold has been shown to have acceptable sensitivity and specificity.[22] By approaching adherence in a comprehensive fashion by defining good adherence as overall ≥80% adherence, we also accounted for selective adherence, which is common in HF patients. In our study, only one third of patients reported good adherence with HF self-care. Additionally, when assessed in a more rigorous fashion requiring ≥80% adherence to each individual recommendation, less than one in ten patients were adherent, indicating that over 90% of patients demonstrate selective adherence. These data suggest alarmingly low adherence with self-care and a high rate of selective adherence. These results may explain why HF self-management trials have largely failed to demonstrate significant impact on outcomes.[32]
Previous studies have suggested that while most HF patients have less difficulty adhering to medications, majority have difficulty adhering to exercise.[33] Likewise, in our study the highest adherence was reported with medications followed by symptom monitoring, and the lowest with exercise. Although not possible to ascertain if this is related to debilitating symptoms or lack of effort, these results are nevertheless concerning since exercise training is shown to be safe and associated with improved outcomes.[34, 35] These results provide insight into the complex and personal nature of selective adherence and suggest the need for a deeper understanding of individuals’ motivations and adherence behavior in order to inform the appropriate individualized intervention to improve outcomes.[36–38] It is unlikely that healthcare quality improvement efforts will realize their full potential without complete engagement by patients.
We found several associations between patient characteristics and self-care adherence, however only age and education level were independently associated with good adherence. Conflicting data exists between age and adherence.[39, 40] As the prevalence of HF increases with aging population,[1] the issue of HF self-care adherence among the elderly will become even more important. Other studies have correlated higher education levels with improved adherence[39, 41] as well as fewer emergency department visits,[15] and low health literacy has been associated with poor self-care and outcomes.[42] Other previous studies evaluating characteristics of the adherent vs. non-adherent patient have reported mixed results.[43–45] While it is possible that adherence may vary by gender, race, and co-morbidity burden, in our study, other demographic and social characteristics were not associated with good self-care. We however do highlight that younger and less educated patients represent a particularly vulnerable population that may benefit from targeted interventions. Because adherence was related to hospitalizations, these associations are especially important as HF hospitalizations have reached an all-time high[46] and account for over half of the $39 billion annual cost of HF care.[47]
Although, in our study, depression was not associated with adherence in multivariate analysis, depression is certainly a barrier to engaging in HF self-care behaviors and is a topic deserving of special emphasis. Although few studies have evaluated the role of depression in HF self-care, some have found greater depression in patients with poor HF self-care.[48, 49] As depression is the most common mood disturbance in persons with HF, ranging in prevalence from 13% to 77%, the association between depression and adherence to self-care behaviors is an important area of investigation that deserves further study.[50, 51]
Previous studies evaluating associations between self-care and HF outcomes have shown varied results.[13, 52] Over half of all HF hospitalizations have been linked to some form of non-adherence with self-care.[4, 53] Some trials have demonstrated improved self-care through interventions,[54] with promising associations with outcomes.[55] However, most studies have concentrated on only specific aspects of self-care, typically medication or dietary.[5, 15] Our results highlight the importance of addressing adherence comprehensively in order to discourage selective focus on particular self-care measure at the expense of others.
In our study, self-care adherence was not associated with the clinical event rate. We caution the interpretation of these results due to the small number of clinical events in the limited study follow-up. Other studies have demonstrated that adherence to prescribed therapy was associated with mortality reduction.[56] Interestingly, mortality reductions with placebo in trials likely reflect upon overall pattern of self-care. We did find an association between self-care and resource utilization including, emergency department visits, all-cause and HF-specific hospitalization rates and overall number of days spent in the hospital. Also, adherent patients had improved overall health status. These results in conjunction with previous studies suggest that better self-care is likely to improve both resource and patient-centered outcomes.
Although the problems related to sub-optimal self-care are evident, how to improve upon them is difficult. Despite consistent education regarding proper self-care by trained HF nurses and standardized self-care instructional videos, adherence with HF self-care was low. There are no easy answers on how to change patient behavior, though there are data supporting improvement in select behaviors by certain intervention e.g. reminder systems. Future research in improving HF self-care adherence should focus on skill development, family involvement and behavior change as well as systems of care changes. Our data regarding the poor rates of adherence with self-care highlights one of the foremost obstacles in caring for HF patients and also underscores a major hindrance in improving outcomes. Further clinical and research effort is needed to understand reasons underlining selective adherence in order to improve self-care and associated outcomes among HF patients.
Our study has several limitations. Our results represent data from tertiary care specialty clinics with rigorous self-care education provided by HF nurses. It is possible that adherence is different in the community setting or that independently verified adherence is even worse than patient-reported adherence. Because eligibility for the study was contingent upon the ability to comprehend and sign the written informed consent, this study does not adequately represent adherence rates among individuals who did not meet this criterion, leaving open the possibility that the adherence rates are even lower among such individuals. Also, the 80% threshold to define good adherence is arbitrary. Whether to define a different threshold, use varying threshold for various populations, or devise a modified questionnaire to quantify adherence, needs further study. Finally, all methods of assessing adherence have limitations and must be considered when interpreting these results. This is especially true for self-report since it is particularly vulnerable to recall and selection biases.[29] It is however likely that the actual adherence to self-care is even lower and not much higher than self-report.
In conclusion, adherence with self-care is alarmingly low among HF patients, and selective adherence to various recommendations is common. Better adherence is associated with improved health status and reduced resource utilization. These results highlight a major opportunity for further clinical and research effort in understanding and improving self-care adherence to optimize HF outcomes.
Acknowledgments
This project was funded by an Emory University Heart and Vascular Board, and supported in part by PHS grant (UL1 RR025008, KL2 RR025009 or TL1 RR025010) from the Clinical and Translational Science Award program, National Institutes of Health, National Center for Research Resources. The funding sources had no role in the design of the study or the analysis and interpretation of the data.
ABBREVIATIONS
- HF
Heart failure
- KCCQ
Kansas City Cardiomyopathy Questionnaire
- MOS-SAS
Medical Outcomes Study Specific Adherence Scale
- CABG
Coronary artery bypass surgery
Footnotes
Conflict of Interest: None
Disclosures
None
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 3.Masoudi FA, Havranek EP, Krumholz HM. The burden of chronic congestive heart failure in older persons: magnitude and implications for policy and research. Heart Fail Rev. 2002;7:9–16. doi: 10.1023/a:1013793621248. [DOI] [PubMed] [Google Scholar]
- 4.Michalsen A, Konig G, Thimme W. Preventable causative factors leading to hospital admission with decompensated heart failure. Heart. 1998;80:437–41. doi: 10.1136/hrt.80.5.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsuyuki RT, McKelvie RS, Arnold JM, Avezum A, Jr, Barretto AC, Carvalho AC, et al. Acute precipitants of congestive heart failure exacerbations. Arch Intern Med. 2001;161:2337–42. doi: 10.1001/archinte.161.19.2337. [DOI] [PubMed] [Google Scholar]
- 6.Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, et al. HFSA 2010 Comprehensive Heart Failure Practice Guideline. Journal of Cardiac Failure. 2010;16:e1–194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Masoudi FA, Baillie CA, Wang Y, Bradford WD, Steiner JF, Havranek EP, et al. The complexity and cost of drug regimens of older patients hospitalized with heart failure in the United States, 1998–2001. Arch Intern Med. 2005;165:2069–76. doi: 10.1001/archinte.165.18.2069. [DOI] [PubMed] [Google Scholar]
- 8.Hunt SA. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2005;46:e1–82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 9.Riegel B, Moser DK, Anker SD, Appel LJ, Dunbar SB, Grady KL, et al. State of the science: promoting self-care in persons with heart failure: a scientific statement from the American Heart Association. Circulation. 2009;120:1141–63. doi: 10.1161/CIRCULATIONAHA.109.192628. [DOI] [PubMed] [Google Scholar]
- 10.McAlister FA, Stewart S, Ferrua S, McMurray JJ. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized trials. Journal of the American College of Cardiology. 2004;44:810–9. doi: 10.1016/j.jacc.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 11.Jovicic A, Holroyd-Leduc JM, Straus SE. Effects of self-management intervention on health outcomes of patients with heart failure: a systematic review of randomized controlled trials. BMC cardiovascular disorders. 2006;6:43. doi: 10.1186/1471-2261-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee CS, Moser DK, Lennie TA, Riegel B. Event-free survival in adults with heart failure who engage in self-care management. Heart & lung: the journal of critical care. 2011;40:12–20. doi: 10.1016/j.hrtlng.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leventhal MJ, Riegel B, Carlson B, De Geest S. Negotiating compliance in heart failure: remaining issues and questions. Eur J Cardiovasc Nurs. 2005;4:298–307. doi: 10.1016/j.ejcnurse.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Chung ML, Lennie TA, de Jong M, Wu JR, Riegel B, Moser DK. Patients differ in their ability to self-monitor adherence to a low-sodium diet versus medication. J Card Fail. 2008;14:114–20. doi: 10.1016/j.cardfail.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Hope CJ, Wu J, Tu W, Young J, Murray MD. Association of medication adherence, knowledge, and skills with emergency department visits by adults 50 years or older with congestive heart failure. Am J Health Syst Pharm. 2004;61:2043–9. doi: 10.1093/ajhp/61.19.2043. [DOI] [PubMed] [Google Scholar]
- 16.Ni H, Nauman D, Burgess D, Wise K, Crispell K, Hershberger RE. Factors influencing knowledge of and adherence to self-care among patients with heart failure. Arch Intern Med. 1999;159:1613–9. doi: 10.1001/archinte.159.14.1613. [DOI] [PubMed] [Google Scholar]
- 17.Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. European Heart Journal. 2007;28:2539–50. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 18.DiMatteo MR, Sherbourne CD, Hays RD, Ordway L, Kravitz RL, McGlynn EA, et al. Physicians’ characteristics influence patients’ adherence to medical treatment: results from the Medical Outcomes Study. Health Psychol. 1993;12:93–102. doi: 10.1037/0278-6133.12.2.93. [DOI] [PubMed] [Google Scholar]
- 19.DiMatteo MR, Hays RD, Sherbourne CD. Adherence to cancer regimens: implications for treating the older patient. Oncology. 1992;6:50–7. [PubMed] [Google Scholar]
- 20.Hays RD, Kravitz RL, Mazel RM, Sherbourne CD, DiMatteo MR, Rogers WH, et al. The impact of patient adherence on health outcomes for patients with chronic disease in the Medical Outcomes Study. J Behav Med. 1994;17:347–60. doi: 10.1007/BF01858007. [DOI] [PubMed] [Google Scholar]
- 21.Kravitz RL, Hays RD, Sherbourne CD, DiMatteo MR, Rogers WH, Ordway L, et al. Recall of recommendations and adherence to advice among patients with chronic medical conditions. Archives of Internal Medicine. 1993;153:1869–78. [PubMed] [Google Scholar]
- 22.Hansen RA, Kim MM, Song L, Tu W, Wu J, Murray MD. Comparison of methods to assess medication adherence and classify nonadherence. Ann Pharmacother. 2009;43:413–22. doi: 10.1345/aph.1L496. [DOI] [PubMed] [Google Scholar]
- 23.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. Journal of general internal medicine. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma R, Anker SD. The 6-minute walk test and prognosis in chronic heart failure--the available evidence. Eur Heart J. 2001;22:445–8. doi: 10.1053/euhj.2000.2451. [DOI] [PubMed] [Google Scholar]
- 25.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–55. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 26.Ortega T, Diaz-Molina B, Montoliu MA, Ortega F, Valdes C, Rebollo P, et al. The utility of a specific measure for heart transplant patients: reliability and validity of the Kansas City Cardiomyopathy Questionnaire. Transplantation. 2008;86:804–10. doi: 10.1097/TP.0b013e318183eda4. [DOI] [PubMed] [Google Scholar]
- 27.Kosiborod M, Soto GE, Jones PG, Krumholz HM, Weintraub WS, Deedwania P, et al. Identifying heart failure patients at high risk for near-term cardiovascular events with serial health status assessments. Circulation. 2007;115:1975–81. doi: 10.1161/CIRCULATIONAHA.106.670901. [DOI] [PubMed] [Google Scholar]
- 28.van der Wal MH, Jaarsma T. Adherence in heart failure in the elderly: problem and possible solutions. Int J Cardiol. 2008;125:203–8. doi: 10.1016/j.ijcard.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 29.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–97. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 30.Walsh JC, Mandalia S, Gazzard BG. Responses to a 1 month self-report on adherence to antiretroviral therapy are consistent with electronic data and virological treatment outcome. AIDS. 2002;16:269–77. doi: 10.1097/00002030-200201250-00017. [DOI] [PubMed] [Google Scholar]
- 31.Gehi AK, Ali S, Na B, Whooley MA. Self-reported medication adherence and cardiovascular events in patients with stable coronary heart disease: the heart and soul study. Arch Intern Med. 2007;167:1798–803. doi: 10.1001/archinte.167.16.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Powell LH, Calvin JE, Jr, Richardson D, Janssen I, Mendes de Leon CF, Flynn KJ, et al. Self-management counseling in patients with heart failure: the heart failure adherence and retention randomized behavioral trial. JAMA. 2010;304:1331–8. doi: 10.1001/jama.2010.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Wal MH, Jaarsma T, Moser DK, Veeger NJ, van Gilst WH, van Veldhuisen DJ. Compliance in heart failure patients: the importance of knowledge and beliefs. Eur Heart J. 2006;27:434–40. doi: 10.1093/eurheartj/ehi603. [DOI] [PubMed] [Google Scholar]
- 34.Flynn KE, Pina IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, et al. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1451–9. doi: 10.1001/jama.2009.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reed SD, Whellan DJ, Li Y, Friedman JY, Ellis SJ, Pina IL, et al. Economic evaluation of the HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) randomized controlled trial: an exercise training study of patients with chronic heart failure. Circ Cardiovasc Qual Outcomes. 2010;3:374–81. doi: 10.1161/CIRCOUTCOMES.109.907287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dickson VV, Buck H, Riegel B. A qualitative meta-analysis of heart failure self-care practices among individuals with multiple comorbid conditions. Journal of Cardiac Failure. 2011;17:413–9. doi: 10.1016/j.cardfail.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 37.Dickson VV, McCarthy MM, Howe A, Schipper J, Katz SM. Sociocultural Influences on Heart Failure Self-care Among an Ethnic Minority Black Population. J Cardiovasc Nurs. 2012 doi: 10.1097/JCN.0b013e31823db328. [DOI] [PubMed] [Google Scholar]
- 38.Riegel B, Dickson VV, Kuhn L, Page K, Worrall-Carter L. Gender-specific barriers and facilitators to heart failure self-care: a mixed methods study. Int J Nurs Stud. 2010;47:888–95. doi: 10.1016/j.ijnurstu.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 39.Rockwell JM, Riegel B. Predictors of self-care in persons with heart failure. Heart Lung. 2001;30:18–25. doi: 10.1067/mhl.2001.112503. [DOI] [PubMed] [Google Scholar]
- 40.Riegel B, Driscoll A, Suwanno J, Moser DK, Lennie TA, Chung ML, et al. Heart failure self-care in developed and developing countries. J Card Fail. 2009;15:508–16. doi: 10.1016/j.cardfail.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burke LE, Dunbar-Jacob JM, Hill MN. Compliance with cardiovascular disease prevention strategies: a review of the research. Ann Behav Med. 1997;19:239–63. doi: 10.1007/BF02892289. [DOI] [PubMed] [Google Scholar]
- 42.Baker DW, Asch SM, Keesey JW, Brown JA, Chan KS, Joyce G, et al. Differences in education, knowledge, self-management activities, and health outcomes for patients with heart failure cared for under the chronic disease model: the improving chronic illness care evaluation. J Card Fail. 2005;11:405–13. doi: 10.1016/j.cardfail.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 43.Bagchi AD, Esposito D, Kim M, Verdier J, Bencio D. Utilization of, and adherence to, drug therapy among medicaid beneficiaries with congestive heart failure. Clin Ther. 2007;29:1771–83. doi: 10.1016/j.clinthera.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 44.George J, Shalansky SJ. Predictors of refill non-adherence in patients with heart failure. Br J Clin Pharmacol. 2007;63:488–93. doi: 10.1111/j.1365-2125.2006.02800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee CS, Riegel B, Driscoll A, Suwanno J, Moser DK, Lennie TA, et al. Gender differences in heart failure self-care: a multinational cross-sectional study. Int J Nurs Stud. 2009;46:1485–95. doi: 10.1016/j.ijnurstu.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DeFrances CJ, Lucas CA, Buie VC, Golosinskiy A. 2006 National Hospital Discharge Survey. Natl Health Stat Report. 2008:1–20. [PubMed] [Google Scholar]
- 47.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 48.Morgan AL, Masoudi FA, Havranek EP, Jones PG, Peterson PN, Krumholz HM, et al. Difficulty taking medications, depression, and health status in heart failure patients. J Card Fail. 2006;12:54–60. doi: 10.1016/j.cardfail.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 49.Riegel B, Vaughan Dickson V, Goldberg LR, Deatrick JA. Factors associated with the development of expertise in heart failure self-care. Nurs Res. 2007;56:235–43. doi: 10.1097/01.NNR.0000280615.75447.f7. [DOI] [PubMed] [Google Scholar]
- 50.Thomas SA, Friedmann E, Khatta M, Cook LK, Lann AL. Depression in patients with heart failure: physiologic effects, incidence, and relation to mortality. AACN clinical issues. 2003;14:3–12. doi: 10.1097/00044067-200302000-00002. [DOI] [PubMed] [Google Scholar]
- 51.Vaccarino V, Kasl SV, Abramson J, Krumholz HM. Depressive symptoms and risk of functional decline and death in patients with heart failure. J Am Coll Cardiol. 2001;38:199–205. doi: 10.1016/s0735-1097(01)01334-1. [DOI] [PubMed] [Google Scholar]
- 52.Hauptman PJ. Medication Adherence in Heart Failure. Heart Failure Review. 2008;13:99–106. doi: 10.1007/s10741-007-9020-7. [DOI] [PubMed] [Google Scholar]
- 53.Ghali JK, Kadakia S, Cooper R, Ferlinz J. Precipitating factors leading to decompensation of heart failure. Traits among urban blacks. Arch Intern Med. 1988;148:2013–6. [PubMed] [Google Scholar]
- 54.McAlister FA, Lawson FM, Teo KK, Armstrong PW. A systematic review of randomized trials of disease management programs in heart failure. Am J Med. 2001;110:378–84. doi: 10.1016/s0002-9343(00)00743-9. [DOI] [PubMed] [Google Scholar]
- 55.Ferrante D, Varini S, Macchia A, Soifer S, Badra R, Nul D, et al. Long-term results after a telephone intervention in chronic heart failure: DIAL (Randomized Trial of Phone Intervention in Chronic Heart Failure) follow-up. J Am Coll Cardiol. 2010;56:372–8. doi: 10.1016/j.jacc.2010.03.049. [DOI] [PubMed] [Google Scholar]
- 56.Granger BB, Swedberg K, Ekman I, Granger CB, Olofsson B, McMurray JJ, et al. Adherence to candesartan and placebo and outcomes in chronic heart failure in the CHARM programme: double-blind, randomised, controlled clinical trial. Lancet. 2005;366:2005–11. doi: 10.1016/S0140-6736(05)67760-4. [DOI] [PubMed] [Google Scholar]